Abstract
In the present investigation, metabolites of Streptomyces sp. MTN14 and Trichoderma harzianum ThU significantly enhanced biomass yield (3.58 and 3.48 fold respectively) in comparison to the control plants. The secondary metabolites treatments also showed significant augmentation (0.75–2.25 fold) in withanolide A, a plant secondary metabolite. Lignin deposition, total phenolic and flavonoid content in W. somnifera were maximally induced in treatment having T. harzianum metabolites. Also, Trichoderma and Streptomyces metabolites were found much better in invoking in planta contents and antioxidants compared with their live culture treatments. Therefore, identification of new molecular effectors from metabolites of efficient microbes may be used as biopesticide and biofertilizer for commercial production of W. somnifera globally.
Electronic supplementary material
The online version of this article (doi:10.1007/s12298-016-0359-x) contains supplementary material, which is available to authorized users.
Keywords: Microbes, Lignifications, Secondary metabolites, Withania somnifera
Introduction
Withania somnifera L. commonly referred Indian ginseng is a chief medicinal herb of paramount importance grown copiously in India and other parts of the world. It has a well recorded history of usage as an anti-tumor, antioxidant, hepatoprotective, anti-stress and also have anti-inflammatory activities (Mishra et al. 2000). The medicinal attributes and therapeutic actions of W. somnifera have been accredited to opulence of various lactones of steroidal nature (withanolides and withaferins) along with acyl steryl glycosides (sitoindosides VII–X) (Bhattacharya et al. 1997). However, inspite being widely cultivated over an area of 10,780 ha in India with a production of 8429 tones, the annual demand of 9127 tonnes (2004–2005) could not be achieved (Shrivastava and Sahu 2013). To meet up the growing demand and keeping in view yield as an important objective, chemical fertilizer are being continuously used (Rajasekar and Elango 2011). Though chemical additives have no doubt increased the productivity, but they have detrimental effects not only on soil heath but also on the phytochemical and pharmacological profile of the plant as well (Kothari et al. 2003). Thus, over the last few years at this critical juncture there has been an increased awareness in the usage of bioinoculants with regard to value addition and sustainable agriculture.
Microbes inhabiting the rhizospheric zone of plants are one of the most propitious groups of microorganisms providing ecological fitness to their host plant by plethora of mechanisms involving solubilization of mineral phosphates and other nutrients, production of phytohormones, vitamins, enzyme, siderophores and by synthesis of antibiotics compounds etc. (Lugtenberg and Kamilova 2009; Sarma et al. 2015). Further there are reports that induced defense signalling via priming with microbes have indirect effect on the concentration of various biomolecules having medicinal properties like antioxidant compounds and polyphenols (Ahuja et al. 2012; Wang and Zheng 2005). Thus, since last decade, in order to harness the beneficial biological activities of the microbes they have been actively used as live cultures in the fields. However, the biggest drawback in attaining the agricultural sustainability has been failure of these microbes in fields owing to a range of factors like varying environmental conditions, niche specificity and species specificity. Thus, recent investigations have suggested development and usage of innovative bioformulates based on microbial metabolites in order to maximize the advantageous effects along with the reduction in the risks linked with the dissemination of microorganisms into the environment. Thus, the present investigation was done with the intent to find out the effect of three different microbial genera namely Streptomycetes spp. (actinomycetes), Trichoderma harzianum (fungus) and Bacillus subtilis (bacteria) active cultures as well as their secondary metabolites on withanolide and withaferin content along with other phytochemical parameters viz., total chlorophyll, carotenoids, total phenolic, flavonoids, free radical scavenging activity and total antioxidant capacity.
Materials and methods
Microbial strains and culture conditions
Bacillus subtilis (JQ713565), Streptomyces sp. MTN14 (KF699062) and T. harzianum ThU were individually maintained on Luria–Bertani (LB) agar, glucose yeast malt (GYM) agar, and potato dextrose agar (PDA) respectively at room temperature and subcultured monthly. The selection of cultures was done on the basis of their plant growth enhancing traits (Gupta et al. 2015b; Gupta and Pandey 2015).
Inoculum preparation
A single pure colony of B. subtilis and Streptomyces sp. were individually inoculated in LB broth and GYM broth respectively. Cultures were incubated at 30 ± 2 °C on a rotary shaker at 120 rpm for 1 day (for bacteria) and 7 days (for actinobacteria) for full growth. The centrifugation at 6000×g for 10 min was done and the pellet obtained was dissolved in saline (0.85 %) to adjust the cell density to 2 × 108 colony forming units (CFU) ml−1. Similarly, the fungal inoculant T. harzianum ThU conidial suspension was subcultured using PD broth. For uniform growth the culture was incubated on a rotary shaker at 120 rpm for 7 days at 28 ± 2 °C. Conidia attached on the mycelial mat was harvested, and homogenously dissolved in 100 mL of 0.1 M phosphate buffer (K2HPO4, KH2PO4). Cell density for Trichoderma was fixed at 1.8 × 106 CFU mL−1 before inoculating in plants.
Culture filtrate preparation
Bacillus subtilis and Streptomyces strains were inoculated by a sterile loop in 250 mL Erlenmeyer flask containing LB broth and GYM broth respectively. A 250 mL Erlenmeyer flask having PD broth was inoculated with 7 mm of actively growing Trichoderma strain. For Bacillus the stationary culture was incubated for 5 days at 25 °C while for Trichoderma and Streptomyces sp. incubation time was kept 30 days. After cultivation of the cultures, metabolites were harvested by centrifugation at 10,000 rpm. Pellets were discarded while the filtrate was kept for further use.
Experimental design
Withania somnifera cv. Poshita, a cultivar reported with high yield was procured from the National Gene Bank for Medicinal and Aromatic Plants at the CSIR-CIMAP, Lucknow, India. Surface sterilization of the seeds was done for 5 min with 1 % (v/v) concentration of sodium hypochlorite solution. Afterwards the seeds were washed with sterile distilled water and submerged in it for 4 h. The healthy looking invariably sized seeds were selected and transferred to plastic pots containing soil sterilized in autoclave. Salubrious seedlings with four leaves were transferred 21 days after sowing into clay pots having 0.5 kg carrying capacity with sterile soil (3:1; soil: vermicompost). Cell pellets (CC) and metabolites (CF) harvested were added @ 10 mL per plant/pot. Following treatments were used for carrying out the experiment; (1) Control (2) STR_CF (3) BS_CF (4) TH_CF (5) STR_CC (6) BS_CC and (7) TH_CC.
In vitro analysis of phytochemical and antioxidant components
Determination of the leaf tissue’s total chlorophyll and carotenoid content was deduced following the protocol of Arnon (1949) and Lichtenthaler and Wellburn (1983) respectively. Folin–Ciocalteu method was used for determining the total phenolic content (TPC) (Singleton and Rossi 1965) and total flavonoid content (TFC) was done following the protocol of Zhishen et al. (1999) using rutin (mg g−1) as standard. Free radical scavenging activity (FRSA) and total antioxidant capacity (TAC) were quantified by the method described by Brand-Williams et al. (1995) and Zheng and Wang (2001), respectively.
Lignification study
Withania somnifera plants were harvested 45 days after application of different treatments. Transverse sections of stem were cut and examined under fluorescent microscope DMI 3000 B (Leica, Wetzlar, Germany) equipped with mercury lamp as a source of radiation of a wide range of wavelengths (250–700 nm) to induce autofluorescence of lignin.
In planta contents
The withanolide A and withaferin A content were estimated by HPLC (Scartezzini et al. 2007). Powdered 100 mg of dried root and leaf tissue were fractionated for 4 h with methanol followed by removal of residue using Whatman filter paper 1. The whole procedure was duplicated thrice and the different methanol extracts obtained were pooled and dried under vacuum using rota evaporator for conducting HPLC analysis. Sample was run in 40:60 (v/v) mixture of acetonitrile amended with 0.1 % (v/v) trifluoroacetic acid and water. The running rate of the sample was maintained at 1 ml min−1, and the detector wavelength was set at 220 nm. For detecting both the alkaloids, standards were purchased from ChromaDex (Irvine, CA).
Data analysis
Means of three replications were used for expressing the experimental data. The analysis of variance (ANOVA) techniques were performed and means were separated by Tukey’s multiple comparison test (P < 0.05) using SPSS package (SPSS V16.0, SPSS Inc., Chicago, USA). Correlation analysis was evaluated among the phytochemical attributes. The principal component analysis (PCA) was applied to produce components suitable to be used as response variables in present analysis.
Results and discussion
The glasshouse experiment showed that inoculation of W. somnifera seedlings with the three microbial cultures and their metabolites resulted in significant (P ≤ 0.05) increase in plant growth when compared with the control plants (Fig. 1). However, plants inoculated with metabolites outperformed the growth promoting effects with their microbial counterparts as maximum increase in plant fresh weight was observed in STR_CF (3.59 fold increase) followed by TH_CF (3.48 fold increase) with respect to control plants (Table 1). Nearly similar stimulatory effects were observed in total plant dry weight with a fold increase of 1.29–2.07 in all the treatments when compared with the control (Table 1). The enhancement in fresh and dry biomass maximally by STR_CF and TH_CF evaluated in this study might be attributed to growth promoting substances produced by the microbes in the culture filtrate which might have acted on plant metabolic processes. Trichoderma, Bacillus, and Streptomyces are rich source of naturally occurring secondary metabolites like cyclic lipopeptides, polypeptides, polyketides, alkaloids, quinines and various volatiles (Reino et al. 2008; Pathma et al. 2011) which not only have plant growth potential but antibiotic properties as well. Tarkka and Hampp (2008) and Prapagdee et al. (2008) reported the role of extracellular secondary metabolites of soil bacterium Streptomycetes in biotic interaction with the host plants. Likewise, the compounds mycosubtilin, rhizocticins, antifungal phosphonate oligopeptides and cyclic lipopeptide produced by different strains of B. subtilis enhanced the organism’s antagonistic and plant growth promotion activities (Leclère et al. 2005; Borisova et al. 2010; Malfanova et al. 2012). Vinale et al. (2008) reported Trichoderma species to be well known producers of compounds that substantially affect the metabolism of the plant. In a study conducted by Verma et al. (2014) T. harzanium culture filtrate at the dose of 3.3 % v/v was able to induce maximum biomass accumulation in embryogenic cell suspensions of latex free Papaver somniferum. Similarly, a significant increment in root elongation and germination was observed by the culture filtrate of an indole acetic acid producing S. viridis NBRC 13373 in maize (Zea mays) and cow pea (Bruguiera parviflora) seeds.
Fig. 1.
Effect of cell pellets (CC) and metabolites (CF) of various treatments (STR_CF, BS_CF, TH_CF, STR_CC, BS_CC and TH_CC) with respect to control on seedling growth of W. somnifera
Table 1.
Effect of microbial inoculants and their secondary metabolites on plant growth parameters and in planta contents of W. somnifera
| Treatments | Fresh plant wt (g) | Dry plant wt (g) | Withanolide A (%) | Withaferin A (%) |
|---|---|---|---|---|
| Control | 5.60 ± 0.68d | 1.40 ± 0.13d | 0.04 ± 0.01bc | 0.02 ± 0.01b |
| STR_CF | 20.11 ± 1.28a | 2.90 ± 0.24a | 0.06 ± 0.01ab | 0.06 ± 0.01ab |
| BS_CF | 11.90 ± 1.55ab | 1.90 ± 0.24cd | 0.03 ± 0.01bc | 0.04 ± 0.01ab |
| TH_CF | 19.50 ± 1.32ab | 2.70 ± 0.48abc | 0.09 ± 0.01a | 0.05 ± 0.01ab |
| STR_CC | 15.30 ± 1.34bc | 2.00 ± 0.20bcd | 0.03 ± 0.00bc | 0.05 ± 0.01ab |
| BS_CC | 15.00 ± 1.41bc | 1.80 ± 0.16d | 0.02 ± 0.01c | 0.08 ± 0.01a |
| TH_CC | 17.00 ± 1.47ab | 2.80 ± 0.15ab | 0.05 ± 0.01bc | 0.06 ± 0.01ab |
Results are means of three replicates ± standard error and means within a column trailed by the same alphabet are not significantly dissimilar (P ≤ 0.05)
A significant (P ≤ 0.05) enhancement of 2.25 fold in withanolide A in W. somnifera extract was observed in TH_CF treatment followed by 1.50 fold in STR_CF over the control. Similarly, withaferin A was also found to be significantly increased in the leaves of microbe treated plants. The maximum increase in withaferin A by 4.00 fold was recorded in BS_CC followed by 3.00 fold in both STR_CF and TH_CC treatments as compared to the control plants (Table 1). Recently, a study showed that application of plant growth promoting microbes significantly enhanced secondary metabolite content in medicinal and aromatic plants like Bacopa monnieri, W. somnifera and Pelargonium graveolens (Rajasekar and Elango 2011; Gupta et al. 2015b, 2016). Increase in antioxidant capacity in TH_CF could also be positively correlated with increased Withanolide A, a well known antioxidant molecule. The results thus indicate that microbial metabolites could be a potential source for alkaloid enhancement in W. somnifera.
Total chlorophyll and carotenoid were increased significantly in all the treatments with respect to control (Fig. 2a, b). In context with the primary pigments, overall the metabolites of the three microbes again outpassed the effects of microbial cultures. In case of total chlorophyll, maximum fold increase of 3.99 with respect to control was observed in TH_CF followed by 2.60 fold increase in STR_CC (Fig. 2a). Similarly, for total carotenoid content, maximum fold increase of 2.66 with respect to control was observed in TH_CF followed by 1.42 fold increase in BS_CF (Fig. 2b). Photosynthesis is regulated by the environmental conditions and during stress conditions primary pigment, chlorophyll and carotenoid are foremost affected. A positive and direct association was found among total chlorophyll and carotenoids (r = 0.957*) (Table 2). In a study conducted by Zhang et al. (2008), plant growth promoting soil bacterium B. subtilis GB03 augmented photosynthetic capability by elevating photosynthetic efficiency and amount of chlorophyll in Arabidopsis.
Fig. 2.
Effect of cell pellets (CC) and metabolites (CF) of various treatments (STR_CF, BS_CF, TH_CF, STR_CC, BS_CC and TH_CC) on a total chlorophyll, b carotenoids, c total phenolic content, and d total flavonoid content with respect to control on biochemical parameters of W. Somnifera. Values used in graphs were average of three independent replicates while the vertical bars were used for denoting standard error of the mean values. Varying alphabets on vertical bars designate significant dissimilarity among treatments (P ≤ 0.05; Tukey’s multiple comparison test). The data presented are from representative experiments that were repeated twice. (1) Control (2) STR_CF (3) BS_CF (4) TH_CF (5) STR_CC (6) BS_CC (7) TH_CC
Table 2.
Correlation analysis among various phytochemical parameters of W. somnifera
| Variables | Total chlorophyll | Carotenoids | TPC | TFC | FRSA | TAC |
|---|---|---|---|---|---|---|
| Total chlorophyll | 1 | 0.957* | 0.826* | 0.933* | 0.683 | 0.728 |
| Carotenoids | 1 | 0.787* | 0.897* | 0.521 | 0.663 | |
| TPC | 1 | 0.685 | 0.679 | 0.933* | ||
| TFC | 1 | 0.749 | 0.685 | |||
| FRSA | 1 | 0.825* | ||||
| TAC | 1 |
TPC total phenolic content, TFC total flavonoid content, FRSA free radical scavenging activity, TAC total antioxidant capacity
* Correlation is significant at P < 0.05
Plants possess various array of antioxidants molecules majorly phenols and flavonoids that mitigate the reactive oxygen species and defend host cells from adverse conditions. In the current study, the microbes and their respective metabolites not only promoted plant growth but also systemically stimulated the production of phenols and flavonoids in the leaves of W. somnifera with respect to control (Fig. 2c, d). The total phenolic content was recorded to be higher in all the treatments than that of the control plants. However, maximum fold increase by 2.85 was observed in TH_CF followed by 2.37 fold increase in treatment having culture filtrate of Streptomyces treatment (Fig. 2c). Similarly, similar results were obtained in case of total flavonoid content where maximum fold increase by 2.22 with respect to control was observed again in TH_CF followed by culture filtrate of Bacillus (BS_CF) (Fig. 2d). Moreover, induction of TPC and TFC were strongly and significantly linked with induced chlorophyll (r = 0.826* and r = 0.787* respectively), and carotenoids (r = 0.933* and r = 0.897* respectively), which are reported to play an important role in plant growth (Table 2). The results obtained suggested that the microbial culture filtrate had certain elicitors which played a vital role in enhancing the phenolic and flavonoid content in W. somnifera leaves. Flavonoids and phenolics acids are the principal groups of secondary metabolites and bioactive compounds in plants known for their role in plant resistance. Recently, the involvement of secondary metabolites in modulating plant health and resistance has been studied thoroughly (Vinale et al. 2008, 2009). The results are also in conformity with the study of other researchers who also showed promising effect of microbes on enhancement of phenolics and flavnoids (Gupta and Pandey 2015; Singh et al. 2015). These results suggest that the cell culture and culture filtrate of microbes play a vital role in boosting the phenolic and flavonoid contents in W. somnifera and can be effectively used for strengthening the plants reactive oxygen species scavenging machinery. In the recent past also there has been tremendous developments relating to the exploitation of Trichoderma spp. where one factor that majorly contributes to their advantageous biological activities is related to the extensive diversity of metabolites that they produce (Vinale et al. 2012). In the present study, Trichoderma metabolites might have acted as elicitors of plant defense response which are reported to contain various classes of proteins, avirulence gene products and low molecular weight compounds (Harman et al. 2004).
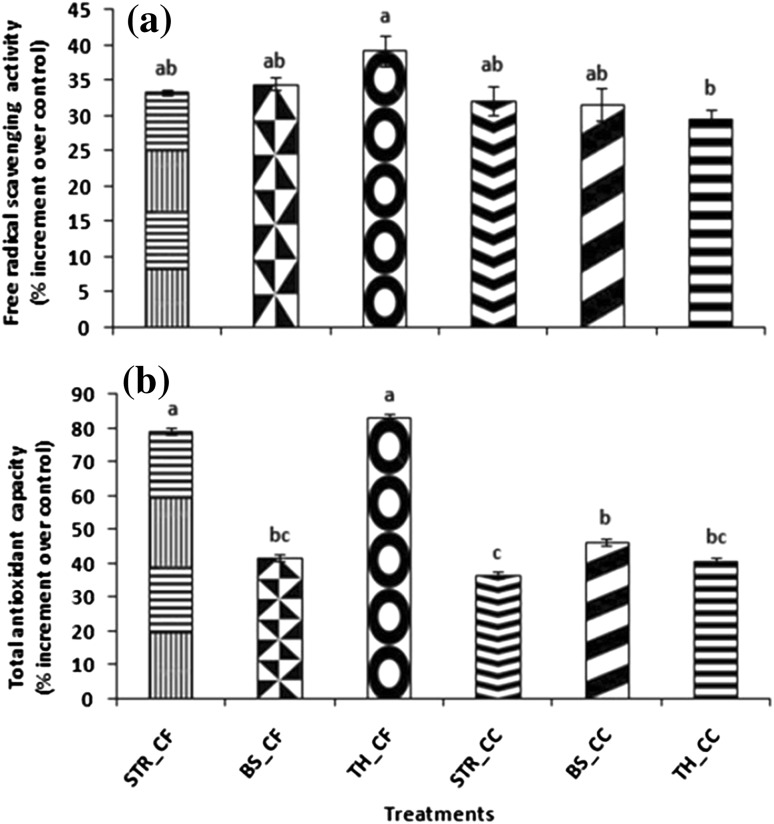
In order to nullify the inimical effects of elevated levels of ROS engendered by stress conditions, plants are endowed with inbuilt range enzymes having potential to scavenge such ROS. However, recently reports have started pouring in with the role of bioinoculants in activating antioxidant system (Nautiyal et al. 2008; Singh et al. 2014; Gupta et al. 2015a). In the present study, all the microbial treatments and their respective metabolites significantly enhanced the free radical scavenging activity and total antioxidant content with respect to control (Fig. 3a, b). However, the results obtained in metabolite treatments were far more effective than microbial culture application as DPPH was maximally increased by 39.21 % in TH_CF followed by BS_CF (39.21 %) with respect to control (Fig. 3a). Similarly, TAC was maximally increased by 83.23 % in TH_CF followed by STR_CF (78.98 %) with respect to control (Fig. 3b). A positive correlation between FRSA and TAC with TPC (r = 0.679 and r = 0.933* respectively) and TFC (r = 0.749 and r = 0.685 respectively) was also observed in the present study (Table 2). The results obtained are in agreement with the previous work of Singh et al. (2014) where beneficial compatible microbes viz. fluorescent pseudomonads (PHU 094), T. harzianum (THU 0816) and Mesorhizobium sp. (RL 091), significantly enhanced antioxidants and free radical scavenging activity in chickpea edible parts. Similarly, in another report Bacillus spp. ameliorated essential oil yield and diminished root-knot infestation in sweet basil grown in fields by augmentation of total antioxidants and free radical scavenging activity (Gupta et al. 2015a). Overall, the augmentation in activity of reactive oxygen species scavenging molecules in treatments particularly Trichoderma metabolites supports the hypothesis that the elicitors present in the metabolites enhance the resistance of plants by activating antioxidant machinery.
Fig. 3.
Effect of cell pellets (CC) and metabolites (CF) of various treatments (STR_CF, BS_CF, TH_CF, STR_CC, BS_CC and TH_CC) on a free radical scavenging activity and b total antioxidant capacity with respect to control on biochemical parameters of W. Somnifera. Values used in graphs were average of three independent replicates while the vertical bars were used for denoting standard error of the mean values. Varying alphabets on vertical bars designate significant dissimilarity among treatments (P ≤ 0.05; Tukey’s multiple comparison test). The data presented are from representative experiments that were repeated twice. (1) Control (2) STR_CF (3) BS_CF (4) TH_CF (5) STR_CC (6) BS_CC (7) TH_CC
Variations in lignin deposition were observed in stem sections of dissimilar treatments when compared with the control set (Fig. S1). Lignin, a polymeric phenylpropanoid compound which autofluoresces blue under fluorescent microscope provides strength to the plant. Maximum and uniform lignin deposition (blue ring) was observed in TH_CF followed by treatment having cell culture of Trichoderma (TH_CC). In the control plants, least lignin deposition was observed as intermittent lignin deposits were observed (Fig. S1). In the present investigation, disparity in lignin deposition in W. somnifera could be accredited to the microbes and their metabolites which might have acted as elicitor in lignifications process. Recently, study conducted by Singh et al. (2013) showed much better deposition of lignin in vascular bundles of chickpea stem in combination of T. harzianum, fluorescent pseudomonads and Rhizobium isolates than their single counterparts.
The results procured from the present experiment was further validated by PCA as congregation of treatments revealed formation of four clusters: control and BS_CF, BS_CC, TH_CC and STR_CC, STR_CF and TH_CF. The STR_CF and TH_CF formed a separate group where various physiological, in planta contents and biochemical parameters of plant were increased significantly. The PCA contributes 85 % of the total variance where PC1 contributed 67 %, and PC2 contributed 18 % of the total variance (Fig. S2). The PCA results showed that attributes such as TPC, TFC, total chlorophyll, caretenoids and withanolide A were significantly increased in TH_CF while the other parameters such as withaferin A, fresh and dry plant weight, free radical scavenging activity and total antioxidant capacity were increased in STR_CF. STR_CF and TH_CF significantly influenced the plant productivity, in planta contents and phytochemicals parameters which in turn contributed to overall plant development and enhanced its economic value. In general, all physiological and biochemical parameters were consistently higher in the TH_CF and STR_CF treatments as compared to the other treatments and control. It might thus be speculated that the metabolites of culture provided all the required nutrients and antioxidants in a much better way than their cell cultures.
Conclusions
The findings of the present work clearly highlighted the role of secondary metabolites especially that of Trichoderma and Streptomyces in invoking defense responses and in planta contents when compared with their live culture treatments. In near future, identification of new molecular effectors from metabolites may support the application of such molecules as new biopesticides and biofertilizers. Also since, the role of beneficial rhizospheric microorganisms in context with medicinal plants and their efficacy on the production of bioactive molecules is still unexplored, it is expected that this work will encourage use of metabolites in medicinal plants particularly the cultivated ones.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
The authors express their sincere gratitude to the Director, CSIR-CIMAP, Lucknow, India for providing necessary facilities during experimental period.
Author contributions
A.S., R.P.: Conceived and designed the experiments, manuscript writing. A.S., R.G.: Performed and conceptualization of the experimental data. M.S., M.M.G.: Performed and analyzed the HPLC data. R.P.: Critical revision of the manuscript. All authors read and approved the final manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that there exists no potential conflict of interest among them.
References
- Ahuja I, Kissen R, Bones AM. Phytoalexins in defense against pathogens. Trends Plant Sci. 2012;17:73–90. doi: 10.1016/j.tplants.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Arnon DI. Copper enzymes in isolated chloroplasts, polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949;24:1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya SK, Satyan KS, Ghosal S. Antioxidant activity of glycowithanolides from Withania somnifera. Ind J Exp Biol. 1997;35:236–239. [PubMed] [Google Scholar]
- Borisova SA, Circello BT, Zhang JK, van der Donk WA, Metcalf WW. Biosynthesis of rhizocticins, antifungal phosphonate oligopeptides produced by Bacillus subtilis ATCC6633. Chem Biol. 2010;17:28–37. doi: 10.1016/j.chembiol.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand-Williams W, Cuvelier ME, Berset CLWT. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci Technol. 1995;28:25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- Gupta R, Pandey R. Microbial interference ameliorates essential oil yield and diminishes root-knot infestation in sweet basil under field conditions. Biocontrol Sci Technol. 2015;25:1165–1179. doi: 10.1080/09583157.2015.1036728. [DOI] [Google Scholar]
- Gupta R, Saikia SK, Pandey R. Bioconsortia augments antioxidant and yield in Matricaria recutita L. against Meloidogyne incognita (Kofoid and White) Chitwood Infestation. Proc Natl Acad Sci Ind Sect B: Biol Sci. 2015 [Google Scholar]
- Gupta R, Tiwari S, Saikia SK, Shukla V, Singh R, Singh SP, Ajay Kumar PV, Pandey R. Exploitation of microbes for enhancing bacoside content and reduction of Meloidogyne incognita infestation in Bacopa monnieri L. Protoplasma. 2015;252:53–61. doi: 10.1007/s00709-014-0657-5. [DOI] [PubMed] [Google Scholar]
- Gupta R, Singh A, Pandey R. Microbes based technology ameliorates glandular trichomes, secondary metabolites and antioxidant in Pelargonium graveolens L’Her. J Sci Food Agric. 2016 doi: 10.1002/jsfa.7617. [DOI] [PubMed] [Google Scholar]
- Harman GE, Howell CR, Viterbo A, Chet I, Lorito M. Trichoderma species—opportunistic, avirulent plant symbionts. Nat Rev Microbiol. 2004;2:43–56. doi: 10.1038/nrmicro797. [DOI] [PubMed] [Google Scholar]
- Kothari SK, Singh CP, Vijay Kumar Y, Singh K. Morphology, yield and quality of ashwagandha (Withania somnifera L. Dunal) roots and its cultivation economics as influenced by tillage depth and plant population density. J Horticul Sci Biotechnol. 2003;78:422–425. doi: 10.1080/14620316.2003.11511642. [DOI] [Google Scholar]
- Leclère V, Béchet M, Adam A, Guez JS, Wathelet B, Ongena M, Thonart P, Gancel F, Chollet-Imbert M, Jacques P. Mycosubtilin overproduction by Bacillus subtilis BBG100 enhances the organism’s antagonistic and biocontrol activities. Appl Environ Microbiol. 2005;71:4577–4584. doi: 10.1128/AEM.71.8.4577-4584.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenthaler HK, Wellburn AR. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem Soc Trans. 1983;11:591–592. doi: 10.1042/bst0110591. [DOI] [Google Scholar]
- Lugtenberg B, Kamilova F. Plant-growth-promoting rhizobacteria. Ann Rev Microbiol. 2009;63:541–556. doi: 10.1146/annurev.micro.62.081307.162918. [DOI] [PubMed] [Google Scholar]
- Malfanova N, Franzil L, Lugtenberg B, Chebotar V, Ongena M. Cyclic lipopeptide profile of the plant-beneficial endophytic bacterium Bacillus subtilis HC8. Arch Microbiol. 2012;194:893–899. doi: 10.1007/s00203-012-0823-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra LC, Singh BB, Dagenais S. Scientific basis for the therapeutic use of Withania somnifera (ashwagandha): a review. Alter Med Rev. 2000;5:334–346. [PubMed] [Google Scholar]
- Nautiyal CS, Govindarajan R, Lavania M, Pushpangadan P. Novel mechanism of modulating natural antioxidants in functional foods: involvement of plant growth promoting rhizobacteria NRRL B-30488. J Agric Food Chem. 2008;56:4474–4481. doi: 10.1021/jf073258i. [DOI] [PubMed] [Google Scholar]
- Pathma J, Rahul GR, Kamaraj KR, Subashri R, Sakthivel N. Secondary metabolite production by bacterial antagonists. J Biol Control. 2011;25:165–181. [Google Scholar]
- Prapagdee B, Kuekulvong C, Mongkolsuk S. Antifungal potential of extracellular metabolites produced by Streptomyces hygroscopicus against phytopathogenic fungi. Int J Biol Sci. 2008;4:330–337. doi: 10.7150/ijbs.4.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasekar S, Elango R. Effect of microbial consortium on plant growth and improvement of alkaloid content in Withania somnifera (Ashwagandha) Curr Bot. 2011;2:27–30. [Google Scholar]
- Reino JL, Guerrero RF, Hernández-Galán R, Collado IG. Secondary metabolites from species of the biocontrol agent Trichoderma. Phytochem Rev. 2008;7:89–123. doi: 10.1007/s11101-006-9032-2. [DOI] [Google Scholar]
- Sarma BK, Yadav SK, Singh S, Singh HB. Microbial consortium-mediated plant defense against phytopathogens: readdressing for enhancing efficacy. Soil Biol Biochem. 2015;87:25–33. doi: 10.1016/j.soilbio.2015.04.001. [DOI] [Google Scholar]
- Scartezzini P, Antognoni F, Conte L, Maxia A, Troia A, Poli F. Genetic and phytochemical difference between some Indian and Italian plants of Withania somnifera (L.) Dunal. Nat Prod Res. 2007;21:923–932. doi: 10.1080/14786410701500169. [DOI] [PubMed] [Google Scholar]
- Shrivastava AK, Sahu PK. Economics of yield and production of alkaloid of Withania somnifera (L.) Dunal. Am J Plant Sci. 2013;4:2023–2030. doi: 10.4236/ajps.2013.410253. [DOI] [Google Scholar]
- Singh A, Sarma BK, Upadhyay RS, Singh HB. Compatible rhizosphere microbes mediated alleviation of biotic stress in chickpea through enhanced antioxidant and phenylpropanoid activities. Microbiol Res. 2013;168:33–40. doi: 10.1016/j.micres.2012.07.001. [DOI] [PubMed] [Google Scholar]
- Singh A, Jain A, Sarma BK, Upadhyay RS, Singh HB. Beneficial compatible microbes enhance antioxidants in chickpea edible parts through synergistic interactions. LWT Food Sci Technol. 2014;56:390–397. doi: 10.1016/j.lwt.2013.11.030. [DOI] [Google Scholar]
- Singh SP, Gupta R, Gaur R, Srivastava AK. Antagonistic actinomycetes mediated resistance in Solanum lycopersicon Mill. against Rhizoctonia solani Kühn. Proc Natl Acad Sci Ind Sect B: Biol Sci. 2015 [Google Scholar]
- Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Viticul. 1965;16:144–158. [Google Scholar]
- Tarkka M, Hampp R. Secondary metabolites of soil streptomycetes in biotic interactions. In: Karlovsky P, editor. Secondary metabolites in soil ecology. Berlin, Heidelberg: Springer; 2008. pp. 107–126. [Google Scholar]
- Verma P, Khan SA, Mathur AK, Ghosh S, Shanker K, Kalra A. Improved sanguinarine production via biotic and abiotic elicitations and precursor feeding in cell suspensions of latex-less variety of Papaver somniferum with their gene expression studies and upscaling in bioreactor. Protoplasma. 2014;251:1359–1371. doi: 10.1007/s00709-014-0638-8. [DOI] [PubMed] [Google Scholar]
- Vinale F, Sivasithamparam K, Ghisalberti EL, Marra R, Barbetti MJ, Li H, Woo SL, Lorito M. A novel role for Trichoderma secondary metabolites in the interactions with plants. Physiol Mol Plant Pathol. 2008;72:80–86. doi: 10.1016/j.pmpp.2008.05.005. [DOI] [Google Scholar]
- Vinale F, Ghisalberti EL, Sivasithamparam K, Marra R, Ritieni A, Ferracane R, Lorito M. Factors affecting the production of Trichoderma harzianum secondary metabolites during the interaction with different plant pathogens. Lett Appl Microbiol. 2009;48:705–711. doi: 10.1111/j.1472-765X.2009.02599.x. [DOI] [PubMed] [Google Scholar]
- Vinale F, Sivasithamparam K, Ghisalberti EL, Ruocco M, Wood S, Lorito M. Trichoderma secondary metabolites that affect plant metabolism. Nat Prod Commun. 2012;7:1545–1550. [PubMed] [Google Scholar]
- Wang SY, Zheng W. Preharvest application of methyl jasmonate increases fruit quality and antioxidant capacity in raspberries. Intern J Food Sci Technol. 2005;40:187–195. doi: 10.1111/j.1365-2621.2004.00930.x. [DOI] [Google Scholar]
- Zhang H, Xie X, Kim MS, Kornyeyev DA, Holaday S, Paré PW. Soil bacteria augment Arabidopsis photosynthesis by decreasing glucose sensing and abscisic acid levels in planta. Plant J. 2008;56:264–273. doi: 10.1111/j.1365-313X.2008.03593.x. [DOI] [PubMed] [Google Scholar]
- Zheng W, Wang SY. Antioxidant activity and phenolic compounds in selected herbs. J Agric Food Chem. 2001;49:5165–5170. doi: 10.1021/jf010697n. [DOI] [PubMed] [Google Scholar]
- Zhishen J, Mengcheng T, Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64:555–559. doi: 10.1016/S0308-8146(98)00102-2. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.