Abstract
Adherence to antiretroviral medications is usually expressed in terms of the proportion of doses taken. However, the timing of doses taken may also be an important dimension to overall adherence. Little is known about whether patients who mistime doses are also more likely to skip doses. Using data from the completed Adherence for Life randomized controlled trial, we created visual and statistical models to capture and analyze dose timing data collected longitudinally with electronic drug monitors (EDM). From scatter plots depicting dose time versus calendar date, we identified dominant patterns of dose taking and calculated key features [slope of line over calendar date; residual mean standard error (RMSE)]. Each was assessed for its ability to categorize subjects with ‘suboptimal’ (<95 % of doses taken) using area under the receiver operating characteristic (AROC) curve analysis. Sixty eight subjects contributed EDM data, with ~300 to 400 observations/subject. While regression line slopes did not predict ‘sub-optimal’ adherence (AROC 0.51, 95 % CI 0.26–0.75), the variability in dose timing (RMSE) was strongly predictive (AROC 0.79, 95 % CI 0.62–0.97). Compared with the lowest quartile of RMSE (minimal dose time variability), each successive quartile roughly doubled the odds of ‘sub-optimal’ adherence (OR 2.1, 95 % CI 1.3–3.4). Patterns of dose timing and mistiming are strongly related to overall adherence behavior. Notably, individuals who skip doses are more likely to mistime doses, with the degree of risk positively correlated with the extent of dose timing variability.
Keywords: Electronic drug monitors, Dose timing, Adherence, Statistical methods, EDM
Introduction
Electronic drug monitors (EDM) are generally considered to be the most accurate tools for measuring adherence [1–3]. In addition to providing objective measures of ‘proportion of doses taken’, uniquely among all adherence measurement tools, EDM provide detailed data regarding the timing (or mistiming) of doses that were taken. Mistiming of doses is considered to be a subtle form of non-adherence [4]. However, with several notable exceptions that used sophisticated mathematical modeling [5–7], the dose timing capacity of EDM has rarely been exploited in the adherence literature. Instead, most studies only report adherence in terms of the percentage of doses taken, an outcome that may be highly specific for non-adherence but which may overly simplify a complex set of behaviors into a single, de-nuanced, summary metric.
Further, we hypothesize that ‘micro’ non-adherence (i.e., mistiming of doses) may well predict more serious ‘macro’ non-adherence (i.e., missing doses entirely). This relationship is analogous to the ‘Broken Window’ theory promulgated by the sociologists Wilson and Kelling (and subsequently embraced by law enforcement) [8]. This theory holds that failing to intervene in response to petty misdemeanors (e.g. vandalism) fosters an environment conducive to more serious crimes. Extending this analogy to HIV care generates a testable question: is micro non-adherence to ART a predictor of macro non-adherence? The recent advent of ‘real time’ electronic monitoring devices emphasizes the timeliness of this question [9–12]. Such devices monitor adherence continuously and report dose timing and skipped doses wirelessly, and can deliver a reminder message to a subject whose dose time has just elapsed [9, 11]. Such interventions presuppose the importance of micro non-adherence, though evidence supporting that assumption is still needed.
In response, we analyzed longitudinal EDM adherence data from a cohort of Chinese HIV patients on ART. Our analysis had three main objectives. First, given the exploratory nature of this work, we summarize key observations regarding the analytic processes that we developed. Second, as a descriptive analysis we comment on several unusual data artifacts within the longitudinal dose timing data sets that we identified, along with typical patterns of dose timing and mistiming at the individual subject level. Lastly, we explored how well different dose timing patterns or summary metrics identified subjects with sub-optimal adherence (defined as taking <95 % of prescribed doses). As an aid to other researchers, we provide the complete library of two versions of dose timing scatter plots for all subjects and the SAS codes used to generate these plots.
Methods
Data for this analysis came from the now completed Adherence For Life (AFL) study, a randomized controlled trial conducted between 2006 and 2007 that showed that systematically integrating objective adherence data from EDM into clinician-patient counseling sessions significantly improved adherence [13, 14]. AFL enrolled subjects living in Old Dali City, Yunnan Province, China, all of whom were receiving a twice-daily, 3-drug ART regimen consisting of AZT (or D4T), 3tC, and nevirapine (or efavirenz). At each monthly study visit, EDM data were downloaded and the patient’s medications were refilled. During the first six-month (pre-intervention) period, subjects’ adherence was continuously monitored by EDM without sharing these data with the subjects or their care providers. During the next 6 months, subjects were randomized to have their EDM data un-blinded (intervention) or to remain blinded (control); the effect of systematically incorporating the EDM adherence data (dubbed ‘EDM feedback’) on their adherence was then assessed across multiple outcomes, and found to have a potent impact on improving adherence rates [14]. Except as noted otherwise, the current analysis used data from the first 6-month, blinded, pre-intervention period.
The EDM system used in AFL was ‘ECaps’ (Information Mediary Corp, Ottawa, Canada). ECaps are electronic pill bottles with a pressure-activated microchip embedded in the bottle’s screw cap that records the time and date of every opening, providing a proxy for doses taken. Data from ECaps are transferred to a computer using a radiofrequency transponder where they can be visualized directly using the proprietary ECaps software, or downloaded to other formats (e.g., Excel) for further analysis.
Descriptive Analyses
In AFL, we had defined a dose as ‘on time’ if it fell within a compliance window of ±1 h of the prescribed dose time, which varied from subject to subject. Thus, for a scheduled dose time of 9 AM, the compliance window extended from 8 to 10 AM. Because all dose times are relative to individual subject’s personal dose schedules, by definition doses can be at most 6 h early or late. This is irrespective of whether a given dose was preceded by a skipped dose, i.e., if a dose was taken 1 h after the dose time but preceded by a skipped dose, we would not categorize this dose as 13 h late, but rather a skipped dose followed by a dose that was 1 h late.
We created two scatter plot models to display longitudinal dose timing data at the individual subject level, as demonstrated in Fig. 1. The SAS codes used to generate these scatter plots are provided in Appendix A in Online Supplementary Information.
Fig. 1.
Examples of uni- and bi-modal longitudinal scatter plots at the level of individual subjects. The figure provides two theoretical examples of a uni-modal (top) and bi-modal (bottom) scatter plot. In the uni-modal plot, calendar date is on the Y-axis, with ‘0’ at the origin representing the date when EDM monitoring commenced and then proceeding up in 1 day increments. The X-axis plots the time that doses were taken relative to when the dose had been prescribed on an absolute scale. For example, a subject who was scheduled to take a dose at 9 AM, but took it at 9:30 AM would be plotted as +0.5 h from the origin. Because the uni-modal plot is on an absolute scale, a dose taken at 8:30 AM would also fall on the same point (i.e., +0.5 h from the origin). By contrast, the bi-modal plots flip the calendar date and time of opening, such that calendar date, starting with the first day EDM monitoring commenced and proceeding from left to right in 1 day increments, is on the X-axis. The Y-axis now depicts that doses were taken relative to when they were prescribed. What distinguishes this from the uni-modal plot is that the delta of actual vs. prescribed dose timing can be early or late, meaning it is on a relative, not absolute, scale. The uni-modal plots have the advantage of being somewhat easier to interpret, and tend to accentuate the slope of the scatter plot. The bi-modal plots allow one to draw inferences about early vs. late dose timings as separate behaviors
Arbitrarily, in the first model we expressed calendar date on the Y-axis, with the origin being the first date that the subject began using the EDM device. On the X-axis we displayed how far away in hours the actual dose time was from the prescribed dose time. Thus, if a patient was scheduled to take his/her at 9:00 AM, but took it either at 8:30 AM or at 9:30 AM (i.e., −30 min or +30 min), the absolute dose time would be 30 min from scheduled. Because this uses an absolute scale, allowing dose time variation to occur only on one direction, we defined these as ‘uni-modal plots’ (see Appendix B in Online Supplementary Information).
The second model expressed dose timing on a relative scale. In this version, we expressed calendar date on the X-axis, displaying actual dose time before or after the scheduled dose time on the Y-axis, making these ‘bimodal plots’ (see Appendix C in Online Supplementary Information).
Uni-modal plots have the advantage of being somewhat simpler to interpret, yielding a single linear expression capturing variations in dose timing. The advantage to the bi-modal plot is that it allowed us to consider early versus late mistiming potentially as different behavioral phenomena, as summarized below.
From the bi-modal plots, we sorted subjects’ dose-taking behavior into three categories:
Early dose timers: ≥60 % of doses taken prior to the prescribed dose time (regardless of whether these were inside the ±1 h compliance window or not).
Late dose timers: ≥60 % of doses taken after the prescribed dose time (regardless of whether these were inside the ±1 h compliance window or not).
Symmetrical dose timers (a default category): neither of these two conditions met.
We also assessed dose mistiming, here focusing only on those events that occurred outside of the ±1 h compliance window.
Early dose mistimers: ≥60 % of mistimed doses occurring 1 h before the compliance window.
Late dose mistimers: ≥60 % of mistimed doses occurring 1 h after the compliance window.
Symmetrical mistimers (default category): neither of these two conditions met.
For both sets, the threshold of ‘≥60 %’ was selected with the intent to capture a dominant pattern of dose timing, i.e., having a ≥10 % asymmetry to the data. Stated another way, with 50 % of doses early/late defining perfect symmetry, we accepted as symmetrical a pattern in which the excess of early or late doses must be less than 60 % and greater than 40 %—hence the use of 10 % asymmetry as the cut point.
Statistical Analyses
Inspection of the bi-modal scatter plots suggested several explanatory variables that could be useful for predicting ‘sub-optimal adherence’. First, there was the slope of the regression line through the scatter plot and its R-squared statistic, which measure how well the deviations in the slope from ‘0’ in either the positive or negative direction explain the data (range −1.0 to +1.0). A line that runs parallel to the calendar date axis (i.e., a zero slope) connotes a subject whose dose taking behavior is stable over time. Conversely a positive or negative slope on a bi-modal plot connotes a subject whose behavior is shifting over time.
Second, there was the extent of variation of individual dose events. This statistic was captured as the ‘residual mean standard error’ (RMSE) from each subject’s regression line. The RMSE is calculated from the sum of squares of the distance separating the observed from the predicted dose times, indexed against the total number of events. Conceptually, this approach is analogous to that used by Ferguson et al. in their analysis of ‘inter-dose frequency’ as a predictor of HIV biological endpoints [7].
In addition, we assessed various patterns or dose timing and mistiming. For dose timing we focused simply on whether dose-taking fell in a dominant early pattern or dominant late pattern—regardless of whether within the compliance window or not; or a symmetrical pattern. For the analysis of mistiming, we assessed only the subset of dose events that occurred outside of the compliance window, again using a threshold of ≥60 % of events occurring in the early or late pattern, respectively; symmetrical mistiming meant that neither early nor late conditions were met. In addition to these dichotomous analyses, we assessed the following continuous variables (all from bi-modal plots): proportion of all doses taken early/late; proportion of mistimed doses early/late; RMSE; and the slope of the regression line.
Our primary statistical method used Area under the Receiver Operator Characteristic (AROC) curves, adjusting sensitivity and specificity for each explanatory variable for identifying ‘sub-optimal adherence’. We defined ‘suboptimal adherence’ as taking ‘<95 % of prescribed doses’. A sensitivity analysis using ‘<90 % of prescribed doses’ was attempted but proved impossible due to low numbers of subjects falling below this threshold. All data manipulations and analyses were conducted using SAS version 9.4 (The SAS Institute, Cary, NC).
Results
Study Subjects
Sixty eight subjects each provided 6 months of continuous twice daily EDM data for this analysis, totaling ~300–400 dose taking events per subject (Table 1). Subjects were predominantly male (50/68, 74 %), and had acquired HIV through injectable drug use (35/68, 55 %). Only 19/68 (28 %) were ART-naïve prior to enrollment in AFL. On average, the AFL cohort took 86 % of doses over the six-month pre-intervention period.
Table 1.
Defining characteristics of dose taking patterns from bimodal plots
| Grouping variables | n/N | Percent |
|---|---|---|
| Proportion of doses taken | ||
| ≥95 % | 54/64 | 84.4 |
| 90–94 % | 7/64 | 10.9 |
| 85–89 % | 3/64 | 4.7 |
| Dose timing patternsa | ||
| Symmetrical dose timers | 5/64 | 7.8 |
| Early dose timers | 3/64 | 4.7 |
| Late dose timers | 56/64 | 87.5 |
| Dose mistiming patternsb | ||
| Symmetrical dose mistimers | 13/64 | 20.3 |
| Early dose mistimers | 10/64 | 15.6 |
| Late dose mistimers | 41/64 | 64.1 |
Dose timing categories defined by whether there was a preponderance (>60 %) of dose events falling before or after the scheduled dose time. For example, a subject who took 392 doses with a schedule of 9 AM/9 PM, of which 238 occurred after the scheduled dose time, would be categorized as a late dose timer. This is irrespective of whether those doses occurred within the compliance window. The symmetrical category is the default, meeting neither of the early or late definitions
By contrast, dose-mistiming categories are defined solely by the events that fell outside of the compliance window. Assuming the same patient above took 392 doses, of which 49 were >1 h early or late, the patient would be categorized as an early or late mistimer based on whether >60 % of the 49 off time doses occurred before or after the compliance window. The symmetrical category is the default, meeting neither of the early or late definitions
EDM Data Artifacts
The de-identified individual subject uni- and bi-modal scatter plots are provided in web appendices B and C, respectively. Inspection of the scatter plots allowed us to identify several probable artifacts related to the EDM system itself. First, by expanding the dose timing scale, we noted that openings only occurred at ~1.5-min intervals, rather than continuously, suggesting that events are either rounded up or down around this interval (Fig. 2a). Theoretically, this could introduce a slight though random misclassification of adherence (e.g., if a subject opened their bottle shortly before the compliance window elapsed, and the system rounded up), though the net effect of this averaged over hundreds of openings should be minimal, and would not introduce a systematic bias.
Fig. 2.
Examples of data artifacts. a Artifact one: The limit of precision of the electronic drug monitor. By expanding the dose time axis on this bi-modal plot, so that we are looking just at a single 15-min interval, it is clear that the electronic drug monitor concatenated dose events captured over time and either rounded up or rounded down events that occurred in between these intervals. The smallest time interval that could be measured was just under 0.025 h (or ~1.5 min). This makes clear that there is a limit to the granularity of this particular system. b Artifact two: Systematic drift in dose time. Examples of systematic drift in dose time, clearly observed in these two subjects whose dose time adherence was exceptionally regular. The bottom subject changed his/her dose time at the mid point of the study, and yet the slopes of the drift artifact remained exactly parallel in both periods. In all cases, on this scale, the slopes on the uni-modal plots measured 5° from the Y-axis. Over a 12-month period, the error totaled up to ~0.2 h (12 min, or 720 s), or a gain of ~2 s per subject per day. c Artifact three: Trending to new a baseline dose time, cause unknown. Bi-modal plot of a subject with a linear shift in dose timing over a several week span. The shift is presumed to be an artifact, though it is possible that the subject in question intentionally shifted their dose-timing forwards by several minutes each day. The ‘drift’ artifact is also quite easy to see in this subject, even on a bi-modal plot. d Artifact four: Linear artifact, presumably due to malfunctioning EDM device. Bi-modal plot of a series of linear artifacts, followed by a period when no data were recorded. It was not possible to re-contact the subject to determine whether a battery failure occurred, or whether the battery was changed or a new device assigned in January 2007. Note also that around the middle of January 2007 this subject abruptly moved his/her scheduled dose time about 30 min earlier
Second, when examining subjects with consistently excellent adherence, we observed a recurring slope trending away from the dose-time axis. This was particularly evident in the uni-modal plots, where we measured it as a 5-degree drift from the Y-axis (see Fig. 2b). Because it was implausible that so many subjects would display precisely the same drift over time, a more logical explanation was that the ECaps’ internal clock was not perfectly accurate, but ran slightly fast by a few seconds each day. To better visualize this, we examined data over the full 12 months of observation for two subjects whose adherence was highly consistent, and measured the rate of gain at ~2 s per day. While this represents a very small error, given a sufficiently long duration of observation, it would introduce a systematic bias that underestimates dose timing compliance. For example over 12 months, this created a drift of 12 min. We have alerted the ECaps manufacturer to this phenomenon.
Lastly, in two subjects we observed shifting patterns of adherence that appeared artifactual. One showed a linear change in dose time over a period of about a month (as if the subject changed their behavior by about 1 min per day) (Fig. 2c). A second subject another showed a series of parallel dose timing lines over a several week period (Fig. 2d). The linearity of these events strongly argued against this being actual patient behavior (intentionally creating this pattern would have required meticulous planning by the subject in question). Moreover, the linear artifacts were followed by several weeks during which no openings were recorded. We hypothesize that a failing ECaps device or battery was to blame.
Common Patterns of Dose Timing/Mistiming
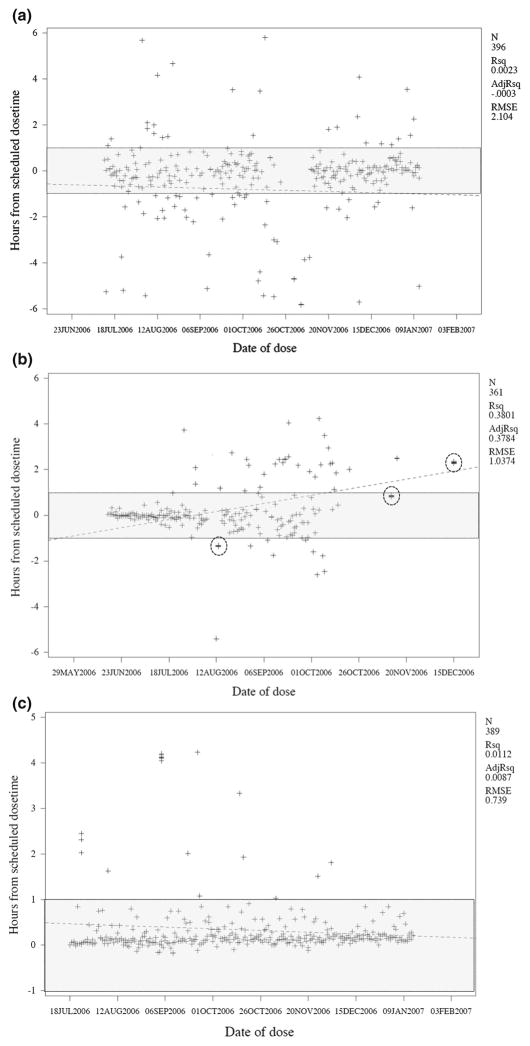
Figure 2a–c are also examples of subjects with extremely consistent dose timing. The subjects in 2a and 2c had no events outside the compliance window, and the subject in 2b had only two events outside the compliance window. By contrast, Fig. 3a, b provide bi-modal plot examples of two subjects with highly variable dose timing. The first had consistently erratic timing throughout the period of observation, with the persistence of this behavior reflected by a regression line nearly parallel to the calendar date axis (slope = −0.002, R-squared = 0.002). By contrast, the subject’s dose timing in Fig. 3b became increasingly erratic after the first 2 months of observation, reflected as a positively sloped regression line (slope = +0.015, R-squared = 0.38). Similarly, Fig. 3c depicts a subject whose dose timing improved over time, reflected as a descending regression line (slope = −0.002, R-squared = 0.01). Additionally, the subjects in 3b and 3c display a clear tendency towards late mistiming, whereas the subject in 3a has a symmetrical pattern of early and late mistimings. For each of these three subjects, the extent of erratic dose timing is reflected in the RMSE statistics (ranging from 0.74 to 2.1). The subject in 3b presents an additional artifact: multiple dose events clustered within minutes of each other. This could reflect a subject ‘playing’ with the EDM bottle, opening and closing it in rapid succession.
Fig. 3.
Representative examples of subjects with erratic dose timing. For reference, we have included the zone of compliance in shading and also statistics related to the scatter plot (the number of data points (N), and the R-squared and residual mean standard error (RMSE) statistics for the regression line (shown as a dotted line). a A subject with consistently poor dose timing. b Subject with erratic dose timing that grows worse over time. Note also that this subject has multiple superimposed dose-taking events (circled in the figure), which correspond to repeated opening and closing of the EDM device within a very narrow period of time. c Subject with erratic dose timing, but improving over time. The improvement in dose timing is evident despite the steady decay in adherence due to the systematic drift artifact described earlier in Fig. 1b. As before, the compliance zone is shown in shading and the slope of the scatter plot as a dotted line
By contrast, Fig. 4a is a bi-modal plot of a subject whose dose timing rarely deviated from the compliance window, and whose dose timing was stable throughout the 6 months of observation (slope = 0.0001, R-squared 0.0002, RMSE of 0.35). Figure 4b depicts an intervention subject captured in both the pre- and post-intervention periods. In this example, dose timing was quite erratic prior to the EDM feedback intervention, and then became much more stable over the next six months. During the pre-intervention period the slope was −0.003 and R-squared 0.03; during the intervention period, the slope was −0.0002, and R-squared 0.001.
Fig. 4.
Examples of subjects with excellent or significantly improving dose timing. a Bimodal plot of a subject with minimal deviation in dose timing. This subject had remarkably good adherence in terms of dose timing: only four mistimed doses were recorded during this six-month period. b Uni-modal plot of an intervention subject in the pre-and post-intervention periods, showing a marked improvement in dose timing. Pre-EDM, this subject frequently took doses off time (many doses outside the compliance window). Post-EDM, the subject had a total of three off timed doses, all of which concentrated in the first 2 months of the intervention period. c Uni-modal plot of a subject whose AM/PM doses are not separated by exactly 12 h. As in b, this intervention group subject shows a marked improvement in dose timing following the start of the intervention half way through the observation period. In addition, this subject appears to have adopted a schedule whereby the two daily doses are systematically separated by 11.5 h instead of 12, as intended (e.g., 9 AM and 8:30 PM)
Figure 4c also presents a uni-modal plot of a subject whose dose timing became more stable during the AFL intervention period. However, the notable feature here is the ‘Y’ shaped pattern of dose timings that emerged during the second half of the observation period. As with all study subjects, this individual was taking a twice-daily regimen in which each dose was scheduled at 12-h intervals. In this case, however, the patient shows two clusters of doses, one of which is consistently off cycle by about 30 min, e.g., a 9:00 AM/9:30 PM schedule.
Descriptive Analysis of Dose Timing and Mistiming
Using the bi-modal plots, we categorized subject does timing patterns depending on whether doses were taken symmetrically relative to the prescribed dose time, or whether they clustered in the pre- or post-dose period. Similarly, we classified mistimed doses into symmetrical mistimers, versus early or late dominant patterns (Table 1). Several points merit attention. First, the most common pattern observed was late dose timing, which characterized 88 % of the cohort. Moreover, the majority of mistiming subjects (64 %) were late dose mistimers. Thus it appears that being late for doses was more the rule than the exception in this cohort. By contrast, early dose timing and mistiming patterns were quite uncommon (4.7 and 15.6 %, respectively). Symmetrical patterns of dose timing and mistiming were also uncommon.
Association Between Dose Timing or Dose Mistiming and ‘Optimal Adherence’
Moving from analysis of individual subjects to the cohort as a whole, Table 2 provides the AROC results for each of our predictor variables. Starting with the overall pattern of dose timing (regardless of whether doses were taken within the compliance window), none of the three categories accurately predicted sub-optimal adherence (AROCs varied from 0.53 to 0.58). By contrast, when categorizing subjects based solely on mistimed doses, stronger associations with sub-optimal adherence were observed (AROCs varied from 0.53 to 0.64). Of note, the pattern of symmetrically mistimed doses had the strongest association of these dichotomous patterns (AROC 0.64).
Table 2.
Predictive accuracy of differing patterns of dose timing and mistiming on whether subjects achieve ‘optimal’ adherence (≥95 % of prescribed doses taken)
| Grouping variables | <95 % of doses taken | |
|---|---|---|
| AROC curvea | 95 % CI
|
|
| Dose timing patterns | ||
| Early dose taking, yes/nob | 0.53 | 0.50–0.56 |
| Late dose taking, yes/nob | 0.56 | 0.41–0.71 |
| Symmetrical dose taking, yes/nob | 0.58 | 0.44–0.73 |
| Dose mistiming patterns | ||
| Early mistimed dose pattern, yes/noc | 0.53 | 0.41–0.65 |
| Late mistimed dose pattern, yes/noc | 0.61 | 0.43–0.80 |
| Symmetrical mistiming, yes/noc | 0.64 | 0.46–0.82 |
| Continuous variables | ||
| Proportion of all doses taken earlyd | 0.74 | 0.59–0.88 |
| Proportion of all doses taken lated | 0.74 | 0.59–0.89 |
| Proportion of mistimed doses taken earlyd | 0.65 | 0.48–0.82 |
| Proportion of mistimed doses taken lated | 0.65 | 0.48–0.82 |
| Slope of regression line for dose events over calendar timee | 0.51 | 0.26–0.75 |
| RMSE of regression linef | 0.79 | 0.62–0.97 |
AROC curve area under the receiver operating characteristic curve, RMSE residual mean standard error
The AROC statistic indicates how well the predictor variable classifies the outcome (optimal adherence). An AROC of 0.5 provides no discriminant information; an AROC of 1.0 connotes a perfect (errorless) test
Defined as >60 % of doses (regardless of whether within the 1 h compliance window) falling early or late, or, in the case of the symmetric pattern, meeting neither of these definitions
Defined as >60 % of mistimed doses taken early or late, or in the case of a symmetrical mistiming pattern, meeting neither of these definitions
Similar analysis to those described in items 2 and 3 above, except defining variables are now continuous instead of dichotomous
This is the slope of the dose taking events over calendar time, i.e., whether the individual’s behavior is stable (zero slope) or shifting with time (positive or negative slope)
The residual mean standard error measures the extent of dose time variability from the regression line for each scatter plot. Individuals whose doses all fall very close to the regression line (few doses early or late) have lower RMSE; those with a high degree of scatter (many doses taken early or late), have larger RMSE
Following this same approach, we re-ran these analyses treating the dose timing/mistiming data as continuous rather than dichotomous variables. Here, the probability of sub-optimal adherence increased in proportion to the extent of early or late dose timings, both of which were characterized by an AROC of 0.74.
The coefficient for the slope of the regression lines reflecting the change in dose timing over calendar date had no predictive value. By contrast, the RMSE, reflecting the extent of dose timing variability, was the measure that best predicted sub optimal adherence (AROC 0.79). In logistic regression analysis, dividing RMSE into quartiles, with the lowest quartile as the reference category, each increment in RMSE quartile approximately doubled the odds of suboptimal adherence (OR 2.1, 95 % CI 1.3–3.4). Thus, subjects in the highest quartile (highest degree of variation) were 9.3 times more likely to have sub-optimal adherence than those in the lowest quartile (least variation in dose timing). Including other predictive variables in this model (early mistiming, late mistiming, slope of the trend over time) did not improve the accuracy of the model (data not shown).
Discussion
Whereas the ‘proportion of doses taken’ draws inferences from the number of doses that were missed relative to the ideal of 100 % adherence, and is therefore focused on events that did not occur, ‘timing of doses’ draws inferences from events that did occur, but with a high degree of variability. To use our earlier metaphor of the ‘Broken Window’ hypothesis, micro non-adherence is predictive of macro non-adherence. Longitudinal studies connecting differential rates of adherence show that sub-optimal adherence can be separated from drops in CD4 or viremic break through by months—or even years [15, 16]. Therefore, the ability to identify risky patterns of medication taking early on and direct interventions to improve such behaviors before they lead to adverse biologic consequences has evident practical value.
These findings could be interpreted in several ways. First, mistiming of doses could be a marker for a pattern of sub-optimal medication taking behaviors that includes missing doses. Alternatively, mistiming doses could be on a causal pathway to missing doses in the future. In fact, these concepts are not mutually exclusive, and from a practical perspective, this distinction may be less important, because in either case a pattern of mistiming doses should prompt an intervention by the patient’s care giver about ways in which dose timing behavior can be stabilized.
Why does this matter? The literature suggests that current 1st line ART regimens, consisting of drugs with excellent pharmacokinetics and long plasma half lives, are far more forgiving of imperfect adherence than older regimens [17–19]. From the perspective of PK/PD, so long as drug concentrations remain above the minimum inhibitory concentration for a sensitive strain of HIV, it probably doesn’t matter whether a dose was taken an hour (or two, or three?) early or late. Rather, the importance of mistiming doses is less about sub-therapeutic drug levels than it is about identifying patients who may be struggling to manage their disease effectively. And even in this era of improved ART drugs, non-adherence remains the leading cause of treatment failure. This is particularly important to patients on older or second line ART regimens or those newly infected with resistant strains of HIV [20–22].
Several other interesting observations emerged from this analysis.
First, we have identified several technical limitations associated with the EDM system used in the AFL study. The most important of these was that the ECaps system’s internal clock was not perfectly accurate, but gained a few seconds each day: given enough time, this would result in substantial misclassification of subject’s adherence, particularly if using pre-defined cut points for on-time/off-time dosing. While it is possible that such artifacts are unique to this particular marketed brand of EDM (ECaps), researchers should be alert to systematic errors intrinsic to the tool used.
Second, we observed that different patterns of dose mistiming (though not dose timing) were themselves predictive of sub-optimal adherence. A symmetrical pattern of mistiming, while unusual in this cohort, was most strongly associated with sub-optimal adherence. Early mistiming of doses was the least common pattern.
Third, the risk for sub-optimal adherence (macro non-adherence) increased significantly as dose timing fell progressively early or late relative to the prescribed dose time. Strongest of all was the relationship between the extent of dose time variation and sub-optimal adherence, as reflected in the RMSE statistic. The RMSE is easily quantified and simple to measure statistically. But more pragmatically, the extent of dose variability is immediately obvious when looking at a subject’s scatter plot without needing any statistics at all. Compared with subjects with the most consistent dose timing, subjects with the most erratic dose timing were roughly nine fold more likely to have suboptimal adherence.
Our study had several limitations. First, our cohort was quite small, though each subject contributed a large amount of data—roughly 300–400 observations per individual. This is a reminder of how much information is captured using EDM, and how these data can be meaningful even at the level of individual subjects. Repeating this kind of analysis in other cohorts and cultural contexts would support the generalizability of these observations. Second, due to the small size of our cohort, our analysis was limited to looking at behavioral relationships, and could not assess viral loads or CD4 counts. Repeating this analysis with data from a larger cohort, followed longer over time, and with a higher rate of detectable viral load at baseline, could be instructive.
In conclusion, electronic drug monitors provide a wealth of granular data that are informative beyond merely dichotomizing individuals as optimally adherent or not, or measuring the proportion of doses taken. As described elegantly by Sherr and colleagues, medication adherence is a deceptively simple concept that belies a complex set of interrelated bio psychosocial inputs and outputs [23]. Analysis of dose timing reveals a great deal about patterns of adherence at the individual patient level and at the population level. Extending the metaphor of the ‘Broken Window’ hypothesis, seemingly minor deviations in dose timing identify subjects who are prone to skipping doses entirely. At present, electronic drug monitors are used almost exclusively as tools in adherence research. These finding suggests that an expanded role for EDM in HIV clinical care may be justified, to enable timely, patient- and context-specific interventions in response to early manifestations of non-adherence [9, 11, 12].
Supplementary Material
Acknowledgments
We wish to thank Ka Lai Poon for her assistance in generating the library of subject-by-subject scatter plots for this analysis. The AFL study was supported by a cooperative agreement (GHS-A-00-03-00030-00) between Boston University and the Office of Health and Nutrition of the United States Agency for International Development (USAID), with additional support from the World Health Organization (WHO) and CDC-GAP/China. Dr. Gill’s work was supported by NIH/NIAID K23 AI 62208. This study was supported by a cooperative agreement (GHS-A-00-03-00030-00) between Boston University and the Office of Health and Nutrition of the United States Agency for International Development (USAID), with additional support from the World Health Organization (WHO) and the United States Centers for Disease Control and Prevention.
Footnotes
Conflict of interest: All authors declare no conflicts of interest.
Electronic supplementary material: The online version of this article (doi:10.1007/s10461-015-1065-3) contains supplementary material, which is available to authorized users.
References
- 1.Liu H, Golin CE, Miller LG, Hays RD, Beck CK, Sanandaji S, et al. A comparison study of multiple measures of adherence to HIV protease inhibitors. Ann Intern Med. 2001;134(10):968–77. doi: 10.7326/0003-4819-134-10-200105150-00011. [DOI] [PubMed] [Google Scholar]
- 2.Gross R, Bilker WB, Friedman HM, Strom BL. Effect of adherence to newly initiated antiretroviral therapy on plasma viral load. Aids. 2001;15(16):2109–17. doi: 10.1097/00002030-200111090-00006. [DOI] [PubMed] [Google Scholar]
- 3.Wagner GJ. Predictors of antiretroviral adherence as measured by self-report, electronic monitoring, and medication diaries. AIDS Patient Care STDS. 2002;16(12):599–608. doi: 10.1089/108729102761882134. [DOI] [PubMed] [Google Scholar]
- 4.Wilson IB, Tchetgen E, Spiegelman D. Patterns of adherence with antiretroviral medications: an examination of between-medication differences. J Acquir Immune Defic Syndr. 2001;28(3):259–63. doi: 10.1097/00042560-200111010-00009. [DOI] [PubMed] [Google Scholar]
- 5.Knafl GJ, Bova CA, Fennie KP, O’Malley JP, Dieckhaus KD, Williams AB. An analysis of electronically monitored adherence to antiretroviral medications. AIDS Behav. 2010;14(4):755–68. doi: 10.1007/s10461-008-9512-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knafl GJ, Fennie KP, Bova C, Dieckhaus K, Williams AB. Electronic monitoring device event modelling on an individual-subject basis using adaptive Poisson regression. Stat Med. 2004;23(5):783–801. doi: 10.1002/sim.1624. [DOI] [PubMed] [Google Scholar]
- 7.Ferguson NM, Donnelly CA, Hooper J, Ghani AC, Fraser C, Bartley LM, et al. Adherence to antiretroviral therapy and its impact on clinical outcome in HIV-infected patients. J R Soc Interface. 2005;2(4):349–63. doi: 10.1098/rsif.2005.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilson JQ, Kelling GL. Broken Windows: the police and neighborhood safety. Atlantic: 1982. [Google Scholar]
- 9.Bachman Desilva M, Gifford AL, Keyi X, Li Z, Feng C, Brooks M, et al. Feasibility and acceptability of a real-time adherence device among HIV-positive IDU patients in China. AIDS Res Treat. 2013;2013:957862. doi: 10.1155/2013/957862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haberer JE, Robbins GK, Ybarra M, Monk A, Ragland K, Weiser SD, et al. Real-time electronic adherence monitoring is feasible, comparable to unannounced pill counts, and acceptable. AIDS Behav. 2012;16(2):375–82. doi: 10.1007/s10461-011-9933-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haberer JE, Kiwanuka J, Nansera D, Muzoora C, Hunt PW, So J, et al. Realtime adherence monitoring of antiretroviral therapy among hiv-infected adults and children in rural uganda. Aids. 2013;27(13):2166–8. doi: 10.1097/QAD.0b013e328363b53f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haberer JE, Kahane J, Kigozi I, Emenyonu N, Hunt P, Martin J, et al. Real-time adherence monitoring for HIV antiretroviral therapy. AIDS Behav. 2010;14(6):1340–6. doi: 10.1007/s10461-010-9799-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sabin LL, Desilva MB, Hamer DH, Keyi X, Yue Y, Wen F, et al. Barriers to adherence to antiretroviral medications among patients living with HIV in southern China: a qualitative study. AIDS Care. 2008;20(10):1242–50. doi: 10.1080/09540120801918651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sabin LL, DeSilva MB, Hamer DH, Xu K, Zhang J, Li T, et al. Using electronic drug monitor feedback to improve adherence to antiretroviral therapy among HIV-positive patients in China. AIDS Behav. 2010;14(3):580–9. doi: 10.1007/s10461-009-9615-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wools-Kaloustian K, Kimaiyo S, Diero L, Siika A, Sidle J, Yiannoutsos CT, et al. Viability and effectiveness of large-scale HIV treatment initiatives in sub-Saharan Africa: experience from western Kenya. Aids. 2006;20(1):41–8. doi: 10.1097/01.aids.0000196177.65551.ea. [DOI] [PubMed] [Google Scholar]
- 16.Nachega JB, Hislop M, Dowdy DW, Chaisson RE, Regensberg L, Maartens G. Adherence to nonnucleoside reverse transcriptase inhibitor-based HIV therapy and virologic outcomes. Ann Intern Med. 2007;146(8):564–73. doi: 10.7326/0003-4819-146-8-200704170-00007. [DOI] [PubMed] [Google Scholar]
- 17.Bangsberg DR. Less than 95% adherence to nonnucleoside reverse-transcriptase inhibitor therapy can lead to viral suppression. Clin Infect Dis. 2006;43(7):939–41. doi: 10.1086/507526. [DOI] [PubMed] [Google Scholar]
- 18.Mocroft A, Horban A, Clumeck N, Stellbrink HJ, Monforte ADA, Zilmer K, et al. Comparison of single and boosted protease inhibitor versus nonnucleoside reverse transcriptase inhibitor-containing cART regimens in antiretroviral-naive patients starting cART after January 1, 2000. HIV Clin Trials. 2006;7(6):271–84. doi: 10.1310/hct0706-271. [DOI] [PubMed] [Google Scholar]
- 19.Weiser SD, Guzman D, Riley ED, Clark R, Bangsberg DR. Higher rates of viral suppression with nonnucleoside reverse transcriptase inhibitors compared to single protease inhibitors are not explained by better adherence. HIV Clin Trials. 2004;5(5):278–87. doi: 10.1310/LNHD-K1R7-HQP5-HJCQ. [DOI] [PubMed] [Google Scholar]
- 20.Price MA, Wallis CL, Lakhi S, Karita E, Kamali A, Anzala O, et al. Transmitted HIV type 1 drug resistance among individuals with recent HIV infection in East and Southern Africa. AIDS Res Hum Retrovir. 2011;27(1):5–12. doi: 10.1089/aid.2010.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Phanuphak P, Sirivichayakul S, Jiamsakul A, Sungkanuparph S, Kumarasamy N, Lee MP, et al. Transmitted drug resistance and antiretroviral treatment outcomes in non-subtype B HIV-1-infected patients in South East Asia. J Acquir Immune Defic Syndr. 2014;66(1):74–9. doi: 10.1097/QAI.0000000000000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mantovani NP, Azevedo RG, Rabelato JT, Sanabani S, Diaz RS, Komninakis SV. Analysis of transmitted resistance to raltegravir and selective pressure among HIV-1-infected patients on a failing HAART in Sao Paulo, Brazil. J Clin Microbiol. 2012;50(6):2122–5. doi: 10.1128/JCM.00539-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sherr L, Lampe F, Norwood S, Leake Date H, Harding R, Johnson M, et al. Adherence to antiretroviral treatment in patients with HIV in the UK: a study of complexity. AIDS Care. 2008;20(4):442–8. doi: 10.1080/09540120701867032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.