Abstract
Although optic pathway gliomas are the most common brain tumors associated with neurofibromatosis type 1 (NF1), extra-optic gliomas occur and may behave more aggressively with outcomes that differ by age. A retrospective case-control study was designed to describe the clinical course of adult NF1 patients with progressive extra-optic pilocytic astrocytomas (PAs) and compare to a pediatric cohort. Data for patients treated at the Johns Hopkins Comprehensive Neurofibromatosis Center from 2003 to 2013 were reviewed to identify cases (adults, age >18) and controls (pediatric, age <18) with clinically or radiographically progressive extra-optic PAs. Demographic, clinical, histologic, and radiographic data were collected. Three adult NF1 cases and four pediatric NF1 controls were identified. Mean age was 32.3 ± 9.5 years, 66% male (cases); 12.8±4.2 years, 100% male (controls). Symptomatic progression occurred in two-of-three adults (67%) while the majority of pediatric patients presented with isolated radiographic progression (n=3, 75%). Onset tended to be more rapid in adults (4±1 vs. 14±8.3 months, P=0.10). Subtotal resection was the treatment for all pediatric patients. Radiotherapy (n=2), chemotherapy (n=2), and targeted, biologic agents (n=2) were administered in adults. Although all pediatric patients are living, outcomes were universally poor in adults with progression to death in all (median survival 17.1 months, range 6.6–30.3). In conclusion, despite grade I histology, all three adult NF1 patients with progressive extra-optic PAs suffered an aggressive clinical course which was not seen in pediatric patients. Clinicians should be aware of this clinico-histologic discrepancy when counseling and managing adult NF1 patients with progressive extra-optic PAs.
Keywords: neurofibromatosis type 1, pilocytic astrocytoma, extra-optic glioma, adult, pediatric, brain tumor
INTRODUCTION
Neurofibromatosis type 1 (NF1) is an autosomal dominant tumor suppressor syndrome predisposing to neoplasms of the central and peripheral nervous systems (CNS and PNS). CNS tumors occur in up to 20% of NF1 patients [Rosser and Packer, 2002; Rosenfeld et al., 2010]. Approximately 70% of these CNS tumors arise along the optic pathway (i.e., optic pathway gliomas, OPGs). Extra-optic gliomas also occur most commonly in the brainstem and posterior fossa (67%), cerebrum (28%), and rarely in the spine (5%) [Guillamo et al., 2003]. Most frequently, CNS neoplasms in NF1 follow an indolent course. In children with OPGs, up to two-thirds do not require therapy and some will regress over time [Perilongo et al., 1999; Parsa et al., 2001; Mikaeloff et al., 2002; King et al., 2003]. Brainstem lesions have also been studied in NF1 children with multiple studies reporting an indolent clinical course, with cases of regression over time, and typically without the need for therapeutic intervention [Molloy et al., 1996; Pollack et al., 1996; Ullrich et al., 2007]. High-grade gliomas (HGGs) are rare in NF1, though when they occur, studies have suggested significantly more favorable outcomes in both NF1 children and adults than in sporadic cases [Huttner et al., 2010]. A study of five NF1 children with glioblastoma reported a 9.25 year median overall survival which was significantly longer than 1.08 years in a non-NF1 comparison cohort [Huttner et al., 2010]. In five adult, NF1 patients with recurrent HGGs, median overall survival was 72.6 months which is also substantially longer than the reported median survival of 15–20 months in non-NF1 cohorts [Stupp et al., 2005; Grossman et al., 2010; Theeler et al., 2014].
Despite more favorable outcomes with the majority of NF1-associated CNS neoplasms, there is a suggestion that extra-optic gliomas in NF1 adults may behave differently. One study demonstrated significantly greater mortality from extra-optic lesions with an adult predominance, although it was not clear whether this was driven by the greater likelihood of these lesions being of higher histologic grade or other factors [Guillamo et al., 2003]. Over the past decade, we have encountered a series of adult NF1 patients with symptomatic extra-optic gliomas that, although histologically consistent with World Health Organization (WHO) grade I pilocytic astrocytomas (PAs), have behaved aggressively. Herein, we review our experience.
MATERIALS AND METHODS
A retrospective case-control study was designed of patients treated at the Johns Hopkins Comprehensive Neurofibromatosis Center (JHCNC) between January 2003 and December 2013. After institutional review board approval was obtained, the JHCNC Database was queried first to identify all patients meeting NIH diagnostic criteria for NF1 who were also documented to have a symptomatic or progressive extra-optic WHO grade I PA confirmed by neuro-imaging to arise outside the optic pathway. Adult patients (age >18) were identified as cases and compared to pediatric controls (age <18). Comprehensive medical record review was performed including clinic notes, operative notes, radiological images and results, and pathology reports. Radiological images and pathology results performed at outside facilities were reviewed and confirmed by experienced neuroradiologists and neuropathologists at JHH when available. Clinical characteristics including gender, ethnicity, age at NF1 diagnosis, NIH criteria met at the time of NF1 diagnosis, other prominent NF1 stigmata, age at tumor diagnosis, tumor location, indication for tumor evaluation, timeline of progressive symptoms prior to tumor diagnosis, extent of surgery, and subsequent treatments were collected. The date of tumor diagnosis was defined as the first date of histopathologic confirmation of primary brain tumor. Extent of surgery was determined by radiology and clinical notes. Details of treatment including radiation therapy, total radiation dose, all chemotherapy types, dosages, and durations were recorded. Dates of last contact were defined as the date of death or last follow up (if alive at data analysis). Overall survival (OS) was defined as the time from the date of diagnosis to the date of last contact. All statistical analyses were performed using Stata/IC v13.1 (StataCorp. College Station, TX. 2014). Descriptive statistics were performed. Demographic, clinical, and treatment characteristics were calculated and compared between cases and controls using χ2 and Fisher’s exact tests for categorical variables and Student’s t-test for continuous variables.
RESULTS
Progressive Extra-Optic Gliomas in NF1 Adults
Of the 535 patients contained in the JHCNC database over the 10 years of study, a total of three cases were identified for inclusion (Table I); two male. All received a clinical diagnosis of NF1 in childhood. The presence of >6 caf'e au-lait macules (at least 5 mm in maximum diameter) and >2 dermal neurofibromas was the most common NIH criterion met at NF1 diagnosis. Additional stigmata included intertriginous freckling (n=1), asymptomatic optic pathway glioma (n=1), and developmental delay (n=2). No patient had a family history of NF1, bony dysplasia, major vascular malformations, known plexiform neurofibromas, or prior malignant peripheral nerve sheath tumor. Mean age at diagnosis of extra-optic glioma was 32±9.5 years. Initial diagnosis was by neuroimaging in all; serial imaging or comparative imaging from childhood was not available to determine duration of tumor existence. Tumor location was posterior fossa in all patients. Indication for surgery was clinical progression in two (Patient 1: hydrocephalus; Patient 3: ataxia and left hemiparesis) and radio-graphic progression in the other (Patient 2: increase in enhancement). Time from first clinical symptom to surgery was 4–7 months. All patients underwent subtotal resection (STR). Histologic diagnosis was WHO grade I PA in two and WHO grade I PA with anaplasia in the other (Table II). Second surgery was not performed in any of these patients.
TABLE 1.
Characteristics of NF1 Adults With Progressive Extra-Optic Pilocytic Astrocytomas
| Pt | Age at NF1 Dx |
Clinical criteria at NF1 Dx |
Other NF1 stigmata |
Decade of age at tumor Dx |
Tumor histology |
Tumor location |
Indication for evaluation |
Timeline of symptomatic progression (months) |
Treatment | Survival from symptom onset (days) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Infancy | CALM, dermal NF |
DD | 40’s | PA | Medulla | Clinical progression |
4 | STR, RT | 330 |
| 2 | Childhood | CALM, freckling, LN |
DD | 20’s | PAa | Brainstem | Radiographic progression |
5 | STR, Carbo/Vinc, RT+Bev |
611 |
| 3 | 5 yrs | CALM, dermal NF |
OPG | 30’s | PA | Cerebellum | Clinical progression |
7 | STR, Carbo/Vinc, everolimus+Bev |
844 |
Dx, diagnosis; NF1, neurofibromatosis type 1; CALM, café au lait macules; NF, neurofbroma; LN, Lisch nodule; DD, developmental delay; OPG, optic pathway glioma; PA, pilocytic astrocytoma; STR, subtotal resection; Carbo, carboplatinum; Vinc, vincristine; RT, radiotherapy; Bev, bevacizumab.
Denotes that histopathologic diagnosis was pilocytic astrocytoma with anaplastic features as detailed in Table II.
TABLE II.
Histopathologic Characteristics of Progressive Extra-Optic Pilocytic Astrocytomas in NF1 Adults
| Pt | Histologic diagnosis |
Infiltration (Y/N) |
Rosenthal fibers (Y/N) |
EGBs (Y/N) |
Myxoid background (Y/N) |
Mitotic activity (Low/Mod) |
Cytologic atypia (Mild/Mod) |
Cell density (Mild/Mod) |
Necrosis (Y/N) |
Microvascular proliferation (Y/N) |
OD-like areas (Y/N) |
MIB-1a |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | PA | Y | Y | Y | Y | Low | Low | Modb | N | N | N | 5 |
| 2 | PAc | Y | Y | Y | N | Mod | Mod | Mod | Y | N | Y | 20 |
| 3 | PA | Y | Y | N | N | Low | Mild | Mild | N | N | N | 2 |
EGBs, eosinophilic granular bodies; OD-like areas, oligodendroglial-like areas; Y, yes; N, no; mod, moderate; min, minimum.
Description of histologic characteristics of the three adult NF1 patients.
Mib-1 was based on minimum cell count of 400.
Focally high areas of cell density were noted in this specimen.
Anaplasia was noted histologically in this specimen.
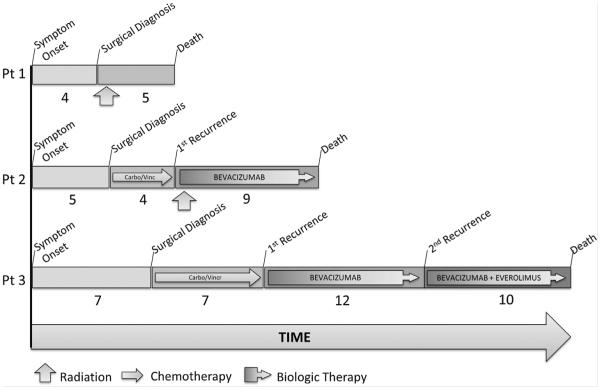
Clinical treatments included radiotherapy (RT, n=2), chemotherapy (n=2), and targeted biologic drugs (n=2). Patient outcomes are summarized in Figures 1 and 2. Patient 1 suffered continued postoperative progressive dysarthria and intractable hiccups and elected to pursue adjuvant intensity modulated RT (IMRT, 5220 Gy) over 6 weeks. He developed symptoms consistent with progressive increased intracranial pressure requiring diuretics and analgesics but not shunting. After symptoms stabilized for the first 3 months following RT, he suffered progressive clinical decline and radiographic progression and was transitioned to hospice. He died approximately 6 months after surgery.
FIG. 1.
Clinical course of three NF1 adults with progressive extra-optic gliomas. Clinical course for the three adult NF1 patients with progressive extra-optic pilocytic astrocytomas. Carbo, carboplatin; Vincr, vincristine.
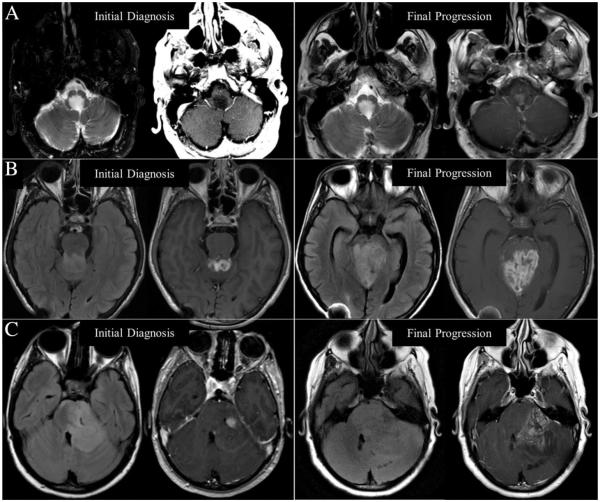
FIG. 2.
Radiographic progression of extra-optic gliomas in three NF1 adults. Neuroimaging findings at initial diagnosis and final progression of disease in Patient 1 (A), Patient 2 (B), and Patient 3 (C) including T2-weighted (left image) and gadolinium enhanced T1-weighted images (right image) at each time point demonstrating increased enhancement in all cases and significant increase in T2-weighted/FLAIR hyperintensity with regional mass effect in Patients 2 and 3.
Patient 2, after undergoing STR for progressive radiographic disease, was offered adjuvant carboplatin and vincristine according to published protocol [Packer et al., 1997]. Clinicoradiographic progression secondary to brainstem compression was observed after only two cycles and the decision was made to transition to conformal external-beam RT with concurrent bevacizumab (10 mg/kg, every 2 weeks). His disease remained stable on bevacizumab for 9 months after which he suffered rapid clinical and radiographic decline and died 1 month later, approximately 14 months after surgery.
Patient 3 suffered post-operative hydrocephalus requiring ventriculoperitoneal shunt placement. After recovery, she also underwent adjuvant carboplatin and vincristine [Packer et al., 1997]. Her disease remained stable for 7 months after which she developed progressive left hemiparesis and was transitioned to bevacizumab monotherapy (10 mg/kg, every 2 weeks). She initially remained clinically stable with partial radiographic response; however, she progressed after 12 months and everolimus (10 mg daily) was added. She continued to suffer progressive clinical decline and died 3 years from the time of initial symptom onset.
Comparison of Adult and Pediatric NF1 Patients With Progressive Extra-Optic Gliomas
A total of four controls were identified; mean age at tumor diagnosis 13 ± 4.2 years; all male. Clinical criteria for NF1 were similar to adults: caf'e au-lait macules (4), freckling (4), or family history (2). Tumor location was brainstem (n=2), third ventricle (n=1), and right parietal (n=1) with subtotal resection as the only treatment performed in all.
Significant differences in clinical presentation and outcome were observed (Table III). Isolated radiographic progression was common in children where three patients (75%) had radiographic progression without clinical correlate. In contrast, clinical symptoms were present in two adult (67%) and tended to occur over a shorter interval than in children (4±1 vs. 14±8.3 months, P=0.10). Outcomes were universally favorable in children with clinical improvement or stabilization occurring in all four children. In adults despite maximum available treatment, outcomes were universally poor with all patients suffering progressive clinical decline and death with median overall survival of 17.1 months (range 6.6–30.3).
TABLE III.
Comparison of Clinical Presentation and Outcome in NF1 Children and Adults With Progressive Extra-Optic Gliomas
| Adult (n=3) | Pediatric (n=4) | P-value | |
|---|---|---|---|
| Gender (M, %) | 2 (66%) | 4 (100%) | 0.42 |
| Age at tumor diagnosis (yrs, mean, stdev) | 32.3 (9.5) | 12.8 (4.2) | 0.01 |
| Histologic diagnosis (n, %) | 0.43 | ||
| PA | 2 (67) | 4 (100) | |
| PA with anaplasia | 1 (33) | ||
| Progression (n, %) | 0.26 | ||
| Radiographic findings | 1 (33) | 3 (75) | |
| Clinicoradiographic findings | 0 | 1 (25) | |
| Clinical symptoms | 2 (67) | 0 | |
| Timeline of progression (months, stdev) | 4 (1) | 14 (8.3) | 0.10 |
| Therapeutic interventions (n, %) | 0.03 | ||
| Surgery | 3 (100) | 4 (100) | |
| Radiotherapy | 2 (67) | ||
| Chemotherapy | 2 (67) | ||
| Targeted/biologic agent | 2 (67) | ||
| Clinical response to intervention (n, %) | 0.06 | ||
| Decline | 3 (100) | 0 | |
| Stabilization | 0 | 1 (25) | |
| Improvement | 0 | 3 (75) | |
| Long-term outcome (n, %) | 0.03 | ||
| Deceaseda | 3 (100) | 0 | |
| Alive | 0 | 4 (100) |
M, male; yrs, years; stdev, standard deviation; PA, pilocytic astrocytoma.
Comparison of clinical and demographic features of three adults NF1 patients and four pediatric patients with progressive extra-optic pilocytic astrocytomas. When chemotherapy was administered, carboplatin and vincristine were administered in all patients. Targeted biologic agents included bevacizumab alone in one patient and bevacizumab and everolimus in another.
Median overall survival was 17.1 months (range 6.6–30.3 months).
DISCUSSION
In this study, we describe an aggressive clinical course in three adult NF1 patients with progressive brainstem PAs. Despite a grade I histologic diagnosis, all experienced an unrelenting clinicoradiographic decline to death even with maximum therapeutic intervention. This clinical course contrasted substantially with that observed in a comparable group of pediatric patients treated over a similar time interval with symptomatic extra-optic gliomas who all experienced universally favorable outcomes despite only subtotal resection. In contrast to the favorable outcomes that have been reported in NF1 children with low-grade gliomas [Molloy et al., 1996; Pollack et al., 1996; Ullrich et al., 2007] and relatively more favorable outcomes in NF1 children and adults with highgrade extra-optic gliomas [Huttner et al., 2010; Theeler et al., 2014], brainstem pilocytic astrocytomas in NF1 adults are different. Despite their benign histology, they appear to behave aggressively.
Brainstem gliomas are the second most common CNS neoplasm in NF1 [Pollack et al., 1996; Guillamo et al., 2003]. Although these lesions occur more commonly in children [Pollack et al., 1996; Rosser and Packer, 2002], risk extends into adulthood. This risk also appears to be significantly greater than non-NF1 adults. Based on a review of death certificate data from the National Center for Health Statistics, adults with NF1 were reported to be five times more likely to have a malignant neoplasm of the brain listed on their death certificate than those without NF1 [Rasmussen et al., 2001]. Based on data from the National Neurofibromatosis Foundation International Database, the prevalence of symptomatic extra-optic brain tumors in adult patients with NF1 was reported to be more than 100 times that expected by age in the general US population [Gutmann et al., 2002]. Hence, prior studies have suggested that clinical suspicion remain high for brainstem glioma in symptomatic adults with NF1 and the current study supports a prompt evaluation given the more rapid clinicoradiographic progression observed in these patients.
Prior studies have also suggested that CNS tumors may behave differently in adults and children with NF1. In the largest retrospective study of NF1 associated optic and extra-optic gliomas, extra-optic location (HR 5.84, 95%CI 1.36–25.13), diagnosis in adulthood (12.59, 2.75–57.64), and symptoms at the time of diagnosis (12.23, 2.84–52.62) were independently associated with poorer survival [Guillamo et al., 2003]. In this study, 21% of the extra-optic gliomas were high grade (grade III–IV), and histology was not included in the multivariate analysis limiting conclusions about the contribution of histology. Furthermore, this study only included one adult NF1 patient with an extra-optic pilocytic astrocytoma. The current study specifically queried NF1 adults with extra-optic PAs and thus reports on the largest single cohort with these clinically aggressive WHO grade I neoplasms.
Despite efforts to identify features which may predict a more aggressive course in NF1-associated gliomas, considerable uncertainty remains. Comprehensive histopathologic evaluation of non-NF1 optic pathway gliomas suggested that p53 and Mib-1 labeling may identify a more aggressive phenotype [Cummings et al., 2000]. However, this was not confirmed in a study of 100 NF1-associated CNS tumors of which 25% were OPGs [Rodriguez et al., 2008]. Tumor location has consistently predicted poor outcomes with OPGs but other neuroimaging features have not been found to predict clinical course [Balcer et al., 2001; Segal et al., 2010; Fisher et al., 2012]. Histopathology studies have shown that anaplasia predicts a more aggressive phenotype in PAs [Rodriguez et al., 2010]. In a series of 34 patients with PA with anaplasia, a significant subset (24%) occurred in NF1 patients and occurred outside of the optic pathway with a majority occurring in adults. In the current study, one patient was found to have histologic features which supported anaplasia, although this was not apparent in the other two and other routine histologic characteristics including mitotic activity, cell density, necrosis, microvascular proliferation, and Mib-1 index did not provide consistent evidence of a more aggressive phenotype.
Importantly, the results from the current study have implications for postoperative counseling and treatment selection in adult NF1 patients with progressive WHO grade I brainstem gliomas. Clinicians should exercise caution when assuming a clinically benign prognosis based on WHO grade I histology. Clinical behavior is more aggressive and care should be taken to advise appropriate postoperative management. The role of chemotherapy, radiotherapy, and biologic therapy in NF1 has been debated given the concerns that alkylating chemotherapy and radiation may be associated with increased morbidity, secondary neoplasm, and malignant transformation in patients harboring an underlying genetic disorder of a tumor suppressor [Evans et al., 2006; Sharif et al., 2006; Nakamura et al., 2011; Choi et al., 2012; Braunstein and Nakamura, 2013].
The utility of radiation therapy in patients with NF1 associated malignancy is controversial. Reports have consistently demonstrated an association between radiation and an increased risk of malignant peripheral nerve sheath tumor development [D’Agostino et al., 1963; Ducatman et al., 1986; Loree et al., 2000; Evans et al., 2012] that may be dose dependent [Nakamura et al., 2011]. Others have demonstrated concerning rates of new CNS malignancy with a threefold increased risk of in-field neoplasm compared to non-irradiated NF1 patients [Ducatman et al., 1986; Sharif et al., 2006]. In addition, non-neoplastic risks include vasculopathy, cardiovascular complications, and hormone deficiency [Evans et al., 2006]. Two of the three adult patients included in our cohort were treated with radiation therapy. In one, although serial neuroimaging revealed findings suggestive of transformation to higher grade lesion, autopsy revealed no evidence of active CNS neoplasm and substantial treatment related changes raising suspicion for possible radiation toxicity. All died as a result of progressive clinical decline. Causal associations cannot be drawn given the retrospective nature of this study and the small populations, and further study to define the risks of RT in benign tumor syndromes, particularly in the setting of aggressive disease are essential.
This small retrospective study does have limitations. Adult NF1 patients with extra-optic gliomas are rare. Although our sample size is small, given the rarity of these tumors, we are aware of no prior series comprehensively reporting on more than one adult NF1 patient with progressive extra-optic PAs. Pooled databases and international collaboration will be necessary to further support the hypothesis-generating findings in this study. Additionally, prospective studies employing varying strategies of therapeutic selection will be required to determine optimal treatment algorithms for these patients and in particular the utility of radiation for clinically aggressive progressive non-optic pathway gliomas in NF1.
CONCLUSION
CNS gliomas are common in patients with NF1. High-grade gliomas appear to behave more favorably in NF1 patients than in non-NF1 counterparts, and although PAs follow an indolent course in children with NF1, brainstem PAs in NF1 adults are different. Although histologically benign, these WHO grade I tumors behave more aggressively. Caution should be taken when counseling postoperatively. Radiation should be carefully considered and possibly deferred and clinicians should be cautious when considering alkylating based chemotherapy given the risk of treatment associated morbidity and mortality in this tumor suppressor genetic syndrome.
Footnotes
Conflicts of interest: The authors declare they have no conflicts of interest to disclose.
REFERENCES
- Balcer LJ, Liu GT, Heller G, Bilaniuk L, Volpe NJ, Galetta SL, Molloy PT, Phillips PC, Janss AJ, Vaughn S, Maguire MG. Visual loss in children with neurofibromatosis type 1 and optic pathway gliomas. Am J Ophthalmol. 2001;131:442–445. doi: 10.1016/s0002-9394(00)00852-7. [DOI] [PubMed] [Google Scholar]
- Braunstein S, Nakamura JL. Radiotherapy-induced malignancies: Review of clinical features, pathobiology, and evolving approaches for mitigating risk. Front Oncol. 2013;3:73. doi: 10.3389/fonc.2013.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi G, Huang B, Pinarbasi E, Braunstein SE, Horvai AE, Kogan S, Bhatia S, Faddegon B, Nakamura JL. Genetically mediated Nf1 loss in mice promotes diverse radiation-induced tumors modeling second malignant neoplasms. Cancer Res. 2012;72:6425–6434. doi: 10.1158/0008-5472.CAN-12-1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings TJ, Provenzale JM, Hunter SB, Friedman a H, Klintworth GK, Bigner SH, McLendon RE. Gliomas of the optic nerve: Histological, immunohistochemical (MIB-1 and p53), and MRI analysis. Acta Neuropathol. 2000;99:563–570. doi: 10.1007/s004010051161. [DOI] [PubMed] [Google Scholar]
- D’Agostino A, Soule E, Miller R. Primary malignant neoplasms of nerves (malignant neurilemomas) in patients without manifestations of multiple neurofibromatosis (von Rechlinghausen’s disease) Cancer. 1963;16:1003–1014. doi: 10.1002/1097-0142(196308)16:8<1003::aid-cncr2820160807>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Ducatman B, Scheithauer B, Piepgras D, Reiman H, Istrup D. Malignant peripheral nerve sheath tumors. A clinicopathological study of 120 cases. Cancer. 1986;57:2006–2021. doi: 10.1002/1097-0142(19860515)57:10<2006::aid-cncr2820571022>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Evans DGR, Birch JM, Ramsden RT, Sharif S, Baser ME. Malignant transformation and new primary tumours after therapeutic radiation for benign disease: Substantial risks in certain tumour prone syndromes. J Med Genet. 2006;43:289–294. doi: 10.1136/jmg.2005.036319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DGR, Huson SM, Birch JM. Malignant peripheral nerve sheath tumours in inherited disease. Clin Sarcoma Res. 2012;2:17. doi: 10.1186/2045-3329-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher MJ, Loguidice M, Gutmann DH, Listernick R, Ferner RE, Ullrich NJ, Packer RJ, Tabori U, Hoffman RO, Ardern-Holmes SL, Hummel TR, Hargrave DR, Bouffet E, Charrow J, Bilaniuk LT, Balcer LJ, Liu GT. Visual outcomes in children with neurofibromatosis type 1-associated optic pathway glioma following chemotherapy: A multicenter retrospective analysis. Neuro Oncol. 2012;14:790–797. doi: 10.1093/neuonc/nos076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman S a, Ye X, Piantadosi S, Desideri S, Nabors LB, Rosenfeld M, Fisher J. Survival of patients with newly diagnosed glioblastoma treated with radiation and temozolomide in research studies in the United States. Clin Cancer Res. 2010;16:2443–2449. doi: 10.1158/1078-0432.CCR-09-3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillamo JS, Créange A, Kalifa C, Grill J, Rodriguez D, Doz F, Barbarot S, Zerah M, Sanson M, Bastuji-Garin S, Wolkenstein P. Prognostic factors of CNS tumours in neurofibromatosis 1 (NF1): A retrospective study of 104 patients. Brain. 2003;126:152–160. doi: 10.1093/brain/awg016. [DOI] [PubMed] [Google Scholar]
- Gutmann DH, Rasmussen S a, Wolkenstein P, MacCollin MM, Guha A, Inskip PD, North KN, Poyhonen M, Birch PH, Friedman JM. Gliomas presenting after age 10 in individuals with neurofibromatosis type 1 (NF1) Neurology. 2002;59:759–761. doi: 10.1212/wnl.59.5.759. [DOI] [PubMed] [Google Scholar]
- Huttner A, Kieran M, Yao X, Ladner J, Quayle K, Goumnerova L, Irons M, Ullrich N. Clinicopathologic study of glioblastoma in children with neurofibromatosis type 1. Pediatr Blood Cancer. 2010;54:890–896. doi: 10.1002/pbc.22462. [DOI] [PubMed] [Google Scholar]
- King A, Listernick R, Charrow J, Piersall L, Gutmann DH. Optic pathway gliomas in neurofibromatosis type 1: The effect of presenting symptoms on outcome. Am J Med Genet A. 2003;122A:95–99. doi: 10.1002/ajmg.a.20211. [DOI] [PubMed] [Google Scholar]
- Loree TR, North JH, Werness BA, Nangia R, Mullins AP, Hicks WL. Malignant peripheral nerve sheath tumors of the head and neck: Analysis of prognostic factors. Otolaryngol Head Neck Surg. 2000;122:667–672. doi: 10.1016/S0194-5998(00)70193-8. [DOI] [PubMed] [Google Scholar]
- Mikaeloff Y, Chaix Y, Grill J, Adamsbaum C, Bursztyn J, Rubie H, Sevely A, Jambaqué I, Kalifa C, Ponsot G, Carriére JC, Rodriguez D. Optic pathway gliomas in neurofibromatosis type I. Longitudinal study of 30 cases in two multidisciplinary practices. Arch Pediatr. 2002;9:797–804. doi: 10.1016/s0929-693x(01)00991-5. [DOI] [PubMed] [Google Scholar]
- Molloy PT, Bilaniuk LT, Vaughan SN, Needle MN, Liu GT, Phillips PC. Brainstern tumors in patients with neurofibrornatosis type 1. Neurology. 1996;45:1897–1902. doi: 10.1212/wnl.45.10.1897. [DOI] [PubMed] [Google Scholar]
- Nakamura JL, Phong C, Pinarbasi E, Kogan SC, Vandenberg S, Horvai AE, Faddegon BA, Fiedler D, Shokat K, Houseman BT, Chao R, Pieper RO, Shannon K. Dose-dependent effects of focal fractionated irradiation on secondary malignant neoplasms in Nf1 mutant mice. Cancer Res. 2011;71:106–115. doi: 10.1158/0008-5472.CAN-10-2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer RJ, Ater J, Allen J, Phillips P, Geyer R, Nicholson HS, Jakacki R, Kurczynski E, Needle M, Finlay J, Reaman G, Boyett JM. Carboplatin and vincristine chemotherapy for children with newly diagnosed progressive low-grade gliomas. J Neurosurg. 1997;86:747–754. doi: 10.3171/jns.1997.86.5.0747. [DOI] [PubMed] [Google Scholar]
- Parsa CF, Hoyt CS, Lesser RL, Weinstein JM, Strother CM, Muci-Mendoza R, Ramella M, Manor RS, Fletcher WA, Repka MX, Garrity JA, Ebner RN, Monteiro ML, McFadzean RM, Rubtsova IV, Hoyt WF. Spontaneous regression of optic gliomas. JAMA. 2001;119:516–529. doi: 10.1001/archopht.119.4.516. [DOI] [PubMed] [Google Scholar]
- Perilongo G, Moras P, Carollo C, Battistella A, Clementi M, Laverda A, Murgia A. Spontaneous partial regression of low-grade glioma in children with neurofibromatosis-1: A real possibility. J Child Neurol. 1999;14:352–356. doi: 10.1177/088307389901400602. [DOI] [PubMed] [Google Scholar]
- Pollack IF, Shultz B, Mulvihill JJ. The management of brainstem gliomas in patients with neurofibromatosis 1. Neurology. 1996;46:1652–1660. doi: 10.1212/wnl.46.6.1652. [DOI] [PubMed] [Google Scholar]
- Rasmussen S a, Yang Q, Friedman JM. Mortality in neurofibromatosis 1: An analysis using U.S. death certificates. Am J Hum Genet. 2001;68:1110–1118. doi: 10.1086/320121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez FJ, Perry A, Gutmann DH, Patrick B, Neill O, Leonard J, Bryant S, Giannini C. Gliomas in neurofibromatosis type 1: A clinico-pathologic study of 100 patients. J Neuropathol Exp Neurol. 2008;67:240–249. doi: 10.1097/NEN.0b013e318165eb75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez FJ, Scheithauer BW, Burger PC, Jenkins S, Giannini C. Anaplasia in pilocytic astrocytoma predicts aggressive behavior. Am J Surg Pathol. 2010;34:147–160. doi: 10.1097/PAS.0b013e3181c75238. [DOI] [PubMed] [Google Scholar]
- Rosenfeld A, Listernick R, Charrow J, Goldman S. Neurofibromatosis type 1 and high-grade tumors of the central nervous system. Childs Nerv Syst. 2010;26:663–667. doi: 10.1007/s00381-009-1024-2. [DOI] [PubMed] [Google Scholar]
- Rosser T, Packer RJ. Intracranial neoplasms in children with neurofibromatosis 1. J Child Neurol. 2002;17:630–637. doi: 10.1177/088307380201700815. [DOI] [PubMed] [Google Scholar]
- Segal L, Darvish-Zargar M, Dilenge ME, Ortenberg J, Polomeno RC. Optic pathway gliomas in patients with neurofibromatosis type 1: Follow-up of 44 patients. J AAPOS. 2010;14:155–158. doi: 10.1016/j.jaapos.2009.11.020. [DOI] [PubMed] [Google Scholar]
- Sharif S, Ferner R, Birch JM, Gillespie JE, Gattamaneni HR, Baser ME, Evans DGR. Second primary tumors in neurofibromatosis 1 patients treated for optic glioma: Substantial risks after radiotherapy. J Clin Oncol. 2006;24:2570–2575. doi: 10.1200/JCO.2005.03.8349. [DOI] [PubMed] [Google Scholar]
- Stupp R, Mason W, van den Bent M, Weller M, Fisher B, Taphoorn MJB, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin S, Gorlia T, Allgeier A, Lacombe D, Cairncross J, Eisenhauer E, Mirimanoff R, Groups, EO for R and T of CBT and R, Group, NCI of CCT Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. NEJM. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- Theeler BJ, Ellezam B, Yust-Katz S, Slopis JM, Loghin ME, De Groot JF. Prolonged survival in adult neurofibromatosis type i patients with recurrent high-grade gliomas treated with bevacizumab. J Neurol. 2014;261:1559–1564. doi: 10.1007/s00415-014-7292-0. [DOI] [PubMed] [Google Scholar]
- Ullrich NJ, Raja AI, Irons MB, Kieran MW, Goumnerova L. Brainstem lesions in neurofibromatosis type 1. Neurosurgery. 2007;61:762–767. doi: 10.1227/01.NEU.0000298904.63635.2D. [DOI] [PubMed] [Google Scholar]