Abstract
Idiopathic pulmonary fibrosis (IPF) is an aging-associated recalcitrant lung disease with historically limited therapeutic options. The recent approval of two drugs, pirfenidone and nintedanib, by the United States Food and Drug Administration (FDA) in 2014 has heralded a new era in its management. Both drugs demonstrated efficacy in Phase III clinical trials by retarding the rate of progression of IPF; neither drug appears to be able to completely arrest disease progression. Advances in the understanding of IPF pathobiology have led to an unprecedented expansion in the number of potential therapeutic targets. Drugs targeting several of these are under investigation in various stages of clinical development. Here, we provide a brief overview of the drugs currently approved, and others in Phase II clinical trials. Future therapeutic opportunities that target novel pathways, including some that are associated with the biology of aging, are examined. A multi-targeted approach, potentially with combination therapies, and the identification of individual (or subsets of) patients who may respond more favorably to specific agents are likely to be more effective.
1. Introduction
Idiopathic pulmonary fibrosis (IPF) is a chronic, progressive lung disease with high mortality and morbidity [1–3]. The world-wide prevalence of IPF continues to rise [4, 5]. Early disappointments with several anti-fibrotic agents [6–9] have led to clinical guidelines strongly recommending against use of most drugs previously studied [10]. Significant advances have been made in our understanding of the pathobiology of lung fibrosis [11–13]. These insights have led to an unprecedented expansion in the number of therapeutic targets that have undergone testing in clinical trials. Two drugs – pirfenidone and nintedanib – were approved by the United States Food and Drug Administration (FDA) in 2014. Here, we review data supporting the use of these drugs for the treatment of IPF, and look ahead to emerging drugs and novel therapeutic targets for this recalcitrant disease.
2. Aging as a paradigm in IPF pathogenesis
Our understanding of the pathogenesis of IPF has evolved over the past three decades, and the role of aging in this disease process is gaining greater attention [14, 15]. The diagnosis of IPF is typically made beyond the fifth decade of life, and there is an increase in both the incidence and prevalence of the disease with advancing age [4, 5, 16–18]. The biological hallmarks of aging [19], namely, genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis (including impaired autophagy), deregulated nutrient sensing, mitochondrial dysfunction, cellular senescence, stem cell exhaustion, and altered intercellular communication, are being linked to key pathobiological processes in fibrosis [15].
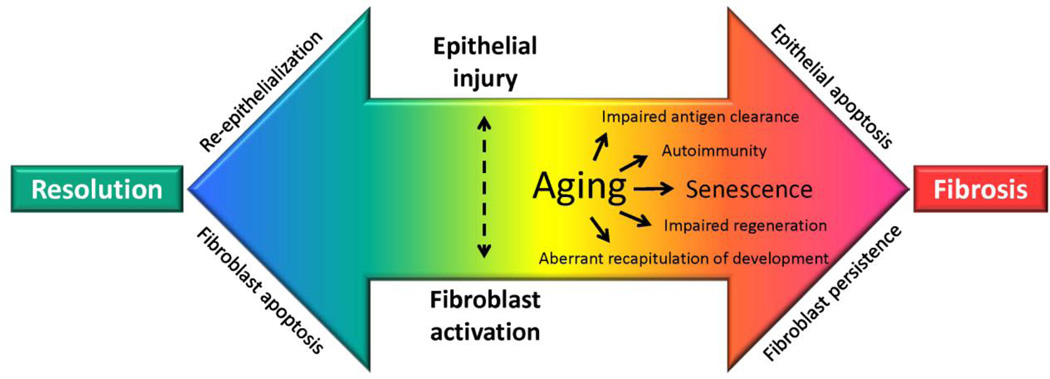
Both clinicians and scientists intuitively approach fibrosis as a pathological process; however, it can be argued that fibrosis serves an adaptive host response function [20]. Accordingly, fibrosis may be viewed as a physiological response conserved through evolution to survive tissue injury, even at the cost of a loss in organ structure/function. This “trade-off” would be predicted to select fibrotic repair over “perfect” organ regeneration in environments of limited bioenergetic resources. Progressive fibrosis may occur when the normal bidirectional signaling between the epithelium and mesenchyme (fibroblasts) that coordinates repair becomes aberrant in the context of chronic injury and aging. This aberrant signaling may result from several factors such as elevated oxidative stress, impaired fibrinolysis, and alterations in cytokines, chemokines, growth factors, and eicosanoids [21]. Ultimately, the causes of pathological fibrosis likely involve impaired ability to clear antigens, autoimmunity, impaired regeneration, and the aberrant activation of developmental or wound healing genes [20]. In the context of aging, senescence of both the epithelium and mesenchyme may be predicted to give rise to cell phenotypes and fates that are characteristic of non-resolving fibrosis (Fig. 1). Consequently, the putative therapeutic targets are myriad and provide opportunity for a multipronged approach to intervene along a spectrum, from halting the progression of fibrosis to reversing remodeling to achieve normal organ structure and function.
Figure 1. Proposed mechanisms for the predilection of fibrosis with aging.
Aging may result in “immunosenescence” that results in impaired antigen clearance and autoimmunity; additionally, senescence of epithelial cells and fibroblasts result in impaired regeneration and aberrant recapitulation of developmental genes. These processes may perpetuate epithelial injury/apoptosis and fibroblast activation that results in failed re-epithelialization, fibroblast persistence and progressive fibrosis.
3. Review of current drugs
3.1. Pirfenidone
About 3 years after its approval in Europe in 2011, pirfenidone has been approved by the United States FDA for the treatment of IPF. Two decades have passed since the first report in the mid-1990s of its potential efficacy in amelioration of experimental lung fibrosis in hamsters [22]. Since then, it has been studied extensively in several animal models and found to be effective in preventing/treating fibrosis involving the lungs, heart, liver, and kidneys [23]. Recent studies have employed local delivery of pirfenidone-containing nanoparticles to treat lung fibrosis in mice [24]. Interestingly, the precise mechanisms of action of pirfenidone that mediate anti-fibrotic effects in vivo are ill-defined, although purported to be via multiple pathways, including down-regulation of inflammatory cytokines (such as tumor necrosis factor-α), pro-fibrotic cytokines (such as transforming growth factor-β, TGF-β), and oxidative stress [25].
In the large multicenter Phase III clinical (ASCEND) trial that ultimately led to the approval of pirfenidone in the U.S., treatment with the drug (compared to placebo) significantly reduced the decline in lung function (as measured by an absolute decrease in forced vital capacity, FVC) and exercise tolerance, and improved progression-free survival in patients with IPF [26]. In this study of 555 patients, 278 received pirfenidone, and 277 received placebo. While this trial failed to show a significant mortality benefit, pooling the data with earlier multicenter Phase III (CAPACITY) trials [27] revealed statistically significant reduction in all-cause mortality (hazard ratio 0.52), as well as IPF-related mortality (hazard ratio 0.32), in patients receiving pirfenidone.
The target dosing of pirfenidone is 2403 mg/day in 3 divided doses. Adverse effects are common with pirfenidone therapy. In the ASCEND trial, among patients receiving pirfenidone, 36% had nausea (vs. 13.4% placebo), and 28% had a rash (vs. 8.7% placebo). Other known adverse effects include upper gastrointestinal (GI) symptoms such as vomiting, dyspepsia and esophageal reflux, in addition to elevated hepatic enzymes, asthenia and weight loss [26, 27].
3.2. Nintedanib
Nintedanib is a multiple tyrosine kinase inhibitor known to inhibit the receptor tyrosine kinases of platelet-derived growth factor (PDGF), fibroblast growth factor (FGF), and vascular endothelial growth factor (VEGF) [28]. There are relatively fewer studies of the efficacy of nintedanib in pre-clinical animal models [29]. Originally used in trials to treat various cancers [30], nintedanib was studied in a Phase II (TOMORROW) trial for IPF [31]. Encouraging results from this trial formed the basis for larger Phase III (INPULSIS) trials that led to its approval for use in IPF in Europe and USA [32]. Recent studies have attempted to define the mechanisms of the anti-fibrotic action of nintedanib via its inhibitory effect on receptor tyrosine kinases leading to inhibition of fibroblast proliferation and migration, myofibroblast differentiation, extracellular matrix (ECM) synthesis, and inflammation [33, 34]. We have recently reported that nintedanib mediates other actions on fibroblasts, including induction of autophagy and direct inhibition of TGF-β receptor signaling [35].
In the two large multicenter INPULSIS trials, a total of 1061 patients with mild-moderate IPF (FVC of 50% or more of predicted; diffusion capacity of the lung for carbon monoxide, DLCO of 30–79% of predicted) were studied; 638 received nintedanib and 423 received placebo [32]. In both studies, nintedanib significantly decreased the rate of decline in FVC. Nintedanib also decreased the incidence of acute exacerbations in INPULSIS 2, but not in INPULSIS 1.
The dosing of nintedanib is at 300 mg/day in two divided doses. Adverse effects are common with Nintedanib therapy. In the INPULSIS trials, among patients treated with nintedanib, >60% reported diarrhea (vs. ~18% placebo). Other known adverse effects include elevated hepatic enzymes, and weight loss [32]. Overall, higher rates of drug discontinuation due to adverse effects were noted in drug groups than placebo groups (21% vs 10.8% in INPULSIS 1, 17.6% vs 15.1% in INPULSIS 2).
3.3. Impact of the approved drugs on the treatment paradigm in IPF
Approval of pirfenidone and nintedanib has provided much needed options to treat patients with IPF. However, these come with a set of new questions and uncertainties. First, the criteria for selecting one drug over the other for a particular patient are unclear [36]. An informed decision-making process is necessary on the part of both physician and patient. In our experience, this decision is currently being driven primarily by the side-effect profile of each drug. Adverse effects leading to potential drug discontinuation has been considered in the 'conditional recommendation with moderate confidence in estimates of effect' for both drugs in the current guidelines for management of IPF [10]. Second, it is unknown if a combination of pirfenidone and nintedanib would confer added benefit over either drug alone. Third, among the other unknowns are the utility of either drug in patients with advanced IPF and/or advanced age with varied comorbidities. Fourth, there are concerns that the drugs may not be cost-effective, at least in the prevalent health-care delivery systems in some countries [37]. Lastly, these drugs will alter the design of future studies of newer drugs with the requirement for a new standard-of-care (control) arm in randomized clinical trials.
4. Drug targets/therapeutics in current clinical trials
Several newer agents are currently in Phase II clinical trials for IPF, and these are summarized in Table 1. Here, we discuss the rationale and important pre-clinical data that support the testing of these agents.
Table 1. Agents in current clinical trials for IPF.
NCT, National Clinical Trial (www.clinicaltrials.gov)
| Agent | Target | Purported mechanism of action | NCT Identifier |
|---|---|---|---|
| Tralokinumab | IL-13 | Decreases expression of TGF-β and macrophage CCL2 | NCT01629667 |
| Lebrikizumab | IL-13 | (as above) | NCT01872689 |
| FG-3019 | CTGF | Decreases CTGF-mediated pro-fibrotic actions on fibroblasts | NCT01890265 |
| Simtuzumab | LOXL2 | Decreases extracellular matrix cross-linking | NCT01769196 |
| STX-100 | Integrin αvβ6 | Decreases activation of latent TGF-β | NCT01371305 |
| BMS-986020 | LPA1 receptor | Decreases vascular leak and fibroblast recruitment | NCT01766817 |
| Rituximab | CD20 | Decreases contribution of antibody-mediated autoimmunity | NCT01969409 |
| Carbon Monoxide | Inflammation | Anti-inflammatory, may also suppress fibroblast proliferation | NCT01214187 |
| Azithromycin | Bacteria, inflammation | Antimicrobial, immunomodulatory | NCT02173145 |
| Cotrimoxazole | Pneumocystis jiroveci, bacteria | Antimicrobial | NCT01777737 |
4.1. IL-13
Interleukin-13 (IL-13) is known to be increased in patients with IPF [38] and in animal models of aging-associated progressive fibrosis [39]. IL-13 signaling, via multiple mechanisms, including interplay with TGF-β and macrophage chemokine (C-C motif) ligand 2 (CCL2), drives fibrosis in vitro and in vivo [40–43]. Blocking IL-13 ameliorates experimental fibrosis in animal models [44]. IL-13 signaling axis has been a target of active investigation for pulmonary fibrosis with several anti-IL-13 monoclonal antibodies. A Phase II trial of QAX576 (NCT01266135, clinicaltrials.gov) was terminated with the cause yet unpublished. Two other Phase II randomized, double-blind, placebo controlled trials of monoclonal antibodies against IL-13, tralokinumab (NCT01629667) and lebrikizumab (NCT01872689) are ongoing. Of note, these two monoclonals are also being evaluated in Phase III trials for treatment of severe asthma. Interestingly, blocking the IL-13 receptor α2 (IL-13Rα2) appears to mediate pleiotropic effects in fibrosis. While studies support a pro-fibrotic role for IL-13Rα2 via TGF-β1 signaling [45], IL-13Rα2 may act as a decoy to IL-13 and thus diminish its pro-fibrotic effects [46].
4.2. CCL2
CCL2, also referred to as monocyte chemotactic protein 1 (MCP1), is purported to contribute to the pathogenesis of pulmonary fibrosis via multiple mechanisms, including effector functions downstream of IL-13 signaling [43, 47–49]. Interestingly, CCL2 has been reported to mediate receptor-independent pleiotropic effects [50]. A Phase II clinical trial of anti-CCL2 monoclonal antibody Carlumab (CNTO888) in 126 IPF patients was completed recently, but found no evidence of benefit [51].
4.3. CTGF
Connective tissue growth factor (CTGF) is a matricellular protein that modulates cellular responses to the ECM. CTGF regulates fibroblast functions and mediates TGF-β actions on fibroblasts [52, 53]. In patients with IPF, CTGF expression is increased in bronchoalveolar lavage (BAL) fluid and in lung tissue, specifically type II alveolar epithelial cells and interstitial fibroblasts [54, 55]. In animal models of lung fibrosis, targeting CTGF diminishes fibrosis [56, 57]. A Phase II randomized, double-blind, placebo-controlled trial of CTGF-neutralizing antibody, FG-3019 (Fibrogen), is currently recruiting IPF patients (NCT01890265), with encouraging interim results from an earlier trial reported at the 2014 ATS Conference [58] As of 2014, out of 53 patients studied, 39 had completed the treatment period of 48 weeks; 27 of these had either no decrease or a modest decrease (< 5%) in FVC. In the 19 patients that accepted extended treatment, 9 had demonstrated improved or stable FVC through 81 weeks of treatment.
4.4. Lysyl Oxidase-like 2
Lysyl oxidases (LOX) are matrix cross-linking enzymes that catalyze formation of aldehydes from lysine residues in collagen and elastin. Their normal function helps stabilize the ECM to provide tensile strength to tissues [59, 60]. Increased activity of lysyl oxidase–like 2 (LOXL2) has been found to play a role in fibrotic disease; it is highly expressed in fibrotic regions of IPF lung, and its inhibition attenuates experimental lung fibrosis [61]. Serum LOXL2 has been noted to increase in patients with progressive IPF, suggesting its potential utility as a biomarker pending validation studies [62]. A Phase II trial (NCT01769196) in IPF using simtuzumab, a monoclonal antibody against LOXL2, is underway.
4.5. Integrin αvβ6
TGF-β signaling is central to fibrogenesis involving multiple organ systems [21, 63]. Global TGF-β inhibition may adversely affect its homeostatic functions, including immune suppression and tumor suppression [64]. The activation of latent TGF-β occurs locally in areas of fibrogenesis, and αvβ6 integrin is known to be an activator of such latent TGF-β complexes [65, 66]. Importantly, αvβ6 expression is minimal in normal lungs [66]. Lack of this integrin or loss of its function protects against experimental fibrosis in animal models [67, 68]. An ongoing Phase II study of STX-100, a monoclonal antibody against αvβ6, is currently recruiting patients (NCT01371305).
4.6. LPA1
Lysophosphatidic acid (LPA) is a phospholipid derivative that signals through multiple cell-surface G-protein coupled receptors that participate in pro-fibrotic wound-repair responses, such as fibroblast activation and resistance to apoptosis, epithelial cell apoptosis, and increased vascular permeability [69]. Inhibiting the LPA1 receptor has been shown to prevent fibrosis in various pre-clinical models [70–73]. Importantly, LPA levels are increased in the BAL fluid of IPF patients; LPA1 signaling has potent fibroblast chemoattractant activity [71]. An ongoing Phase II study in IPF patients with BMS-986020, an LPA1 receptor antagonist, is currently recruiting patients (NCT01766817).
4.7. Autoantibodies
It has long been recognized that aging is associated with immunosenescence accompanied by an increase in autoantibodies [74, 75]. Recent pilot data indicate that targeting autoantibodies during acute IPF exacerbations might improve outcomes; strategies to reduce autoantibodies include treatment with rituximab, a monoclonal antibody against CD20 [76]. Studies have shown favorable effects on lung function in patients with scleroderma-induced interstitial lung disease with long term use of rituximab [77], despite concerns that rituximab treatment itself can induce lung fibrosis [78–80]. A Phase II study on Autoantibody Reduction Therapy in patients with Idiopathic Pulmonary Fibrosis (ART-IPF) is currently recruiting patients (NCT01969409).
4.8. Carbon Monoxide
Carbon monoxide is a gaseous molecule with multifunctional actions, and typifies contextual duplicity. While it is clearly toxic at high concentrations, therapeutic beneficial effects of exposure to lower concentrations are emerging in various conditions, including inflammation, sepsis, and acute lung injury [81–85]. Carbon monoxide suppresses in vitro fibroblast proliferation and bleomycin-induced lung injury in mice [86]. A Phase II multicenter trial to study inhaled carbon monoxide in IPF has recently stopped enrolling, and the results are yet to be published (NCT01214187).
4.9. Antimicrobials
Recent reports suggest that infections may contribute to IPF pathogenesis and lead to its progression, as well as acute exacerbations; however, precise cause-effect relationships have not been established [87–89]. There is also emerging interest in the role of the microbiome in IPF [87]. Use of antimicrobials to clear infection/colonization, thereby potentially altering the microbiome and the immune response, is being studied in IPF. In a study using the bleomycin-injury model in mice, azithromycin ameliorated lung fibrosis [90]. A randomized study of azithromycin in IPF is currently recruiting patients (NCT02173145), with the primary goal of assessing its immunomodulatory functions in suppressing cough. In a multi-center, randomized, controlled trial of cotrimoxazole in 181 IPF patients, in those patients adhering to regimen to study completion, drug therapy was noted to confer an all-cause mortality benefit (hazard ratio 0.21), and a reduction in need for increase in oxygen therapy (odds ratio 0.05), although no benefit in the primary outcome of retarding decline in lung function (FVC) or exercise capacity (6-minute walk test) [91]. In the cotrimoxazole group, while a significantly higher number of patients discontinued treatment due to adverse effects (chiefly nausea and skin rash), respiratory infections were significantly less in this group. A larger Phase III trial to test the validity of the treatment of IPF with cotrimoxazole (NCT01777737) is currently recruiting participants.
5. Emerging drug targets/therapeutic strategies
5.1. ROCK
First discovered in 1995 [92], the Rho kinase (ROCK) family members, ROCK1 and ROCK2, are serine/threonine kinases that regulate multiple cellular functions, including fibroblast apoptosis/survival and mechanotransduction [93, 94]. Many of their downstream targets are associated with the regulation of cytoskeletal stability, stress fiber formation, focal adhesion assembly, and cell contractility. Biomechanical stress-induced signal transduction by ROCKs may function as a feed-forward mechanism in the microenvironment of an already stiffened ECM in ongoing fibrosis. Recent studies have shown that inhibition of this pathway can ameliorate experimental fibrosis; more importantly, ROCK activity is known to be increased in areas of active fibrosis in human IPF, and thus, its inhibition may be an effective therapeutic strategy [95]. A Phase II study of an oral selective ROCK2 inhibitor, KD025, to treat IPF is being planned. It is noteworthy that this drug is being evaluated in multiple therapeutic areas, including autoimmune, fibrotic, neurologic, and metabolic diseases.
5.2. NOX4
NADPH oxidase 4 (NOX4) is an oxidant-generating enzyme that mediates myofibroblast activation and fibrogenic responses in multiple organ systems [96–101]. Its biochemical activity was first discovered as a TGF-β-responsive, H2O2-generating flavoenzyme in lung fibroblasts [102], several years prior to the identification and cloning of non-phagocytic NOX family enzymes [103]. NOX4 may demonstrate antagonistic pleiotropy in aging, contributing to excessive oxidative stress, thereby increasing predilection for fibrosis as a response to lung injury [104, 105]. Newer insights in epigenetics have helped better understand the mechanisms involved in NOX4 up-regulation in senescence [106]. Targeting NOX4 by intranasal siRNA as well as a small molecule inhibitor GKT137831, reverses the otherwise persistent fibrosis seen in aged mice [107]. Specific targeting of NOX4 awaits study in human IPF.
5.3. AMPK
AMP-activated protein kinase (AMPK) is a master metabolic sensor and homeostat at the cellular level. AMPK activation is among the few interventions which control the aging process and extend lifespan in animal models [108]. Interestingly, AMPK activation has been reported to mediate antifibrotic effects in experimental models, both in vitro and in vivo, although mechanisms are unclear [109]. AMPK activation leads to enhanced autophagy and metabolic reprogramming, both of which have potential therapeutic value in the context of aging-associated fibrosis [110, 111]. The antidiabetic drug, metformin, used by millions of people worldwide is a potent activator of AMPK, and being cost-effective with a favorable safety profile, is an attractive candidate for repurposing in IPF.
5.4. Other kinases
The concept of targeting protein kinases with tyrosine kinase inhibitors (TKIs) in IPF has been considered for several years [112–115]. The recent results with nintedanib suggest that other TKIs may be as (or even more) effective, and this warrants further testing. In addition, while much has been studied and proposed about the role of receptor tyrosine kinases and their therapeutic targeting in pulmonary fibrosis [116, 117], the role of many non-receptor tyrosine- and serine-threonine kinases are unclear. Recent reports suggest that inhibition of the Src family of non-receptor protein kinases can ameliorate experimental fibrosis in vitro and in vivo [118]. Future studies are likely to inform if several kinase inhibitors that are either already approved or are being tested in treatment of various cancers maybe repurposed for IPF.
5.5. RNA inhibition
MicroRNAs (miRNAs) and small interfering RNAs (siRNAs) are tiny regulatory non-coding RNAs that have similar function but different sources of origin [119]. They decrease levels of target gene transcripts (mRNA) predominantly by mRNA destabilization, although they also modestly reduce translation efficiency [120]. Depending on the target(s) of the siRNA or miRNA, their activity can lead to a pro-fibrotic or anti-fibrotic milieu [121]. For example, miR-29 is known to have anti-fibrotic effects; its levels are suppressed by TGF-β stimulation in vitro and in lungs of bleomycin injured mice [122, 123]. On the other hand, miR-21 is known to promote fibrosis [124]. Strategies to increase levels of anti-fibrotic miRNAs/siRNAs and decrease levels of pro-fibrotic miRNAs would open up a multitude of potent and highly targeted options to treat IPF. Numerous miRNA-based therapeutics are already in development for various diseases such as HCV infection, various cancers, heart failure, and fibrosis [125]. Novel technologies for optimal delivery of siRNAs, miRNA mimics, and antimiR oligonucleotides are being explored [126].
6. Future directions
The year 2014 marks a watershed moment in the history of the care of IPF patients in the U.S. with the FDA approval of the first drugs ever for this disease. However, several questions regarding the use of pirfenidone and nintedanib remain. Can they benefit patients with more advanced disease than were enrolled in the Phase III studies? Can they be given in combination to achieve potentially greater efficacy? How long should either be given to patients who continue to progress despite treatment before they are deemed a treatment failure? Most importantly, given the inherent heterogeneity of IPF, how can we identify patients most likely to benefit from one or both of these drugs?
While the approval of pirfenidone and nintedanib should be considered an important landmark, much work needs to be done. With renewed interest and commitment from both academia and industry, several more drugs and drug targets are likely to follow. We have reviewed some of the emerging candidate drugs that are currently in Phase II trials, and several others that are in earlier stages of clinical or pre-clinical development. Deeper insights into the biology of aging in IPF pathogenesis are likely to uncover an exciting new set of drug targets. The repurposing of drugs has the potential to allow candidate drugs gain quicker access into the clinic. At the same time, drug discovery efforts based on emerging understanding of IPF pathobiology must continue. Coupling of drug discovery with biomarker discovery has the greatest potential to realize the promise of personalized medicine. Discovery of biomarkers that serve as surrogates for clinically relevant end-points, ideally mortality, would facilitate clinical trials over shorter periods and potentially with fewer subjects. Ultimately, such advancements will result in bringing drugs with greater efficacy, safety, and tolerability to patients with IPF, a disease once thought to be a death-sentence.
Key Points.
Approval of pirfenidone and nintedanib has provided much needed options to treat patients with IPF.
Several new therapeutic agents are currently being studied in Phase II trials.
Advances in the understanding of IPF pathobiology have led to an unprecedented expansion in the number of novel therapeutic targets being investigated in pre-clinical studies.
Footnotes
Compliance with ethical standards
Tracy Luckhardt participated in the advisory board for Intermune Inc. (manufacturer of pirfenidone) in June 2014 and received an honorarium and travel expenses.
The other authors have no conflicts of interest to report.
References
- 1.Thannickal VJ, Flaherty KR, Martinez FJ, Lynch JP., 3rd Idiopathic pulmonary fibrosis: emerging concepts on pharmacotherapy. Expert Opin Pharmacother. 2004 Aug;5(8):1671–1686. doi: 10.1517/14656566.5.8.1671. [DOI] [PubMed] [Google Scholar]
- 2.American Thoracic Society. Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS) Am J Respir Crit Care Med. 2000 Feb;161(2 Pt 1):646–664. doi: 10.1164/ajrccm.161.2.ats3-00. [DOI] [PubMed] [Google Scholar]
- 3.American Thoracic Society, European Respiratory Society. American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med. 2002 Jan 15;165(2):277–304. doi: 10.1164/ajrccm.165.2.ats01. [DOI] [PubMed] [Google Scholar]
- 4.Nalysnyk L, Cid-Ruzafa J, Rotella P, Esser D. Incidence and prevalence of idiopathic pulmonary fibrosis: review of the literature. Eur Respir Rev. 2012 Dec 1;21(126):355–361. doi: 10.1183/09059180.00002512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raghu G, Weycker D, Edelsberg J, Bradford WZ, Oster G. Incidence and prevalence of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2006 Oct 1;174(7):810–816. doi: 10.1164/rccm.200602-163OC. [DOI] [PubMed] [Google Scholar]
- 6.Idiopathic Pulmonary Fibrosis Clinical Research Network. Raghu G, Anstrom KJ, King TE, Jr, Lasky JA, Martinez FJ. Prednisone, azathioprine, and N-acetylcysteine for pulmonary fibrosis. N Engl J Med. 2012 May 24;366(21):1968–1977. doi: 10.1056/NEJMoa1113354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.King TE, Jr, Brown KK, Raghu G, du Bois RM, Lynch DA, Martinez F, et al. BUILD-3: a randomized, controlled trial of bosentan in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011 Jul 1;184(1):92–99. doi: 10.1164/rccm.201011-1874OC. [DOI] [PubMed] [Google Scholar]
- 8.King TE, Jr, Albera C, Bradford WZ, Costabel U, Hormel P, Lancaster L, et al. Effect of interferon gamma-1b on survival in patients with idiopathic pulmonary fibrosis (INSPIRE): a multicentre, randomised, placebo-controlled trial. Lancet. 2009 Jul 18;374(9685):222–228. doi: 10.1016/S0140-6736(09)60551-1. [DOI] [PubMed] [Google Scholar]
- 9.Idiopathic Pulmonary Fibrosis Clinical Research Network. Zisman DA, Schwarz M, Anstrom KJ, Collard HR, Flaherty KR, et al. A controlled trial of sildenafil in advanced idiopathic pulmonary fibrosis. N Engl J Med. 2010 Aug 12;363(7):620–628. doi: 10.1056/NEJMoa1002110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raghu G, Rochwerg B, Zhang Y, Garcia CA, Azuma A, Behr J, et al. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline: Treatment of Idiopathic Pulmonary Fibrosis. An Update of the 2011 Clinical Practice Guideline. Am J Respir Crit Care Med. 2015 Jul 15;192(2):e3–e19. doi: 10.1164/rccm.201506-1063ST. [DOI] [PubMed] [Google Scholar]
- 11.Ding Q, Luckhardt T, Hecker L, Zhou Y, Liu G, Antony VB, et al. New insights into the pathogenesis and treatment of idiopathic pulmonary fibrosis. Drugs. 2011 May 28;71(8):981–1001. doi: 10.2165/11591490-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahluwalia N, Shea BS, Tager AM. New therapeutic targets in idiopathic pulmonary fibrosis. Aiming to rein in runaway wound-healing responses. Am J Respir Crit Care Med. 2014 Oct 15;190(8):867–878. doi: 10.1164/rccm.201403-0509PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woodcock HV, Maher TM. The treatment of idiopathic pulmonary fibrosis. F1000Prime Rep. 2014;6:16. doi: 10.12703/P6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thannickal VJ, Murthy M, Balch WE, Chandel NS, Meiners S, Eickelberg O, et al. Blue journal conference. Aging and susceptibility to lung disease. Am J Respir Crit Care Med. 2015 Feb 1;191(3):261–269. doi: 10.1164/rccm.201410-1876PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thannickal VJ. Mechanistic links between aging and lung fibrosis. Biogerontology. 2013 Dec;14(6):609–615. doi: 10.1007/s10522-013-9451-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raghu G, Chen SY, Yeh WS, Maroni B, Li Q, Lee YC, et al. Idiopathic pulmonary fibrosis in US Medicare beneficiaries aged 65 years and older: incidence, prevalence, and survival, 2001–11. Lancet Respir Med. 2014 Jul;2(7):566–572. doi: 10.1016/S2213-2600(14)70101-8. [DOI] [PubMed] [Google Scholar]
- 17.Fell CD, Martinez FJ, Liu LX, Murray S, Han MK, Kazerooni EA, et al. Clinical predictors of a diagnosis of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2010 Apr 15;181(8):832–837. doi: 10.1164/rccm.200906-0959OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Collard HR. The age of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2010 Apr 15;181(8):771–772. doi: 10.1164/rccm.201001-0049ED. [DOI] [PubMed] [Google Scholar]
- 19.Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013 Jun 6;153(6):1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thannickal VJ, Zhou Y, Gaggar A, Duncan SR. Fibrosis: ultimate and proximate causes. J Clin Invest. 2014 Nov;124(11):4673–4677. doi: 10.1172/JCI74368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thannickal VJ, Toews GB, White ES, Lynch JP, 3rd, Martinez FJ. Mechanisms of pulmonary fibrosis. Annu Rev Med. 2004;55:395–417. doi: 10.1146/annurev.med.55.091902.103810. [DOI] [PubMed] [Google Scholar]
- 22.Iyer SN, Wild JS, Schiedt MJ, Hyde DM, Margolin SB, Giri SN. Dietary intake of pirfenidone ameliorates bleomycin-induced lung fibrosis in hamsters. J Lab Clin Med. 1995 Jun;125(6):779–785. [PubMed] [Google Scholar]
- 23.Schaefer CJ, Ruhrmund DW, Pan L, Seiwert SD, Kossen K. Antifibrotic activities of pirfenidone in animal models. Eur Respir Rev. 2011 Jun;20(120):85–97. doi: 10.1183/09059180.00001111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trivedi R, Redente EF, Thakur A, Riches DW, Kompella UB. Local delivery of biodegradable pirfenidone nanoparticles ameliorates bleomycin-induced pulmonary fibrosis in mice. Nanotechnology. 2012 Dec 21;23(50):505101. doi: 10.1088/0957-4484/23/50/505101. [DOI] [PubMed] [Google Scholar]
- 25.Maher TM. Pirfenidone in idiopathic pulmonary fibrosis. Drugs Today (Barc) 2010 Jul;46(7):473–482. doi: 10.1358/dot.2010.46.7.1488336. [DOI] [PubMed] [Google Scholar]
- 26.King TE, Jr, Bradford WZ, Castro-Bernardini S, Fagan EA, Glaspole I, Glassberg MK, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014 May 29;370(22):2083–2092. doi: 10.1056/NEJMoa1402582. [DOI] [PubMed] [Google Scholar]
- 27.Noble PW, Albera C, Bradford WZ, Costabel U, Glassberg MK, Kardatzke D, et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet. 2011 May 21;377(9779):1760–1769. doi: 10.1016/S0140-6736(11)60405-4. [DOI] [PubMed] [Google Scholar]
- 28.Hilberg F, Roth GJ, Krssak M, Kautschitsch S, Sommergruber W, Tontsch-Grunt U, et al. BIBF 1120: triple angiokinase inhibitor with sustained receptor blockade and good antitumor efficacy. Cancer Res. 2008 Jun 15;68(12):4774–4782. doi: 10.1158/0008-5472.CAN-07-6307. [DOI] [PubMed] [Google Scholar]
- 29.Chaudhary NI, Roth GJ, Hilberg F, Muller-Quernheim J, Prasse A, Zissel G, et al. Inhibition of PDGF, VEGF and FGF signalling attenuates fibrosis. Eur Respir J. 2007 May;29(5):976–985. doi: 10.1183/09031936.00152106. [DOI] [PubMed] [Google Scholar]
- 30.McCormack PL. Nintedanib: first global approval. Drugs. 2015 Jan;75(1):129–139. doi: 10.1007/s40265-014-0335-0. [DOI] [PubMed] [Google Scholar]
- 31.Richeldi L, Costabel U, Selman M, Kim DS, Hansell DM, Nicholson AG, et al. Efficacy of a tyrosine kinase inhibitor in idiopathic pulmonary fibrosis. N Engl J Med. 2011 Sep 22;365(12):1079–1087. doi: 10.1056/NEJMoa1103690. [DOI] [PubMed] [Google Scholar]
- 32.Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, et al. Efficacy and Safety of Nintedanib in Idiopathic Pulmonary Fibrosis. N Engl J Med. 2014 May 29;370(22):2071–2082. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- 33.Wollin L, Maillet I, Quesniaux V, Holweg A, Ryffel B. Antifibrotic and Anti-inflammatory Activity of the Tyrosine Kinase Inhibitor Nintedanib in Experimental Models of Lung Fibrosis. J Pharmacol Exp Ther. 2014 May;349(2):209–220. doi: 10.1124/jpet.113.208223. [DOI] [PubMed] [Google Scholar]
- 34.Wollin L, Wex E, Pautsch A, Schnapp G, Hostettler KE, Stowasser S, et al. Mode of action of nintedanib in the treatment of idiopathic pulmonary fibrosis. Eur Respir J. 2015 May;45(5):1434–1445. doi: 10.1183/09031936.00174914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rangarajan S, Kurundkar A, Kurundkar D, Bernard K, Sanders YY, Ding Q, et al. Novel Mechanisms for the Anti-Fibrotic Action of Nintedanib. Am J Respir Cell Mol Biol. 2015 Jun 13; doi: 10.1165/rcmb.2014-0445OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Loveman E, Copley VR, Scott DA, Colquitt JL, Clegg AJ, O'Reilly KM. Comparing new treatments for idiopathic pulmonary fibrosis--a network meta-analysis. BMC Pulm Med. 2015;15:37. doi: 10.1186/s12890-015-0034-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Loveman E, Copley VR, Colquitt JL, Scott DA, Clegg AJ, Jones J, et al. The effectiveness and cost-effectiveness of treatments for idiopathic pulmonary fibrosis: systematic review, network meta-analysis and health economic evaluation. BMC Pharmacol Toxicol. 2014;15:63. doi: 10.1186/2050-6511-15-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hancock A, Armstrong L, Gama R, Millar A. Production of interleukin 13 by alveolar macrophages from normal and fibrotic lung. Am J Respir Cell Mol Biol. 1998 Jan;18(1):60–65. doi: 10.1165/ajrcmb.18.1.2627. [DOI] [PubMed] [Google Scholar]
- 39.Cieslik KA, Taffet GE, Carlson S, Hermosillo J, Trial J, Entman ML. Immune-inflammatory dysregulation modulates the incidence of progressive fibrosis and diastolic stiffness in the aging heart. J Mol Cell Cardiol. 2011 Jan;50(1):248–256. doi: 10.1016/j.yjmcc.2010.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee CG, Homer RJ, Zhu Z, Lanone S, Wang X, Koteliansky V, et al. Interleukin-13 induces tissue fibrosis by selectively stimulating and activating transforming growth factor beta(1) J Exp Med. 2001 Sep 17;194(6):809–821. doi: 10.1084/jem.194.6.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee JH, Kaminski N, Dolganov G, Grunig G, Koth L, Solomon C, et al. Interleukin-13 induces dramatically different transcriptional programs in three human airway cell types. Am J Respir Cell Mol Biol. 2001 Oct;25(4):474–485. doi: 10.1165/ajrcmb.25.4.4522. [DOI] [PubMed] [Google Scholar]
- 42.Zhu Z, Ma B, Zheng T, Homer RJ, Lee CG, Charo IF, et al. IL-13-induced chemokine responses in the lung: role of CCR2 in the pathogenesis of IL-13-induced inflammation and remodeling. J Immunol. 2002 Mar 15;168(6):2953–2962. doi: 10.4049/jimmunol.168.6.2953. [DOI] [PubMed] [Google Scholar]
- 43.Murray LA, Argentieri RL, Farrell FX, Bracht M, Sheng H, Whitaker B, et al. Hyper-responsiveness of IPF/UIP fibroblasts: interplay between TGFbeta1, IL-13 and CCL2. Int J Biochem Cell Biol. 2008;40(10):2174–2182. doi: 10.1016/j.biocel.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 44.Murray LA, Zhang H, Oak SR, Coelho AL, Herath A, Flaherty KR, et al. Targeting interleukin-13 with tralokinumab attenuates lung fibrosis and epithelial damage in a humanized SCID idiopathic pulmonary fibrosis model. Am J Respir Cell Mol Biol. 2014 May;50(5):985–994. doi: 10.1165/rcmb.2013-0342OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fichtner-Feigl S, Strober W, Kawakami K, Puri RK, Kitani A. IL-13 signaling through the IL-13alpha2 receptor is involved in induction of TGF-beta1 production and fibrosis. Nat Med. 2006 Jan;12(1):99–106. doi: 10.1038/nm1332. [DOI] [PubMed] [Google Scholar]
- 46.Lumsden RV, Worrell JC, Boylan D, Walsh SM, Cramton J, Counihan I, et al. Modulation of pulmonary fibrosis by IL-13Ralpha2. Am J Physiol Lung Cell Mol Physiol. 2015 Apr 1;308(7):L710–L718. doi: 10.1152/ajplung.00120.2014. [DOI] [PubMed] [Google Scholar]
- 47.Baran CP, Opalek JM, McMaken S, Newland CA, O'Brien JM, Jr, Hunter MG, et al. Important roles for macrophage colony-stimulating factor, CC chemokine ligand 2, and mononuclear phagocytes in the pathogenesis of pulmonary fibrosis. Am J Respir Crit Care Med. 2007 Jul 1;176(1):78–89. doi: 10.1164/rccm.200609-1279OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu X, Das AM, Seideman J, Griswold D, Afuh CN, Kobayashi T, et al. The CC chemokine ligand 2 (CCL2) mediates fibroblast survival through IL-6. Am J Respir Cell Mol Biol. 2007 Jul;37(1):121–128. doi: 10.1165/rcmb.2005-0253OC. [DOI] [PubMed] [Google Scholar]
- 49.Moore BB, Murray L, Das A, Wilke CA, Herrygers AB, Toews GB. The role of CCL12 in the recruitment of fibrocytes and lung fibrosis. Am J Respir Cell Mol Biol. 2006 Aug;35(2):175–181. doi: 10.1165/rcmb.2005-0239OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kalderen C, Stadler C, Forsgren M, Kvastad L, Johansson E, Sydow-Backman M, et al. CCL2 mediates anti-fibrotic effects in human fibroblasts independently of CCR2. Int Immunopharmacol. 2014 May;20(1):66–73. doi: 10.1016/j.intimp.2014.02.020. [DOI] [PubMed] [Google Scholar]
- 51.Raghu G, Martinez FJ, Brown KK, Costabel U, Cottin V, Wells AU, et al. A Phase II, Randomized, Double-Blind, Placebo-Controlled, Parallel-Group, Dose-Ranging Study Of The Safety And Efficacy Of CNTO 888 (Carlumab) In Patients With Idiopathic Pulmonary Fibrosis [abstract] Am J Respir Crit Care Med. 2013;187:A3376. [Google Scholar]
- 52.Duncan MR, Frazier KS, Abramson S, Williams S, Klapper H, Huang X, et al. Connective tissue growth factor mediates transforming growth factor beta-induced collagen synthesis: down-regulation by cAMP. FASEB J. 1999 Oct;13(13):1774–1786. [PubMed] [Google Scholar]
- 53.Grotendorst GR. Connective tissue growth factor: a mediator of TGF-beta action on fibroblasts. Cytokine Growth Factor Rev. 1997 Sep;8(3):171–179. doi: 10.1016/s1359-6101(97)00010-5. [DOI] [PubMed] [Google Scholar]
- 54.Allen JT, Knight RA, Bloor CA, Spiteri MA. Enhanced insulin-like growth factor binding protein-related protein 2 (Connective tissue growth factor) expression in patients with idiopathic pulmonary fibrosis and pulmonary sarcoidosis. Am J Respir Cell Mol Biol. 1999 Dec;21(6):693–700. doi: 10.1165/ajrcmb.21.6.3719. [DOI] [PubMed] [Google Scholar]
- 55.Pan LH, Yamauchi K, Uzuki M, Nakanishi T, Takigawa M, Inoue H, et al. Type II alveolar epithelial cells and interstitial fibroblasts express connective tissue growth factor in IPF. Eur Respir J. 2001 Jun;17(6):1220–1227. doi: 10.1183/09031936.01.00074101. [DOI] [PubMed] [Google Scholar]
- 56.Lasky JA, Ortiz LA, Tonthat B, Hoyle GW, Corti M, Athas G, et al. Connective tissue growth factor mRNA expression is upregulated in bleomycin-induced lung fibrosis. Am J Physiol. 1998 Aug;275(2 Pt 1):L365–L371. doi: 10.1152/ajplung.1998.275.2.L365. [DOI] [PubMed] [Google Scholar]
- 57.Lipson KE, Wong C, Teng Y, Spong S. CTGF is a central mediator of tissue remodeling and fibrosis and its inhibition can reverse the process of fibrosis. Fibrogenesis Tissue Repair. 2012;5(Suppl 1):S24. doi: 10.1186/1755-1536-5-S1-S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Raghu G, Scholand MB, De Andrade J, Lancaster L, Mageto YN, Goldin JG, et al. Safety And Efficacy Of Anti-CTGF Monoclonal Antibody FG-3019 For Treatment Of Idiopathic Pulmonary Fibrosis (IPF): Results Of Phase 2 Clinical Trial Two Years After Initiation [abstract] Am J Respir Crit Care Med. 2014;189:A1426. [Google Scholar]
- 59.Pinnell SR, Martin GR. The cross-linking of collagen and elastin: enzymatic conversion of lysine in peptide linkage to alpha-aminoadipic-delta-semialdehyde (allysine) by an extract from bone. Proc Natl Acad Sci U S A. 1968 Oct;61(2):708–716. doi: 10.1073/pnas.61.2.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Siegel RC, Pinnell SR, Martin GR. Cross-linking of collagen and elastin. Properties of lysyl oxidase. Biochemistry. 1970 Nov 10;9(23):4486–4492. doi: 10.1021/bi00825a004. [DOI] [PubMed] [Google Scholar]
- 61.Barry-Hamilton V, Spangler R, Marshall D, McCauley S, Rodriguez HM, Oyasu M, et al. Allosteric inhibition of lysyl oxidase-like-2 impedes the development of a pathologic microenvironment. Nat Med. 2010 Sep;16(9):1009–1017. doi: 10.1038/nm.2208. [DOI] [PubMed] [Google Scholar]
- 62.Chien JW, Richards TJ, Gibson KF, Zhang Y, Lindell KO, Shao L, et al. Serum lysyl oxidase-like 2 levels and idiopathic pulmonary fibrosis disease progression. Eur Respir J. 2014 May;43(5):1430–1438. doi: 10.1183/09031936.00141013. [DOI] [PubMed] [Google Scholar]
- 63.Border WA, Noble NA. Transforming growth factor beta in tissue fibrosis. N Engl J Med. 1994 Nov 10;331(19):1286–1292. doi: 10.1056/NEJM199411103311907. [DOI] [PubMed] [Google Scholar]
- 64.Pardali K, Moustakas A. Actions of TGF-beta as tumor suppressor and pro-metastatic factor in human cancer. Biochim Biophys Acta. 2007 Jan;1775(1):21–62. doi: 10.1016/j.bbcan.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 65.Munger JS, Harpel JG, Gleizes PE, Mazzieri R, Nunes I, Rifkin DB. Latent transforming growth factor-beta: structural features and mechanisms of activation. Kidney Int. 1997 May;51(5):1376–1382. doi: 10.1038/ki.1997.188. [DOI] [PubMed] [Google Scholar]
- 66.Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, et al. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999 Feb 5;96(3):319–328. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- 67.Puthawala K, Hadjiangelis N, Jacoby SC, Bayongan E, Zhao Z, Yang Z, et al. Inhibition of integrin alpha(v)beta6, an activator of latent transforming growth factor-beta, prevents radiation-induced lung fibrosis. Am J Respir Crit Care Med. 2008 Jan 1;177(1):82–90. doi: 10.1164/rccm.200706-806OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Horan GS, Wood S, Ona V, Li DJ, Lukashev ME, Weinreb PH, et al. Partial inhibition of integrin alpha(v)beta6 prevents pulmonary fibrosis without exacerbating inflammation. Am J Respir Crit Care Med. 2008 Jan 1;177(1):56–65. doi: 10.1164/rccm.200706-805OC. [DOI] [PubMed] [Google Scholar]
- 69.Shea BS, Tager AM. Role of the lysophospholipid mediators lysophosphatidic acid and sphingosine 1-phosphate in lung fibrosis. Proc Am Thorac Soc. 2012 Jul;9(3):102–110. doi: 10.1513/pats.201201-005AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pradere JP, Klein J, Gres S, Guigne C, Neau E, Valet P, et al. LPA1 receptor activation promotes renal interstitial fibrosis. J Am Soc Nephrol. 2007 Dec;18(12):3110–3118. doi: 10.1681/ASN.2007020196. [DOI] [PubMed] [Google Scholar]
- 71.Tager AM, LaCamera P, Shea BS, Campanella GS, Selman M, Zhao Z, et al. The lysophosphatidic acid receptor LPA1 links pulmonary fibrosis to lung injury by mediating fibroblast recruitment and vascular leak. Nat Med. 2008 Jan;14(1):45–54. doi: 10.1038/nm1685. [DOI] [PubMed] [Google Scholar]
- 72.Castelino FV, Seiders J, Bain G, Brooks SF, King CD, Swaney JS, et al. Amelioration of dermal fibrosis by genetic deletion or pharmacologic antagonism of lysophosphatidic acid receptor 1 in a mouse model of scleroderma. Arthritis Rheum. 2011 May;63(5):1405–1415. doi: 10.1002/art.30262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sakai N, Chun J, Duffield JS, Wada T, Luster AD, Tager AM. LPA1-induced cytoskeleton reorganization drives fibrosis through CTGF-dependent fibroblast proliferation. FASEB J. 2013 May;27(5):1830–1846. doi: 10.1096/fj.12-219378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hirokawa K. Autoimmunity and aging. Concepts Immunopathol. 1985;1:251–288. [PubMed] [Google Scholar]
- 75.Prelog M. Aging of the immune system: a risk factor for autoimmunity? Autoimmun Rev. 2006 Feb;5(2):136–139. doi: 10.1016/j.autrev.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 76.Donahoe M, Valentine VG, Chien N, Gibson KF, Raval JS, Saul M, et al. Autoantibody-Targeted Treatments for Acute Exacerbations of Idiopathic Pulmonary Fibrosis. PLoS One. 2015;10(6):e0127771. doi: 10.1371/journal.pone.0127771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Daoussis D, Liossis SN, Tsamandas AC, Kalogeropoulou C, Paliogianni F, Sirinian C, et al. Effect of long-term treatment with rituximab on pulmonary function and skin fibrosis in patients with diffuse systemic sclerosis. Clin Exp Rheumatol. 2012 Mar-Apr;30(2 Suppl 71):S17–S22. [PubMed] [Google Scholar]
- 78.Leon RJ, Gonsalvo A, Salas R, Hidalgo NC. Rituximab-induced acute pulmonary fibrosis. Mayo Clin Proc. 2004 Jul;79(7):949. doi: 10.1016/S0025-6196(11)62171-X. 53. [DOI] [PubMed] [Google Scholar]
- 79.Chaumais MC, Garnier A, Chalard F, Peuchmaur M, Dauger S, Jacqz-Agrain E, et al. Fatal pulmonary fibrosis after rituximab administration. Pediatr Nephrol. 2009 Sep;24(9):1753–1755. doi: 10.1007/s00467-009-1195-9. [DOI] [PubMed] [Google Scholar]
- 80.Rathi M, Ramachandran R, Gundlapalli S, Agarwal R, Das A, Kumar V, et al. Rituximab induced pulmonary fibrosis in a patient with lupus nephritis. Lupus. 2012 Sep;21(10):1131–1134. doi: 10.1177/0961203312444892. [DOI] [PubMed] [Google Scholar]
- 81.Ryter SW, Choi AM. Therapeutic applications of carbon monoxide in lung disease. Curr Opin Pharmacol. 2006 Jun;6(3):257–262. doi: 10.1016/j.coph.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 82.Kohmoto J, Nakao A, Kaizu T, Tsung A, Ikeda A, Tomiyama K, et al. Low-dose carbon monoxide inhalation prevents ischemia/reperfusion injury of transplanted rat lung grafts. Surgery. 2006 Aug;140(2):179–185. doi: 10.1016/j.surg.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 83.Hoetzel A, Dolinay T, Vallbracht S, Zhang Y, Kim HP, Ifedigbo E, et al. Carbon monoxide protects against ventilator-induced lung injury via PPAR-gamma and inhibition of Egr-1. Am J Respir Crit Care Med. 2008 Jun 1;177(11):1223–1232. doi: 10.1164/rccm.200708-1265OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hoetzel A, Schmidt R, Vallbracht S, Goebel U, Dolinay T, Kim HP, et al. Carbon monoxide prevents ventilator-induced lung injury via caveolin-1. Crit Care Med. 2009 May;37(5):1708–1715. doi: 10.1097/CCM.0b013e31819efa31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chiang N, Shinohara M, Dalli J, Mirakaj V, Kibi M, Choi AM, et al. Inhaled carbon monoxide accelerates resolution of inflammation via unique proresolving mediator-heme oxygenase-1 circuits. J Immunol. 2013 Jun 15;190(12):6378–6388. doi: 10.4049/jimmunol.1202969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhou Z, Song R, Fattman CL, Greenhill S, Alber S, Oury TD, et al. Carbon monoxide suppresses bleomycin-induced lung fibrosis. Am J Pathol. 2005 Jan;166(1):27–37. doi: 10.1016/S0002-9440(10)62229-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Han MK, Zhou Y, Murray S, Tayob N, Noth I, Lama VN, et al. Lung microbiome and disease progression in idiopathic pulmonary fibrosis: an analysis of the COMET study. Lancet Respir Med. 2014 Jul;2(7):548–556. doi: 10.1016/S2213-2600(14)70069-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Molyneaux PL, Cox MJ, Willis-Owen SA, Mallia P, Russell KE, Russell AM, et al. The role of bacteria in the pathogenesis and progression of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2014 Oct 15;190(8):906–913. doi: 10.1164/rccm.201403-0541OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wootton SC, Kim DS, Kondoh Y, Chen E, Lee JS, Song JW, et al. Viral infection in acute exacerbation of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011 Jun 15;183(12):1698–1702. doi: 10.1164/rccm.201010-1752OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wuyts WA, Willems S, Vos R, Vanaudenaerde BM, De Vleeschauwer SI, Rinaldi M, et al. Azithromycin reduces pulmonary fibrosis in a bleomycin mouse model. Exp Lung Res. 2010 Dec;36(10):602–614. doi: 10.3109/01902148.2010.492895. [DOI] [PubMed] [Google Scholar]
- 91.Shulgina L, Cahn AP, Chilvers ER, Parfrey H, Clark AB, Wilson EC, et al. Treating idiopathic pulmonary fibrosis with the addition of co-trimoxazole: a randomised controlled trial. Thorax. 2013 Feb;68(2):155–162. doi: 10.1136/thoraxjnl-2012-202403. [DOI] [PubMed] [Google Scholar]
- 92.Leung T, Manser E, Tan L, Lim L. A novel serine/threonine kinase binding the Ras-related RhoA GTPase which translocates the kinase to peripheral membranes. J Biol Chem. 1995 Dec 8;270(49):29051–29054. doi: 10.1074/jbc.270.49.29051. [DOI] [PubMed] [Google Scholar]
- 93.Riento K, Ridley AJ. Rocks: multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol. 2003 Jun;4(6):446–456. doi: 10.1038/nrm1128. [DOI] [PubMed] [Google Scholar]
- 94.Street CA, Bryan BA. Rho kinase proteins--pleiotropic modulators of cell survival and apoptosis. Anticancer Res. 2011 Nov;31(11):3645–3657. [PMC free article] [PubMed] [Google Scholar]
- 95.Zhou Y, Huang X, Hecker L, Kurundkar D, Kurundkar A, Liu H, et al. Inhibition of mechanosensitive signaling in myofibroblasts ameliorates experimental pulmonary fibrosis. J Clin Invest. 2013 Mar 1;123(3):1096–1108. doi: 10.1172/JCI66700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang P, Tang F, Li R, Zhang H, Chen S, Liu P, et al. Contribution of different Nox homologues to cardiac remodeling in two-kidney two-clip renovascular hypertensive rats: effect of valsartan. Pharmacol Res. 2007 May;55(5):408–417. doi: 10.1016/j.phrs.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 97.Masamune A, Kikuta K, Watanabe T, Satoh K, Hirota M, Shimosegawa T. Hypoxia stimulates pancreatic stellate cells to induce fibrosis and angiogenesis in pancreatic cancer. Am J Physiol Gastrointest Liver Physiol. 2008 Oct;295(4):G709–G717. doi: 10.1152/ajpgi.90356.2008. [DOI] [PubMed] [Google Scholar]
- 98.Hecker L, Vittal R, Jones T, Jagirdar R, Luckhardt TR, Horowitz JC, et al. NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat Med. 2009 Sep;15(9):1077–1081. doi: 10.1038/nm.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Amara N, Goven D, Prost F, Muloway R, Crestani B, Boczkowski J. NOX4/NADPH oxidase expression is increased in pulmonary fibroblasts from patients with idiopathic pulmonary fibrosis and mediates TGFbeta1-induced fibroblast differentiation into myofibroblasts. Thorax. 2010 Aug;65(8):733–738. doi: 10.1136/thx.2009.113456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kuroda J, Ago T, Matsushima S, Zhai P, Schneider MD, Sadoshima J. NADPH oxidase 4 (Nox4) is a major source of oxidative stress in the failing heart. Proc Natl Acad Sci U S A. 2010 Aug 31;107(35):15565–15570. doi: 10.1073/pnas.1002178107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sancho P, Mainez J, Crosas-Molist E, Roncero C, Fernandez-Rodriguez CM, Pinedo F, et al. NADPH oxidase NOX4 mediates stellate cell activation and hepatocyte cell death during liver fibrosis development. PLoS One. 2012;7(9):e45285. doi: 10.1371/journal.pone.0045285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Thannickal VJ, Fanburg BL. Activation of an H2O2-generating NADH oxidase in human lung fibroblasts by transforming growth factor beta 1. J Biol Chem. 1995 Dec 22;270(51):30334–30338. doi: 10.1074/jbc.270.51.30334. [DOI] [PubMed] [Google Scholar]
- 103.Cheng G, Cao Z, Xu X, van Meir EG, Lambeth JD. Homologs of gp91phox: cloning and tissue expression of Nox3, Nox4, and Nox5. Gene. 2001 May 16;269(1–2):131–140. doi: 10.1016/s0378-1119(01)00449-8. [DOI] [PubMed] [Google Scholar]
- 104.Thannickal VJ. Aging, antagonistic pleiotropy and fibrotic disease. Int J Biochem Cell Biol. 2010 Sep;42(9):1398–1400. doi: 10.1016/j.biocel.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Thannickal VJ. Mechanisms of pulmonary fibrosis: role of activated myofibroblasts and NADPH oxidase. Fibrogenesis Tissue Repair. 2012 Jun 6;5(Suppl 1):S23. doi: 10.1186/1755-1536-5-S1-S23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sanders YY, Liu H, Liu G, Thannickal VJ. Epigenetic mechanisms regulate NADPH oxidase-4 expression in cellular senescence. Free Radic Biol Med. 2015 Feb;79:197–205. doi: 10.1016/j.freeradbiomed.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 107.Hecker L, Logsdon NJ, Kurundkar D, Kurundkar A, Bernard K, Hock T, et al. Reversal of persistent fibrosis in aging by targeting Nox4-Nrf2 redox imbalance. Sci Transl Med. 2014 Apr 9;6(231):231ra47. doi: 10.1126/scitranslmed.3008182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Salminen A, Kaarniranta K. AMP-activated protein kinase (AMPK) controls the aging process via an integrated signaling network. Ageing Res Rev. 2012 Apr;11(2):230–241. doi: 10.1016/j.arr.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 109.Rangarajan S, Liu Y, Park DW, Zmijewska A, Abraham E, Thannickal VJ, et al. AMP-Activated Protein Kinase Activation Diminishes the Severity of Experimental Lung Fibrosis [abstract] Am J Respir Crit Care Med. 2015;191:A3474. [Google Scholar]
- 110.Mihaylova MM, Shaw RJ. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol. 2011 Sep;13(9):1016–1023. doi: 10.1038/ncb2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Burkewitz K, Zhang Y, Mair WB. AMPK at the nexus of energetics and aging. Cell Metab. 2014 Jul 1;20(1):10–25. doi: 10.1016/j.cmet.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Daniels CE, Wilkes MC, Edens M, Kottom TJ, Murphy SJ, Limper AH, et al. Imatinib mesylate inhibits the profibrogenic activity of TGF-beta and prevents bleomycin-mediated lung fibrosis. J Clin Invest. 2004 Nov;114(9):1308–1316. doi: 10.1172/JCI19603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Daniels CE, Lasky JA, Limper AH, Mieras K, Gabor E, Schroeder DR, et al. Imatinib treatment for idiopathic pulmonary fibrosis: Randomized placebo-controlled trial results. Am J Respir Crit Care Med. 2010 Mar 15;181(6):604–610. doi: 10.1164/rccm.200906-0964OC. [DOI] [PubMed] [Google Scholar]
- 114.Vittal R, Horowitz JC, Moore BB, Zhang H, Martinez FJ, Toews GB, et al. Modulation of prosurvival signaling in fibroblasts by a protein kinase inhibitor protects against fibrotic tissue injury. Am J Pathol. 2005 Feb;166(2):367–375. doi: 10.1016/S0002-9440(10)62260-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Vittal R, Zhang H, Han MK, Moore BB, Horowitz JC, Thannickal VJ. Effects of the protein kinase inhibitor, imatinib mesylate, on epithelial/mesenchymal phenotypes: implications for treatment of fibrotic diseases. J Pharmacol Exp Ther. 2007 Apr;321(1):35–44. doi: 10.1124/jpet.106.113407. [DOI] [PubMed] [Google Scholar]
- 116.Garneau-Tsodikova S, Thannickal VJ. Protein kinase inhibitors in the treatment of pulmonary fibrosis. Curr Med Chem. 2008;15(25):2632–2640. doi: 10.2174/092986708785908969. [DOI] [PubMed] [Google Scholar]
- 117.Grimminger F, Gunther A, Vancheri C. The role of tyrosine kinases in the pathogenesis of idiopathic pulmonary fibrosis. Eur Respir J. 2015 May;45(5):1426–1433. doi: 10.1183/09031936.00149614. [DOI] [PubMed] [Google Scholar]
- 118.Hu M, Che P, Han X, Cai GQ, Liu G, Antony V, et al. Therapeutic Targeting of Src Kinase in Myofibroblast Differentiation and Pulmonary Fibrosis. J Pharmacol Exp Ther. 2014 Jul 21;351(1):87–95. doi: 10.1124/jpet.114.216044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004 Jan 23;116(2):281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 120.Guo H, Ingolia NT, Weissman JS, Bartel DP. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature. 2010 Aug 12;466(7308):835–840. doi: 10.1038/nature09267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cui H, Xie N, Thannickal VJ, Liu G. The code of non-coding RNAs in lung fibrosis. Cell Mol Life Sci. 2015 Sep;72(18):3507–3519. doi: 10.1007/s00018-015-1939-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cushing L, Kuang PP, Qian J, Shao F, Wu J, Little F, et al. miR-29 is a major regulator of genes associated with pulmonary fibrosis. Am J Respir Cell Mol Biol. 2011 Aug;45(2):287–294. doi: 10.1165/rcmb.2010-0323OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yang T, Liang Y, Lin Q, Liu J, Luo F, Li X, et al. miR-29 mediates TGFbeta1-induced extracellular matrix synthesis through activation of PI3K-AKT pathway in human lung fibroblasts. J Cell Biochem. 2013 Jun;114(6):1336–1342. doi: 10.1002/jcb.24474. [DOI] [PubMed] [Google Scholar]
- 124.Liu G, Friggeri A, Yang Y, Milosevic J, Ding Q, Thannickal VJ, et al. miR-21 mediates fibrogenic activation of pulmonary fibroblasts and lung fibrosis. J Exp Med. 2010 Aug 2;207(8):1589–1597. doi: 10.1084/jem.20100035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.van Rooij E, Kauppinen S. Development of microRNA therapeutics is coming of age. EMBO Mol Med. 2014 Jul;6(7):851–864. doi: 10.15252/emmm.201100899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Morry J, Ngamcherdtrakul W, Gu S, Goodyear SM, Castro DJ, Reda MM, et al. Dermal delivery of HSP47 siRNA with NOX4-modulating mesoporous silica-based nanoparticles for treating fibrosis. Biomaterials. 2015 Oct;66:41–52. doi: 10.1016/j.biomaterials.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]