Abstract
Background
India has a large population of HIV-positive individuals, including men who have sex with men (MSM) and the incidence of human papillomavirus (HPV)-related cancers is high. In developed countries, HIV-positive MSM exhibit the highest prevalence of anal HPV infection and incidence of anal cancer. Little is known about anal HPV infection in HIV-positive Indian MSM.
Methods
We evaluated 300 HIV-positive MSM from two cities in India. Men were tested for anal HPV infection using L1-HPV DNA PCR with probes specific for 29 types and a mixture of 10 additional types. CD4+ level and plasma HIV viral load were measured. Participants completed an interviewer-administered questionnaire including a sexual history.
Results
The prevalence of anal HPV was 95% (95% CI 91%-97%). The three most common types were HPV 35 (20%), HPV 16 (13%) and HPV 6/11 (13%). History of taking antiretroviral medications decreased risk of anal HPV 16 infection (RR: 0.6 (0.4-1.0). Having an increased number of vaginal sex partners lowered risk of any anal HPV infection. Ever having receptive sex increased risk of any anal HPV (RR: 1.2 (1.1-1.4) and anal HPV 16 (RR: 6.5 1.8-107).
Conclusions
Almost all Indian HIV-positive MSM had anal HPV infection. The prevalence of HPV 16 was lower and the prevalence of other oncogenic HPV types was higher than in similar populations in North America and Europe. Vaccine based prevention strategies for HPV infection in India should consider potential differences in HPV type distribution among HIV-infected MSM when designing interventions.
Keywords: Human Papillomavirus, HPV, HIV/AIDS, anal cancer, MSM, India
Introduction
Men who have sex with men (MSM) are at greater risk for anal cancer and its precursor lesion, anal high-grade squamous intraepithelial lesions (HSIL) than men in the general population1,2. The incidence of anal cancer among HIV-negative MSM in the US is estimated to be up to 37/100,000, which is similar to the incidence of cervical cancer among women in developing countries, including India2. HIV-positive MSM in the US are at even greater risk of developing anal cancer than HIV-negative MSM, with an estimated incidence rate of 131/100,0003,4. The high rates of anal cancer in the US population have not been reduced by antiretroviral therapy (ARV), and several studies have documented that the incidence of anal cancer has stayed high or has continued to rise, even after the introduction of ARVs4-6. As with cervical cancer, the etiological agent that causes most anal cancers is human papillomavirus (HPV). HIV-positive MSM are also at greater risk of anal HPV infection than HIV-negative MSM7,8.
There is little information available on HPV infection and HPV-associated disease in Indian men and almost nothing is known about HPV infection in either HIV-positive or HIV-negative Indian MSM. There is evidence that the incidence of several HPV-related cancers is high among both Indian men and women compared with the incidence in Western countries. Cervical cancer is the most common cancer among Indian women, with an estimated 132,000 new cases of cervical cancer each year 9. The incidence of penile cancer in India varies from 0·7 to 3·0 /100,000 10 and represents more than 6% of all cancers in Indian men 10.
Limited data indicate that HIV-positive men in India may have higher incidence rates of anal cancer than HIV-negative men. In a 2008 study conducted in Mumbai among patients registered in an HIV Cancer Clinic, the proportional incidence ratio in HIV-positive males for anal cancer was 10.311. This study did not report data on the sexual behaviors of participants and the risk of anal cancer in HIV-positive MSM specifically is not known. Given that the prevalence of HIV is very high (7-22%) among Indian MSM and up to 63% in male-to-female transgendered individuals who are commonly commercial sex workers12-14, there may be a large population of Indian men at high risk of developing anal cancer. In addition, many Indian MSM are behaviorally bisexual and therefore may serve as a bridge population to their female partners. Understanding the prevalence of anal HPV infection among HIV-positive MSM, is a critical first step in addressing this emerging public health issue.
We conducted a cross-sectional study in two demographically distinct cities in India. Our objective was to determine the prevalence and risk factors for anal HPV infection in a diverse population of Indian HIV-positive MSM.
Methods
HIV-positive MSM were recruited from two study sites, Christian Medical College (CMC), Vellore, (a large research and teaching institution) and Humsafar Trust (HT), Mumbai (a male sexual health non-governmental organization (NGO)). Men were recruited through outreach workers and local HIV/AIDS support groups, and were also referred from other NGOs. Enrollment occurred from September 2009 to August 2010. Men were eligible for the study if they had had sexual contact with another man in the preceding six months and were HIV-positive. Participants completed a questionnaire in the local language administered by a male interviewer. They had a clinical examination, including collection of an anal swab for HPV testing. Blood was collected for a CD4+ lymphocyte count which was measured by standardized two- or three-color fluorescence methods. Plasma HIV viral load (HIV VL) was measured using the COBAS® Amplicor HIV-1 Monitor test, Version 1·5 (Roche, CA, USA). All procedures were performed after obtaining written informed consent from each participant. The study was approved by the Committee on Human Research of UCSF, and the Institutional Review Boards of CMC and HT.
Testing for anal HPV infection was performed as described previously using the polymerase chain reaction (PCR) with L1 consensus primers and probes specific for 29 HPV types and a combined probe mixture of ten additional types for a total of 39 HPV types 8. We used a combined probe for HPV types: 6/11, 32/42, 86/87, 89/102, and 90/106. Beta-globin-negative samples were excluded from analysis. Any anal HPV was defined by a sample positive with the consensus probe mixture or at least one type-specific probe. For our risk factor analysis, we considered two outcomes, “any anal HPV infection” and “anal HPV 16 infection”.
Assessment of potential risk factors
Demographic, lifestyle characteristics, medical history, use of ARV, and history of sexual behavior were collected by self-report. We asked about sexual behaviors over the participant's lifetime and in the past 30 days. Men were queried about sex with men and women, “insertive” anal intercourse (participant inserts his penis into partner's anus), “receptive” anal intercourse (participant receives his partner's penis into his anus), oral-anal contact (rimming, participant's anus receives oral contact).
Statistical analysis
Characteristics thought to be related to either of the two outcomes were examined for bivariable association using Fisher's Exact Test for categorical variables and analysis of variance (ANOVA) or ranked ANOVAs for continuous variables. Variables were considered significant if the p-value was <0.05.
To calculate relative risks (RRs) to assess strength of association with our two outcomes, we constructed log linear models specifying a binomial distribution. Both outcomes were compared with men who had no HPV infection (n=14). Because we had so few men with no HPV infection, it was not possible to construct multivariable models or adjust for potential confounders.
Results
Three hundred HIV-positive MSM were enrolled into the study; of these 34 (11%) had samples that were beta-globin negative and these results were excluded from further analyses. Table 1 includes a description of the study population.
Table 1.
Socio-demographic and lifestyle characteristics of Indian HIV-positive MSM (N=266).
| Characteristic | N | (%) |
|---|---|---|
| Demographic factors | ||
| Age (years) | ||
| 18-25 | 42 | (16) |
| 26-35 | 111 | (42) |
| >35 | 113 | (42) |
| Highest year of school completed | ||
| None | 45 | (17) |
| 1-10 | 171 | (64) |
| >10 | 50 | (19) |
| Monthly income (INR), median (IQR) | 3500 | (2000-6000) |
| Married | 126 | (47) |
| Religion | ||
| Hindu | 207 | (78) |
| Muslim | 37 | (14) |
| Other | 22 | (8) |
| Substance use | ||
| Smoked more than 100 lifetime cigarettes | 81 | (30) |
| Chew tobacco regularly | 89 | (33) |
| Ever consumed alcoholic beverage | 155 | (58) |
| HIV disease status | ||
| CD4+ level (cells/uL) | ||
| <200 | 35 | (13) |
| 200-500 | 125 | (48) |
| >500 | 103 | (39) |
| Undetectable HIV viral load level | 94 | (36) |
| Ever taken antiretroviral therapy | 118 | (44) |
| Currently taking antiretroviral therapy (ARV) | 116 | (44) |
| Participant knew name of ARV medication taken | 33 | (28)* |
| Nucleotide reverse transcriptase inhibitors use | 33 | (28)* |
| Non-nucleotide reverse transcriptase inhibitors use | 29 | (25)* |
| Protease inhibitors use | 0 | (0)* |
| Sexual behavior | ||
| Lifetime number of women vaginal sex partners | ||
| 0 | 102 | (38) |
| 1-4 | 87 | (33) |
| 5-39 | 37 | (14) |
| ≥40 | 39 | (15) |
| Age first had sex with another male (years), median (IQR) | 16 | (13-20) |
| Lifetime total receptive male partners | ||
| 0-10 | 55 | (21) |
| 11-100 | 37 | (14) |
| 101-1000 | 94 | (35) |
| >1000 | 79 | (30) |
Data from 34 participants were excluded due to beta-globin DNA-negative anal specimens. When totals do not reach 266, data were missing. SD, standard deviation.
Percentage out of 116 current ARV users.
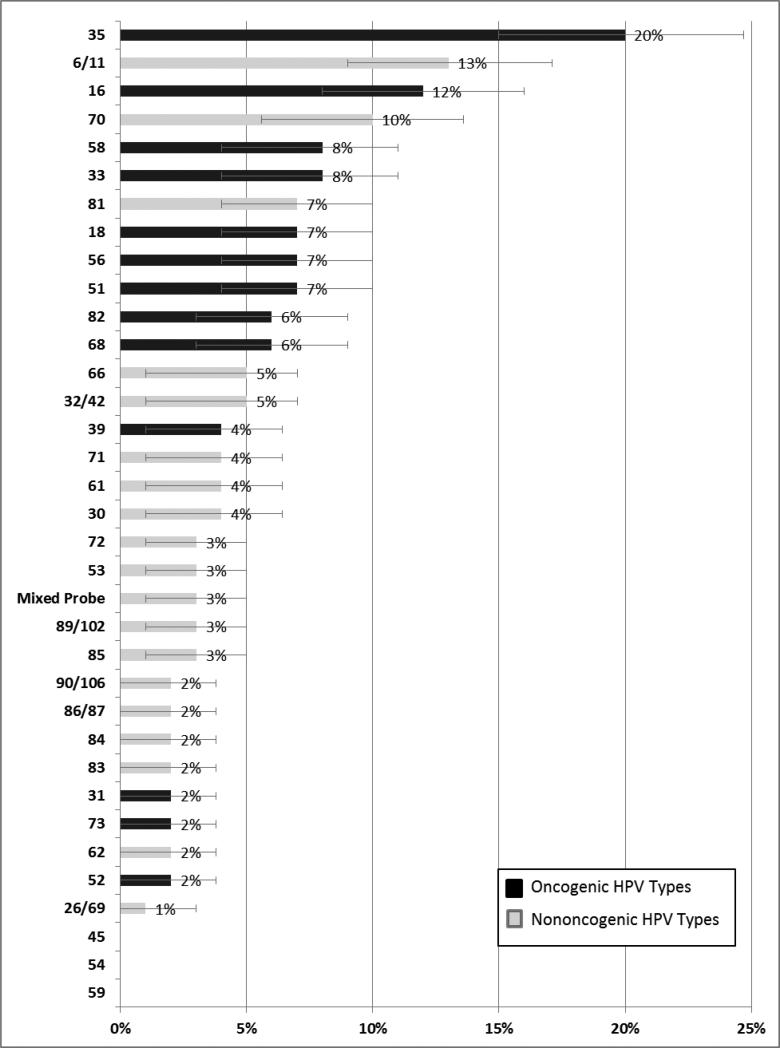
Two hundred and fifty two men (95%; 95% CI: 91%-97%) had any anal HPV infection and 49% had an oncogenic anal HPV infection. The five most common HPV types were HPV 35 (20%), HPV 16 (13%), HPV 6/11 (13%), HPV 70 (10%), and HPV 58 (8%) (Figure 1). Neither the prevalence of anal HPV infection nor the type distribution differed by study site or sexual identity.
Figure 1.
Type-specific prevalence of anal HPV infection among Indian MSM (n=266). Percentage out of ß-globin positive participants; error bars represent exact binomial 95% confidence intervals (CI); Black bars represent oncogenic HPV types; Grey bars represent non-oncogenic HPV types 54 includes subtypes; n/n represents a combined probe.
The mean number of types among men who had any anal HPV infection was 2.5 and the mean number of oncogenic types among men who had oncogenic types was 1.7 and the mean did not differed by CD4+ level or HIV VL.
Associations with any anal HPV infection
Men with any anal HPV infection had a younger median age than men with no anal HPV infection (33 vs. 46 years, p=0·005). Unmarried men had a higher prevalence of any anal HPV infection (98% vs. 91%, p=0·02). Having 5-39 and ≥40 lifetime vaginal sex partners (compared to 0-4) both reduced risk of anal HPV infection by approximately 10% (Table 3). Having two or more vaginal sex partners in the past 30 days (compared with none) was also associated with a 10% lower risk of any anal HPV infection. Ever having receptive was also associated with a small risk increase.
Table 3.
Association sexual risk factors, any anal HPV infection, and anal HPV 16 infection.
| Overall | No HPV Infection | Any HPV Infection | RR (95% CI) | HPV 16 Infection | RR (95% CI)a | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | N | (%) | N | (%) | N | (%) | N | (%) | ||
| Sex with Female Partners | ||||||||||
| Lifetime vaginal sex partners | ||||||||||
| 0 | 102 | (38) | 0 | (0) | 102 | (100) | 1.0b | 13 | (13) | 1.0b |
| 1-4 | 87 | (33) | 4 | (5) | 83 | (95) | 1.0b | 10 | (11) | 1.0b |
| 5-39 | 37 | (14) | 5 | (14) | 32 | (86) | 0.88 (0.8-0.97) | 5 | (14) | 0.6 (0.3-0.97) |
| ≥40 | 39 | (15) | 5 | (13) | 34 | (87) | 0.89 (0.8-0.98) | 3 | (8) | 0.4 (0.1-0.9) |
| Vaginal sex partners, past 30 days | ||||||||||
| 0 | 188 | (71) | 6 | (3) | 182 | (97) | 10 | 21 | (11) | 1.0 |
| 1 | 42 | (16) | 2 | (5) | 40 | (95) | 1.0 (0.9 – 1.1) | 6 | (14) | 1.0 (0.5 – 1.4) |
| ≥2 | 36 | (14) | 6 | (17) | 30 | (83) | 0.9 (0.7 – 0.97) | 4 | (11) | 0.5 (0.2 – 1.0) |
| Sex with Male Partners | ||||||||||
| Lifetime male partners | ||||||||||
| 1-100 | 67 | (25) | 7 | (10) | 60 | (90) | 1.0 | 4 | (6) | 1.0 |
| 101-1000 | 102 | (38) | 3 | (3) | 99 | (97) | 1.1 (1.0 – 1.2) | 16 | (16) | 2.3 (1.2 – 6.6) |
| >1000 | 96 | (36) | 4 | (4) | 92 | (96) | 1.1 (1.0 – 1.2) | 11 | (11) | 2.0 (1.0 – 5.8) |
| Ever receptive sex | ||||||||||
| Yes | 231 | (87) | 7 | (3) | 224 | (97) | 1.2 (1.1 – 1.5) | 30 | (13) | 6.5 (1.8 - 107) |
| No | 35 | (13) | 7 | (20) | 28 | 1.0 | 1 | (3) | 1.0 | |
| Lifetime partners with whom participant is the receptive partner | ||||||||||
| 0-10 | 55 | (21) | 7 | (13) | 48 | (87) | 1.0 | 2 | (4) | 1.0 |
| 11-100 | 37 | (14) | 2 | (5) | 35 | (95) | 1.1 (1.0 - 1.2) | 4 | (11) | 3.0 (0.8 - 17) |
| 101-1000 | 94 | (63) | 2 | (2) | 92 | (96) | 1.1 (1.0 – 1.3) | 7 | (13) | 3.9 (1.5 - 22) |
| >1000 | 79 | (30) | 3 | (4) | 76 | (96) | 1.1 (1.0 – 1.2) | 18 | (15) | 3.5 (1.3 - 20) |
| Partner uses condom during receptive anal sexc | ||||||||||
| Not always use condoms | 183 | (79) | 5 | (3) | 178 | (97) | 1.0 | 26 | (84) | 1.0 |
| Almost always/Always used condoms | 48 | (21) | 2 | (4) | 46 | (96) | 1.0 (0.9 – 1.0) | 4 | (67) | 1.3 (0.8 -3.0) |
| Receptive sex partners, past 30 days | ||||||||||
| No | 71 | (27) | 8 | (11) | 63 | (89) | 1.0 | 5 | (7) | 1.0 |
| Yes | 195 | (73) | 6 | (3) | 189 | (97) | 1.1 (1.0 – 1.2) | 26 | (13) | 2.1 (1.2 – 5.1) |
p-value, RR (95% CI) for comparison between having anal HPV 16 (N=31) infection and having no anal HPV infection (N=14); p-value for categorical variable from Fisher's Exact Test, and from ANOVA or ranked ANOVA for continuous variables; significance of relative risks determined from general linear model from likelihood ratio test.
groups for “0 partners” and “1-4 partners” combined as reference group.
among those who report ever having receptive anal sex
Bold text =p-value <0.05.
Associations with anal HPV 16 infection
History of ARV use was associated with a decreased risk of anal HPV 16 (Table 2). As with any anal HPV infection, sex with female partners was associated with reduced risk of anal HPV 16 infection (Table 3) and reporting receptive anal sex was associated with increased risk of anal HPV 16. Ever having receptive anal sex had increased risk of anal HPV 16, having higher number of lifetime partners with whom the participant was the receptive partner, and having receptive sex in the past 30 days were all associated with increases in risk of anal HPV 16 infection.
Table 2.
Association of HIV disease status, any anal HPV infection, anal HPV 16 infection among HIV-Positive MSM
| Overall | No Anal HPV Infection | Any Anal HPV Infection | RR (95% CI) | Anal HPV 16 Infection | RR (95% CI)a | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | N | (%) | N | (%) | N | (%) | N | (%)* | ||
| Total N | 266 | (100) | 14 | (5) | 252 | (95) | 31 | (12) | ||
| Years since first HIV+ test | 4 | (±4) | 3 | (±2) | 4 | (±4) | 1.0 (1.0 – 1.0) | 3 | (±3) | 1.0 (0.8 – 1.1) |
| Mean CD4+ level | 460 | (±253) | 381 | (±222) | 465 | (±255) | 1.0 (1.0 – 1.0) | 467 | (±256) | 1.0 (1.0 – 1.0) |
| <200 | 35 | (13) | 3 | (9) | 32 | (91) | 0.95 (0·8 – 1.0) | 3 | (9) | 0.7 (0.2 – 1.3) |
| 200-500 | 125 | (48) | 7 | (6) | 118 | (94) | 0·98 (0.9 – 1.1) | 15 | (12) | 0.9 (0.6 – 1.4) |
| >500 | 103 | (39) | 4 | (4) | 99 | (96) | 1·0 | 13 | (13) | 1.0 |
| Lowest CD4 count (self-report) | 315 | (±234) | 218 | (±214) | 321 | (±234) | 1.0 (1.0 – 1.0) | 331 | (±235) | 1.0 (1.0 – 1.0) |
| HIV viral load level | ||||||||||
| Undetectable | 94 | (36) | 6 | (6) | 88 | (94) | 1.0 | 9 | (10) | 1.0 |
| Detectable | 170 | (64) | 8 | (5) | 162 | (95) | 1.0 (1.0-1.1) | 22 | (73) | 1.2 (0.8-2.2) |
| Ever taken ARVs | ||||||||||
| Yes | 118 | (44) | 9 | (8) | 109 | (92) | 0.96 (0.9 – 1.0) | 9 | (8) | 0.6 (0.3 – 0.95) |
| No | 148 | (56) | 5 | (3) | 143 | (97) | 1.0 | 22 | (15) | 1.0 |
| Currently taking ARVs | ||||||||||
| Yes | 116 | (44) | 8 | (7) | 108 | (93) | 0.97 (0.9 – 1.0) | 9 | (7) | 0.7 (0.3 – 1.04) |
| No | 150 | (56) | 6 | (4) | 144 | (96) | 1.0 | 22 | (15) | 1.0 |
| Years since first initiating ARV | 2 | (±2) | 3 | (±2) | 2 | (±2) | 0.99 (1.0 – 1.0) | 1 | (±1) | 0.8 (0.5 – 1.01) |
p-value, RR (95% CI) for comparison between having anal HPV 16 infection and having no anal HPV infection; p-value for categorical variable Fisher's Exact Test, from ANOVA or ranked ANOVA for continuous variables; significance of relative risks determined from general linear model likelihood ratio test.
Bold text =p-value <0.05; NE, not estimable.
Discussion
This is the first report of anal HPV infection among HIV-positive Indian MSM. Almost 95% of our participants were positive for anal HPV infection. This high prevalence is within the range reported for other populations of HIV-positive MSM worldwide. A recent meta-analysis calculated a summary prevalence estimate of 92.6% (90.8-94.5) 15 from 21 studies of HIV-positive MSM conducted mostly in North America and Europe.
The construct of MSM does not apply as clearly in India as it does in North America and Europe. Concepts of sexual identity are more fluid in India than in the West and Indian MSM have been described as having from four to over 20 categories. Many Indian MSM are behaviorally bisexual and may marry women and 2-15% of Indian men report exploring same-sex behavior over their lifetime16-20. We did not see a difference in anal HPV infection by self-reported sexual identity. Given that many Indian MSM have female partners, they are an important bridge population from male to female partners and vice versa. These high prevalences of anal HPV infection may represent increased risk for anal cancer among Indian MSM, but also increased transmission of anal, penile and cervical HPV infection for their male and female sexual partners.
The distribution of anal HPV types that we found in our study was different than in Western populations. Although HPV 16 was the second most common anal HPV type detected its prevalence was much lower than that found in other HIV-positive MSM populations. The summary prevalence for HPV-16 calculated from a recent meta-analysis was 35%15 (compared with our finding of 12%). Other oncogenic types not typically found at high proportions among other HIV-positive MSM were high in our study. Our most prevalent type was oncogenic HPV 35 at 20%. In contrast, in studies in San Francisco the prevalence of HPV 35 ranged from only 3-5% 8. Oncogenic HPV types 58 and 33 also had high prevalences (>8%). Although these other oncogenic types (33 and 58) have rarely been detected in anal cancer specimens21,22, they are known to be associated with cervical cancer and may be important in anal disease in HIV-positive populations. It is possible that these other oncogenic types are responsible for a greater proportion of anal cancers in India than they are in Western populations. The high prevalence of HPV 35 is also important because the recently approved 9-valent HPV vaccine does not include HPV 3523. Vaccinating Indian HIV-positive MSM may not be as effective in preventing anal cancer if a larger proportion of disease is caused by types common in the population but not included in the vaccine.
We were limited in our ability to conduct risk factor analyses with only 13 men in our comparison group having no anal HPV infection. We did not have the power to detect differences between two groups in many cases, and we were unable to adjust analyses for potential confounders. Younger age was associated with both any anal HPV infection and anal HPV 16 infection, perhaps because younger men are more sexually active than older men in this population. Anal HPV infection may be cleared over time. Similarly, unmarried men were more likely to have any HPV infection and this may be because married men have fewer male partners than unmarried men or practice safer sexual behaviors with their extra-marital partners. While a higher number of female vaginal sex partners was associated with a lower prevalence of anal HPV infection, this relationship could not be evaluated controlling for number male receptive partners.
Of the sexual risk factors examined, receptive anal intercourse was the most common behavior associated with any anal HPV infection and anal HPV 16 infection. Ever having had receptive anal intercourse and a higher number of receptive male partners both increased the prevalence of both any anal HPV infection and anal HPV 16 infection. This finding is consistent with other studies of men that have shown that receptive anal intercourse is a risk factor for anal HPV infection8,24,25.
Although only 36% of our participants had an undetectable HPV VL and 44% ever reported using ARVs the only marker of HIV disease status that showed a significant association in our analyses was self-reported history of ARV use, and this was associated with a reduced prevalence of anal HPV 16 infection. The similar association between current ARV use and HPV16 did not reach statistical significance; there were only 2 men who had a history of ARV use but were no longer currently using ARVs. Neither current nor history of ARV use was associated with any anal HPV. Again, because of our high anal HPV prevalence, we may not have had the power to evaluate the association of ARVS and anal HPV infection. A reduction in prevalence with ARV use is consistent with findings of a reduction in other opportunistic infections following initiation of ARVs 26,27. However, the relationship between ARVs and HPV is not as clear as the relationship with other opportunistic infections. Anal cancer incidence have declined after the introduction of ARV therapy 2,3. Studies of HSIL among HIV-positive individuals talking ARVs have had mixed results. Some have shown no increase in regression of HSIL rates or reduction in progression of HSIL rates 26. However, a recent study on HIV-positive MSM who had been receiving their current ARV regime for >4 years had a reduced risk of progression to HSIL28. Prospective studies of the relationship between use of ARVs and incident HPV infection and HSIL are necessary to elucidate this relationship among Indian HIV-positive MSM.
This study had several limitations. As this is the first study of anal HPV in this population, it was designed as a cross-sectional study. We do not know if the sexual behavior occurred before the anal HPV infection. Another potential limitation to the study is that we did not have a random sample of Indian HIV-positive MSM. The results may be generalizeable to Indian MSM living in urban centers with access to medical services but not necessarily to all HIV-positive Indian MSM. Since MSM behavior and HIV-positive status are both highly stigmatized in India it is unlikely that other sampling strategies would have yielded a more representative sample while ensuring participant confidentiality. Finally, the self-reported nature of the data is subject to errors in recall.
Our study has confirmed our hypothesis that anal HPV infection is very common in HIV-positive Indian MSM. However, the type distribution is different from HPV type distribution seen in other similar populations with HPV type 16 prevalence lower and other oncogenic types higher than seen in North America and Europe. Given the large number of Indian MSM living with HIV, this high prevalence of anal HPV infection indicates that many Indian men are at risk of developing anal cancer, which is potentially preventable through the HPV vaccination and early treatment of anal HSIL. HIV-positive Indian MSM should be considered for HPV vaccination, similar to U.S. guidelines that recommend routine vaccination for both HIV-positive and HIV-negative MSM up to the age of 26 years 29
Acknowledgments
This work was supported by National Cancer Institute's AIDS Malignancy Consortium grant number AMC - U01 CA121947. This study was also funded by the Indian Council of Medical Research Grant No: HIV/INDO-US/32/2007- ECD-II. Dr. Hernandez was also supported through the University of California, Berkeley, Fogarty International AIDS/HIV International Training and Research grant (AITRP) 1-D43-TW000003.
The authors thank Dr. Hernandez's dissertation committee for review and editing of the manuscript and the HPV Infection in Indian Men (HIIM) study staff and the study participants for their time and dedication.
Footnotes
Author contributions: All authors contributed to the interpretation of data and the overall intellectual content of the paper, and drafting and editing of the manuscript. ALH, JP, DM, and ARK also contributed to the study design. RK and MS also contributed to data collection. ALH, SL, JYL and JP also contributed to data analysis. AR, MG, RK, JP and PA also contributed to collecting, analyzing and interpreting laboratory data.
Meetings at which parts of the data were presented;
The 27th International Papillomavirus Conference, Berlin Germany, September 17-21 2011
Conflicts of Interest
No conflicts of interests were declared by any of the authors.
Contributor Information
Alexandra L. Hernandez, Department of Medicine, University of California, San Francisco, AND School of Public Health, Department of Epidemiology, University of California, Berkeley.
Rajiv Karthik, Department of Medicine, Christian Medical College, Vellore, India.
Murugesan Sivasubramanian, The Humsafar Trust, Mumbai, India.
Anantharam Raghavendran, Department of Clinical Virology, Christian Medical College, Vellore, India.
Manu Gnanamony, Department of Cancer Biology and Pharmacology, University of Illinois Chicago.
Shelly Lensing, Department of Biostatistics, University of Arkansas for Medical Sciences.
Jeannette Y. Lee, Department of Biostatistics, University of Arkansas for Medical Sciences.
Rajesh Kannangai, Department of Clinical Virology, Christian Medical College, Vellore India.
Priya Abraham, Department of Clinical Virology, Christian Medical College, Vellore, India.
Dilip Mathai, Apollo Institute of Medical Sciences and Research, Hyderabad, India.
Joel M. Palefsky, Department of Medicine, University of California, San Francisco.
References
- 1.Frisch M, Biggar RJ, Engels EA, Goedert JJ. Association of cancer with AIDS-related immunosuppression in adults. JAMA : the journal of the American Medical Association. 2001 Apr 4;285(13):1736–1745. doi: 10.1001/jama.285.13.1736. [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM. The global health burden of infection-associated cancers in the year 2002. International journal of cancer. Journal international du cancer. 2006 Jan 10;118(12):3030–3044. doi: 10.1002/ijc.21731. [DOI] [PubMed] [Google Scholar]
- 3.Frisch M, Biggar RJ, Goedert JJ. Human papillomavirus-associated cancers in patients with human immunodeficiency virus infection and acquired immunodeficiency syndrome. J Natl Cancer Inst. 2000 Sep 2;92(18):1500–1510. doi: 10.1093/jnci/92.18.1500. [DOI] [PubMed] [Google Scholar]
- 4.Silverberg MJ, Lau B, Justice AC, et al. Risk of Anal Cancer in HIV-Infected and HIV Uninfected Individuals in North America. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2012 Feb 13; doi: 10.1093/cid/cir1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bower M, Palmieri C, Dhillon T. AIDS-related malignancies: changing epidemiology and the impact of highly active antiretroviral therapy. Curr Opin Infect Dis. 2006 Feb;19(1):14–19. doi: 10.1097/01.qco.0000200295.30285.13. [DOI] [PubMed] [Google Scholar]
- 6.Chiao EY, Krown SE, Stier EA, Schrag D. A population-based analysis of temporal trends in the incidence of squamous anal canal cancer in relation to the HIV epidemic. J Acquir Immune Defic Syndr. 2005 Dec 1;40(4):451–455. doi: 10.1097/01.qai.0000159669.80207.12. [DOI] [PubMed] [Google Scholar]
- 7.de Pokomandy A, Rouleau D, Ghattas G, et al. Prevalence, clearance, and incidence of anal human papillomavirus infection in HIV-infected men: the HIPVIRG cohort study. The Journal of infectious diseases. 2009 Apr 1;199(7):965–973. doi: 10.1086/597207. [DOI] [PubMed] [Google Scholar]
- 8.Palefsky JM, Holly EA, Ralston ML, Jay N. Prevalence and risk factors for human papillomavirus infection of the anal canal in human immunodeficiency virus (HIV)-positive and HIV-negative homosexual men. The Journal of infectious diseases. 1998;177(2):361–367. doi: 10.1086/514194. [DOI] [PubMed] [Google Scholar]
- 9.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005 Mar-Apr;55(2):74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 10.(ICMR) ICfMR. Consolidated Report of the Population based cancer registries 1990–1996. Indian Council for Medical Research; New Delhi: 2001. National Cancer Registry Programme. [Google Scholar]
- 11.Dhir AA, Sawant S, Dikshit RP, et al. Spectrum of HIV/AIDS related cancers in India. Cancer causes & control : CCC. 2008 Mar;19(2):147–153. doi: 10.1007/s10552-007-9080-y. [DOI] [PubMed] [Google Scholar]
- 12.NACO. Department of AIDS Control Ministry of Health and Family Welfare. Indian National AIDS Control Organization; 2009-1010. [Google Scholar]
- 13.Setia MS, Lindan C, Jerajani HR, et al. Men who have sex with men and transgenders in Mumbai, India: an emerging risk group for STIs and HIV. Indian J Dermatol Venereol Leprol. 2006 Nov-Dec;72(6):425–431. doi: 10.4103/0378-6323.29338. [DOI] [PubMed] [Google Scholar]
- 14.Solomon SS, Srikrishnan AK, Sifakis F, et al. The Emerging HIV Epidemic among Men Who have Sex with Men in Tamil Nadu, India: Geographic Diffusion and Bisexual Concurrency. AIDS Behav. 2010 May 1; doi: 10.1007/s10461-010-9711-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Machalek DA, Poynten M, Jin F, et al. Anal human papillomavirus infection and associated neoplastic lesions in men who have sex with men: a systematic review and meta-analysis. Lancet Oncol. 2012 May;13(5):487–500. doi: 10.1016/S1470-2045(12)70080-3. [DOI] [PubMed] [Google Scholar]
- 16.Aggarwal O, Sharma AK, Chhabra P. Study in sexuality of medical college students in India. J Adolesc Health. 2000 Mar;26(3):226–229. doi: 10.1016/s1054-139x(98)00083-4. [DOI] [PubMed] [Google Scholar]
- 17.Dwivedi M, Misra SP. Motilal Nehru Medical College, Allahabad. Natl Med J India. 1997 Jan-Feb;10(1):43–44. [PubMed] [Google Scholar]
- 18.Rodrigues JJ, Mehendale SM, Shepherd ME, et al. Risk factors for HIV infection in people attending clinics for sexually transmitted diseases in India. Bmj. 1995 Jul 29;311(7000):283–286. doi: 10.1136/bmj.311.7000.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Go VF, Srikrishnan AK, Sivaram S, et al. High HIV prevalence and risk behaviors in men who have sex with men in Chennai, India. J Acquir Immune Defic Syndr. 2004 Mar 1;35(3):314–319. doi: 10.1097/00126334-200403010-00014. [DOI] [PubMed] [Google Scholar]
- 20.Thomas BE, Rehman F, Malaisamy M, et al. A study of condom acceptability among men in an urban population in South India. AIDS Behav. 2004 Jun;8(2):215–220. doi: 10.1023/B:AIBE.0000030252.01514.ba. [DOI] [PubMed] [Google Scholar]
- 21.Alemany L, Saunier M, Alvarado-Cabrero I, et al. Human papillomavirus DNA prevalence and type distribution in anal carcinomas worldwide. International journal of cancer. Journal international du cancer. 2015 Jan 1;136(1):98–107. doi: 10.1002/ijc.28963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baricevic I, He X, Chakrabarty B, et al. High-sensitivity human papilloma virus genotyping reveals near universal positivity in anal squamous cell carcinoma: different implications for vaccine prevention and prognosis. European journal of cancer. 2015 Apr;51(6):776–785. doi: 10.1016/j.ejca.2015.01.058. [DOI] [PubMed] [Google Scholar]
- 23.Van de Velde N, Boily MC, Drolet M, et al. Population-Level Impact of the Bivalent, Quadrivalent, and Nonavalent Human Papillomavirus Vaccines: A Model-Based Analysis. J Natl Cancer Inst. 2012 Oct 27; doi: 10.1093/jnci/djs395. [DOI] [PubMed] [Google Scholar]
- 24.Nyitray AG, Carvalho da Silva RJ, Baggio ML, et al. Age-specific prevalence of and risk factors for anal human papillomavirus (HPV) among men who have sex with women and men who have sex with men: the HPV in men (HIM) study. The Journal of infectious diseases. 2011 Jan 1;203(1):49–57. doi: 10.1093/infdis/jiq021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Critchlow CW, Hawes SE, Kuypers JM, et al. Effect of HIV infection on the natural history of anal human papillomavirus infection. AIDS. 1998 Jul 9;12(10):1177–1184. doi: 10.1097/00002030-199810000-00010. [DOI] [PubMed] [Google Scholar]
- 26.Palefsky J. Biology of HPV in HIV Infection. Adv Dent Res. 2006;19(1):99–105. doi: 10.1177/154407370601900120. [DOI] [PubMed] [Google Scholar]
- 27.Cameron JE, Hagensee ME. Human papillomavirus infection and disease in the HIV+ individual. Cancer Treat Res. 2007;133:185–213. doi: 10.1007/978-0-387-46816-7_7. [DOI] [PubMed] [Google Scholar]
- 28.de Pokomandy A, Rouleau D, Ghattas G, et al. HAART and progression to high-grade anal intraepithelial neoplasia in men who have sex with men and are infected with HIV. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2011 May;52(9):1174–1181. doi: 10.1093/cid/cir064. [DOI] [PubMed] [Google Scholar]
- 29.Recommendations on the use of quadrivalent human papillomavirus vaccine in males--Advisory Committee on Immunization Practices (ACIP), 2011. MMWR. Morbidity and mortality weekly report. 2011 Dec 2;60(50):1705–1708. [PubMed] [Google Scholar]