Abstract
Background
The purpose of this study was to compare the surgical outcomes of robotic thyroidectomy (RT) using bilateral axillo-breast approach (BABA) with conventional open thyroidectomy (OT) in papillary thyroid carcinoma patients.
Methods
Between January 2009 and December 2013, 815 patients who had received thyroidectomy for papillary thyroid carcinoma were enrolled. Of these, 126 patients received RT and 689 patients underwent OT. Age, gender, body mass index, extent of surgery, tumor size, multiplicity, bilaterality, extrathyroidal extension, and tumor stage were used for the propensity score matching analysis. One hundred and nine patients were selected in each group, and surgical outcomes were compared between the two groups.
Results
The RT group showed a significantly longer operating time (290.6 ± 74.4 vs. 107.9 ± 30.8 min, P < 0.001). However, the mean hospital stay after surgery (3.6 ± 0.8 vs. 3.4 ± 1.2 days, P = 0.293), postoperative complication rates (major and minor, P = 0.754 and P = 0.852), and pain score (postoperative day, P = 0.669; postoperative day 1, P = 0.952) were comparable between the two groups. There was no difference in the number of metastatic lymph nodes, but the mean number of retrieved lymph nodes in the RT group was lesser than that in the OT group (3.5 ± 3.5 vs. 5.3 ± 5.2, P = 0.002).
Conclusions
Robotic thyroidectomy via the BABA may be a safe and acceptable surgical technique. But, further development that resolves the limitation of central node dissection is needed.
Keywords: Robotic surgical procedure, Thyroid neoplasm, Thyroidectomy, Treatment outcome
Background
Since the time minimally invasive endoscopic thyroid surgery has been introduced [1], various methods have been developed to improve accessibility and cosmetic satisfaction. Thereafter, minimally invasive surgery was extended to the field of papillary thyroid carcinoma (PTC) and many studies have reported favorable surgical outcomes including outstanding cosmetic results [2–4]. But, endoscopic thyroidectomy has several limitations such as restricted motion of surgical instruments and two-dimensional camera view. These limitations were reduced by using the da Vinci S robotic system (Intuitive Surgical, Sunnyvale, CA) that offered several advantages to the surgeon with a highly magnified three-dimensional view, fine motor scaling, tremor-free, and endo-wrist function [5, 6]. A number of studies showing promising surgical outcomes of robotic thyroidectomy (RT) have been published [7–26]. But, there has been no randomized study for RT and any other robotic surgery, and it may be difficult to perform such a study in the near future because of the fundamental limitation about the cost issues. In particular, the issue concerning the oncological safety of RT in PTC patients is debatable and there is no worldwide consensus on the definite indication for RT as yet. Therefore, we analyzed the results of RT in our hospital for the last 5 years and compared the surgical and oncological safety of RT with conventional open thyroidectomy (OT) for analyzing the feasibility of RT in PTC patients.
Methods
This retrospective cohort study was carried out between January 2009 and December 2013 in the Department of Surgery, Kyung Hee University Medical Center, Seoul, South Korea. We included 815 PTC patients who underwent thyroidectomy with or without central node dissection (CND). Of these, 689 patients received conventional OT and 126 patients underwent RT using the bilateral axillary breast approach (BABA) method (Fig. 1).
Fig. 1.
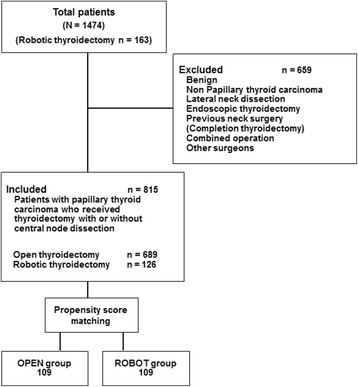
Patient selection
The decision regarding the operation methods, whether robotic or conventional open thyroidectomy, was made based on the patients’ preferences. Randomization of operative methods was not possible because the cost of robotic surgery is not reimbursed by national health insurance; hence, the hospital cost for RT is two to three times higher.
We conducted a propensity score matching analysis to reduce treatment selection bias and potential confounding effects [27]. We selected the following 10 factors that could affect the surgical outcomes: age, sex, body mass index (BMI), extent of surgery, extent of lymph node dissection, tumor size, multiplicity, bilaterality, extrathyroidal extension, and cancer stage [28]. One hundred and nine patients were selected in each group, and surgical outcomes were compared between the two groups.
RT was not recommended in cases with tumors >4 cm or clinically evident lateral lymph node metastasis or tumors located on the dorsal aspect of the thyroid with suspicion of invasion into adjacent organs such as the esophagus, trachea, or recurrent laryngeal nerve.
Unilateral or bilateral CND was conducted for prophylactic or therapeutic purposes. Bilateral CND was performed for bilateral tumors or in patients with contralateral suspicious lymph node enlargement on preoperative evaluation or intraoperative gross morphology. Lobectomy without CND was performed only when there was a single tumor <5 mm without extrathyroidal extension.
All patients were diagnosed with PTC or suspicious for PTC by fine needle aspiration cytology. We performed ultrasonography (US), computed tomography (CT), and thyroid function tests in all patients.
Surgical outcomes
Details of surgery such as operation time, status of parathyroid glands, and postoperative complications were compared between the two groups. Transient hypocalcemia was defined as the patients who received calcium replacement to treat hypocalcemic symptoms and who had a serum parathyroid hormone (PTH) level <13 pg/mL regardless of their serum calcium levels. We measured the PTH level at postoperative day (POD) 2. The patients who had hoarseness with vocal cord palsy confirmed by laryngoscopy were defined as having transient hoarseness. Permanent hypocalcemia was defined as PTH level <13 pg/mL and the need for calcium or vitamin D supplements for more than 6 months after thyroidectomy. Permanent recurrent laryngeal nerve palsy was diagnosed when they lasted over 6 months.
A drain was placed in the thyroid bed, and it was removed when the amount of drainage was less than 40 mL in a day. Postoperative pain was evaluated using an 11-point visual analog scale (VAS). Pain score was checked at 1 h after surgery and once in a day until discharge. Hospital cost was determined based on the inpatient charges. The evaluation of an abnormal sequelae or complication was routinely carried out at 2 weeks after the operation and continued until 1 year after the operation in an outpatient setting. Radioactive iodine (RAI) ablation therapy was performed on the basis of cancer stage and risk factors, according to the American Thyroid Association (ATA) guidelines [29]. TSH-stimulated thyroglobulin (Tg) levels in patients who received radioiodine therapy were analyzed to compare surgical completeness between the two groups. TSH-stimulated Tg levels were checked after withdrawal of levothyroxine or injection of recombinant human thyrotropin, while TSH levels were >30 μIU/mL. The stimulated Tg and TSH levels were measured on the day of RAI ablation therapy before RAI administration. The RAI dose ranged from 30 mCi 131-I to 150 mCi 131-I. Patients received RAI ablation therapy after 8–12 weeks after thyroidectomy.
Surgical procedures
All surgeries were performed by a single surgeon. We used the BABA technique that was introduced by Lee et al. [5] for RT. For the detailed procedure, please refer to the bibliography [5]. The only difference was that we injected normal saline instead of diluted epinephrine (1:200,000) for flap elevation.
Statistical analysis
We used the Kolmogorov-Smirnov test to assess normality of data distribution. Baseline clinicopathologic characteristics between the OT and RT groups were compared by using the Fisher’s exact test for categorical variables and the independent sample t test or Mann-Whitney U test for continuous variables. After propensity score matching, the two groups were compared in terms of baseline clinicopathologic characteristics and surgical outcomes by using the McNemar test for categorical variables and the paired t test for continuous variables. All statistical tests were two-sided, and a P value <0.05 was considered statistically significant. Statistical analysis was performed using SPSS® version 19.0 (IBM Co., Armonk, NY, USA). The study protocol was approved by our Institutional Review Board.
Results
Baseline characteristics of the study groups before cohort matching
Table 1 shows the baseline clinicopathologic characteristics of the two groups before propensity score matching. The mean age was lower in the RT group than that in the OT group (39.86 ± 10.29 years vs. 52.15 ± 12.06 years, P < 0.001). The BMI was lower in the RT group (23.40 [range, 15.45–37.77] vs. 24.45 [range, 17.10–37.79], P = 0.001). The proportions of stage III disease and total thyroidectomy were significantly lower in the RT group than that in the OT group (P < 0.001, P = 0.027).
Table 1.
Baseline characteristics of patients before propensity score matching
| Variables | Open group | Robot group | P value |
|---|---|---|---|
| n = 689 | n = 126 | ||
| Age, years (mean ± SD) | 52.15 ± 12.06 | 39.86 ± 10.29 | < 0.001 |
| Gender | 0.351 | ||
| Male (%) | 110 (16.0) | 16 (12.7) | |
| Female (%) | 579 (84.0) | 110 (87.3) | |
| BMI (median (range)) | 24.45 (17.10–37.79) | 23.40 (15.45–37.77) | 0.001 |
| Extent of surgery | 0.027 | ||
| Total thyroidectomy (%) | 583 (84.6) | 99 (78.6) | |
| Subtotal thyroidectomy (%) | 1 (0.1) | 2 (1.6) | |
| Lobectomy (%) | 105 (15.2) | 25 (19.8) | |
| Lymph nodes dissection | 0.400 | ||
| None (%) | 67 (9.7) | 21 (16.7) | |
| Unilateral (%) | 461 (66.9) | 83 (65.9) | |
| Bilateral (%) | 161 (23.4) | 22 (17.5) | |
| No. of metastatic lymph nodes (mean ± SD (range)) | 0.90 ± 2.07 (0–19) | 0.70 ± 1.54 (0–9) | 0.611 |
| No. of retrieved lymph nodes (mean ± SD (range)) | 5.12 ± 4.52 (0–35) | 3.35 ± 3.48 (0–17) | < 0.001 |
| Tumor size (cm, median (range)) | 0.76 (0.1–4.7) | 0.74 (0.2–2.5) | 0.389 |
| Multiplicity (%) | 201 (29.2) | 31 (24.6) | 0.334 |
| Bilaterality (%) | 157 (26.9, 157/584) | 23 (22.8, 23/101) | 0.294 |
| Extrathyroidal extension (%) | 281 (40.8) | 45 (35.7) | 0.323 |
| Stage (%) | < 0.001 | ||
| I | 405 (58.8) | 109 (86.5) | |
| II | 6 (0.9) | 0 (0) | |
| III | 278 (40.3) | 17 (13.5) | |
| IV | 0 (0) | 0 (0) |
Baseline characteristics of the study groups after cohort matching
Table 2 shows the baseline clinicopathologic characteristics of the two groups after propensity score matching. After cohort matching, 109 pairs of patients were selected in the two groups. The 10 covariates that could affect the surgical outcomes were used to calculate the propensity score, and significant differences in covariates such as age, BMI, extent of surgery, and stage which were observed before the matching were no longer present.
Table 2.
Baseline characteristics of patients, after propensity score matching
| Variables | Open group | Robot group | P value |
|---|---|---|---|
| (n = 109) | (n = 109) | ||
| Age, years (mean ± SD) | 40.81 ± 10.84 | 41.78 ± 9.36 | 0.259 |
| Sex, female (%) | 91 (83.5) | 94 (86.2) | 0.700 |
| BMI (median (range)) | 23.73 (17.58–33.37) | 24.53 (16.63–37.77) | 0.984 |
| Extent of surgery (total thyroidectomy [%]) | 93 (85.1) | 88 (80.7) | 0.278 |
| Lymph nodes dissection | 0.940 | ||
| None (%) | 15 (13.8) | 14 (12.8) | |
| Unilateral (%) | 72 (66.1) | 75 (68.8) | |
| Bilateral (%) | 22 (20.2) | 20 (18.3) | |
| Tumor size (cm, median (range)) | 0.7 (0.2–2.5) | 0.7 (0.2–2.5) | 0.331 |
| Multiplicity (%) | 25 (22.9) | 28 (25.7) | 0.760 |
| Bilaterality (%) | 20 (18.3) | 21 (19.3) | 1.000 |
| Extrathyroidal extension (%) | 36 (33.0) | 38 (34.9) | 0.888 |
| Stage (I/III, %) | 92 (89.0)/12 (11.0) | 93 (85.3)/16 (14.7) | 0.317 |
Comparison of surgical outcomes
Table 3 shows the comparison of surgical outcomes between the two groups. The operation time was longer in the RT group (P < 0.001), and the total amount of hospital cost was higher in the RT group than that in the OT group (P < 0.001). There were no significant differences between the two groups in the length of hospital stay (P = 0.293) and postoperative pain score (P = 0.669). Postoperative complications showed no differences between the two groups (minor complications [P = 0.852], major complications [P = 0.754]). The number of cases that showed identification of the parathyroid gland with permanent pathology (P = 1.000) and the number of parathyroid glands saved during the operation (P = 0.160) were not different between the two groups.
Table 3.
Comparison of the surgical outcomes between two groups, after propensity score matching
| Variables | Open group | Robot group | P value |
|---|---|---|---|
| n = 109 | n = 109 | ||
| Operation time (min, mean ± SD (range)) | 107.94 ± 30.84 (60–280) | 290.57 ± 74.37 (160–715) | < 0.001 |
| Hospital stay (days, mean ± SD (range)) | 3.40 ± 1.24 (2–11) | 3.56 ± 0.83 (2–8) | 0.293 |
| Cost ($, mean ± SD) | 2995 ± 695 | 7,632 ± 1,282 | < 0.001 |
| Pain score, operation day (mean ± SD (range)) | 3.84 ± 1.11 (0–7) | 3.76 ± 1.22 (0–7) | 0.669 |
| Pain score, postoperative 1 day (mean ± SD (range)) | 2.36 ± 0.99 (0–5) | 2.38 ± 1.06 (0–5) | 0.952 |
| Parathyroid gland in pathology | 42 (38.5) | 41 (37.6) | 1.000 |
| Identified number of parathyroid glands during surgery (mean ± SD (range)) | 2.68 ± 0.88 (0–4) | 2.54 ± 0.87 (1–4) | 0.160 |
| Minor complication (%) | 37 (34) | 43 (39) | 0.852 |
| Transient hypocalcemia | 29 | 36 | |
| Transient hoarseness | 6 | 7 | |
| Wound seroma | 1 | 0 | |
| Wound infection | 1 | 0 | |
| Major complication (%) | 6 (5.5) | 4 (3.7) | 0.754 |
| Bleeding | 1 | 1 | |
| Chyle leakage | 2 | 0 | |
| Permanent hoarseness | 1 | 1 | |
| Permanent hypocalcemia | 2 | 2 | |
| Radioiodine ablation (%) | 52 (47.7) | 67 (61.5) | 0.033 |
| Stimulated thyroglobulin (ng/mL, median (range)) | 0.25 (0–66.7) | 0.20 (0–6.8) | 0.954 |
| No. of metastatic lymph nodes (mean ± SD (range)) | 1.26 ± 3.07 (0–19) | 0.60 ± 1.26 (0–5) | 0.133 |
| No. of retrieved lymph nodes (mean ± SD (range)) | 5.29 ± 5.25 (0–29) | 3.50 ± 3.55 (0–17) | 0.002 |
Radioiodine ablation therapy was conducted in 61.5 % of patients in the RT group (67/109 patients) and in 47.7 % of patients in the OT group (52/109 patients). The mean TSH-stimulated Tg level was not different between the two groups (P = 0.954).
There was no difference between the two groups in the mean number of metastatic lymph nodes, but the RT group showed less number of retrieved lymph nodes than the OT group (3.50 ± 3.55 [range, 0–17] vs. 5.29 ± 5.25 [range, 0–29]).
Discussion
We conducted this study to analyze our initial experiences of BABA robotic thyroidectomy for the last 5 years and to compare the surgical outcomes between RT and OT for assessing the feasibility of robotic thyroidectomy for PTC.
In our study, baseline clinicopathologic characteristics were different between the two groups. The RT group showed a lower mean age, lower mean BMI, higher proportion of lobectomy than total thyroidectomy, and lower stage (UICC/AJCC seventh edition), although the tumor size was not different. These differences may be due to a greater desire to avoid a visible anterior neck scar in younger patients, and RT was not recommended in the patients with clinically suspected lymph node metastases. Thus, the findings of this study were inevitably influenced by several confounding factors including a selection bias between the RT and OT groups. The patient’s preferences and narrow indication for RT in our hospital may be the major causes of selection bias. We think that the economic burden of robotic surgery is the main reason why we cannot conduct a randomized study.
The propensity score analysis was used to reduce the confounding factors [27]. Several clinical features and surgical outcomes were compared between the paired 109 patients in both groups after propensity score matching analysis.
The RT group showed a significantly longer operating time. The main contributing factors are the process of creating the flap and robotic docking, which are required for the robotic system operation, and most of the other studies showed similar results [8–24]. But, the robotic operative time is likely to decrease with accumulation of experience and overcoming the learning curve [7].
As expected, the hospital cost in the RT group was about three times higher than that in the OT group. Although we cannot ignore the fact that robotic surgery causes an increase in the total health cost, from a personal point of view, this problem can be solved by lowering the price via competing with other suppliers. In addition, the problem of high cost may be naturally resolved when robotic surgery is popularized like laparoscopic surgery. Most importantly, it can be affordable enough on considering additional excellent cosmetic benefits [10, 11].
On the assessment of safety of RT, there was no significant difference in complication rates between the two groups. This may be an important result on considering the advantage of robotic surgery like fine movement and magnification view. Equivalence of complication rates is enough to demonstrate the safety of RT, considering the low incidence of serious complications after OT.
TSH-stimulated Tg level measured for assessing surgical completeness in papillary thyroid carcinoma was not different between the two groups. TSH-stimulated Tg is one of the important clinical parameters that reflect surgical completeness [30]. The study that particularly analyzed surgical completeness of RT showed a similar result [12], and there is a study that showed superiority of surgical completeness of RT [13]. In this study, while there was no difference in the TNM stage between the two groups, the rates of RAI ablation therapy was higher in the RT group (Table 3). This difference might have resulted from aggressive treatment policy at our institution. We performed RAI ablation therapy in accordance with the ATA guidelines [29] in most cases, but selected patients with stage I disease who received RAI ablation therapy, especially those with angiolymphatic invasion, multifocal disease, nodal disease, and aggressive histology.
There was no difference in the number of metastatic lymph nodes between the two groups, but the number of retrieved lymph nodes was lower in the RT group. To date, there is no consensus about the prognostic implications of lymph node ratio in PTC. The recently published seventh UICC/AJCC staging criteria of thyroid carcinoma do not evaluate lymph node ratio [28]. But, the importance of the LN ratio in PTC has been reported [31–35] and it is likely to have a greater oncological significance in PTC, as in cases of other solid organ cancers [36–38]. Although the follow-up period was short (range, 22–68 months), there was no case of recurrence in the RT group.
Although the absolute value of the retrieved lymph nodes seemed to show a marginal difference, similar results were observed in other studies and meta-analysis [14–16]. The limitation of central node dissection was also reported with a trans-axillary approach [17, 18]. Despite the strong advantages of the robotic arm multi-articulated joint system, directional rigidity of the scope and restricted view of the lower part of the neck are considered to be the most important causes of limitation of central node dissection and it is a matter that needs to be carefully considered as a limitation of RT.
However, several studies reported that the number of retrieved lymph nodes in central node dissection is similar in both robotic and open thyroidectomies [19, 20], and various methods are being attempted to overcome the limitation of the field of view in the lower part of the neck (e.g., make widening the camera view by applying elastic bandage at the lower breast and change the operation table to reverse Trendelenburg position) [15]. The most important part to ensure good visibility of the lower part of the neck is secure sufficient space at the lower neck region during the process of creating the flap.
In our experience, with the sense of incompatibility, the great feature of the robot joint function was not fully utilized at the beginning of robotic surgery. This could simply indicate the learning curve, but familiarity of conventional endoscopic or laparoscopic equipment can act as additional difficulties for expert surgeons. And, the result of this study with a lower number of retrieved lymph node might have been influenced by 30~40 of cases of the early period. It will be explained with further analysis after the experience has accumulated.
Currently, BABA RT has not been accepted as a standard surgical method for PTC, but results of recent studies including a meta-analysis generally show favorable surgical outcomes of RT [21–23]. Accordingly, recent interests are being focused on functional benefits of RT. We analyzed postoperative pain with respect to functional benefit, and there was no significant difference between the two groups. Results for cosmetic satisfaction [10, 11], sensory change in the anterior neck region [24], swallowing discomfort [25], and voice impairments [26] were similar or better with RT compared to OT as well as pain.
Conclusions
In conclusion, our observational study showed that BABA RT is feasible in terms of surgical safety and surgical completeness that are estimated by postoperative complications and TSH-stimulated Tg, respectively, when compared with OT after adjusting for the selection bias by propensity score matching analysis. But, we could not confirm the oncological safety of BABA RT because it showed limitations in the central compartment LN dissection. In order to establish a clear surgical indication for RT, more evidences are needed that can ensure both surgical and oncological safety.
Abbreviations
BABA, bilateral axillary breast approach; CND, central node dissection; OT, open thyroidectomy; POD, postoperative days; PTC, papillary thyroid carcinoma; PTH, parathyroid hormone; RAI, radioactive iodine; RT, robotic thyroidectomy; Tg, thyroglobulin
Acknowledgements
None.
Source of funding
None.
Availability of data and materials
The datasets supporting the conclusions of this article are not shared.
Authors’ contributions
WS Park, JN Cho, and SY Min contributed to the conception and design of the study. JN Cho contributed to the data collection and data analysis. WS Park and JN Cho wrote, reviewed, and revised the manuscript. WS Park supervised the study. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
This study was approved by Kyung Hee University Hospital Institutional Review Board (IRB No. KMC IRB 1602-04).
Contributor Information
Jeong Nam Cho, Email: jmfor@naver.com.
Won Seo Park, Phone: +82-2-958-8259, Email: pwsmd@hanmail.net.
Sun Young Min, Email: minishan@naver.com.
Sang-Ah Han, Email: drhsa.khmc@gmail.com.
Jeong-Yoon Song, Email: jeonguni@khu.ac.kr.
References
- 1.Hüscher CS, Chiodini S, Napolitano C, Recher A. Endoscopic right thyroid lobectomy. Surg Endosc. 1997;11:877. doi: 10.1007/s004649900476. [DOI] [PubMed] [Google Scholar]
- 2.Miccoli P, Elisei R, Materazzi G, Capezzone M, Galleri D, Pacini F, et al. Minimally invasive video-assisted thyroidectomy for papillary carcinoma: a prospective study of its completeness. Surgery. 2002;132:1070–3. doi: 10.1067/msy.2002.128694. [DOI] [PubMed] [Google Scholar]
- 3.Chung YS, Choe J-H, Kang K-H, Kim SW, Chung K-W, Park KS, et al. Endoscopic thyroidectomy for thyroid malignancies: comparison with conventional open thyroidectomy. World J Surg. 2007;31:2302–6. doi: 10.1007/s00268-007-9117-0. [DOI] [PubMed] [Google Scholar]
- 4.Jeong JJ, Kang S-W, Yun J-S, Sung TY, Lee SC, Lee YS, et al. Comparative study of endoscopic thyroidectomy versus conventional open thyroidectomy in papillary thyroid microcarcinoma (PTMC) patients. J Surg Oncol. 2009;100:477–80. doi: 10.1002/jso.21367. [DOI] [PubMed] [Google Scholar]
- 5.Lee KE, Rao J, Youn Y-K. Endoscopic thyroidectomy with the da Vinci robot system using the bilateral axillary breast approach (BABA) technique: our initial experience. Surg Laparosc Endosc Percutan Tech. 2009;19:e71–5. doi: 10.1097/SLE.0b013e3181a4ccae. [DOI] [PubMed] [Google Scholar]
- 6.Kang S-W, Jeong JJ, Yun J-S, Sung TY, Lee SC, Lee YS, et al. Robot-assisted endoscopic surgery for thyroid cancer: experience with the first 100 patients. Surg Endosc. 2009;23:2399–406. doi: 10.1007/s00464-009-0366-x. [DOI] [PubMed] [Google Scholar]
- 7.Kim WW, Jung JH, Park HY. A single surgeon’s experience and surgical outcomes of 300 robotic thyroid surgeries using a bilateral axillo-breast approach. J Surg Oncol. 2015;111:135–40. doi: 10.1002/jso.23793. [DOI] [PubMed] [Google Scholar]
- 8.Noureldine SI, Abdelghani R, Saeed A, Cortes N, Abbas A, Aslam R, et al. Is robotic hemithyroidectomy comparable to its conventional counterpart? Surgery. 2013;154:363–8. doi: 10.1016/j.surg.2013.04.066. [DOI] [PubMed] [Google Scholar]
- 9.Yi O, Yoon JH, Lee Y-M, Sung T-Y, Chung K-W, Kim TY, et al. Technical and oncologic safety of robotic thyroid surgery. Ann Surg Oncol. 2013;20:1927–33. doi: 10.1245/s10434-012-2850-0. [DOI] [PubMed] [Google Scholar]
- 10.Song CM, Ji YB, Bang HS, Park CW, Kim DS, Tae K. Quality of life after robotic thyroidectomy by a gasless unilateral axillary approach. Ann. Surg. Oncol. 2014;21:4188-94. [DOI] [PubMed]
- 11.Lee S, Kim HY, Lee CR, Park S, Son H, Kang S-W, et al. A prospective comparison of patient body image after robotic thyroidectomy and conventional open thyroidectomy in patients with papillary thyroid carcinoma. Surgery. 2014;156:117–25. doi: 10.1016/j.surg.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 12.Lee KE, Koo DH, Im HJ, Park SK, Choi JY, Paeng JC, et al. Surgical completeness of bilateral axillo-breast approach robotic thyroidectomy: comparison with conventional open thyroidectomy after propensity score matching. Surgery. 2011;150:1266–74. doi: 10.1016/j.surg.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 13.Lee S, Lee CR, Lee SC, Park S, Kim HY, Son H, et al. Surgical completeness of robotic thyroidectomy: a prospective comparison with conventional open thyroidectomy in papillary thyroid carcinoma patients. Surg Endosc. 2014;28:1068–75. doi: 10.1007/s00464-013-3303-y. [DOI] [PubMed] [Google Scholar]
- 14.Kwak HY, Kim HY, Lee HY, Jung SP, Woo SU, Son GS, et al. Robotic thyroidectomy using bilateral axillo-breast approach: comparison of surgical results with open conventional thyroidectomy. J Surg Oncol. 2015;111:141–145. doi: 10.1002/jso.23674. [DOI] [PubMed] [Google Scholar]
- 15.Kim BS, Kang KH, Kang H, Park SJ. Central neck dissection using a bilateral axillo-breast approach for robotic thyroidectomy: comparison with conventional open procedure after propensity score matching. Surg Laparosc Endosc Percutan Tech. 2014;24:67–72. doi: 10.1097/SLE.0b013e3182a4bfec. [DOI] [PubMed] [Google Scholar]
- 16.Son SK, Kim JH, Bae JS, Lee SH. Surgical safety and oncologic effectiveness in robotic versus conventional open thyroidectomy in thyroid cancer: a systematic review and meta-analysis. Ann Surg Oncol. 2015;22:3022–32. doi: 10.1245/s10434-015-4375-9. [DOI] [PubMed] [Google Scholar]
- 17.Tae K, Ji YB, Cho SH, Lee SH, Kim DS, Kim TW. Early surgical outcomes of robotic thyroidectomy by a gasless unilateral axillo-breast or axillary approach for papillary thyroid carcinoma: 2 years’ experience. Head Neck. 2012;34:617–25. doi: 10.1002/hed.21782. [DOI] [PubMed] [Google Scholar]
- 18.Lee S, Ryu HR, Park JH, Kim KH, Kang S-W, Jeong JJ, et al. Early surgical outcomes comparison between robotic and conventional open thyroid surgery for papillary thyroid microcarcinoma. Surgery. 2012;151:724–30. doi: 10.1016/j.surg.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 19.Kim WW, Kim JS, Hur SM, Kim SH, Lee S-K, Choi JH, et al. Is robotic surgery superior to endoscopic and open surgeries in thyroid cancer? World J Surg. 2011;35:779–84. doi: 10.1007/s00268-011-0960-7. [DOI] [PubMed] [Google Scholar]
- 20.Tae K, Song CM, Ji YB, Kim KR, Kim JY, Choi YY. Comparison of surgical completeness between robotic total thyroidectomy versus open thyroidectomy. Laryngoscope. 2014;124:1042–7. doi: 10.1002/lary.24511. [DOI] [PubMed] [Google Scholar]
- 21.Lang BH-H, Wong CKH, Tsang JS, Wong KP, Wan KY. A systematic review and meta-analysis comparing surgically-related complications between robotic-assisted thyroidectomy and conventional open thyroidectomy. Ann Surg Oncol. 2014;21:850–61. doi: 10.1245/s10434-013-3406-7. [DOI] [PubMed] [Google Scholar]
- 22.Lee SG, Lee J, Kim MJ, Choi JB, Kim TH, Ban EJ, et al. Long-term oncologic outcome of robotic versus open total thyroidectomy in PTC: a case-matched retrospective study. Surg Endosc. 2015 doi: 10.1007/s00464-015-4632-9. [DOI] [PubMed] [Google Scholar]
- 23.Kandil E, Hammad AY, Walvekar RR, Hu T, Masoodi H, Mohamed SE, et al. Robotic thyroidectomy versus nonrobotic approaches: a meta-analysis examining surgical outcomes. Surg Innov. 2016;23:317–25. doi: 10.1177/1553350615613451. [DOI] [PubMed] [Google Scholar]
- 24.Lee J, Nah KY, Kim RM, Ahn YH, Soh E-Y, Chung WY. Differences in postoperative outcomes, function, and cosmesis: open versus robotic thyroidectomy. Surg Endosc. 2010;24:3186–94. doi: 10.1007/s00464-010-1113-z. [DOI] [PubMed] [Google Scholar]
- 25.Tae K, Kim KY, Yun BR, Ji YB, Park CW, Kim DS, et al. Functional voice and swallowing outcomes after robotic thyroidectomy by a gasless unilateral axillo-breast approach: comparison with open thyroidectomy. Surg Endosc. 2012;26:1871–7. doi: 10.1007/s00464-011-2116-0. [DOI] [PubMed] [Google Scholar]
- 26.Lee J, Na KY, Kim RM, Oh Y, Lee JH, Lee J, et al. Postoperative functional voice changes after conventional open or robotic thyroidectomy: a prospective trial. Ann Surg Oncol. 2012;19:2963–70. doi: 10.1245/s10434-012-2253-2. [DOI] [PubMed] [Google Scholar]
- 27.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivar Behav Res. 2011;46:399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC Cancer Staging Manual. 7th ed. New York: Springer-Verlag; 2010.
- 29.American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Cooper DS, Doherty GM, Haugen BR, Hauger BR, Kloos RT, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid Off J Am Thyroid Assoc. 2009;19:1167–214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 30.Sawka AM, Orlov S, Gelberg J, Stork B, Dowar M, Shaytzag M, et al. Prognostic value of postsurgical stimulated thyroglobulin levels after initial radioactive iodine therapy in well-differentiated thyroid carcinoma. Head Neck. 2008;30:693–700. doi: 10.1002/hed.20755. [DOI] [PubMed] [Google Scholar]
- 31.Ryu IS, Song CI, Choi S-H, Roh J-L, Nam SY, Kim SY. Lymph node ratio of the central compartment is a significant predictor for locoregional recurrence after prophylactic central neck dissection in patients with thyroid papillary carcinoma. Ann Surg Oncol. 2014;21:277–83. doi: 10.1245/s10434-013-3258-1. [DOI] [PubMed] [Google Scholar]
- 32.Min HS. N stage: controversies and recent issues. J Korean Thyroid Assoc. 2012;5:109. doi: 10.11106/jkta.2012.5.2.109. [DOI] [Google Scholar]
- 33.Vas Nunes JH, Clark JR, Gao K, Chua E, Campbell P, Niles N, et al. Prognostic implications of lymph node yield and lymph node ratio in papillary thyroid carcinoma. Thyroid Off J Am Thyroid Assoc. 2013;23:811–6. doi: 10.1089/thy.2012.0460. [DOI] [PubMed] [Google Scholar]
- 34.Kang SY, Kim SK, Youn HJ, Jung SH. The prognostic significance of the metastatic lymph node ratio in patients with papillary thyroid carcinoma. Korean J Korean J Endocr Surg. 2015;15:67–72. doi: 10.16956/kaes.2015.15.3.67. [DOI] [Google Scholar]
- 35.Jeon MJ, Yoon JH, Han JM, Yim JH, Hong SJ, Song DE, et al. The prognostic value of the metastatic lymph node ratio and maximal metastatic tumor size in pathological N1a papillary thyroid carcinoma. Eur J Endocrinol Eur Fed Endocr Soc. 2013;168:219–25. doi: 10.1530/EJE-12-0744. [DOI] [PubMed] [Google Scholar]
- 36.Slidell MB, Chang DC, Cameron JL, Wolfgang C, Herman JM, Schulick RD, et al. Impact of total lymph node count and lymph node ratio on staging and survival after pancreatectomy for pancreatic adenocarcinoma: a large, population-based analysis. Ann Surg Oncol. 2008;15:165–74. doi: 10.1245/s10434-007-9587-1. [DOI] [PubMed] [Google Scholar]
- 37.Rosenberg R, Friederichs J, Schuster T, Gertler R, Maak M, Becker K, et al. Prognosis of patients with colorectal cancer is associated with lymph node ratio: a single-center analysis of 3,026 patients over a 25-year time period. Ann Surg. 2008;248:968–78. doi: 10.1097/SLA.0b013e318190eddc. [DOI] [PubMed] [Google Scholar]
- 38.Marchet A, Mocellin S, Ambrosi A, de Manzoni G, Di Leo A, Marrelli D, et al. The prognostic value of N-ratio in patients with gastric cancer: validation in a large, multicenter series. Eur J Surg Oncol. 2008;34:159–65. doi: 10.1016/j.ejso.2007.04.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets supporting the conclusions of this article are not shared.


