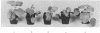Abstract
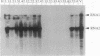
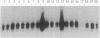
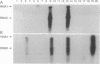
The 3' proximal portion of the gene encoding the 201-kDa putative replicase protein from the Tobravirus pea early browning virus (PEBV) can potentially be expressed separately as a 54-kDa protein. Nicotiana benthamiana plants transformed with the open reading frame (ORF) encoding the 54-kDa protein, designated 54K ORF, were resistant to infection by purified PEBV at inoculum doses of up to 1 mg/ml, the highest concentration tested. However, resistance was abolished by the introduction into the 54K ORF of mutations that would cause premature termination of translation. This suggests that the resistance mechanism requires the involvement of an intact 54-kDa protein. The 54K ORF-transformed plants were also resistant to infection by broad bean yellow band virus and an uncharacterized isolate of British PEBV (PGRO R) but were not resistant to infection by two other tobraviruses, pepper ringspot virus and the I6 isolate of tobacco rattle virus. Additionally, two variants of PEBV which overcame 54K ORF-mediated resistance have been isolated, the analysis of which might provide important information about both the resistance mechanism itself and the process of normal virus replication.
Full text
PDF


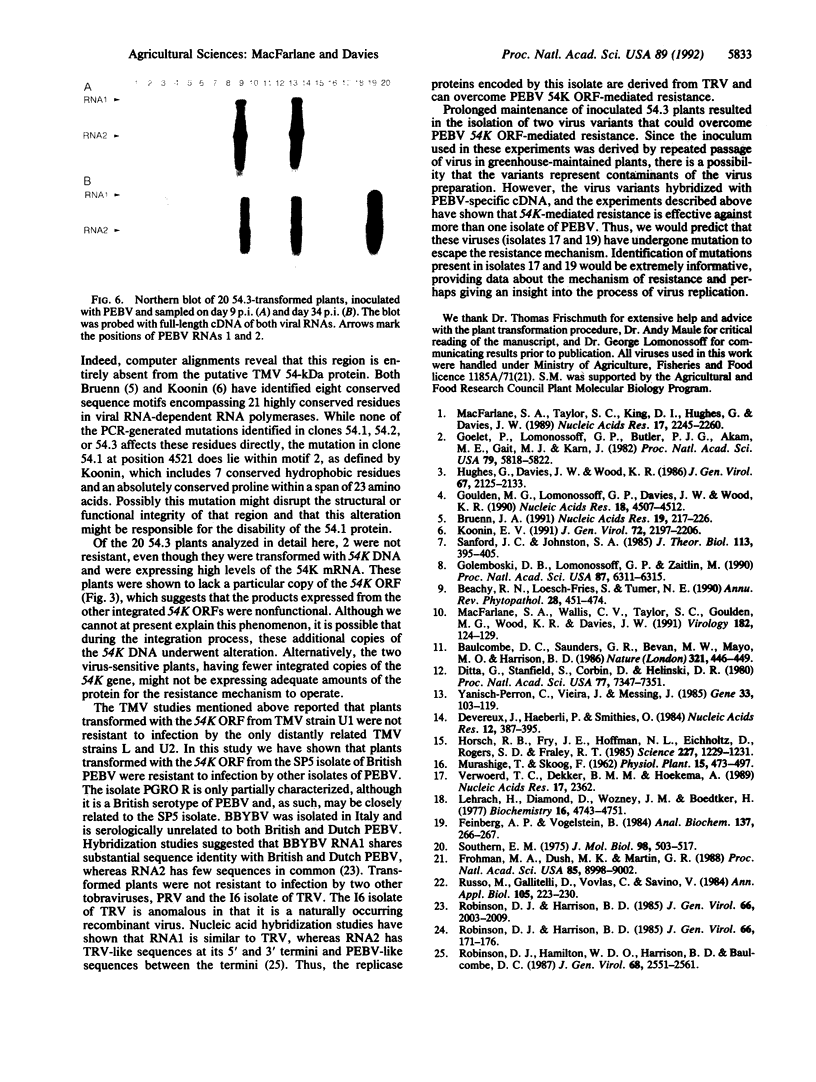
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- A simple and general method for transferring genes into plants. Science. 1985 Mar 8;227(4691):1229–1231. doi: 10.1126/science.227.4691.1229. [DOI] [PubMed] [Google Scholar]
- Bruenn J. A. Relationships among the positive strand and double-strand RNA viruses as viewed through their RNA-dependent RNA polymerases. Nucleic Acids Res. 1991 Jan 25;19(2):217–226. doi: 10.1093/nar/19.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditta G., Stanfield S., Corbin D., Helinski D. R. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7347–7351. doi: 10.1073/pnas.77.12.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Frohman M. A., Dush M. K., Martin G. R. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8998–9002. doi: 10.1073/pnas.85.23.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goelet P., Lomonossoff G. P., Butler P. J., Akam M. E., Gait M. J., Karn J. Nucleotide sequence of tobacco mosaic virus RNA. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5818–5822. doi: 10.1073/pnas.79.19.5818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golemboski D. B., Lomonossoff G. P., Zaitlin M. Plants transformed with a tobacco mosaic virus nonstructural gene sequence are resistant to the virus. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6311–6315. doi: 10.1073/pnas.87.16.6311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulden M. G., Lomonossoff G. P., Davies J. W., Wood K. R. The complete nucleotide sequence of PEBV RNA2 reveals the presence of a novel open reading frame and provides insights into the structure of tobraviral subgenomic promoters. Nucleic Acids Res. 1990 Aug 11;18(15):4507–4512. doi: 10.1093/nar/18.15.4507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gualdi Russo E. Biological distances in dermatoglyphics of beta-thalassemic subjects. Acta Anthropogenet. 1984;8(3-4):223–230. [PubMed] [Google Scholar]
- Koonin E. V. The phylogeny of RNA-dependent RNA polymerases of positive-strand RNA viruses. J Gen Virol. 1991 Sep;72(Pt 9):2197–2206. doi: 10.1099/0022-1317-72-9-2197. [DOI] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- MacFarlane S. A., Taylor S. C., King D. I., Hughes G., Davies J. W. Pea early browning virus RNA1 encodes four polypeptides including a putative zinc-finger protein. Nucleic Acids Res. 1989 Mar 25;17(6):2245–2260. doi: 10.1093/nar/17.6.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacFarlane S. A., Wallis C. V., Taylor S. C., Goulden M. G., Wood K. R., Davies J. W. Construction and analysis of infectious transcripts synthesized from full-length cDNA clones of both genomic RNAs of pea early browning virus. Virology. 1991 May;182(1):124–129. doi: 10.1016/0042-6822(91)90655-u. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Verwoerd T. C., Dekker B. M., Hoekema A. A small-scale procedure for the rapid isolation of plant RNAs. Nucleic Acids Res. 1989 Mar 25;17(6):2362–2362. doi: 10.1093/nar/17.6.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]