Abstract
Although the synergy between erythropoietin and thrombopoietin was previously pointed out, the clonal demonstration of a human bipotent Erythroid/Megakaryocytic progenitor (MEP) was first published in ExpHem (April 1996) and later in the same year by Debili et al in Blood. This demonstration, and the fact that both bipotent and monopotent erythroid or megakaryocytic progenitors co-express markers of both lineages and respond to both lineage specific transcription factors, has provided a background for the extensive use of MEP assessment by FACS in many subsequent studies. Beyond this, the demonstration of shared regulatory elements and the presence of single mutations affecting both lineages have inspired further studies to decipher how the shift in transcription factor networks occurs from one lineage to the other. Furthermore, in addition to shared effects, erythropoietin and thrombopoietin have additional independent effects. Most notable for thrombopoietin is its effect on hematopoietic stem cells demonstrated by in vitro and in vivo approaches.
Functional evaluation of the newly cloned ligand for the proto-oncogene cytokine receptor c-Mpl in 1994 established the existence of the long-sought hematopoietic growth factor thrombopoietin (TPO) [1], the purported primary regulator of platelet production. This event was met with much fanfare [2]. From the initial few studies it was clear that thrombopoietin was a powerful stimulus of megakaryocytopoiesis, especially in the additional presence of interleukin-3 or stem cell factor [3], and that in vivo it was by far and away the most powerful stimulus of thrombopoiesis [1]. However, subsequent attention was turned to its potential for a more expanded biological role, either developmentally early or late in the hematopoietic hierarchy. Although prior to its cloning, TPO, like erythropoietin (EPO), was regarded as a “late-acting cytokine”, there was ample precedence derived from the functional characterization of other late-acting cytokines that exert an influence on additional cell types to rationalize this effort. Therefore, experiments in our University of Washington laboratories were initiated to assess the effects of TPO either on more primitive hematopoietic cells [4, 5], or on later lineages i.e., erythroid cells, and the functional relationship between erythropoiesis and megakaryopoiesis [6], the focus of this brief commentary.
There were tantalizing prior data from the 1980’s suggesting a close relationship between erythropoiesis and megakaryopoiesis. For example, all erythroleukemia cell lines described thus far displayed in addition to erythroid characteristics, megakaryocytic features [7, 8]; EPO enhances CFU-Mk colony growth in vitro (3); effects on both lineages were noted following in vivo EPO treatment [9]; GPIIa−/− mice displayed effects on both erythroid and megakaryocytic lineages [10]; and a number of transcription factors and surface antigens also seem to be shared by both Ery or Meg Progenitor cells [11–13]. Nevertheless it was unclear whether this relationship relied on influencing an early common progenitor cell capable of generating both lineages, or the effect was exerted on cells later in their differentiation pathway, or due to actions on both developmentally early and later erythroid cells.
Compared to when many of these initial studies were conducted, cell culture conditions supporting the growth of both megakaryocytic and erythroid progenitors had greatly improved, allowing us to design experiments to test whether in the presence of EPO or TPO, or both, colony growth of the “corresponding” progenitors, or both progenitor types were affected; whether this effect relied on the presence of specific surface antigens, and whether the effects were preferentially addressed to more primitive or late progenitors, peaking at different points in culture.
Experiments described in Experimental Hematology [6] using EPO or TPO or both to stimulate CD34+ cells re-emphasized the concept of functional synergy exercised by the two cytokines on erythropoiesis and megakaryopoiesis. This cytokine combination increased the generation of both CD41+ cells and of globin+ cells in vitro in suspension cultures, and this increase was preceded by an increase in both erythroid (BFU-E) and megakaryocytic (CFU-Mk) progenitors. These data suggested that the proliferative effects of the combination of the two cytokines were exerted at the progenitor cell level. Beyond that, our data revealed that both erythroid and megakaryocytic progenitors were enriched at the subset of CD34+ cells that were negative for CD45RA (CD34+/CD45RA−), quite distinct from CFU-GM type progenitors which were enriched in the CD34+/CD45RA+ subset [6]. Furthermore, within the CD34+/Cd45RA− subtype, CFU-Mk progenitors were highly enriched on the subset that was positive for CD41 (34+/45RA−/41+), whereas erythroid progenitors were present in significant numbers in both CD41+ and CD41− subsets.
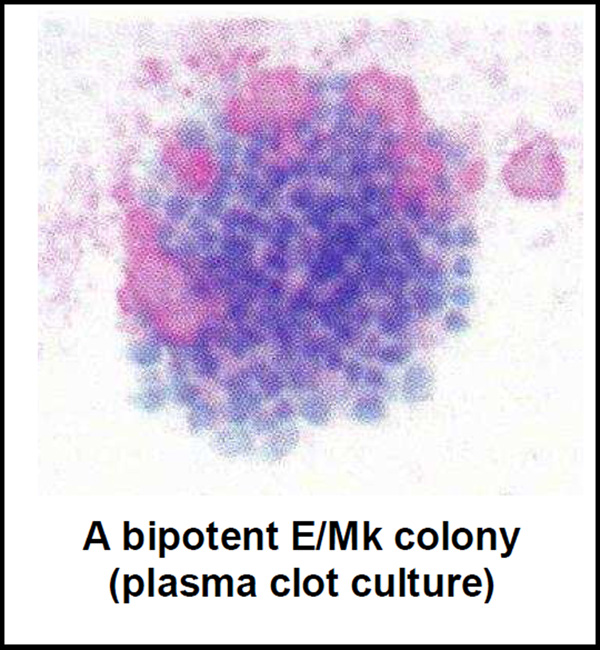
What was unclear from these data was whether the erythroid and megakaryocytic progenitors we were assessing were all derived from a common, more primitive bipotent progenitor, and whether such a progenitor was present under our culture conditions. We reasoned that if this were the case, this type of progenitor should be sought among the CD34+/CD45RA−/Cd41+ compared to CD41− subset. To test this hypothesis we initiated single cell clonal cultures from these two subsets and evaluated the presence of erythroid and megakaryocytic cells in each colony growth. It is important to emphasize that in our plasma clot system, in contrast to the more commonly used methyl cellulose culture system, we could efficiently identify both erythroid and megakaryocytic clonal growth (also myeloid ones). In these cultures in addition to pure erythroid colonies represented in greater numbers, we also identified pure megakaryocytic colonies and in much smaller numbers, mixed erythroid/megakaryocytic colonies. In every case the bipotent progenitor gave rise to smaller colonies when compared to the large pure erythroid colonies. In view of these data, although for the first time we documented the presence, at the clonal level, of a human bipotent erythroid/megakaryocytic progenitor giving rise to both cell types. Although previous experiments have documented the clonal presence of bipotent progenitors in mice [14, 15], using human cells, only an earlier hint was available [16]. Nevertheless, because of the absence of early-acting cytokines in these cultures, we could not robustly determine whether the unipotent colonies (i.e., the larger BFU-E-derived colonies or the pure CFU-Mk-derived colonies) were generated from an earlier more primitive bipotent progenitor, an inquiry left to be pursued in subsequent experiments.
As it usually occurs in science, a few months later investigators in France reported [17] the presence of bipotent erythroid/megakaryocytic progenitors in cultures of CD34+ cells stimulated with a cocktail of cytokines (IL-3, SCF, IL-6, EPO, G-CSF, GM-CSF and TPO). These investigators also confirmed the EPO/TPO synergy that we had shown on more mature progenitor cells, and using single cell clonal cultures they identified bipotent E/Mk colonies at a somewhat higher frequency than we reported. Nevertheless it was of interest that the bipotent colonies described in their studies again were smaller and their temporal development peaked earlier in culture (day 12) than the large BFU-E. However, in contrast to our data, they emphasized that only the pure megakaryocytic progenitors were in the CD41+ subset, and not the bipotent progenitors, as we suggested. Subsequent publications however using FACS purification of megakaryocyte/erythroid progenitors used the CD41+/CD45RA− subset [18–21]. Of interest, all studies employing single cell cultures from purified MEP phenotypic subsets showed that the majority of clonal growth was represented by pure large erythroid or pure megakaryocytic colonies, rather than bipotent MEP [6, 17, 22].
Several subsequent studies have addressed questions pertaining to how unilineage cells, i.e., erythroid or Mk, are selected from earlier cells on the basis of phenotypic marker expression [19, 23–27]. Although thus far it has been agreed that bipotent E/Mk progenitor cells branched out separately from G/GM progenitors, a distinction marked by the appearance of high levels of erythroid/megakaryocytic cells, or high levels of PU.1 expression in G/GM cells [22], it has not been clear how pure erythroid or pure megakaryocytic progenitors emerge. Are they all derived from a developmentally early bipotent progenitor as advocated, or are both pure and bipotent cells derived from a common progenitor which displays some heterogeneity in the levels of lineage-specific transcription factor expression? It has been shown that 90% or more of megakaryocytic progenitor cells express c-Mpl and 59% coexpress the EPO receptor, and only 7% express KLF-1, which is expressed by 73% of pure erythroid progenitor cells in single cell clonal cultures [22]. Thus one may surmise that cells with higher levels of erythroid factors (EPO receptor, KLF-1) give rise directly to pure erythroid colonies, whereas those with lower KLF-1, but higher c-Mpl and CD41 (and/or von Willebrand factor) give rise to pure megakaryocytic colonies, bypassing the bipotent stage, whereas bipotent colonies arise only from a subset of cells that express both erythroid and megakaryocytic specific factors in somewhat balanced levels. The recent demonstration of erythroid and megakaryocytic markers present in a subset of murine spleen resident erythroid precursor cells, not progenitor cells, has not functionally clarified the picture any further [24].
Beyond these ambiguities recent revisions of phenotypic marker expression and lineage determination in murine hematopoiesis have surfaced. The expression of CD41 or vWF, the platelet/Mk related genes used for MEP markers previously, have been found to mark HSCs with prolonged myelo-erythroid restricted reconstitution in transplantation experiments [25], or to bias HSCs for myeloid vs. lymphoid potential which increases with age [26]. Alternatively, platelet-biased (vWF+) HSCs had both short term and long term myeloid/platelet reconstitution potential, thus placing them at the apex of the hematopoietic hierarchy [27]. A subset of the vWF + HSCs with high expression of many other megakaryocytic markers in addition to vWF was later described [28]. This subset, although not contributing to steady state hematopoiesis, can be triggered by acute inflammation to give rise to mature platelets with accelerated kinetics. The above observations taken together would suggest that HSC subsets exist with expression of either a narrow or more broad expression of megakaryocytic/erythroid transcriptional factors influencing their functional potential in vivo. Beyond the murine hematopoiesis, recent data exploring these issues have also reshaped the lineage generation from human pluripotent stem cells [29]. In the adult, in contrast to fetal hematopoiesis, the revised framework suggests a direct derivation of unipotent progenitors from multipotent stem cells. In vivo functional consequences in mice [4, 30] are congruent with high expression of c-Mpl in HSCs and in humans treatment of aplastic anemia patients with Eltrombopag, a Tpo-R agonist induced trilineage responses [31].
Additional studies have identified key regulators (transcriptional factors, long non-coding RNAs) and their downstream signaling partners with both synergistic and/or cross-antagonistic functions in vertebrate erythropoiesis and megakaryocytopoiesis [32–38]. Genetic mutations affecting some of the common regulators in humans give rise to both anemia and thrombocytopenia [39–41], or post-natal aberrations in common signal intermediates lead to development of myeloproliferative syndromes [42, 43]. Homodimerization of Mpl induced by binding of Tpo to its extracellular domain elicits a distinct signaling cascade. However, when direct homodimerization of its intracellular domain is induced by a chemical-inducer of dimerization (in the absence of Tpo) [21], an alternative signaling is induced. In the latter case, only Akt is upregulated (in contrast to Tpo ligation) inducing a uniquely erythroid biased gene expression cascade in vitro [44] and in vivo [45]. A higher expression of FKBP in erythroid cells would also enhance this effect. Recently a similar approach was used to initiate intracellular Epo-R signaling through CID administration to stimulate erythropoiesis in mice [46]. However, thus far this approach has not been tested clinically in humans.
In summary, despite the recently reshaped human hematopoietic hierarchy, several unsettled issues remain and include: The functional potential of the different purified subsets for generating their respective mature progeny in vivo versus in vitro; the extent of contribution of bipotent erythroid/megakaryocytic progenitors to terminally mature cells at baseline states compared to acute and chronic stress hematopoiesis [47]; the molecular underpinning of functional differences of lineage determination across the developmental landscape of human hematopoiesis; how the revised adult hematopoietic hierarchy [29] accommodates the previously described lineage priming of HSCs, or the synergy and cross-antagonism of transcriptional factors at steady state or in perturbations of adult hematopoiesis.
Figure 1.
A bipotent E/Mk colony (plasma clot culture)
Figure 2.
A large Mk colony (plasma clot culture)
Figure 3.
A large BFU-e derived colony (methyl cellulose culture)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Kaushansky K, Lok S, Holly RD, et al. Promotion of megakaryocyte progenitor expansion and differentiation by the c-Mpl ligand thrombopoietin. Nature. 1994;369:568–571. doi: 10.1038/369568a0. [DOI] [PubMed] [Google Scholar]
- 2.Metcalf D. Blood. Thrombopoietin--at last. Nature. 1994;369:519–520. doi: 10.1038/369519a0. [DOI] [PubMed] [Google Scholar]
- 3.Broudy VC, Lin NL, Kaushansky K. Thrombopoietin (c-mpl ligand) acts synergistically with erythropoietin, stem cell factor, and interleukin-11 to enhance murine megakaryocyte colony growth and increases megakaryocyte ploidy in vitro. Blood. 1995;85:1719–1726. [PubMed] [Google Scholar]
- 4.Kaushansky K, Broudy VC, Grossmann A, et al. Thrombopoietin expands erythroid progenitors, increases red cell production, and enhances erythroid recovery after myelosuppressive therapy. The Journal of clinical investigation. 1995;96:1683–1687. doi: 10.1172/JCI118210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sitnicka E, Lin N, Priestley GV, et al. The effect of thrombopoietin on the proliferation and differentiation of murine hematopoietic stem cells. Blood. 1996;87:4998–5005. [PubMed] [Google Scholar]
- 6.Papayannopoulou T, Brice M, Farrer D, Kaushansky K. Insights into the cellular mechanisms of erythropoietin-thrombopoietin synergy. Experimental hematology. 1996;24:660–669. [PubMed] [Google Scholar]
- 7.Papayannopoulou T, Nakamoto B, Kurachi S, Nelson R. Analysis of the erythroid phenotype of HEL cells: clonal variation and the effect of inducers. Blood. 1987;70:1764–1772. [PubMed] [Google Scholar]
- 8.Vainchenker W, Testa U, Guichard J, Titeux M, Breton-Gorius J. Heterogeneity in the cellular commitment of a human leukemic cell line: K 562. Blood cells. 1981;7:357–375. [PubMed] [Google Scholar]
- 9.McDonald TP, Cottrell MB, Clift RE, Cullen WC, Lin FK. High doses of recombinant erythropoietin stimulate platelet production in mice. Experimental hematology. 1987;15:719–721. [PubMed] [Google Scholar]
- 10.Tronik-Le Roux D, Roullot V, Schweitzer A, Berthier R, Marguerie G. Suppression of erythro-megakaryocytopoiesis and the induction of reversible thrombocytopenia in mice transgenic for the thymidine kinase gene targeted by the platelet glycoprotein alpha IIb promoter. The Journal of experimental medicine. 1995;181:2141–2151. doi: 10.1084/jem.181.6.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Romeo PH, Prandini MH, Joulin V, et al. Megakaryocytic and erythrocytic lineages share specific transcription factors. Nature. 1990;344:447–449. doi: 10.1038/344447a0. [DOI] [PubMed] [Google Scholar]
- 12.Orkin SH. GATA-binding transcription factors in hematopoietic cells. Blood. 1992;80:575–581. [PubMed] [Google Scholar]
- 13.Orkin SH. Transcription factors and hematopoietic development. The Journal of biological chemistry. 1995;270:4955–4958. doi: 10.1074/jbc.270.10.4955. [DOI] [PubMed] [Google Scholar]
- 14.Humphries RK, Eaves AC, Eaves CJ. Characterization of a primitive erythropoietic progenitor found in mouse marrow before and after several weeks in culture. Blood. 1979;53:746–763. [PubMed] [Google Scholar]
- 15.McLeod DL, Shreeve MM, Axelrad AA. Chromosome marker evidence for the bipotentiality of BFU-E. Blood. 1980;56:318–322. [PubMed] [Google Scholar]
- 16.Kanz L, Straub G, Bross KG, Fauser AA. Identification of human megakaryocytes derived from pure megakaryocytic colonies (CFU-M), megakaryocytic-erythroid colonies (CFU-M/E), and mixed hemopoietic colonies (CFU-GEMM) by antibodies against platelet associated antigens. Blut. 1982;45:267–274. doi: 10.1007/BF00320194. [DOI] [PubMed] [Google Scholar]
- 17.Debili N, Coulombel L, Croisille L, et al. Characterization of a bipotent erythro-megakaryocytic progenitor in human bone marrow. Blood. 1996;88:1284–1296. [PubMed] [Google Scholar]
- 18.Edvardsson L, Dykes J, Olofsson T. Isolation and characterization of human myeloid progenitor populations--TpoR as discriminator between common myeloid and megakaryocyte/erythroid progenitors. Experimental hematology. 2006;34:599–609. doi: 10.1016/j.exphem.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 19.Klimchenko O, Mori M, Distefano A, et al. A common bipotent progenitor generates the erythroid and megakaryocyte lineages in embryonic stem cell-derived primitive hematopoiesis. Blood. 2009;114:1506–1517. doi: 10.1182/blood-2008-09-178863. [DOI] [PubMed] [Google Scholar]
- 20.Weissman IL, Shizuru JA. The origins of the identification and isolation of hematopoietic stem cells, and their capability to induce donor-specific transplantation tolerance and treat autoimmune diseases. Blood. 2008;112:3543–3553. doi: 10.1182/blood-2008-08-078220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim WS, Zhu Y, Deng Q, et al. Erythropoiesis from human embryonic stem cells through erythropoietin-independent AKT signaling. Stem cells. 2014;32:1503–1514. doi: 10.1002/stem.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pronk CJ, Rossi DJ, Mansson R, et al. Elucidation of the phenotypic, functional, and molecular topography of a myeloerythroid progenitor cell hierarchy. Cell stem cell. 2007;1:428–442. doi: 10.1016/j.stem.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 23.Forsberg EC, Serwold T, Kogan S, Weissman IL, Passegue E. New evidence supporting megakaryocyte-erythrocyte potential of flk2/flt3+ multipotent hematopoietic progenitors. Cell. 2006;126:415–426. doi: 10.1016/j.cell.2006.06.037. [DOI] [PubMed] [Google Scholar]
- 24.Vannucchi AM, Paoletti F, Linari S, et al. Identification and characterization of a bipotent (erythroid and megakaryocytic) cell precursor from the spleen of phenylhydrazine-treated mice. Blood. 2000;95:2559–2568. [PubMed] [Google Scholar]
- 25.Miyawaki K, Arinobu Y, Iwasaki H, et al. CD41 marks the initial myelo-erythroid lineage specification in adult mouse hematopoiesis: redefinition of murine common myeloid progenitor. Stem cells. 2015;33:976–987. doi: 10.1002/stem.1906. [DOI] [PubMed] [Google Scholar]
- 26.Gekas C, Graf T. CD41 expression marks myeloid-biased adult hematopoietic stem cells and increases with age. Blood. 2013;121:4463–4472. doi: 10.1182/blood-2012-09-457929. [DOI] [PubMed] [Google Scholar]
- 27.Sanjuan-Pla A, Macaulay IC, Jensen CT, et al. Platelet-biased stem cells reside at the apex of the haematopoietic stem-cell hierarchy. Nature. 2013;502:232–236. doi: 10.1038/nature12495. [DOI] [PubMed] [Google Scholar]
- 28.Haas S, Hansson J, Klimmeck D, et al. Inflammation-Induced Emergency Megakaryopoiesis Driven by Hematopoietic Stem Cell-like Megakaryocyte Progenitors. Cell stem cell. 2015;17:422–434. doi: 10.1016/j.stem.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 29.Notta F, Zandi S, Takayama N, et al. Distinct routes of lineage development reshape the human blood hierarchy across ontogeny. Science. 2015 doi: 10.1126/science.aab2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kohlscheen S, Wintterle S, Schwarzer A, et al. Inhibition of Thrombopoietin/Mpl Signaling in Adult Hematopoiesis Identifies New Candidates for Hematopoietic Stem Cell Maintenance. PloS one. 2015;10:e0131866. doi: 10.1371/journal.pone.0131866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Desmond R, Townsley DM, Dumitriu B, et al. Eltrombopag restores trilineage hematopoiesis in refractory severe aplastic anemia that can be sustained on discontinuation of drug. Blood. 2014;123:1818–1825. doi: 10.1182/blood-2013-10-534743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pina C, May G, Soneji S, Hong D, Enver T. MLLT3 regulates early human erythroid and megakaryocytic cell fate. Cell stem cell. 2008;2:264–273. doi: 10.1016/j.stem.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 33.Randrianarison-Huetz V, Laurent B, Bardet V, Blobe GC, Huetz F, Dumenil D. Gfi-1B controls human erythroid and megakaryocytic differentiation by regulating TGF-beta signaling at the bipotent erythro-megakaryocytic progenitor stage. Blood. 2010;115:2784–2795. doi: 10.1182/blood-2009-09-241752. [DOI] [PubMed] [Google Scholar]
- 34.Mancini E, Sanjuan-Pla A, Luciani L, et al. FOG-1 and GATA-1 act sequentially to specify definitive megakaryocytic and erythroid progenitors. The EMBO journal. 2012;31:351–365. doi: 10.1038/emboj.2011.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Foudi A, Kramer DJ, Qin J, et al. Distinct, strict requirements for Gfi-1b in adult bone marrow red cell and platelet generation. The Journal of experimental medicine. 2014;211:909–927. doi: 10.1084/jem.20131065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paralkar VR, Mishra T, Luan J, et al. Lineage and species-specific long noncoding RNAs during erythro-megakaryocytic development. Blood. 2014;123:1927–1937. doi: 10.1182/blood-2013-12-544494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bianchi E, Bulgarelli J, Ruberti S, et al. MYB controls erythroid versus megakaryocyte lineage fate decision through the miR-486-3p-mediated downregulation of MAF. Cell death and differentiation. 2015;22:1906–1921. doi: 10.1038/cdd.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuvardina ON, Herglotz J, Kolodziej S, et al. RUNX1 represses the erythroid gene expression program during megakaryocytic differentiation. Blood. 2015;125:3570–3579. doi: 10.1182/blood-2014-11-610519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu C, Niakan KK, Matsushita M, Stamatoyannopoulos G, Orkin SH, Raskind WH. X-linked thrombocytopenia with thalassemia from a mutation in the amino finger of GATA-1 affecting DNA binding rather than FOG-1 interaction. Blood. 2002;100:2040–2045. doi: 10.1182/blood-2002-02-0387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Del Vecchio GC, Giordani L, De Santis A, De Mattia D. Dyserythropoietic anemia and thrombocytopenia due to a novel mutation in GATA-1. Acta haematologica. 2005;114:113–116. doi: 10.1159/000086586. [DOI] [PubMed] [Google Scholar]
- 41.Neuwirtova R, Fuchs O, Holicka M, et al. Transcription factors Fli1 and EKLF in the differentiation of megakaryocytic and erythroid progenitor in 5q- syndrome and in Diamond-Blackfan anemia. Annals of hematology. 2013;92:11–18. doi: 10.1007/s00277-012-1568-1. [DOI] [PubMed] [Google Scholar]
- 42.James C, Ugo V, Le Couedic JP, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434:1144–1148. doi: 10.1038/nature03546. [DOI] [PubMed] [Google Scholar]
- 43.Bellucci S, Michiels JJ. The role of JAK2 V617F mutation, spontaneous erythropoiesis and megakaryocytopoiesis, hypersensitive platelets, activated leukocytes, and endothelial cells in the etiology of thrombotic manifestations in polycythemia vera and essential thrombocythemia. Seminars in thrombosis and hemostasis. 2006;32:381–398. doi: 10.1055/s-2006-942759. [DOI] [PubMed] [Google Scholar]
- 44.Jin L, Siritanaratkul N, Emery DW, et al. Targeted expansion of genetically modified bone marrow cells. Proceedings of the National Academy of Sciences. 1998;95:8093–8097. doi: 10.1073/pnas.95.14.8093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Okazuka K, Beard BC, Emery DW, et al. Long-term regulation of genetically modified primary hematopoietic cells in dogs. Molecular therapy : the journal of the American Society of Gene Therapy. 2011;19:1287–1294. doi: 10.1038/mt.2011.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Suzuki N, Mukai HY, Yamamoto M. In vivo regulation of erythropoiesis by chemically inducible dimerization of the erythropoietin receptor intracellular domain. PloS one. 2015;10:e0119442. doi: 10.1371/journal.pone.0119442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sanchez M, Weissman IL, Pallavicini M, et al. Differential amplification of murine bipotent megakaryocytic/erythroid progenitor and precursor cells during recovery from acute and chronic erythroid stress. Stem cells. 2006;24:337–348. doi: 10.1634/stemcells.2005-0023. [DOI] [PubMed] [Google Scholar]