Abstract
Background
BRCA1 interacting protein C-terminal helicase 1 (BRIP1) is one of the Fanconi Anaemia Complementation (FANC) group family of DNA repair proteins. Biallelic mutations in BRIP1 are responsible for FANC group J, and previous studies have also suggested that rare protein truncating variants in BRIP1 are associated with an increased risk of breast cancer. These studies have led to inclusion of BRIP1 on targeted sequencing panels for breast cancer risk prediction.
Methods
We evaluated a truncating variant, p.Arg798Ter (rs137852986), and 10 missense variants of BRIP1, in 48 144 cases and 43 607 controls of European origin, drawn from 41 studies participating in the Breast Cancer Association Consortium (BCAC). Additionally, we sequenced the coding regions of BRIP1 in 13 213 cases and 5242 controls from the UK, 1313 cases and 1123 controls from three population-based studies as part of the Breast Cancer Family Registry, and 1853 familial cases and 2001 controls from Australia.
Results
The rare truncating allele of rs137852986 was observed in 23 cases and 18 controls in Europeans in BCAC (OR 1.09, 95% CI 0.58 to 2.03, p=0.79). Truncating variants were found in the sequencing studies in 34 cases (0.21%) and 19 controls (0.23%) (combined OR 0.90, 95% CI 0.48 to 1.70, p=0.75).
Conclusions
These results suggest that truncating variants in BRIP1, and in particular p.Arg798Ter, are not associated with a substantial increase in breast cancer risk. Such observations have important implications for the reporting of results from breast cancer screening panels.
INTRODUCTION
Susceptibility to breast cancer is known to be mediated through a very large number of genetic variants conferring a wide range of disease risks relative to population incidence rates.1 These variants include rare mutations in high-penetrance genes (fourfold or higher risk), notably BRCA1 and BRCA2, mutations in genes conferring more moderate risks of breast cancer (twofold to fourfold higher risks), and approximately 100 common susceptibility variants (SNPs) conferring modest risks of the disease (typically 1.1–1.2-fold). Clinical genetic testing for breast cancer has largely focused on the high-risk genes. However, with the increasing use of high-throughput sequencing, genetic testing is being extended to larger panels of genes, including those in the ‘moderate-risk’ category.2
The known genes in the moderate-risk category encode proteins involved in DNA repair. One of the genes involved in DNA repair that has been proposed as a breast cancer susceptibility gene is BRIP1. BRIP1 (BRCA1-interacting protein 1, also known as BACH1) encodes a helicase-like protein that was identified via its direct binding to the BRCA1 BRCT domains, and is known to contribute to DNA repair via homologous recombination.3,4 BRIP1 was shown to be the likely causative gene for Fanconi Anaemia Complementation group J through positional cloning and the identification of germline mutations in nine families from two studies.4,5 The most common truncating mutation identified was c.2392C>T (p.Arg798Ter) in exon 17. Analysis of a cell line from a patient homozygous for this mutation showed complete absence of the full-length BRIP1 protein.4 p.Arg798Ter has been found in patients from diverse populations, suggesting that it is either a relatively ancient founder mutation or is recurrent.
Given the role of BRCA1 and other genes involved in DNA repair in susceptibility to breast and other cancers, it seems reasonable to speculate that germline mutations of BRIP1 might also predispose to breast cancer. Seal et al6 screened the coding sequence of 1212 women with breast cancer having a family history of disease and 2012 controls. They identified mutations predicted to lead to a truncated protein in nine cases versus two in controls and obtained an estimated relative risk of breast cancer, after adjustment for oversampling of cases with a family history, of 2.0 (95% CI 1.2 to 3.2, p=0.012). The most common mutation was p. Arg798Ter, accounting for five of the mutations in cases and one in controls.
Since the Seal et al6 paper, several other studies have identified BRIP1 variants through screening of breast cancer cases for specific mutations,7–12 but no large-scale case–control mutation screening studies have been reported. To evaluate more definitively the evidence that BRIP1 is a breast cancer susceptibility gene, we genotyped the p.Arg798Ter variant and 10 missense variants in >48 000 cases and 43 000 controls in studies participating in the Breast Cancer Association Consortium (BCAC). Additionally, we screened the entire coding sequence of BRIP1 in three large case–control studies comprising >16 000 cases and 8000 controls.
METHODS
Breast Cancer Association Consortium
Breast cancer cases and controls were drawn from 52 studies participating in the BCAC. The analysis was restricted to 48 143 cases and 43 608 controls from 41 studies in populations of European origin (comprising ~87% of the data set) since the sample sizes for Asian and African-American women were too small for separate analysis. The truncating variant p.Arg798Ter and 10 missense variants in BRIP1 (table 1) were genotyped using iCOGS, a custom array of ~200 000 variants.13 Genotypes were subject to standard quality control procedures as described previously.13
Table 1.
Summary of missense variants tested for association with breast cancer risk in Breast Cancer Association Consortium
| rs number | Position* | Substitution | Protein alteration | CADD20 | PolyPhen | SIFT | MAF | OR (95% CI) | p Value |
|---|---|---|---|---|---|---|---|---|---|
| rs4988345 | 59924572 | c.517C>T | p.Arg173Ser | 20.8 | Probably damaging | Deleterious | 0.0043 | 1.05 (0.91 to 1.21) | 0.49 |
| rs4988346 | 59924512 | c.577G>A | p.Val193Ile | 0.342 | Benign | Tolerated | 0.0044 | 1.11 (0.97 to 1.28) | 0.13 |
| rs4988347 | 59924505 | c.584T>C | p.Leu195Pro | 0.578 | Benign | Tolerated | 0.0022 | 1.13 (0.93 to 1.37) | 0.23 |
| rs28997569 | 59885956 | c.790C>T | p.Arg264Trp | 16.72 | Probably damaging | Deleterious | 0.0011 | 1.01 (0.76 to 1.34) | 0.96 |
| rs28997570 | 59885856 | c.890A>G | p.Lys297Arg | 8.669 | Benign | Tolerated | 0.0016 | 1.06 (0.84 to 1.34) | 0.60 |
| rs4988350 | 59861668 | c.1591T>G | p.Phe531Val | 23.8 | Probably damaging | Tolerated | 0 | ||
| rs4988349 | 59861640 | c.1619A>T | p.Gln540Leu | 16.61 | Possibly damaging | Tolerated | 0 | ||
| rs137852986 | 59793412 | c.2392C>T | p.Arg798Ter | 39 | – | – | 0.00021 | 1.09 (0.58 to 2.03) | 0.79 |
| rs28904918 | 59770797 | c.2569A>G | p.Ile857Val | 18.50 | Probably damaging | Tolerated | 6×10−5 | 0.87 (0.21 to 3.66) | 0.85 |
| rs4986764 | 59763347 | c.2755T>C | p.Ser919Pro | 4.321 | Benign | Deleterious | 0.42 | 1.00 (0.98 to 1.01) | 0.66 |
| rs4988356 | 59763298 | c.2804T>G | p.Val935Gly | 1.149 | Benign | Deleterious | 2×10−5 | 0.44 (0.039 to 5.00) | 0.51 |
hg19 (build 37) position.
CADD, Combined Annotation-Dependent Depletion scores; MAF, minor allele frequency.
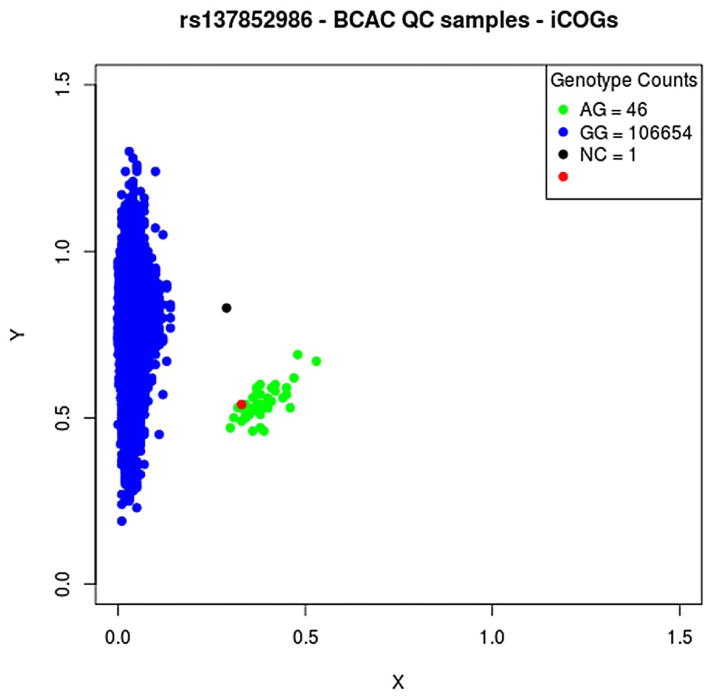
For the purpose of this analysis, we manually recalled the genotypes for BRIP1 p.Arg798Ter using the cluster plot of normalised intensities (figure 1). The experiment included a positive control previously identified as a carrier of the mutant allele through sequencing of a series of prostate cancer cases. This individual was genotyped correctly as a variant carrier. We further confirmed the genotypes through comparison with data from two re-sequencing experiments conducted in Studies of Epidemiology and Risk Factors in Cancer Heredity (SEARCH) and the Breast Cancer Family Registry (BCFR), for which individuals were also genotyped using iCOGS (see below). Thirteen individuals in the former study and two in the latter study were identified as carrying the variant allele at p.Arg798Ter; genotypes determined by the two methods were 100% concordant.
Figure 1.
Cluster plot for genotype intensities for rs137852986 on the iCOGS array. Normalised intensities for the variant and wild-type allele for each individual are given by the X and Y coordinates, respectively. Individuals called as p.Arg798Ter carriers are indicated by green dots and non-carriers by blue dots. The red dot indicates a positive control individual known to carry the variant from prior sequencing. BCAC, Breast Cancer Association Consortium; NC, no call.
SEARCH study
Subjects
Cases were drawn from SEARCH, a population-based study of breast cancer in the region covered by the Eastern Cancer Registration and Information Centre, UK.14 SEARCH recruited patients diagnosed with invasive breast cancer before the age of 55 years since 1991 and still alive at the start of the study in 1996 (prevalent cases; median age 48 years), together with all those diagnosed before 70 years of age between 1996 and 2014. The study was approved by the Cambridgeshire Research Ethics Committee. The present analysis is based on data from 13 824 case participants. Controls were drawn from the EPIC-Norfolk study, a population-based cohort study of diet and health women attending general practitioner (GP) practices, frequency matched to cases by age and geographic region (2003–present),14 and women attending breast screening as part of the National Health Service Breast Screening Program participating in the Sisters in Breast Screening study.15 The final analyses were based on 13 213 cases and 5242 controls that passed QC filters (see below).
Mutation screening
Target enrichment was accomplished using the 48.48 Fluidigm Access Array system. This approach employed multiplexed microfluidic PCR reactions to first amplify targeted regions and then ligate one of 1536 unique barcodes and sequencing adapters. To cover the 19 protein-coding exons and associated splice junctions of BRIP1, we designed 45 PCR amplicons that were 133–199 bp in length, which together produced unique coverage of 3750 bp, as part of a larger multiplex panel involving ~500 amplicons. The amplicon designs covered 100% of the targeted regions. Fourteen 1536-sample sequencing libraries were produced according to the manufacturer’s protocol (Fluidigm, San Francisco, California, USA) and assayed with the KAPA library quantification kit with specific probes for the ends of the adapters (KapaBiosystems, Boston, Massachusetts, USA). Libraries were sequenced in paired end mode on the Illumina HiSeq 2000 and CASAVA was used to construct demultiplexed sequence files, according to the manufacturer’s protocols (Illumina, San Diego, California, USA). Cutadapt V.1.5 was used to remove primer sequences from both ends of each read, and untrimmed reads were discarded.16 Reads were aligned to the hg19 human genome reference sequence using BWA-MEM V.0.7,17 and GATK V.3.3-0-g37228af was used for base quality score recalibration and indel realignment, and for deriving quality and depth metrics.18 BRIP1 was segmented into intervals of 2–7 exons, and the GATK UnifiedGenotyper was used to perform SNP and indel discovery and genotyping across all samples simultaneously, according to GATK Best Practices recommendations.19 The samples had a median coverage of 446.4, and a median of 97.47% of the targeted region (coding exons with 6 bp of flanking sequence) covered in each sample. In initial filtering, variants with >20% missing data were removed, and samples with no genotype at >20% of remaining positions were also excluded. Genotypes with depth <20 or genotype quality <13 were re-coded as no genotype. GATK was used to recalculate variant-level metrics without these failed samples and low-confidence genotypes, and positions genotyped in >95% of samples and with quality by depth between 3.0 and 25.0 were retained for further analysis. The remaining variants were annotated with Combined Annotation-Dependent Depletion (CADD) V.1.2,20 and 40 truncating and predicted damaging missense variants were selected for Sanger sequencing. Of these, 39 (positive predictive value 97.5%) variants were successfully confirmed.
iCOGS data were available for 13 133 individuals that were also sequenced. Six rare coding variants (MAF<1%) were polymorphic in the iCOGS data. Of the 357 rare allele carriers identified by iCOGS, the sequencing identified 355 (99.4%), although for two of the variants (p.Val193Ile and p.Arg173Ser), 13/111 and 17/138 of individuals called heterozygotes by iCOGS genotyping were called rare allele homozygotes by sequencing, reflecting bias in PCR amplification. One common coding polymorphism (rs4986764, p.Ser919Pro) was concordant in 99.9% of samples.
BCFR study
Subjects
Eligible participants included women ascertained by population-based sampling by the Australian, Northern Californian and Ontarian sites of the BCFR between 1995 and 2005.21 For the present study, the selection criteria for cases (n=1313) were diagnosis of breast cancer at <45 years of age and self-reported race/ethnicity, plus grandparents’ country of origin information consistent with Caucasian, East Asian, Hispanic/Latino, or African-American racial/ethnic heritage. The controls (n=1123) were frequency-matched to the cases within each centre by racial/ethnic group, with age at selection not more than 10 years older or younger than the age at diagnosis of the cases ascertained at the same centre. The design of this study has been described in detail previously.22–27 Recruitment and genetic studies were approved by the Ethics Committee of the International Agency for Research on Cancer (Lyon, France), the University of Utah Institutional Review Boards (IRBs) and the local IRBs of the BCFR centres from which samples were received. Written informed consent was obtained from each participant.
Mutation screening
Mutation screening was carried out using 30 ng of whole-genome amplified (WGA) DNA and covered the 19 coding exons of BRIP1 (NM_032043.2). The laboratory process has been described in detail for our recent studies of ATM,22 CHEK2,23 XRCC2,24RAD51,25 RINT126 and MRN genes.27 The semi-automated approach relies on mutation scanning by high-resolution melt curve (HRM) analysis followed by direct Sanger sequencing of the individual samples for which an aberrant melt curve profile is indicative of the presence of a sequence variant. In our previous work, we showed, by comparing the results with those obtained with Sanger sequencing,28 that the HRM technique showed high sensitivity and specificity (1.0 and 0.8, respectively, for amplicons of <400 bp) for mutation screening. All rare exonic variants, plus intronic variants that fell within 20 bp of a splice acceptor site or 8 bp of a splice donor site, were independently re-amplified from the two WGA reaction products to confirm the presence of the variant using direct Sanger sequencing. Primer and HRM probe sequences are available from the authors upon request.
Peter MacCallum Cancer Centre study
Subjects
The familial cohort included 1853 index individuals with personal and family histories of breast cancer who were previously assessed at Familial Cancer Centres in Victoria and New South Wales. A total of 979 cases were obtained from the ‘Variants in Practice’ study, which recruited via the combined Familial Cancer Centers in Melbourne, Australia,29 and 874 through the Hunter Area Pathology Service, Newcastle, Australia. All index cases were previously screened through their clinical genetics services and found to be negative for mutations in BRCA1 and BRCA2. Large deletions and duplications in BRCA1 and BRCA2 were included in the mutational analysis as determined by multiplex ligation-dependent probe amplification analysis. The 2001 female controls were accessed through Lifepool (http://www.lifepool.org), which is a cohort of women attending population mammography screening programme in Victoria, Australia. Controls were aged 40 years and above (mean age 64) and were cancer-free at the time of blood collection. This study was approved by the Hunter New England Human Research Ethics Committee and the Peter MacCallum Cancer Centre Human Research Ethics Committee.
Mutation screening
Cases and controls were screened for germline mutations in all 19 exons of BRIP1 on the HiSeq 2500 System (Illumina) using the Haloplex target enrichment system (Agilent) as described previously.30 Paired-end sequence reads were aligned to the human genome (hg19 assembly) using the BWA-MEM software.31 Base quality score recalibration and indel realignment was performed using the GATK software. Single-nucleotide variants and indels were identified using the GATK Unified Genotyper and Variant Quality Score Recalibration.18,19 Variants were annotated with information from Ensembl release 62. The average percentage of bases covered at a depth of ≥10× was 94.8% for cases and 96.1% for controls with all samples having at least 85% of bases sequenced at a depth of ≥10×.
All truncating variants in BRIP1 were validated by Sanger sequencing, as were any missense SNPs with a CADD score >10 that had not been previously reported in any databases. Previously reported SNPs were only validated in selected cases if the variant calling was unclear (quality score <150 or not identified in bidirectional reads).
Statistical analysis
Association between each of the variants in BRIP1 and breast cancer risk was assessed in BCAC using logistic regression, with adjustment for study and seven principal components for women of European ancestry derived from genotypes of SNPs on the iCOGS array, as previously described.13 For the three targeted sequencing studies, we carried out burden analyses, which evaluated the risk associated with carrying any one of a set of likely deleterious variants, since the variants were too rare to be analysed individually, and this is directly relevant to the potential clinical application of the findings of this study. We considered two sets of variants: those predicted to result in a truncated protein product and missense substitutions with a CADD score >20. ORs and 95% CIs were calculated for each of the three individual studies (SEARCH, BCFR and Peter MacCallum) and combined with those for BCAC/iCOGS using fixed effects meta-analysis. Heterogeneity in the OR among studies was assessed using a standard heterogeneity χ2 test and I2 statistic.
The BCAC data set partially overlapped with SEARCH and two of the BCFR studies (Australian Breast Cancer Family Study (ABCFS) and Ontario Familial Breast Cancer Registry (OFBCR)). Since p.Arg798Ter failed the minimum coverage threshold in SEARCH, for simplicity we excluded the p. Arg798Ter variant, and two other missense variants (rs4988345 and rs28997569) that were genotyped on the iCOGS from both the SEARCH and BCFR sequencing data (but retained them in the BCAC data set) when combining the results across all data sets. This resulted in an overlap in the (non-carrier) data sets between the BCAC, and the SEARCH and BCFR sequencing data sets, but the resulting bias in the combined odds ratio would be negligible since the variants are all extremely rare. The most probable haplotypes for markers across the BRIP1 region were generated using SHAPEIT V.2.32
Nonsense-mediated mRNA decay analysis of BRIP1 p.ArgR798Ter
To investigate whether the protein truncating mutation p. Arg798Ter triggers nonsense mediated decay, we treated lymphoblastoid cell lines from a heterozygous carrier and wild-type controls with10 mg/mL cycloheximide for 5 h. We extracted total RNA and DNA from treated and untreated cells with the AllPrep DNA/RNA Micro kit (Qiagen), and then prepared cDNA with the QuantiTect Reverse Transcription Kit (Qiagen). PCR primers for DNA and cDNA analysis can be provided on request. The experiment was carried out in triplicate.
RESULTS
Truncating variants
In analyses restricted to women of European ancestry, the mutant allele was observed in 23 of 47 654 cases (0.050%) and 18 of 43 172 controls (0.04%) (OR 1.09, 95% CI 0.58 to 2.013, p=0.79) (table 2). Consistent results were obtained when analyses were restricted to women with known invasive breast cancer (OR 0.95, 95% CI 0.49 to 1.83). When the analysis was restricted to studies without oversampling of cases with a family history and/or bilaterality, the results were very similar to those for the whole data set (OR 1.09, 95% CI 0.56 to 2.09, p=0.81).
Table 2.
Association between protein truncating variants in BRIP1 and breast cancer risk
| Study | Case carriers/total (%) | Control carriers/total (%) | OR (95% CI) | p Value |
|---|---|---|---|---|
| BCAC | 23/47,654 (0.05%) | 18/43,172 (0.04%) | 1.09 (0.58 to 2.03) | 0.79 |
| SEARCH | 24/13,213 (0.18%) | 13/5242 (0.25%) | 0.73 (0.36 to 1.57) | 0.36 |
| BCFR | 4/1313 (0.30%) | 2/1123 (0.27%) | 1.71 (0.24 to 19.0) | 0.69 |
| PeterMac | 6/1853 (0.38%) | 4/2001 (0.20%) | 1.62 (0.38 to 7.82) | 0.45 |
| Combined | 0.98 (0.62 to 1.54) | 0.93 |
BCAC, Breast Cancer Association Consortium; BCFR, Breast Cancer Family Registry.
In the SEARCH, BCFR and Peter MacCallum Cancer Centre studies, we identified 34 truncating variants in cases (0.21%) and 19 in controls (0.23%) (combined OR 0.90, 95% CI 0.48 to 1.70, p=0.75) (table 2 and online supplementary tables S1–S3) The carrier frequency in controls was similar to that observed in exome sequencing data from 60 706 individuals in the Exome Aggregation Consortium (http://exac.broadinstitute.org/:0.21%). There was no evidence of heterogeneity in the OR among studies (p=0.49, I2=0.0). After elimination of the overlaps between BCAC and the SEARCH and BCFR data sets, the combined OR across all four studies for identified BRIP1 truncating variants was 0.98 (95% CI 0.62 to 1.54, p=0.93) (table 2).
There was weak evidence of an increased risk of oestrogen receptor (ER)-negative breast cancer for p.Arg798Ter carriers in BCAC (OR 2.25, 95% CI 0.93 to 5.46, p=0.07), but no evidence of an association with truncating variants in SEARCH (0.53, 95% CI 0.06 to 2.34, p=0.054; combined OR 1.71, 95% CI 0.77 to 3.80, p=0.19) (table 3). There was also weak evidence of an association with triple (ER/PR/HER2)-negative disease in BCAC (OR 3.62, 95% CI 0.99 to 13.2, p=0.05) but not in SEARCH (combined OR 2.71, 95% CI 0.84 to 8.74, p=0.10); however, these analyses were based on only four and one triple-negative cases carrying the variant in BCAC and SEARCH, respectively. There was no evidence for an association with ER-positive disease in either data set (combined OR 0.61, 95% CI 0.33 to 1.13, p=0.12).
Table 3.
Association between protein truncating variants in BRIP1 and breast cancer risk by subtype
| ER-positive
|
ER-negative
|
Triple negative
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Study | Carrier/total (%) | OR (95% CI) | p Value | Carrier/total (%) | OR (95% CI) | p Value | Carrier/total (%) | OR (95% CI) | p Value |
| BCAC | 4/27,680 (0.01%) | 0.38 (0.13 to 1.15) | 0.09 | 8/7707 (0.10%) | 2.25 (0.93 to 5.46) | 0.07 | 4/2983 (0.13%) | 3.62 (0.99 to 13.2) | 0.05 |
| SEARCH | 14/7391 (0.19%) | 0.76 (0.36 to 1.63) | 0.56 | 2/1521 (0.13%) | 0.53 (0.06 to 2.34) | 0.54 | 1/551 (0.18%) | 0.73 (0.02 to 4.89) | 1.0 |
| Combined | 0.61 (0.33 to 1.13) | 0.12 | 1.71 (0.77 to 3.80) | 0.19 | 2.71 (0.84 to 8.74) | 0.10 | |||
BCAC, Breast Cancer Association Consortium; ER, oestrogen receptor.
Nonsense-mediated decay
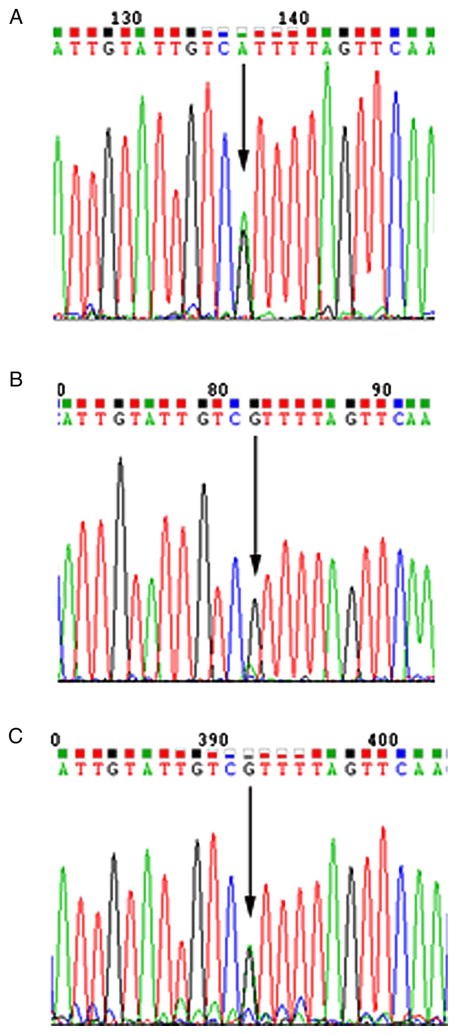
We performed Sanger sequencing on both cDNA and DNA of cycloheximide-treated and untreated wild-type and p.Arg798Ter lymphoblastoid cell lines (figure 2). Sequencing chromatograms showed that the rare, truncating allele was much less abundant than the wild-type allele in cDNA from untreated cells, but not in the treated cells, consistent with the inhibition of nonsense-mediated decay with cycloheximide.
Figure 2.
Sequencing of cDNA from a cycloheximide-treated and untreated lymphoblastoid cell line from a BRIP1 p.Arg798Ter carrier. Forward sequence of (A) cDNA from cycloheximide-treated lymphoblastoid cell line, (B) cDNA from the untreated lymphoblastoid cell line and (C) DNA sequence from the same cell line.
Missense variants
We considered missense variants with a CADD score >20 as the most likely deleterious variants. There was no evidence for association between carrying one of these missense variants, as a set, with breast cancer risk in the combined data set (OR 1.08, 95% CI 0.95 to 1.24, p=0.25; table 4), though there was some weak evidence of association in the Peter MacCallum Cancer Centre study. One variant, p.Arg173Ser, accounted for the majority of carriers of likely deleterious variants in the sequencing studies; it was also genotyped in BCAC and showed no evidence of association (combined OR 1.07, 95% CI 0.93 to 1.23, p=0.35). None of the other missense variants genotyped in BCAC showed evidence for association (table 1).
Table 4.
Association between missense variants in BRIP1 with Combined Annotation-Dependent Depletion score >20 and breast cancer risk
| Study | Case carriers/total (%) | Control carriers/total (%) | OR (95% CI) | p Value |
|---|---|---|---|---|
| BCAC | 429/47,666 (0.90%) | 370/43,176 (0.86%) | 1.06 (0.92 to 1.22) | 0.43 |
| SEARCH | 276/13,213 (2.1%) | 107/5242 (2.0%) | 1.06 (0.85 to 1.32) | 0.66 |
| BCFR | 0/1313 (0%) | 1/1123 (0.09%) | – | |
| PeterMac | 40/1853 (2.2%) | 28/2001 (1.4%) | 1.68 (1.02 to 2.82) | 0.03 |
| Combined | 1.08 (0.95 to 1.24) | 0.25 |
BCAC, Breast Cancer Association Consortium; BCFR, Breast Cancer Family Registry.
Distribution of p.Arg798Ter by population
Among European populations, there was substantial variation in the frequency of the p.Arg798Ter allele by country (p<0.0001); the carrier frequency was approximately 0.1% in the UK, Ireland and Australia, but virtually absent elsewhere in Europe. Also, 41 of the 42 carriers shared a common haplotype of 21 markers across 150 kb (see online supplementary figure s1 and supplementary table S4). In addition, we observed two occurrences among 12 893 women of Asian ancestry, both from a Malaysian study (MYBRCA) and both carrying the common haplotype in Europeans, and two occurrences among 2048 African-American women, one of which carried the founder European haplotype. These results suggest that the variant has arisen multiple times but that the majority of the carriers of p. Arg798Ter in Europeans have a common ancestral origin.
DISCUSSION
BRIP1 is included on many cancer gene sequencing panels and has been generally regarded as a ‘moderate-risk’ breast cancer susceptibility gene, together with other genes, including ATM, CHEK2 and PALB2.2 The evidence that deleterious mutations in these latter three genes confer an increased breast cancer risk is unequivocal, supported by large case–control, kin–cohort and segregation studies.22,33–37 In the case of BRIP1, however, it is notable that no large systematic studies have been published since the original study by Seal et al6 (see online supplementary table S1), although clear evidence of an association between truncating mutations and ovarian cancer risk has emerged.38,39 We sought to evaluate the evidence that protein truncating mutations in BRIP1 are associated with breast cancer, taking advantage of the large body of data generated as part of the iCOGS genotyping array. This allowed us to genotype one such variant, p.Arg798Ter, shown to be relatively frequent in previous studies, in >48 000 cases and 43 000 controls of European origin. In addition, we sequenced the coding region of BRIP1 in >16 000 cases and 8000 controls, predominantly of European origin, from three studies. We found no evidence of an association with breast cancer risk either for p. Arg798Ter or for carrying any truncating variant in the gene. The upper 95% confidence limit (1.54) excludes a twofold risk of breast cancer, often taken as a lower threshold for a moderate-risk allele.2
We found weak evidence of an association between p. Arg798Ter and ER-negative disease and triple-negative disease in BCAC, but not for truncating variants in the combined analysis. A recent study found eight BRIP1 truncating variants in 1853 triple-negative breast cancer cases, slightly higher than the frequency observed in our sequence analysis.40 Assuming that there is association for triple-negative breast cancer, a sample size of ~1400 triple-negative cases, that is approximately threefold larger than the current data set, would be required to exclude an OR of 3 (upper 95% CI), assuming a large control set. Thus, while an association of this magnitude may exist for triple-negative disease, this should be resolvable by larger studies.
It remains possible that some subset variants in BRIP1 do confer more substantial risks of breast cancer. p.Arg798Ter is a classic protein truncating mutation, which we showed undergoes nonsense-mediated decay. Rare homozygotes, with complete loss of the BRIP1 protein, are associated with Fanconi Anaemia.4 Although the results from the sequence analyses found no other truncating variants of comparable frequency to pArg798Ter, additional founder mutations might exist at similar or greater frequency in other European or non-European populations. We also found no evidence of association for missense variants, defined as potentially deleterious by CADD score; again the upper 95% confidence limit in this analysis excludes a twofold risk, though it remains possible that individual missense variants might confer a more substantial risk, as occurs in ATM.41–43
It also remains possible that truncating (or missense) variants are associated with a smaller (less than twofold) risk of breast cancer (perhaps with a higher relative risk for certain disease subtypes). However, in this case even larger studies would be required to establish the association and to provide reliable risk estimates. Moreover, this would place such variants in the same category as common risk SNPs and other modest risk variants, such as CHEK2 p.Ile157Thr and BRCA2 p.Lys3326Ter. If this were the case, the clinical implications would be quite different from those of established susceptibility genes since the risks conferred by the variant would only be substantial if combined with other risk factors.
These results highlight the importance of very large systematic studies to estimate disease risks associated with genetic variants. We conclude that there is no clear evidence for an association between protein truncating variants in BRIP1 and breast cancer risk. While BRIP1 screening might have utility for ovarian cancer risk prediction, in combination with other risk factors,39 such variants should not be used for breast cancer risk prediction.
Supplementary Material
Acknowledgments
The authors thank Sue Healey and Laura Sarimaa for technical assistance, and Heather Thorne, Eveline Niedermayr, all the kConFab research nurses and staff, the heads and staff of the Family Cancer Clinics, and the Clinical Follow Up Study (which has received funding from the NHMRC, the National Breast Cancer Foundation, Cancer Australia, and the National Institute of Health (USA)) for their contributions to this resource, and the many families who contribute to kConFab. kConFab is supported by a grant from the National Breast Cancer Foundation, and previously by the National Health and Medical Research Council (NHMRC), the Queensland Cancer Fund, the Cancer Councils of New South Wales, Victoria, Tasmania and South Australia, and the Cancer Foundation of Western Australia. The Peter MacCallum Cancer Centre study was supported by the Victorian Breast Cancer Research Consortium, the National Breast Cancer Foundation, the Victorian Cancer Agency, and the NHMRC. Lifepool is supported by an infrastructure grant from the National Breast Cancer Foundation, Australia. The authors thank Lisa Devereux for assistance in access to the Lifepool resource. The authors wish to thank all participants in the BCFR for their contribution to the study. The BCFR case–control mutation screening study was supported by the United States National Institutes of Health (NIH) National Cancer Institute (NCI) grant R01 CA121245, by the Canadian Institutes of Health Research (CIHR) for the CIHR Team in Familial Risks of Breast Cancer programme, by the Government of Canada through Genome Canada and the Canadian Institutes of Health Research, and the Ministère de l’enseignement supérieur, de la recherche, de la science, et de la technologie du Québec through Génome Québec. The BCFR was supported by grant UM1 CA164920 from the USA National Cancer Institute. The content of this manuscript does not necessarily reflect the views or policies of the National Cancer Institute or any of the collaborating centres in the Breast Cancer Family Registry (BCFR), nor does mention of trade names, commercial products, or organisations imply endorsement by the US government or the BCFR. The work also benefited from the Huntsman Cancer Institute’s Bioinformatics Shared Resource, which is supported by NCI grant P30 CA042014. The COGS study would not have been possible without the contributions of the following: Andrew Berchuck (OCAC), Rosalind A. Eeles, Ali Amin Al Olama, Zsofia Kote-Jarai (PRACTICAL), Antonis Antoniou, and Ken Offit (CIMBA), Andrew Lee, and Ed Dicks (Cambridge), the staff of the CNIO genotyping unit, Jacques Simard and Daniel C. Tessier, Francois Bacot, Daniel Vincent, Sylvie LaBoissière and Frederic Robidoux and the staff of the McGill University and Génome Québec Innovation Centre, Sune F. Nielsen, Borge G. Nordestgaard, and the staff of the Copenhagen DNA laboratory, and Julie M. Cunningham, Sharon A. Windebank, Christopher A. Hilker, Jeffrey Meyer and the staff of Mayo Clinic Genotyping Core Facility. The authors thank Zsofia Kote-Jarai for providing the positive control sample. BCAC(acknowledgments by study) (ABCFS) Maggie Angelakos, Judi Maskiell, Gillian Dite. (ABCS) C Ellen van der Schoot, Sanquin Amsterdam. (ACP) The ACP study wishes to thank the participants in the Thai Breast Cancer study. Special Thanks also go to the Thai Ministry of Public Health (MOPH), doctors and nurses who helped with the data collection process. Finally, the study participants would like to thank Dr Prat Boonyawongviroj, the former Permanent Secretary of MOPH and Dr Pornthep Siriwanarungsan, the Department Director-Generalof Disease Control who have supported the study throughout. (BBCS) Eileen Williams, Elaine Ryder-Mills, Kara Sargus (BIGGS) Niall McInerney, Gabrielle Colleran, Andrew Rowan, Angela Jones. (BSUCH) Peter Bugert, Medical Faculty Mannheim (CGPS) Staff and participants of the Copenhagen General Population Study. For the excellent technical assistance: Dorthe Uldall Andersen, Maria Birna Arnadottir, Anne Bank, Dorthe Kjeldgård Hansen (CNIO-BCS) Guillermo Pita, Charo Alonso, Daniel Herrero, Nuria Álvarez, Pilar Zamora, Primitiva Menendez, the Human Genotyping-CEGEN Unit (CNIO). (CTS) The CTS Steering Committee includes Leslie Bernstein, Susan Neuhausen, James Lacey, Sophia Wang, Huiyan Ma, Yani Lu, and Jessica Clague DeHart at the Beckman Research Institute of City of Hope, Dennis Deapen, Rich Pinder, Eunjung Lee, and Fred Schumacher at the University of Southern California, Pam Horn-Ross, Peggy Reynolds, Christina Clarke Dur and David Nelson at the Cancer Prevention Institute of California, and Hoda Anton-Culver, Argyrios Ziogas, and Hannah Park at the University of California Irvine. (ESTHER) Hartwig Ziegler, Christa Stegmaier, Sonja Wolf, Volker Hermann. (GC-HBOC) Heide Hellebrand, Stefanie Engert and GC-HBOC (Supported by Deutsche Krebshilfe). (GENICA) The GENICA Network: Dr Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, and University of Tübingen, Germany (HB, Wing-Yee Lo, Christina Justenhoven), German Cancer Consortium (DKTK) and German Cancer Research Center (DKFZ) (HB), Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany (Yon-Dschun Ko, Christian Baisch), Institute of Pathology, University of Bonn, Germany (Hans-Peter Fischer), Molecular Genetics of Breast Cancer, Deutsches Krebsforschungszentrum (DKFZ), Heidelberg, Germany (UH), Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr University Bochum (IPA), Bochum, Germany (Thomas Brüning, Beate Pesch, Sylvia Rabstein, Anne Lotz); and Institute of Occupational Medicine and Maritime Medicine, University Medical Center Hamburg-Eppendorf, Germany (Volker Harth). (HEBCS) Carl Blomqvist ,Kirsimari Aaltonen, Karl von Smitten, Sofia Khan, Tuomas Heikkinen, Irja Erkkilä. (HMBCS) Natalia Antonenkova, Peter Hillemanns, Hans Christiansen and Johann H. Karstens. (KBCP) Eija Myöhänen, Helena Kemiläinen. (kConFab/AOCS) The authors wish to thank Heather Thorne, Eveline Niedermayr, all the kConFab research nurses and staff, the heads and staff of the Family Cancer Clinics, and the Clinical Follow Up Study (which has received funding from the NHMRC, the National Breast Cancer Foundation, Cancer Australia, and the National Institute of Health (USA)) for their contributions to this resource, and the many families who contribute to kConFab. (LAABC) The authors thank all the study participants and the entire data collection team, especially Annie Fung and June Yashiki (LMBC), Gilian Peuteman, Dominiek Smeets, Thomas Van Brussel and Kathleen Corthouts (MARIE), Petra Seibold, Dieter Flesch-Janys, Judith Heinz, Nadia Obi, Alina Vrieling, Sabine Behrens, Ursula Eilber, Muhabbet Celik, Til Olchers and Stefan Nickels. MCCS cohort recruitment was funded by VicHealth and Cancer Council Victoria. The MCCS was further supported by Australian NHMRC grants 209057, 251553 and 504711 and by infrastructure provided by Cancer Council Victoria. Cases and their vital status were ascertained through the Victorian Cancer Registry (VCR) and the Australian Institute of Health and Welfare (AIHW), including the National Death Index. (MBCSG): Paolo Radice, Bernard Peissel, Daniela Zaffaroni and Jacopo Azzollini of the Fondazione IRCCS Istituto Nazionale dei Tumori (INT), Milan, Italy; Bernardo Bonanni, Monica Barile and Irene Feroce of the Istituto Europeo di Oncologia (IEO), Milan, Italy; and the personnel of the Cogentech Cancer Genetic Test Laboratory, Milan, Italy. (MYBRCA) Phuah Sze Yee, Peter Kang, Kang In Nee, Kavitta Sivanandan, Shivaani Mariapun, Yoon Sook-Yee, Daphne Lee, Teh Yew Ching and Nur Aishah Mohd Taib for DNA Extraction and patient recruitment. (NBCS) Dr Kristine Kleivi, PhD (K.G. Jebsen Centre for Breast Cancer Research, Institute of Clinical Medicine, University of Oslo, Oslo, Norway and Department of Research, Vestre Viken, Drammen, Norway), Dr Lars Ottestad, MD (Department of Genetics, Institute for Cancer Research, Oslo University Hospital Radiumhospitalet, Oslo, Norway), Prof. Em. Rolf Kåresen, MD (Department of Oncology, Oslo University Hospital and Faculty of Medicine, University of Oslo, Oslo, Norway), Dr Anita Langerød, PhD (Department of Genetics, Institute for Cancer Research, Oslo University Hospital Radiumhospitalet, Oslo, Norway), Dr Ellen Schlichting, MD (Department for Breast and Endocrine Surgery, Oslo University Hospital Ullevaal, Oslo, Norway), Dr Marit Muri Holmen, MD (Department of Radiology and Nuclear Medicine, Oslo University Hospital, Oslo, Norway), Prof. Toril Sauer, MD (Department of Pathology at Akershus University hospital, Lørenskog, Norway), Dr Vilde Haakensen, MD (Department of Genetics, Institute for Cancer Research, Oslo University Hospital Radiumhospitalet, Oslo, Norway), Dr Olav Engebråten, MD (Institute for Clinical Medicine, Faculty of Medicine, University of Oslo and Department of Oncology, Oslo University Hospital, Oslo, Norway), Prof. Bjørn Naume, MD (Division of Cancer Medicine and Radiotherapy, Department of Oncology, Oslo University Hospital Radiumhospitalet, Oslo, Norway), Dr Cecile E. Kiserud, MD (National Advisory Unit on Late Effects after Cancer Treatment, Department of Oncology, Oslo University Hospital, Oslo, Norway and Department of Oncology, Oslo University Hospital, Oslo, Norway), Dr Kristin V. Reinertsen, MD (National Advisory Unit on Late Effects after Cancer Treatment, Department of Oncology, Oslo University Hospital, Oslo, Norway and Department of Oncology, Oslo University Hospital, Oslo, Norway), Assoc. Prof. Åslaug Helland, MD (Department of Genetics, Institute for Cancer Research and Department of Oncology, Oslo University Hospital Radiumhospitalet, Oslo, Norway), Dr Margit Riis, MD (Dept of Breast- and Endocrine Surgery, Oslo University Hospital, Ullevål, Oslo, Norway), Dr Ida Bukholm, MD (Department of Breast-Endocrine Surgery, Akershus University Hospital, Oslo, Norway and Department of Oncology, Division of Cancer Medicine, Surgery and Transplantation, Oslo University Hospital, Oslo, Norway), Prof. Per Eystein Lønning, MD (Section of Oncology, Institute of Medicine, University of Bergen and Department of Oncology, Haukeland University Hospital, Bergen, Norway), Dr Silje Nord, PhD (Department of Genetics, Institute for Cancer Research, Oslo University Hospital Radiumhospitalet, Oslo, Norway) and Grethe I. Grenaker Alnæs, M.Sc. (Department of Genetics, Institute for Cancer Research, Oslo University Hospital Radiumhospitalet, Oslo, Norway). (NBHS) The authors thank study participants and research staff for their contributions and commitment to this study. (OBCS) Meeri Otsukka, Kari Mononen. (OFBCR) Teresa Selander, Nayana Weerasooriya. (ORIGO) The authors thank E. Krol-Warmerdam, and J. Blom for patient accrual, administering questionnaires, and managing clinical information. The LUMC survival data were retrieved from the Leiden hospital-based cancer registry system (ONCDOC) with the help of Dr J. Molenaar. (PBCS) Louise Brinton, Mark Sherman, Neonila Szeszenia-Dabrowska, Beata Peplonska, Witold Zatonski, Pei Chao, Michael Stagner. (pKARMA) The Swedish Medical Research Counsel. (RBCS) Petra Bos, Jannet Blom, Ellen Crepin, Elisabeth Huijskens, Annette Heemskerk, the Erasmus MC Family Cancer Clinic. (SASBAC) The Swedish Medical Research Counsel. (SBCGS) The authors thank study participants and research staff for their contributions and commitment to this study. (SBCS) Sue Higham, Helen Cramp, Ian Brock, Malcolm W. R. Reed, Sabapathy Balasubramanian and Dan Connley. (SEARCH) The SEARCH and EPIC teams. (SGBCC) The authors thank the participants and research coordinator Kimberley Chua. (SKKDKFZS) German Cancer Research Center (DKFZ), Heidelberg, Germany (Ute Hamann, DT, MK). The authors thank all study participants, clinicians, family doctors, researchers and technicians for their contributions and commitment to this study. (TNBCC) Robert Pilarski and Charles Shapiro were instrumental in the formation of the OSU Breast Cancer Tissue Bank. The authors thank the Human Genetics Sample Bank for processing of samples and providing OSU Columbus area control samples. (UKBGS) The authors thank Breast Cancer Now and the Institute of Cancer Research for support and funding of the Breakthrough Generations Study, and the study participants, study staff, and the doctors, nurses and other health care providers and health information sources who have contributed to the study. The authors acknowledge NHS funding to the Royal Marsden/ICR NIHR Biomedical Research Centre.
Funding Higher-level funding: The COGS project is funded through a European Commission’s Seventh Framework Programme grant (agreement number 223175—HEALTH-F2-2009-223175). BCAC is funded by Cancer Research UK (C1287/A10118, C1287/A12014) and by the European Community’s Seventh Framework Programme under grant agreement number 223175 (grant number HEALTH-F2-2009-223175) (COGS). Funding for the iCOGS infrastructure came from: the European Community’s Seventh Framework Programme under grant agreement n ° 223175 (HEALTH-F2-2009-223175) (COGS), Cancer Research UK (C1287/A10118, C1287/A 10710, C12292/A11174, C1281/A12014, C5047/A8384, C5047/A15007, C5047/A10692, C8197/A16565), the National Institutes of Health (CA128978) and Post-Cancer GWAS initiative (1U19 CA148537, 1U19 CA148065 and 1U19 CA148112—the GAME-ON initiative), the Department of Defense (W81XWH-10-1-0341), the Canadian Institutes of Health Research (CIHR) for the CIHR Team in Familial Risks of Breast Cancer, Komen Foundation for the Cure, the Breast Cancer Research Foundation, and the Ovarian Cancer Research Fund. This study made use of data generated by the Wellcome Trust Case Control consortium. Funding for the project was provided by the Wellcome Trust under award 076113. The results published here are in part based upon data generated by The Cancer Genome Atlas Project established by the National Cancer Institute and National Human Genome Research Institute. Personal support: GC-T is a National Health and Medical Research (NHMRC) Senior Principal Research Fellow. IGC is a National Health and Medical Research (NHMRC) Principal Research Fellow. ERT is a National Breast Cancer Foundation Postdoctoral Fellow. JL is supported by the Susan S. Komen Foundation. Funding of constituent studies: BCAC—The Australian Breast Cancer Family Study (ABCFS) was supported by grant UM1 CA164920 from the National Cancer Institute (USA). The content of this manuscript does not necessarily reflect the views or policies of the National Cancer Institute or any of the collaborating centres in the Breast Cancer Family Registry (BCFR), nor does mention of trade names, commercial products or organisations imply endorsement by the US government or the BCFR. The ABCFS was also supported by the National Health and Medical Research Council of Australia, the New South Wales Cancer Council, the Victorian Health Promotion Foundation (Australia) and the Victorian Breast Cancer Research Consortium. JLH is an NHMRC Senior Principal Research Fellow. MCS. is an NHMRC Senior Research Fellow. The ABCS study was supported by the Dutch Cancer Society (grants NKI 2007-3839; 2009 4363). The ACP study is funded by the Breast Cancer Research Trust, UK. The work of the BBCC was partly funded by ELAN-Fond of the University Hospital of Erlangen. The BBCS is funded by Cancer Research UK and Breakthrough Breast Cancer (recently merged with Breast Cancer Campaign forming Breast Cancer Now) and acknowledges NHS funding to the NIHR Biomedical Research Centre, and the National Cancer Research Network (NCRN). (BIGGS) ES is supported by NIHR Comprehensive Biomedical Research Centre, Guy’s & St. Thomas’ NHS Foundation Trust in partnership with King’s College London, UK. IT is supported by the Oxford Biomedical Research Centre. The BSUCH study was supported by the Dietmar-Hopp Foundation, the Helmholtz Society and the German Cancer Research Center (DKFZ). The CECILE study was funded by Fondation de France, Institut National du Cancer (INCa), Ligue Nationale contre le Cancer, Ligue contre le Cancer Grand Ouest, Agence Nationale de Sécurité Sanitaire (ANSES), Agence Nationale de la Recherche (ANR). The CGPS was supported by the Chief Physician Johan Boserup and Lise Boserup Fund, the Danish Medical Research Council and Herlev Hospital. The CNIO-BCS was supported by the Instituto de Salud Carlos III, the Red Temática de Investigación Cooperativa en Cáncer and grants from the Asociación Española Contra el Cáncer and the Fondo de Investigación Sanitario (PI11/00923 and PI12/00070). The CTS was initially supported by the California Breast Cancer Act of 1993 and the California Breast Cancer Research Fund (contract 97-10500) and is currently funded through the National Institutes of Health (R01 CA77398). Collection of cancer incidence data was supported by the California Department of Public Health as part of the statewide cancer reporting programme mandated by California Health and Safety Code Section 103885. HAC receives support from the Lon V Smith Foundation (LVS39420). The ESTHER study was supported by a grant from the Baden Württemberg Ministry of Science, Research and Arts. Additional cases were recruited in the context of the VERDI study, which was supported by a grant from the German Cancer Aid (Deutsche Krebshilfe). The GC-HBOC was supported by Deutsche Krebshilfe (107 352). The GENICA was funded by the Federal Ministry of Education and Research (BMBF) Germany grants 01KW9975/5, 01KW9976/8, 01KW9977/0 and 01KW0114, the Robert Bosch Foundation, Stuttgart, Deutsches Krebsforschungszentrum (DKFZ), Heidelberg, the Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr University Bochum (IPA), Bochum, as well as the Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany. The HEBCS was financially supported by the Helsinki University Central Hospital Research Fund, Academy of Finland (266528), the Finnish Cancer Society, The Nordic Cancer Union and the Sigrid Juselius Foundation. The HERPACC was supported by a Grant-in-Aid for Scientific Research on Priority Areas from the Ministry of Education, Science, Sports, Culture and Technology of Japan, by a Grant-in-Aid for the Third Term Comprehensive 10-Year Strategy for Cancer Control from Ministry Health, Labour and Welfare of Japan, by Health and Labour Sciences Research Grants for Research on Applying Health Technology from Ministry Health, Labour and Welfare of Japan, National Cancer Center Research and Development Fund and Grant form Takeda Health Foundation. The HMBCS was supported by a grant from the Friends of Hannover Medical School and by the Rudolf Bartling Foundation. Financial support for KARBAC was provided through the regional agreement on medical training and clinical research (ALF) between Stockholm County Council and Karolinska Institutet, the Swedish Cancer Society, The Gustav V Jubilee foundation and Bert von Kantzows foundation. The KBCP was financially supported by the special Government Funding (EVO) of Kuopio University Hospital grants, Cancer Fund of North Savo, the Finnish Cancer Organizations, and by the strategic funding of the University of Eastern Finland. kConFab is supported by a grant from the National Breast Cancer Foundation, and previously by the National Health and Medical Research Council (NHMRC), the Queensland Cancer Fund, the Cancer Councils of New South Wales, Victoria, Tasmania and South Australia, and the Cancer Foundation of Western Australia. Financial support for the AOCS was provided by the United States Army Medical Research and Materiel Command (DAMD17-01-1-0729), Cancer Council Victoria, Queensland Cancer Fund, Cancer Council New South Wales, Cancer Council South Australia, The Cancer Foundation of Western Australia, Cancer Council Tasmania and the National Health and Medical Research Council of Australia (NHMRC; 400413, 400281, 199600). LAABC is supported by grants (1RB-0287, 3PB-0102, 5PB-0018,10PB-0098) from the California Breast Cancer Research Program. Incident breast cancer cases were collected by the USC Cancer Surveillance Program (CSP), which is supported under subcontract by the California Department of Health. The CSP is also part of the National Cancer Institute’s Division of Cancer Prevention and Control Surveillance, Epidemiology, and End Results Program, under contract number N01CN25403. LMBC is supported by the ‘Stichting tegen Kanker’ (232-2008 and 196-2010). Diether Lambrechts is supported by the FWO and the KULPFV/10/016-SymBioSysII. The MARIE study was supported by the Deutsche Krebshilfe e.V. (70-2892-BR I, 106332, 108253, 108419), the Hamburg Cancer Society, the German Cancer Research Center (DKFZ) and the Federal Ministry of Education and Research (BMBF) Germany (01KH0402). (MBCSG) is supported by grants from the Italian Association for Cancer Research (AIRC) and by funds from the Italian citizens who allocated the 5/1000 share of their tax payment in support of the Fondazione IRCCS Istituto Nazionale Tumori, according to Italian laws (INT-Institutional strategic projects “5×1000”). The MCBCS was supported by the NIH grants CA128978, CA116167, CA192393, CA176785 an NIH Specialized Program of Research Excellence (SPORE) in Breast Cancer (CA116201), and the Breast Cancer Research Foundation and a generous gift from the David F. and Margaret T. Grohne Family Foundation .MCCS cohort recruitment was funded by VicHealth and Cancer Council Victoria. The MCCS was further supported by Australian NHMRC grants 209057, 251553 and 504711 and by infrastructure provided by Cancer Council Victoria. Cases and their vital status were ascertained through the Victorian Cancer Registry (VCR). The MEC was support by NIH grants CA63464, CA54281, CA098758 and CA132839. MYBRCA is funded by research grants from the Malaysian Ministry of Science, Technology and Innovation (MOSTI), Malaysian Ministry of Higher Education (UM.C/HlR/MOHE/06) and Cancer Research Initiatives Foundation (CARIF). Additional controls were recruited by the Singapore Eye Research Institute, which was supported by a grant from the Biomedical Research Council (BMRC08/1/35/19/550), Singapore and the National medical Research Council, Singapore (NMRC/CG/SERI/2010). The NBCS has received funding from the K.G. Jebsen Centre for Breast Cancer Research; the Research Council of Norway grant 193387/V50 (to A-L Børresen-Dale and VNK) and grant 193387/H10 (to A-L Børresen-Dale and VNK), South Eastern Norway Health Authority (grant 39346 to A-L Børresen-Dale) and the Norwegian Cancer Society (to A-L Børresen-Dale and VN K). The NBHS was supported by NIH grant R01CA100374. Biological sample preparation was conducted the Survey and Biospecimen Shared Resource, which is supported by P30 CA68485. The OBCS was supported by research grants from the Finnish Cancer Foundation, the Academy of Finland (grant number 250083, 122715 and Center of Excellence grant number 251314), the Finnish Cancer Foundation, the Sigrid Juselius Foundation, the University of Oulu, the University of Oulu Support Foundation and the special Governmental EVO funds for Oulu University Hospital-based research activities. The Ontario Familial Breast Cancer Registry (OFBCR) was supported by grant UM1 CA164920 from the National Cancer Institute (USA). The content of this manuscript does not necessarily reflect the views or policies of the National Cancer Institute or any of the collaborating centres in the Breast Cancer Family Registry (BCFR), nor does mention of trade names, commercial products, or organisations imply endorsement by the USA Government or the BCFR. The ORIGO study was supported by the Dutch Cancer Society (RUL 1997-1505) and the Biobanking and Biomolecular Resources Research Infrastructure (BBMRI-NL CP16). The PBCS was funded by Intramural Research Funds of the National Cancer Institute, Department of Health and Human Services, USA. The pKARMA study was supported by Märit and Hans Rausings Initiative Against Breast Cancer. The RBCS was funded by the Dutch Cancer Society (DDHK 2004-3124, DDHK 2009-4318). The SASBAC study was supported by funding from the Agency for Science, Technology and Research of Singapore (A*STAR), the US National Institute of Health (NIH) and the Susan G. Komen Breast Cancer Foundation. The SBCGS was supported primarily by NIH grants R01CA64277, R01CA148667, and R37CA70867. Biological sample preparation was conducted the Survey and Biospecimen Shared Resource, which is supported by P30 CA68485. The scientific development and funding of this project were, in part, supported by the Genetic Associations and Mechanisms in Oncology (GAME-ON) Network U19 CA148065.The SBCS was supported by Yorkshire Cancer Research S295, S299, S305PA and Sheffield Experimental Cancer Medicine Centre. The SCCS is supported by a grant from the National Institutes of Health (R01 CA092447). Data on SCCS cancer cases used in this publication were provided by the Alabama Statewide Cancer Registry; Kentucky Cancer Registry, Lexington, KY; Tennessee Department of Health, Office of Cancer Surveillance; Florida Cancer Data System; North Carolina Central Cancer Registry, North Carolina Division of Public Health; Georgia Comprehensive Cancer Registry; Louisiana Tumor Registry; Mississippi Cancer Registry; South Carolina Central Cancer Registry; Virginia Department of Health, Virginia Cancer Registry; Arkansas Department of Health, Cancer Registry, 4815 W. Markham, Little Rock, AR 72205. The Arkansas Central Cancer Registry is fully funded by a grant from National Program of Cancer Registries, Centers for Disease Control and Prevention (CDC). Data on SCCS cancer cases from Mississippi were collected by the Mississippi Cancer Registry which participates in the National Program of Cancer Registries (NPCR) of the Centers for Disease Control and Prevention (CDC). The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the CDC or the Mississippi Cancer Registry. SEARCH is funded by a programme grant from Cancer Research UK (C490/A10124) and supported by the UK National Institute for Health Research Biomedical Research Centre at the University of Cambridge. Targeted sequencing in SEARCH was supported by Cancer Research UK grants C1287/A16563 to DFE and C8197/A16565 to AMD. SEBCS was supported by the BRL (Basic Research Laboratory) programme through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (2012-0000347). SGBCC is funded by the NUS start-up Grant, National University Cancer Institute Singapore (NCIS) Centre Grant and the NMRC Clinician Scientist Award. Additional controls were recruited by the Singapore Consortium of Cohort Studies-Multi-ethnic cohort (SCCS-MEC), which was funded by the Biomedical Research Council, grant number: 05/1/21/19/425. SKKDKFZS is supported by the DKFZ. The SZBCS was supported by Grant PBZ_KBN_122/P05/2004. The TBCS was funded by The National Cancer Institute Thailand. TNBCC was supported by: a Specialized Program of Research Excellence (SPORE) in Breast Cancer (CA116201), a grant from the Breast Cancer Research Foundation, a generous gift from the David F. and Margaret T. Grohne Family Foundation, the Hellenic Cooperative Oncology Group research grant (HR R_BG/04) and the Greek General Secretary for Research and Technology (GSRT) Program, Research Excellence II, the European Union (European Social Fund—ESF), and Greek national funds through the Operational Program ‘Education and Lifelong Learning’ of the National Strategic Reference Framework (NSRF)—ARISTEIA. The TWBCS is supported by the Taiwan Biobank project of the Institute of Biomedical Sciences, Academia Sinica, Taiwan. The UKBGS is funded by Breakthrough Breast Cancer and the Institute of Cancer Research (ICR), London. ICR acknowledges NHS funding to the NIHR Biomedical Research Centre.
Footnotes
Additional material is published online only. To view please visit the journal online (http://dx.doi.org/10.1136/jmedgenet-2015-103529).
Twitter Follow Steven Hart at @StevenNHart and Michelle Wong-Brown at @michellewwong
Competing interests
GM reports grants from Cancer Australia during the conduct of the study; personal fees from AstraZeneca outside the submitted work. AS reports grants from Breast Cancer Now (previously Breakthrough Breast Cancer) during the conduct of the study. SVT reports grants from US National Cancer Institute during the conduct of the study and is an inventor on most of the key BRCA1 and BRCA2 patents. The claims in these patents that constrained commercial genetic testing by entities other than Myriad Genetics have been overturned, expired or abandoned. MWB reports grants from ELAN Found during the conduct of the study. DEG has US Patent nos. 5747282; 5710001; 5837942; 6033857 with royalties paid by Myriad Genetics. CV reports grants from NCI during the conduct of the study. PAF reports grants and/or personal fees from Amgen, Novartis, Biomarin, Roche, Pfizer, GSK, TEVA and Genomic Health, outside the submitted work. RNL reports grants from Medical Research Council and Cancer Research UK during the conduct of the study.
Patient consent
Obtained.
Ethics approval
Ethics Committee of the International Agency for Research on Cancer (IARC; Lyon, France), the University of Utah IRB, Cambridgeshire Research Ethics Committee, Hunter New England Human Research Ethics Committee, the Peter MacCallum Cancer Centre Human Research Ethics Committee and the local IRBs of the BCFR and BCAC centres from which samples were received..
Provenance and peer review
Not commissioned; externally peer reviewed.
Contributors DFE coordinated the BCAC project, performed statistical analysis and drafted the manuscript. CL and AMD coordinated the targeted sequencing in SEARCH and genotyping in BCAC. BD and JA performed bioinformatics analysis of the SEARCH sequencing data. KAP assisted in the validation of SEARCH sequencing data. KM performed statistical analysis of the BCAC data. MKB and QW provided data management support for the BCAC. MS provided data management support for SEARCH. PDPP coordinated SEARCH. FLC-K: experimental design, coordination and supervision of the BRIP1 mutation screening for the BCFR study and interpretation of data. NR, GD and NF performed BRIP1 mutation screening for the BCFR study. JA, FD and MP performed BRIP1 mutation screening and contributed to interpretation of data for the BCFR study. CV managed BRIP1 mutation screening data for the BCFR study. NM performed Sanger confirmation of rare BRIP1 variants in the BCFR study. FL contributed to the study design and analysis of the data for the BCFR study, and to the writing of the manuscript. ERT and IGC performed BRIP1 mutation screening and contributed to interpretation of data for the Peter MacCallum study. SVT and DEG responsible for overall study design for BCFR, contributed to data analysis and helped to draft the manuscript. JL performed the NMD analysis. GC-T helped coordinate the study and draft the manuscript. JD, RNL, SA, KA, HA-C, VA, AOCS, CB, MWB, JB, DB, WJB, NVB, SEB, A-LB-D, HB, JC-C, KSC, J-YC, DMC, AC, SSC, KC, HD, PD, ME, PAF, JF, HF, FF, MG-C, GGG, GG, AG-N, PG, CAH, PH, SNH, MH, MJH, C-NH, HI, AJ, PAJ, EJ, NJ , MJ, MK, DK, KCF, V-MK, VK, DL, NL, LI, AL, JiLo, ArLo, JaLu, AM, SM, SaMa, KM, AM, GM, KM, IN, AO, PP, SYP, KP, SMR, SS, RKS, C-YS, X-OS, MCS, HS, AS, SHT, RAEMT, IT, DT, TT, CV, SV, MW-B, WZ, YZ, HN, RJS, ILA, AHW, JLH, FJC, RW, BB, EJS, MKS, AR, TD, HB, UH, SLN, RLM and OF provided DNA samples and/or phenotypic data. All authors read and approved the final manuscript.
References
- 1.Ghoussaini M, Pharoah PD, Easton DF. Inherited genetic susceptibility to breast cancer: the beginning of the end or the end of the beginning? AmJPathol. 2013;183:1038–51. doi: 10.1016/j.ajpath.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 2.Easton DF, Pharoah PD, Antoniou AC, Tischkowitz M, Tavtigian SV, Nathanson KL, Devilee P, Meindl A, Couch FJ, Southey M, Goldgar DE, Evans DG, Chenevix-Trench G, Rahman N, Robson M, Domchek SM, Foulkes WD. Gene-panel sequencing and the prediction of breast-cancer risk. N Engl J Med. 2015;372:2243–57. doi: 10.1056/NEJMsr1501341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cantor SB, Bell DW, Ganesan S, Kass EM, Drapkin R, Grossman S, Wahrer DC, Sgroi DC, Lane WS, Haber DA, Livingston DM. BACH1, a novel helicase-like protein, interacts directly with BRCA1 and contributes to its DNA repair function. Cell. 2001;105:149–60. doi: 10.1016/s0092-8674(01)00304-x. [DOI] [PubMed] [Google Scholar]
- 4.Litman R, Peng M, Jin Z, Zhang F, Zhang J, Powell S, Andreassen PR, Cantor SB. BACH1 is critical for homologous recombination and appears to be the Fanconi anemia gene product FANCJ. Cancer cell. 2005;8:255–65. doi: 10.1016/j.ccr.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 5.Levitus M, Waisfisz Q, Godthelp BC, de Vries Y, Hussain S, Wiegant WW, Elghalbzouri-Maghrani E, Steltenpool J, Rooimans MA, Pals G, Arwert F, Mathew CG, Zdzienicka MZ, Hiom K, De Winter JP, Joenje H. The DNA helicase BRIP1 is defective in Fanconi anemia complementation group J. Nat Genet. 2005;37:934–5. doi: 10.1038/ng1625. [DOI] [PubMed] [Google Scholar]
- 6.Seal S, Thompson D, Renwick A, Elliott A, Kelly P, Barfoot R, Chagtai T, Jayatilake H, Ahmed M, Spanova K, North B, McGuffog L, Evans DG, Eccles D, Easton DF, Stratton MR, Rahman N Breast Cancer Susceptibility C. Truncating mutations in the Fanconi anemia J gene BRIP1 are low-penetrance breast cancer susceptibility alleles. Nat Genet. 2006;38:1239–41. doi: 10.1038/ng1902. [DOI] [PubMed] [Google Scholar]
- 7.Frank B, Hemminki K, Meindl A, Wappenschmidt B, Sutter C, Kiechle M, Bugert P, Schmutzler RK, Bartram CR, Burwinkel B. BRIP1 (BACH1) variants and familial breast cancer risk: a case-control study. BMC Cancer. 2007;7:83. doi: 10.1186/1471-2407-7-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Catucci I, Milgrom R, Kushnir A, Laitman Y, Paluch-Shimon S, Volorio S, Ficarazzi F, Bernard L, Radice P, Friedman E, Peterlongo P. Germline mutations in BRIP1 and PALB2 in Jewish high cancer risk families. Fam Cancer. 2012;11:483–91. doi: 10.1007/s10689-012-9540-8. [DOI] [PubMed] [Google Scholar]
- 9.Wong MW, Nordfors C, Mossman D, Pecenpetelovska G, Avery-Kiejda KA, Talseth-Palmer B, Bowden NA, Scott RJ. BRIP1, PALB2, and RAD51C mutation analysis reveals their relative importance as genetic susceptibility factors for breast cancer. Breast Cancer Res Treat. 2011;127:853–9. doi: 10.1007/s10549-011-1443-0. [DOI] [PubMed] [Google Scholar]
- 10.Kuusisto KM, Bebel A, Vihinen M, Schleutker J, Sallinen SL. Screening for BRCA1, BRCA2, CHEK2, PALB2, BRIP1, RAD50, and CDH1 mutations in high-risk Finnish BRCA1/2-founder mutation-negative breast and/or ovarian cancer individuals. Breast Cancer Res. 2011;13:R20. doi: 10.1186/bcr2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cao AY, Huang J, Hu Z, Li WF, Ma ZL, Tang LL, Zhang B, Su FX, Zhou J, Di GH, Shen KW, Wu J, Lu JS, Luo JM, Yuan WT, Shen ZZ, Huang W, Shao ZM. Mutation analysis of BRIP1/BACH1 in BRCA1/BRCA2 negative Chinese women with early onset breast cancer or affected relatives. Breast Cancer Res Treat. 2009;115:51–5. doi: 10.1007/s10549-008-0052-z. [DOI] [PubMed] [Google Scholar]
- 12.Guénard F, Labrie Y, Ouellette G, Joly Beauparlant C, Simard J, Durocher F BRCAs I. Mutational analysis of the breast cancer susceptibility gene BRIP1/BACH1/FANCJ in high-risk non-BRCA1/BRCA2 breast cancer families. J Hum Genet. 2008;53:579–91. doi: 10.1007/s10038-008-0285-z. [DOI] [PubMed] [Google Scholar]
- 13.Michailidou K, Hall P, Gonzalez-Neira A, Ghoussaini M, Dennis J, Milne RL, Schmidt MK, Chang-Claude J, Bojesen SE, Bolla MK, Wang Q, Dicks E, Lee A, Turnbull C, Rahman N, Fletcher O, Peto J, Gibson L, Dos Santos Silva I, Nevanlinna H, Muranen TA, Aittomaki K, Blomqvist C, Czene K, Irwanto A, Liu J, Waisfisz Q, Meijers-Heijboer H, Adank M, Hereditary B, van der Luijt RB, Hein R, Dahmen N, Beckman L, Meindl A, Schmutzler RK, Muller-Myhsok B, Lichtner P, Hopper JL, Southey MC, Makalic E, Schmidt DF, Uitterlinden AG, Hofman A, Hunter DJ, Chanock SJ, Vincent D, Bacot F, Tessier DC, Canisius S, Wessels LF, Haiman CA, Shah M, Luben R, Brown J, Luccarini C, Schoof N, Humphreys K, Li J, Nordestgaard BG, Nielsen SF, Flyger H, Couch FJ, Wang X, Vachon C, Stevens KN, Lambrechts D, Moisse M, Paridaens R, Christiaens MR, Rudolph A, Nickels S, Flesch-Janys D, Johnson N, Aitken Z, Aaltonen K, Heikkinen T, Broeks A, Veer LJ, van der Schoot CE, Guenel P, Truong T, Laurent-Puig P, Menegaux F, Marme F, Schneeweiss A, Sohn C, Burwinkel B, Zamora MP, Perez JI, Pita G, Alonso MR, Cox A, Brock IW, Cross SS, Reed MW, Sawyer EJ, Tomlinson I, Kerin MJ, Miller N, Henderson BE, Schumacher F, Le Marchand L, Andrulis IL, Knight JA, Glendon G, Mulligan AM, Lindblom A, Margolin S, Hooning MJ, Hollestelle A, van den Ouweland AM, Jager A, Bui QM, Stone J, Dite GS, Apicella C, Tsimiklis H, Giles GG, Severi G, Baglietto L, Fasching PA, Haeberle L, Ekici AB, Beckmann MW, Brenner H, Muller H, Arndt V, Stegmaier C, Swerdlow A, Ashworth A, Orr N, Jones M, Figueroa J, Lissowska J, Brinton L, Goldberg MS, Labreche F, Dumont M, Winqvist R, Pylkas K, Jukkola-Vuorinen A, Grip M, Brauch H, Hamann U, Bruning T, Network G, Radice P, Peterlongo P, Manoukian S, Bonanni B, Devilee P, Tollenaar RA, Seynaeve C, van Asperen CJ, Jakubowska A, Lubinski J, Jaworska K, Durda K, Mannermaa A, Kataja V, Kosma VM, Hartikainen JM, Bogdanova NV, Antonenkova NN, Dork T, Kristensen VN, Anton-Culver H, Slager S, Toland AE, Edge S, Fostira F, Kang D, Yoo KY, Noh DY, Matsuo K, Ito H, Iwata H, Sueta A, Wu AH, Tseng CC, Van Den Berg D, Stram DO, Shu XO, Lu W, Gao YT, Cai H, Teo SH, Yip CH, Phuah SY, Cornes BK, Hartman M, Miao H, Lim WY, Sng JH, Muir K, Lophatananon A, Stewart-Brown S, Siriwanarangsan P, Shen CY, Hsiung CN, Wu PE, Ding SL, Sangrajrang S, Gaborieau V, Brennan P, McKay J, Blot WJ, Signorello LB, Cai Q, Zheng W, Deming-Halverson S, Shrubsole M, Long J, Simard J, Garcia-Closas M, Pharoah PD, Chenevix-Trench G, Dunning AM, Benitez J, Easton DF Breast, Ovarian Cancer Susceptibility C; Ovarian Cancer Research Group N; kConFab I, Australian Ovarian Cancer Study G. Large-scale genotyping identifies 41 new loci associated with breast cancer risk. Nat Genet. 2013;45:353–61. 361e1–2. doi: 10.1038/ng.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barnett GC, Shah M, Redman K, Easton DF, Ponder BA, Pharoah PD. Risk factors for the incidence of breast cancer: do they affect survival from the disease? J Clin Oncol. 2008;26:3310–6. doi: 10.1200/JCO.2006.10.3168. [DOI] [PubMed] [Google Scholar]
- 15.Kataoka M, Antoniou A, Warren R, Leyland J, Brown J, Audley T, Easton D. Genetic models for the familial aggregation of mammographic breast density. Cancer Epidemiol Biomarkers Prev. 2009;18:1277–84. doi: 10.1158/1055-9965.EPI-08-0568. [DOI] [PubMed] [Google Scholar]
- 16.Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnetjournal. 2011;17:10–2. [Google Scholar]
- 17.Li H. Exploring single-sample SNP and INDEL calling with whole-genome de novo assembly. Bioinformatics. 2012;28:1838–44. doi: 10.1093/bioinformatics/bts280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, Garimella K, Altshuler D, Gabriel S, Daly M, DePristo MA. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20:1297–303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DePristo MA, Banks E, Poplin R, Garimella KV, Maguire JR, Hartl C, Philippakis AA, del Angel G, Rivas MA, Hanna M, McKenna A, Fennell TJ, Kernytsky AM, Sivachenko AY, Cibulskis K, Gabriel SB, Altshuler D, Daly MJ. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat Genet. 2011;43:491–8. doi: 10.1038/ng.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kircher M, Witten DM, Jain P, O’Roak BJ, Cooper GM, Shendure J. A general framework for estimating the relative pathogenicity of human genetic variants. Nat Genet. 2014;46:310–15. doi: 10.1038/ng.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.John EM, Hopper JL, Beck JC, Knight JA, Neuhausen SL, Senie RT, Ziogas A, Andrulis IL, Anton-Culver H, Boyd N, Buys SS, Daly MB, O’Malley FP, Santella RM, Southey MC, Venne VL, Venter DJ, West DW, Whittemore AS, Seminara D Breast Cancer Family R. The Breast Cancer Family Registry: an infrastructure for cooperative multinational, interdisciplinary and translational studies of the genetic epidemiology of breast cancer. Breast Cancer Res. 2004;6:R375–89. doi: 10.1186/bcr801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tavtigian SV, Oefner PJ, Babikyan D, Hartmann A, Healey S, Le Calvez-Kelm F, Lesueur F, Byrnes GB, Chuang SC, Forey N, Feuchtinger C, Gioia L, Hall J, Hashibe M, Herte B, McKay-Chopin S, Thomas A, Vallee MP, Voegele C, Webb PM, Whiteman DC, Sangrajrang S, Hopper JL, Southey MC, Andrulis IL, John EM, Chenevix-Trench G Australian Cancer S, Breast Cancer Family R, Kathleen Cuningham Foundation Consortium for Research into Familial Aspects of Breast C. Rare, evolutionarily unlikely missense substitutions in ATM confer increased risk of breast cancer. Am J Hum Genet. 2009;85:427–46. doi: 10.1016/j.ajhg.2009.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Le Calvez-Kelm F, Lesueur F, Damiola F, Vallee M, Voegele C, Babikyan D, Durand G, Forey N, McKay-Chopin S, Robinot N, Nguyen-Dumont T, Thomas A, Byrnes GB, Hopper JL, Southey MC, Andrulis IL, John EM, Tavtigian SV Breast Cancer Family R. Rare, evolutionarily unlikely missense substitutions in CHEK2 contribute to breast cancer susceptibility: results from a breast cancer family registry case-control mutation-screening study. Breast Cancer Res. 2011;13:R6. doi: 10.1186/bcr2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park DJ, Lesueur F, Nguyen-Dumont T, Pertesi M, Odefrey F, Hammet F, Neuhausen SL, John EM, Andrulis IL, Terry MB, Daly M, Buys S, Le Calvez-Kelm F, Lonie A, Pope BJ, Tsimiklis H, Voegele C, Hilbers FM, Hoogerbrugge N, Barroso A, Osorio A, Giles GG, Devilee P, Benitez J, Hopper JL, Tavtigian SV, Goldgar DE, Southey MC Breast Cancer Family R, Kathleen Cuningham Foundation Consortium for Research into Familial Breast C. Rare mutations in XRCC2 increase the risk of breast cancer. Am J Hum Genet. 2012;90:734–9. doi: 10.1016/j.ajhg.2012.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Le Calvez-Kelm F, Oliver J, Damiola F, Forey N, Robinot N, Durand G, Voegele C, Vallee MP, Byrnes G, Registry BC, Hopper JL, Southey MC, Andrulis IL, John EM, Tavtigian SV, Lesueur F. RAD51 and breast cancer susceptibility: no evidence for rare variant association in the Breast Cancer Family Registry study. PLoS ONE. 2012;7:e52374. doi: 10.1371/journal.pone.0052374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park DJ, Tao K, Le Calvez-Kelm F, Nguyen-Dumont T, Robinot N, Hammet F, Odefrey F, Tsimiklis H, Teo ZL, Thingholm LB, Young EL, Voegele C, Lonie A, Pope BJ, Roane TC, Bell R, Hu H, Shankaracharya, Huff CD, Ellis J, Li J, Makunin IV, John EM, Andrulis IL, Terry MB, Daly M, Buys SS, Snyder C, Lynch HT, Devilee P, Giles GG, Hopper JL, Feng BJ, Lesueur F, Tavtigian SV, Southey MC, Goldgar DE. Rare mutations in RINT1 predispose carriers to breast and Lynch syndrome-spectrum cancers. Cancer Discov. 2014;4:804–15. doi: 10.1158/2159-8290.CD-14-0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Damiola F, Pertesi M, Oliver J, Le Calvez-Kelm F, Voegele C, Young EL, Robinot N, Forey N, Durand G, Vallee MP, Tao K, Roane TC, Williams GJ, Hopper JL, Southey MC, Andrulis IL, John EM, Goldgar DE, Lesueur F, Tavtigian SV. Rare key functional domain missense substitutions in MRE11A, RAD50, and NBN contribute to breast cancer susceptibility: results from a Breast Cancer Family Registry case-control mutation-screening study. Breast Cancer Res. 2014;16:R58. doi: 10.1186/bcr3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garritano S, Gemignani F, Voegele C, Nguyen-Dumont T, Le Calvez-Kelm F, De Silva D, Lesueur F, Landi S, Tavtigian SV. Determining the effectiveness of High Resolution Melting analysis for SNP genotyping and mutation scanning at the TP53 locus. BMC Genet. 2009;10:5. doi: 10.1186/1471-2156-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sawyer S, Mitchell G, McKinley J, Chenevix-Trench G, Beesley J, Chen XQ, Bowtell D, Trainer AH, Harris M, Lindeman GJ, James PA. A role for common genomic variants in the assessment of familial breast cancer. J Clin Oncol. 2012;30:4330–6. doi: 10.1200/JCO.2012.41.7469. [DOI] [PubMed] [Google Scholar]
- 30.Thompson ER, Gorringe KL, Rowley SM, Wong-Brown MW, McInerny S, Li N, Trainer AH, Devereux L, Doyle MA, Li J, Lupat R, Delatycki MB, LifePool I, Mitchell G, James PA, Scott RJ, Campbell IG. Prevalence of PALB2 mutations in Australian familial breast cancer cases and controls. Breast Cancer Res. 2015;17:111. doi: 10.1186/s13058-015-0627-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–60. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Delaneau O, Marchini J, Zagury JF. A linear complexity phasing method for thousands of genomes. Nat Methods. 2012;9:179–81. doi: 10.1038/nmeth.1785. [DOI] [PubMed] [Google Scholar]
- 33.Thompson D, Duedal S, Kirner J, McGuffog L, Last J, Reiman A, Byrd P, Taylor M, Easton DF. Cancer risks and mortality in heterozygous ATM mutation carriers. J Natl Cancer Inst. 2005;97:813–22. doi: 10.1093/jnci/dji141. [DOI] [PubMed] [Google Scholar]
- 34.Renwick A, Thompson D, Seal S, Kelly P, Chagtai T, Ahmed M, North B, Jayatilake H, Barfoot R, Spanova K, McGuffog L, Evans DG, Eccles D, Easton DF, Stratton MR, Rahman N Breast Cancer Susceptibility C. ATM mutations that cause ataxia-telangiectasia are breast cancer susceptibility alleles. Nat Genet. 2006;38:873–5. doi: 10.1038/ng1837. [DOI] [PubMed] [Google Scholar]
- 35.Consortium CBCC-C. CHEK2*1100delC and susceptibility to breast cancer: a collaborative analysis involving 10,860 breast cancer cases and 9,065 controls from 10 studies. Am J Hum Genet. 2004;74:1175–82. doi: 10.1086/421251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meijers-Heijboer H, van den Ouweland A, Klijn J, Wasielewski M, de Snoo A, Oldenburg R, Hollestelle A, Houben M, Crepin E, van Veghel-Plandsoen M, Elstrodt F, van Duijn C, Bartels C, Meijers C, Schutte M, McGuffog L, Thompson D, Easton D, Sodha N, Seal S, Barfoot R, Mangion J, Chang-Claude J, Eccles D, Eeles R, Evans DG, Houlston R, Murday V, Narod S, Peretz T, Peto J, Phelan C, Zhang HX, Szabo C, Devilee P, Goldgar D, Futreal PA, Nathanson KL, Weber B, Rahman N, Stratton MR Consortium CH-BC. Low-penetrance susceptibility to breast cancer due to CHEK2(*)1100delC in noncarriers of BRCA1 or BRCA2 mutations. Nat Genet. 2002;31:55–9. doi: 10.1038/ng879. [DOI] [PubMed] [Google Scholar]
- 37.Antoniou AC, Casadei S, Heikkinen T, Barrowdale D, Pylkas K, Roberts J, Lee A, Subramanian D, De Leeneer K, Fostira F, Tomiak E, Neuhausen SL, Teo ZL, Khan S, Aittomaki K, Moilanen JS, Turnbull C, Seal S, Mannermaa A, Kallioniemi A, Lindeman GJ, Buys SS, Andrulis IL, Radice P, Tondini C, Manoukian S, Toland AE, Miron P, Weitzel JN, Domchek SM, Poppe B, Claes KB, Yannoukakos D, Concannon P, Bernstein JL, James PA, Easton DF, Goldgar DE, Hopper JL, Rahman N, Peterlongo P, Nevanlinna H, King MC, Couch FJ, Southey MC, Winqvist R, Foulkes WD, Tischkowitz M. Breast-cancer risk in families with mutations in PALB2. N Engl J Med. 2014;371:497–506. doi: 10.1056/NEJMoa1400382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rafnar T, Gudbjartsson DF, Sulem P, Jonasdottir A, Sigurdsson A, Jonasdottir A, Besenbacher S, Lundin P, Stacey SN, Gudmundsson J, Magnusson OT, le Roux L, Orlygsdottir G, Helgadottir HT, Johannsdottir H, Gylfason A, Tryggvadottir L, Jonasson JG, de Juan A, Ortega E, Ramon-Cajal JM, Garcia-Prats MD, Mayordomo C, Panadero A, Rivera F, Aben KK, van Altena AM, Massuger LF, Aavikko M, Kujala PM, Staff S, Aaltonen LA, Olafsdottir K, Bjornsson J, Kong A, Salvarsdottir A, Saemundsson H, Olafsson K, Benediktsdottir KR, Gulcher J, Masson G, Kiemeney LA, Mayordomo JI, Thorsteinsdottir U, Stefansson K. Mutations in BRIP1 confer high risk of ovarian cancer. Nat Genet. 2011;43:1104–7. doi: 10.1038/ng.955. [DOI] [PubMed] [Google Scholar]
- 39.Ramus SJ, Song H, Dicks E, Tyrer JP, Rosenthal AN, Intermaggio MP, Fraser L, Gentry-Maharaj A, Hayward J, Philpott S, Anderson C, Edlund CK, Conti D, Harrington P, Barrowdale D, Bowtell DD, Alsop K, Mitchell G, Group AS, Cicek MS, Cunningham JM, Fridley BL, Alsop J, Jimenez-Linan M, Poblete S, Lele S, Sucheston-Campbell L, Moysich KB, Sieh W, McGuire V, Lester J, Bogdanova N, Durst M, Hillemanns P, Odunsi K, Whittemore AS, Karlan BY, Dork T, Goode EL, Menon U, Jacobs IJ, Antoniou AC, Pharoah PD, Gayther SA Ovarian Cancer Association C. Germline Mutations in the BRIP1, BARD1, PALB2, and NBN genes in Women with Ovarian Cancer. J Natl Cancer Inst. 2015;107:djv214. doi: 10.1093/jnci/djv214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Couch FJ, Hart SN, Sharma P, Toland AE, Wang X, Miron P, Olson JE, Godwin AK, Pankratz VS, Olswold C, Slettedahl S, Hallberg E, Guidugli L, Davila JI, Beckmann MW, Janni W, Rack B, Ekici AB, Slamon DJ, Konstantopoulou I, Fostira F, Vratimos A, Fountzilas G, Pelttari LM, Tapper WJ, Durcan L, Cross SS, Pilarski R, Shapiro CL, Klemp J, Yao S, Garber J, Cox A, Brauch H, Ambrosone C, Nevanlinna H, Yannoukakos D, Slager SL, Vachon CM, Eccles DM, Fasching PA. Inherited mutations in 17 breast cancer susceptibility genes among a large triple-negative breast cancer cohort unselected for family history of breast cancer. J Clin Oncol. 2015;33:304–11. doi: 10.1200/JCO.2014.57.1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stankovic T, Kidd AM, Sutcliffe A, McGuire GM, Robinson P, Weber P, Bedenham T, Bradwell AR, Easton DF, Lennox GG, Haites N, Byrd PJ, Taylor AM. ATM mutations and phenotypes in ataxia-telangiectasia families in the British Isles: expression of mutant ATM and the risk of leukemia, lymphoma, and breast cancer. Am J Hum Genet. 1998;62:334–45. doi: 10.1086/301706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bernstein JL, Teraoka S, Southey MC, Jenkins MA, Andrulis IL, Knight JA, John EM, Lapinski R, Wolitzer AL, Whittemore AS, West D, Seminara D, Olson ER, Spurdle AB, Chenevix-Trench G, Giles GG, Hopper JL, Concannon P. Population-based estimates of breast cancer risks associated with ATM gene variants c.7271T>G and c. 1066–6T>G (IVS10–6T>G) from the Breast Cancer Family Registry. Hum Mutat. 2006;27:1122–8. doi: 10.1002/humu.20415. [DOI] [PubMed] [Google Scholar]
- 43.Goldgar DE, Healey S, Dowty JG, Da Silva L, Chen X, Spurdle AB, Terry MB, Daly MJ, Buys SM, Southey MC, Andrulis I, John EM, Khanna KK, Hopper JL, Oefner PJ, Lakhani S, Chenevix-Trench G Bcfr, kConFab. Rare variants in the ATM gene and risk of breast cancer. Breast Cancer Res. 2011;13:R73. doi: 10.1186/bcr2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.