Abstract
The hippocampus is not fully developed at birth and, with respect to spatial cognition, only begins to show signs of adult-like function at three postnatal weeks in rodents. Studying the developmental period spanning roughly two to four weeks of age permits an understanding of the neural framework necessary for the emergence of spatial navigation and, quite possibly, human episodic memory. However, due to developmental factors, behavior data collection and interpretation can be severely compromised if inappropriate designs are applied. As such, we propose methodological considerations for the behavioral assessment of hippocampal function in developing rats that take into account animal size, growth rate, and sensory and motor ability. We further summarize recent key interdisciplinary studies that are beginning to unravel the molecular machinery and physiological alterations responsible for hippocampal maturation. In general, hippocampal development is a protracted process during which unique contributions to spatial cognition and complex recognition memory come “on line” at different postnatal ages creating a unique situation for elucidating the neural bases of specific components of higher cognitive abilities.
Keywords: Spatial navigation, Learning and memory, Postnatal development, Hippocampus, Y-maze, AMPAR, NMDAR, Behavioral testing, Novelty, Environment, Place field, Juvenile
1. Introduction
Episodic memory constitutes the “what,” “when” and “where” aspects of personal experiences and is subserved by the hippocampus and associated neural structures (Nyberg et al., 1996). Episodic memory allows us to relate past experiences to current situations, to plan future scenarios, to create narratives and, in large part, defines who we are as individuals. In rodents, the hippocampus sits at the top of a perceptual and cognitive system that permits rapid spatial memory formation and spatial navigation (Dumas and Rudy, 2010). Due to similarities in architecture, cellular physiology and network dynamics across species, research into spatial navigation and spatial memory in rodents serves as an ideal model to understand the physiological bases of episodic memory in humans (Squire, 1992; Squire and Zola, 1998). Moreover, the protracted development of the hippocampus during the late postnatal period allows for examination of discrete hippocampus-dependent functions as they come on line, beginning with contextual encoding at two weeks and ending with the ability to form and retrieve long-term episodic memories at after four weeks of age.
In humans, spatial memory emerges at about three years of age (Aadland et al., 1985; Huttenlocher, 2008). In rodents, as judged by performance in spatial learning and memory tasks, the hippocampus does not show signs of adult-like function until at least the end of the third postnatal week (Blair et al., 2013; Douglas et al., 1973; Dumas, 2004; Kraemer and Randall, 1995; Rudy et al., 1987). Reliance of spatial learning and memory on well-developed motor and distal sensory systems (Rudy, 1992) may partially explain why the hippocampus matures late in the postnatal period, so that information fed to the juvenile hippocampus is an accurate, high fidelity representation of the environment (Fagiolini et al., 1994; Göb et al., 1987; Schachtele et al., 2011; Stanton, 2000). This notion is substantiated by studies that have demonstrated that the trajectory for hippocampal maturation can be modified via experimental manipulation of visual experience (Dumas, 2004; Foreman and Altaha, 1992; Kenny and Turkewitz, 1986), gain-of-function pharmacological treatment (Blair et al., 2013), and genetic mutation of neurotransmitter receptors (Sanders et al., 2013). Such approaches permit creation of a holistic model of hippocampal construction and identification of the specific neural processes that underlie various aspects of spatial cognition and episodic memory.
To fully understand episodic memory in adulthood, it is important to understand how the hippocampus is built during postnatal development and how it gains the ability to influence behavior (Wills et al., 2013). Because developmental approaches to investigation of hippocampal function are on the rise, this mini-review is intended to define appropriate behavioral tests for younger animals (often referring back to pioneering literature) and highlight recent mechanistic experiments that are beginning to create a unified model of the neural bases of hippocampal maturation.
2. Considerations for behavioral tests in immature rodents
Behavioral testing in juvenile rodents is very similar to that performed in adults with a few considerations, including smaller body size and accelerated growth rate of young rodents, continued refinement of sensory and motor systems, and interpretation complications produced by multiple-day training procedures. Attention paid to such details reduces experimental variability and increases the ability to resolve age-related differences in spatial learning and memory.
2.1. Issues related to size, diet, and rapid growth
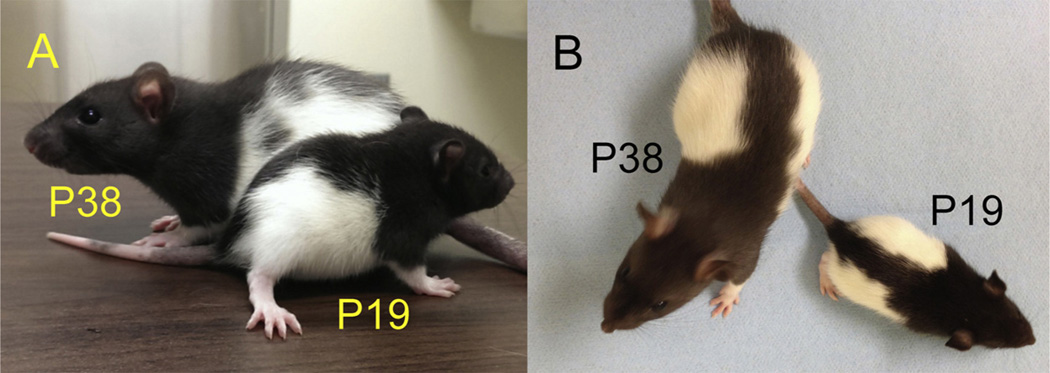
Developing rats and mice are smaller than adults and remain in a phase of rapid growth as they approach three weeks of age (Fig. 1). Because juveniles are smaller, maze dimensions need to be adjusted (Bulut and Altman, 1974; Carman and Mactutus, 2002; Carman et al., 2003; Dumas, 2004). Also, during the third postnatal week, developing rodents still feed from the dam while transitioning to solid food. Thus, appetitive tasks that require food deprivation, whether spatial or not, are likely to produce excessive metabolic and/or psychological stress prior to training, impact growth trajectory, and are not appropriate (Bronstein and Spear, 1972). As well, appetitive rewards that are salient to older animals may not be so to younger animals, impeding analysis of learning and memory abilities (Smith and Bogomolny, 1983). Furthermore, appetitive and aversive tasks that require multi-day training procedures produce temporal confounds that hinder interpretation of results. For instance, if behavior changes from day one to day two of a multi-day procedure, without single exposure controls, it is not possible to know if any behavioral alteration observed during day-two performance was influenced by day-one training or emerged independently on day two.
Fig. 1.
Long–Evans rats at P19 and P38. Both height (A) and length (B) differ dramatically between these age groups. Behavioral testing conditions must account for differences in size and step length in relation to the animal’s age.
2.2. Environmental considerations
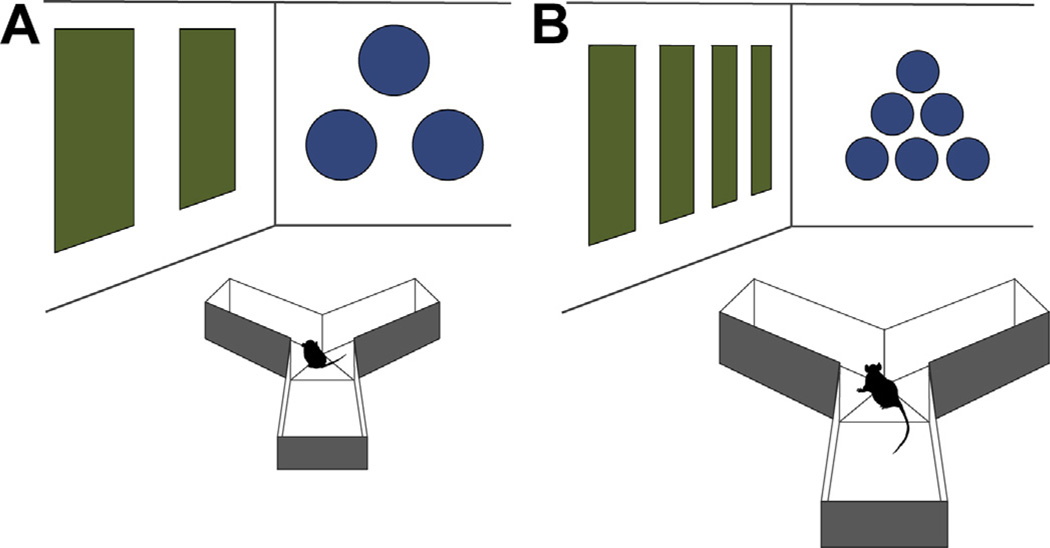
Finally, while sensory and motor abilities are largely well-developed at three weeks of age, some degree of continued refinement is apparent (Moye and Rudy, 1987; Prusky and Douglas, 2003). It is likely that, at three weeks, young animals cannot see as far as more mature animals (Fagiolini et al., 1994; Liao et al., 2004). Thus, given testing environments of the same size, contextual cue patterns should be larger for juveniles (Rudy et al., 1987) (Fig. 2). Finally, three-week-old rodents are not as strong as adults nor have they produced as thick a coat of fur. Therefore, special attention should be paid to environmental temperature and number of trials administered, especially in wet mazes where these animals do not float as well and may be more adversely affected by excessive number or duration of trials and inter-trial temperature changes (Iivonen et al., 2003; Kraemer and Randall, 1995).
Fig. 2.
Y-maze environment for juvenile (A) and adult (B) rodents. Maze dimensions are reduced to accommodate the smaller juvenile. Visual cues for juvenile testing should be larger and separated by a greater distance than their adult counterparts to allow for developmental differences in visual acuity.
2.3. Optimal tasks for probing hippocampal integrity in juveniles
Given the warnings described above, some spatial navigation tasks are more appropriate than others when working with immature rodents. For instance, while the Morris water maze is considered by many to be the gold standard behavioral assay for hippocampal function in adults (Morris, 1984; Vorhees and Williams, 2006), this test is suboptimal for developing animals. The younger the animal, the more effort must be exerted to remain afloat (Kraemer and Randall, 1995), which may produce stress levels that negatively impact learning and memory. Additionally, the younger the test subject, the greater the impact of water and ambient temperature on performance. To the contrary, tests that are performed on dry mazes are confined to one day of training, do not require food restriction or substantial time away from the dam, and are minimally stressful are best suited for studying developmental trajectory of hippocampal maturation. At the top of the list are spontaneous alternation in a Y-maze and tests of novelty detection.
2.3.1. Spontaneous alternation
The use of spontaneous alternation as a behavioral assay for hippocampal integrity has become well established over the past half century (Dember and Fowler, 1958; Douglas, 1975; Kirby, 1967; Lalonde, 2002; Richman et al., 1986). Examination of spontaneous alternation is typically conducted in a T-maze or symmetrical Y-maze having three identical, equally spaced arms with walls. For Y-maze testing, animals are individually introduced to the maze for eight to fifteen minutes and allowed to freely navigate. There is no food deprivation or taxing physical exertion, and animals are almost always active enough beyond postnatal day (P) 16 to collect sufficient data and calculate alternation rate (Dumas, 2004). Spontaneous alternation in a Y-maze may also be preferable to discrete-trials alternation in a T-maze due to the minimal amount of handling needed for data collection (Conrad et al., 1996; Hughes, 2004). Selective sensitivity of spontaneous alternation to hippocampal lesions (Douglas, 1972) and robust age-dependency late in the postnatal period strongly support use of this test to study hippocampal maturation.
Foundational contributions by Robert Douglas and colleagues firmly established the validity of the spontaneous alternation paradigm for assessing hippocampal development. They first showed that in normal adult rats, alternation rate was about 65–70% of total arm choices, which was significantly greater than chance levels (50%, given the observation that re-entry back into an arm just exited is rare) (Douglas and Isaacson, 1965; Douglas, 1972). In rats with hippocampal lesions, alternation rate dropped to 50%. As well, when visual contextual cues were limited, alternation rate was reduced, indicating that maze navigation is guided primarily by visual experience (Douglas, 1966; Means and Douglas, 1970). Douglas and colleagues then used this task to assess the time course of hippocampal maturation and found that alternation rate increased across the third week of postnatal development and reached adult levels by roughly P25 (Douglas et al., 1973; Douglas, 1975). More recent longitudinal studies have replicated this finding (Dumas, 2004) and experiments with discrete testing ages showed that alternation rate is significantly greater at P22–24 than at P17–19 (Blair et al., 2013; Egger et al., 1973), suggesting that navigation based on the constellation of extramaze cues may occur as early as P20. While there is no experimentally defined spatial goal or explicit learning and memory phases in spontaneous alternation, this task very likely necessitates contextual encoding and use of spatial information for navigation and serves well for defining the age at which these latter cognitive abilities first emerge.
2.3.2. Novelty detection
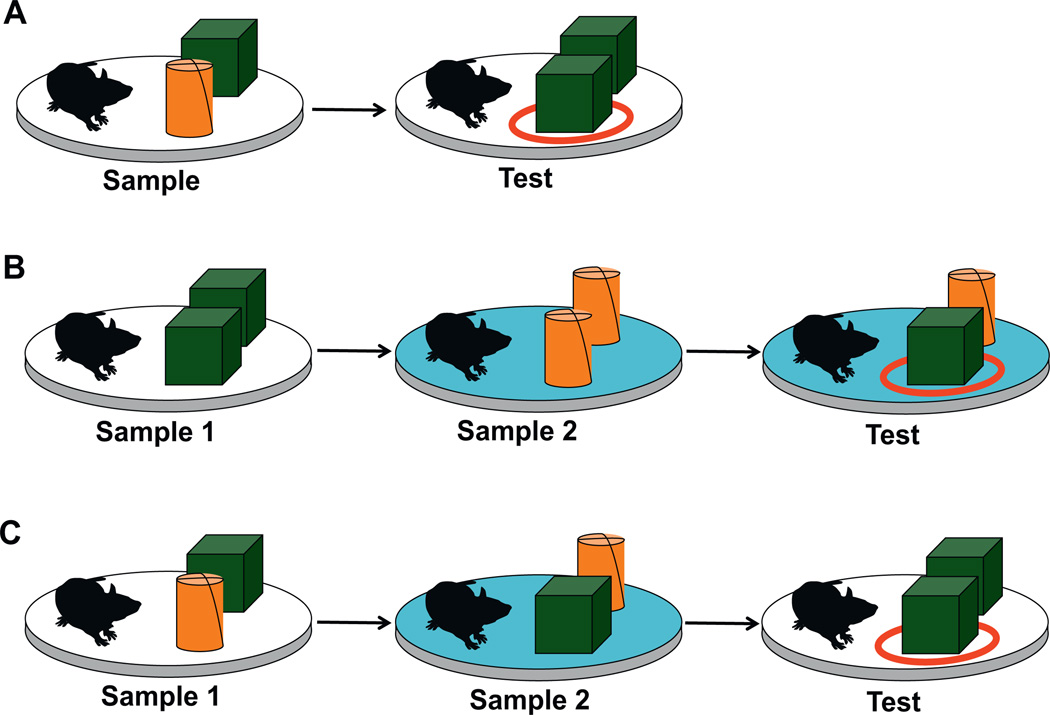
Rodents have an innate desire to explore novel objects and places. Thus, it is no surprise that, without explicit training or reward contingencies, exploration behavior is dictated in large part by novelty (Poucet et al., 1986). Historically, the precise role of the hippocampus in novelty approach and investigation has been debated. Some studies have deemed the hippocampus necessary to varying degrees for novel object recognition (Broadbent et al., 2010; Cohen et al., 2013) and novel place recognition (Barker and Warburton, 2011). However, other studies have shown that hippocampal lesions leave novel object recognition intact, but impair recognition of objects in novel places and novel contexts (Mumby et al., 2002; Piterkin et al., 2008). A comprehensive study by Rosamund Langston and Emma Wood showed that when the hippocampus was completely destroyed bilaterally in adult rats, novel object and novel place recognition abilities remained largely intact (Langston and Wood, 2010). However, object–place–context memory, determined by selective investigation of one of two familiar objects placed in an unexpected location relative to the context in which it was originally presented, was impaired (Fig. 3). Recently, Langston and colleagues applied this approach to developing rats (Langston et al., unpublished results), and found that novel object and novel place recognition mature earlier than novel object–place recognition, which matures earlier than novel object–place–context recognition. The former abilities emerge shortly after the end of the third postnatal week, but the latter does not become apparent until the animals are well over one month of age. Combined, the adult lesion and developmental trajectory studies suggest that complete integration of the hippocampus into brain networks necessary for identification of specific events occurring at distinct places within specific contexts (i.e. episodic memory) emerges later in development than the ability to navigate according to spatial context. Alternatively, differences in age of onset of spatial navigation and novel object–place–context recognition may exist due to delayed maturation and greater involvement of the prefrontal or perirhinal cortex in novel object–place–context recognition (Barker and Warburton, 2011; Browning et al., 2005). In general, temporal separation in the developmental emergence of various hippocampal-dependent cognitive abilities (context encoding, navigating according to context, complex novelty recognition) creates an attractive model for delineation of the individual neural properties that subserve each individual cognitive function.
Fig. 3.
Object–place (A), object–context (B), and object–place–context (C) recognition tasks as described by Langston and Wood (2010). The cylinder and cube represent two different objects. (A) In the sample phase of the object–place task, two different objects (green cube and orange cylinder) are presented to the rat. In the test phase, two objects of the same shape are presented in the same context, such that one object (the circled cube) is in a novel place. (B) In the sample 1 phase of the object–context task, two objects of the same shape are presented in spatial context 1. In the sample 2 phase, two copies of a different object are presented in spatial context 2 (denoted by the blue platform). The test condition maintains context 2 and swaps one object from context 2 with one object from context 1, such that one object (the circled cube) is in a novel object–context configuration. (C) In the sample 1 phase of the object–place–context recognition task, two different objects are presented in context 1. In the sample 2 phase, the context is changed and the object locations are swapped. The test condition returns to the original context and presents a copy of one object from the original context with one object from the second context in the place of the other object, such that one object (the circled cube) is in a novel object–place–context configuration. Typically, there is a two-minute latency between phases of each recognition experiment. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
3. First reports of the neural underpinnings of hippocampal maturation
Arguably, no single individual has contributed more to our understanding of the developmental profiles for learning and memory abilities in rodents than Jerry Rudy. Rudy used cleverly designed behavioral experiments to tease apart the individual trajectories for specific types of learning and memory (reviewed in Dumas and Rudy, 2010), including the design of two or more maze tasks differing in only one procedural aspect to isolate the cognitive mechanism underlying the performance deficit (Moye and Rudy, 1987; Rudy et al., 1987). For instance, to delineate a timeline for various types of conditioned learning, Moye and Rudy performed three behavioral tasks in animals either fifteen or seventeen days of age (1985). They observed a failure to fear condition to a visual cue at P15 but not P17. Upon swapping the visual cue for an auditory cue, fear condition was apparent at P15. Additional tests confirmed detection of the visual stimulus. Combined, the results suggest that the inability to fear condition to a visual cue at P15 was not a function of a basic visual deficit or a basic fear conditioning deficit, but instead was due to a selective impairment of the visual system to associate the light with the shock. However, until fairly recently, direct investigation of the neural substrates of cognitive maturation in developing rodents was limited to lesion and behavior studies. On the rise are interdisciplinary studies that unify behavioral actions and neural processes, tremendously enhancing our understanding of hippocampal development.
3.1. Early eyelid parting
One decade ago, studies on the impact of early eyelid parting in rodents first demonstrated parallels between synaptic modifications in the hippocampus and behavioral adjustments in spatial mazes (Dumas, 2004). In this study, eyelids were parted at P8, nearly a week in advance of normal eyelid parting (P14–15) and spontaneous alternation in a Y-maze and excitatory synaptic transmission in hippocampal slices were investigated. Akin to the work of Douglas, in control animals, spontaneous alternation rate increased steadily from P16 to roughly P28. In animals that underwent early eyelid parting, alternation rate peaked near P22, almost one week earlier than observed in controls. These data suggest that altering the developmental trajectory of visual perception indirectly influences and, in this case, hastens the rate of developmental of the hippocampus. This notion was confirmed by the electrophysiological recordings, which showed accelerated development of fiber pathways leading into the dentate gyrus and area CA1 and earlier maturation of excitatory synaptic transmission in the dentate gyrus with early eyelid parting. This work underscored that the developmental trajectory of the hippocampus is modifiable by external input and is regulated by visual experience.
3.2. Place cell electrophysiology
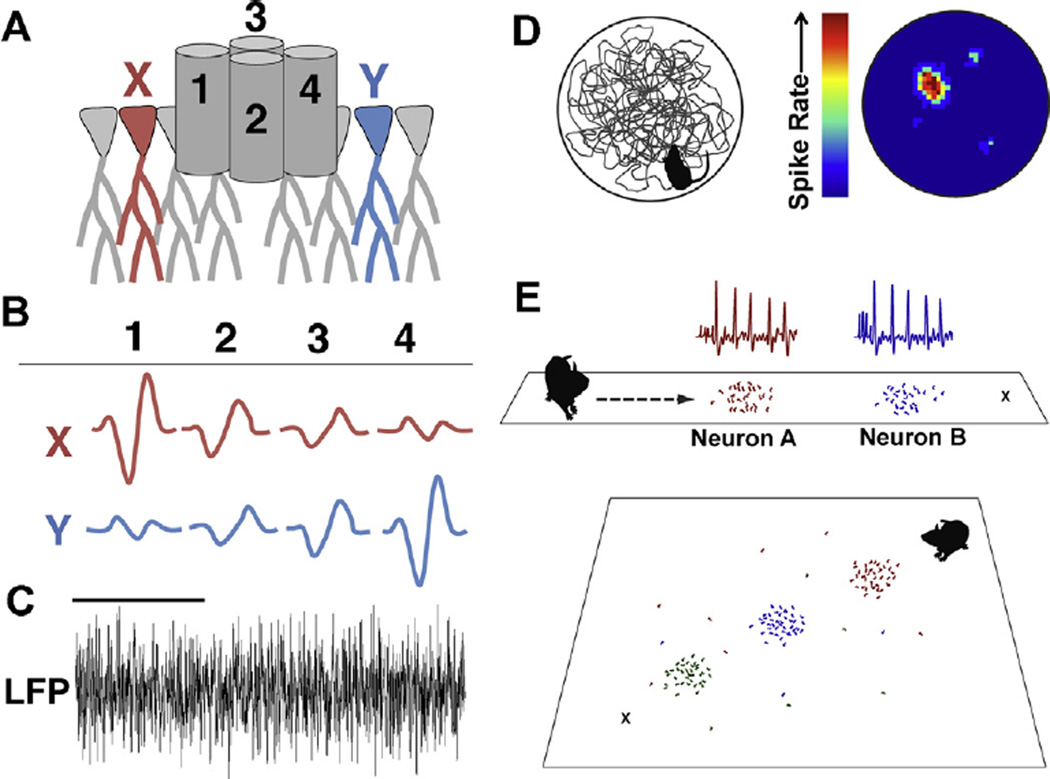
In vivo electrophysiological recording of action potential discharge from the hippocampus in freely behaving rats has been performed since the 1970s. John O’Keefe and colleagues discovered that there were neurons in the hippocampus that discharged action potentials only when the animal was in a particular location in the testing environment (O’Keefe and Dostrovsky, 1971). The cells were termed “place cells” and the region of the maze in which they discharged was called the “place field.” Different place cells have different place fields such that, when combined, populations of place cells encode complete environments or produce a cognitive map (O’Keefe and Nadel, 1979). Place cells have since been shown to do more than map environments, and are also engaged in memory for locations visited in the recent past (Foster and Knierim, 2012; Ji and Wilson, 2008) and planning paths to future intended destinations (prospective encoding) (Ainge et al., 2007, 2012; Ferbinteanu and Shapiro, 2003; Johnson and Redish, 2007; Pfeiffer and Foster, 2013) (Fig. 4).
Fig. 4.
Single units (individual action potential discharge events) and LFPs reflecting population-level events can be measured in real time during arena exploration. (A)Typically, tetrodes are implanted so that the tips of the electrodes reside in the cell body layer of hippocampal area CA1. (B) Signals are high-pass filtered to observe the pattern of responses that individual action potentials produce on each electrode, permitting isolation of multiple distinct units (X and Y) by each tetrode. (C) One or more electrodes can be used to record population LFPs. Power scores can be calculated to determine the amount that any frequency or range of frequencies is represented in the population trace. Oscillatory rhythms in the range of 6–12 (theta) and 45–120 Hz (gamma) are often analyzed with respect to behavior of the animal. The example LFP is filtered at 3–140 Hz. Scale bar is one second. (D) The exploration path of an animal in a circular arena (left) shown with the heat map of discharge frequency for a single unit (right) reveals the place field of a single neuron. Firing rate increases when the animal occupies the place field and different units have different, but sometimes overlapping place fields. (E) During prospective encoding, neurons with established place fields (indicated by the colored dots on the linear maze and waveforms at top) fire transiently in advance of the animal’s movement and sweep from one place field (Neuron A) to the next (Neuron B) toward the animal’s destination (X) (Johnson and Redish, 2007). In a two-dimensional maze, neurons with established place fields (indicated by the colored dots) show prospective encoding by firing sequentially (red, blue, then green) toward the animal’s destination (X). Neurons sweep toward a home location (X) independent of the animal’s previous outbound path (Pfeiffer and Foster, 2013). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
In 2010, the first two reports of in vivo electrophysiological recording in awake and behaving juvenile rats younger than three weeks of age were published and contained highly similar findings with regard to properties of individual units and oscillatory activity in local field potentials (LFPs) (Langston et al., 2010; Wills et al., 2010). That is, stable place cells were observed well before the end of the third postnatal week, as early as P16, in conjunction with adult-like properties of “head-direction cells” in the pre- and parasubiculum and “grid cells” in the medial entorhinal cortex. Approximately half of all hippocampal units monitored at the youngest age passed the criteria for place cell classification (compared to roughly 80% in adults), were theta modulated, and even displayed theta phase precession and place field expansion with continued experience in the same environment. These findings support the presence of a relatively stable geometric reference system and the ability to identify, process, and discern different contexts at this early age. Comparing these physiological data with behavioral profiles, it appears that many spatial properties of large numbers of individual neurons are somewhat mature well in advance of the first behavioral indicators of spatial navigation. However, age-related increases in the number of place cells, spatial coherence and inter-trial stability were noted beyond the end of the third postnatal week, along with increases in theta power measured from LFPs and entorhinal grid stability. Many of these developmental changes were corroborated in a later study highlighting an increase in the proportion of adult-like place cells, average spatial signal, and place field stability from P23 to P35 (Scott et al., 2011). No studies have yet correlated developmental alterations in spatial behavior with place or grid cell metrics or the concomitant increase in theta power (Hasselmo, 2005; Vanderwolf, 1969). Also, more complex place cell properties, like prospective encoding, have not been investigated in developing animals, but are prime candidates as factors that regulate the emergence of hippocampal-dependent behaviors. Specifically, the prospective “sweeping” behavior of extra-field firing (Johnson and Redish, 2007; Pfeiffer and Foster, 2013) may be a necessary developmental pre-requisite for navigating according to spatial context.
3.3. Glutamatergic receptors
Most recently, spatial navigation was explored in developing rodents in combination with pharmacological and genetic manipulations of key neurotransmitter receptors within the hippocampus, namely glutamatergic AMPA and NMDA receptors. As described below, these innovative and highly informative approaches are creating a holistic model of hippocampal maturation.
3.3.1. AMPA receptors
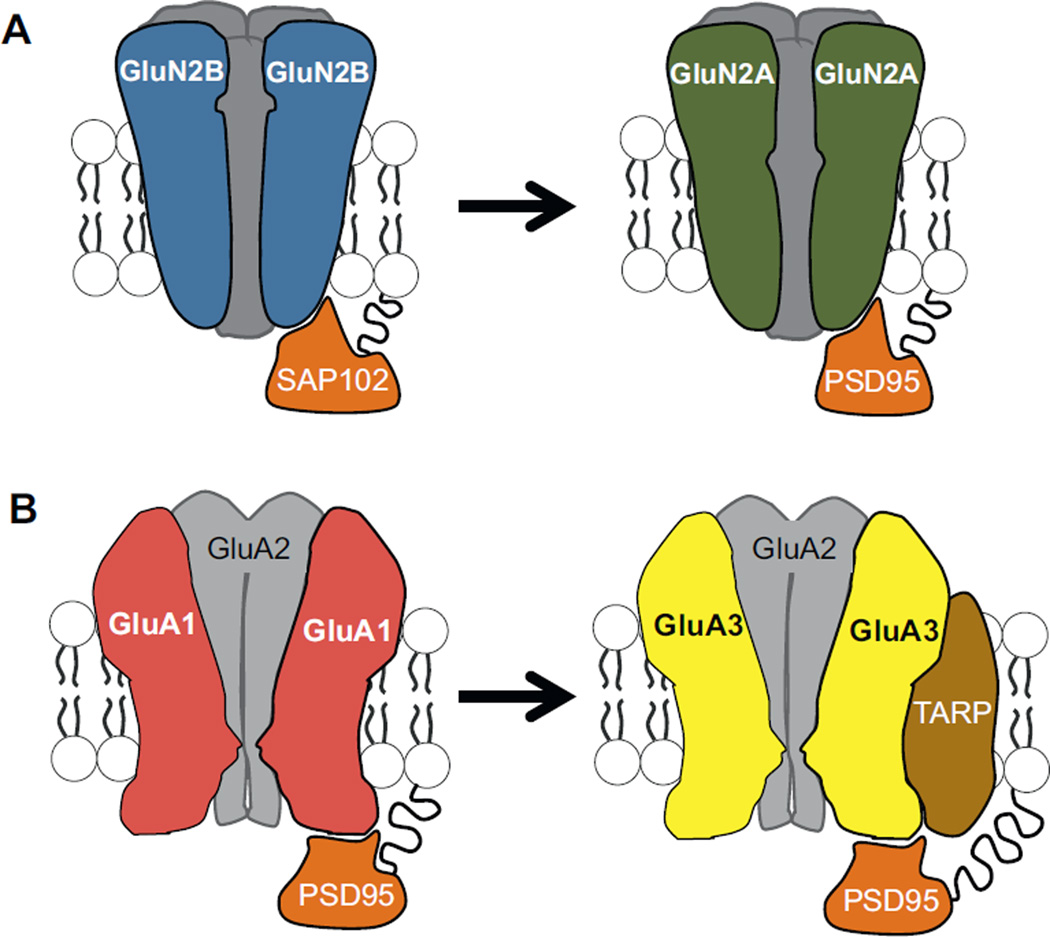
AMPA receptors create the fast postsynaptic depolarization that results from the presynaptic release of the neurotransmitter, glutamate. Relationships between spatial navigation and functional dynamics of AMPA receptors were explored (Blair et al., 2013) through treatment with a positive allosteric modulator that prolongs AMPA receptor-induced postsynaptic depolarization (Arai and Kessler, 2007). Increasing the duration of AMPA receptor depolarization in rats less than three weeks of age increased spontaneous alternation rate. Electrophysiological work showed that prolonging AMPA receptor responses also produced stronger coupling between excitatory synaptic depolarization and postsynaptic discharge and reduced the activity threshold necessary for induction of long-lasting synaptic plasticity. Proteomic analysis of AMPA receptors revealed a change in the composition of subunits from P17–24 that would explain both the developmental increase in excitatory synaptic response duration under control conditions and the decrease in drug efficacy with increasing age. More specifically, higher expression levels for GluA1 shifted to higher expression levels for GluA3 and the transmembrane AMPA receptor regulatory protein (TARP) (Fig. 5). Effects of increasing age on behavior were not mimicked when blocking inhibitory synaptic transmission, nor did allosteric modulation of AMPA receptors alter behavior in a task known to not rely on the hippocampus, specifying the drug-induced enhancement of alternation rate to excitatory synapses in the hippocampus. Combined, the findings promote the notion that subtle molecular alterations to the AMPA receptor protein complex late in postnatal development drive changes in basic functional properties of excitatory transmission that are sufficient to alter hippocampal network function and unmask spatial navigation ability. Molecular modification of the AMPA receptor protein complex might represent a synaptic alteration that provides a more stable network to support long-term memory storage with sufficient plasticity to encode new information (Dumas, 2005a).
Fig. 5.
Developmental modifications to AMPA receptors and NMDA receptors at excitatory synapses in the hippocampus. (A) During the third postnatal week, NMD A receptors with GluN2B subunits (blue) are replaced by NMDA receptors with GluN2A subunits (green). (B) From P17 to P24, expression of the AMPA receptor subunit, GluA1 (red), decreases while expression of GluA3 (yellow) and transmembrane AMPA receptor regulatory protein TARP (brown) increase. Synapse associated protein (SAP102) and postsynaptic density protein (PSD95) are anchoring proteins (orange) for AMPA and NMDA receptors at glutamatergic synapses (Elias et al., 2008). TARP regulates synaptic anchoring and AMPA receptor channel dynamics (Jackson and Nicoll, 2011). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
3.3.2. NMDA receptors
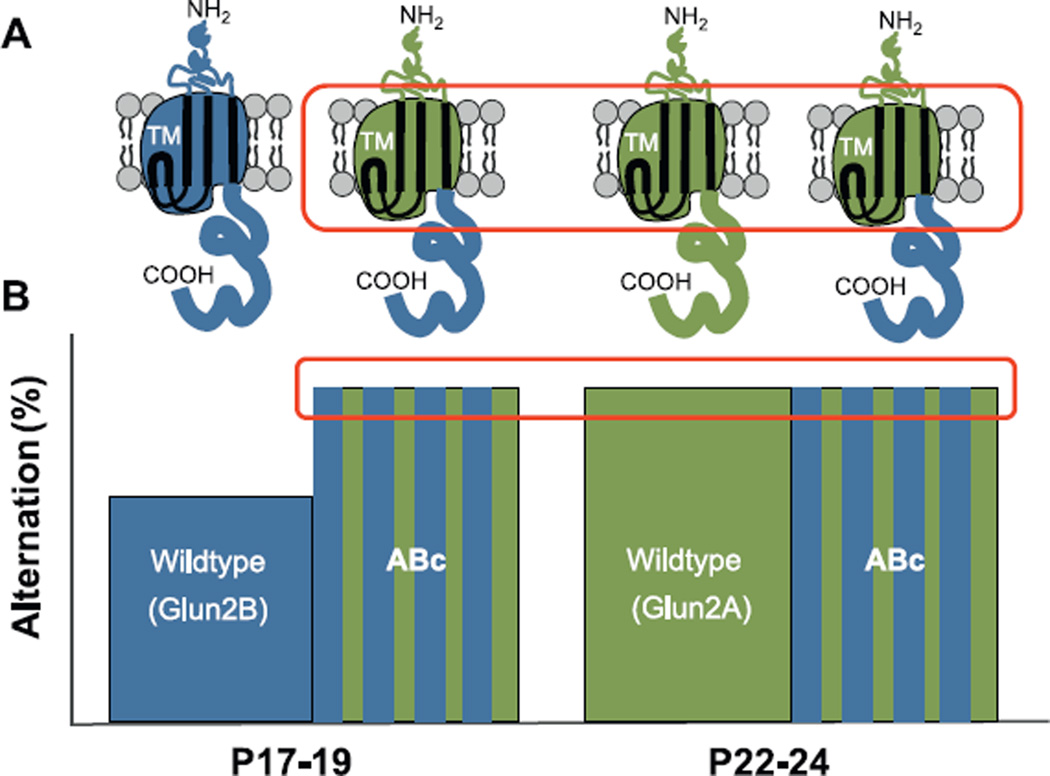
Glutamatergic synapses contain NMDA receptors in combination with AMPA receptors. Activation of NMDA receptors has been shown to be critical for activity-dependent synaptic plasticity in the hippocampus (Collingridge and Singer, 1990; Collingridge, 2003; Morris, 1989; Morris et al., 1990), place cell stability (Kentros et al., 2004), and spatial learning and memory (Butcher et al., 1990; Collingridge, 1987; Morris, 1989; Morris et al., 2013). Modification to NMDA receptor composition during late postnatal development is suspected to play a role in age-related improvements in spatial navigation and spatial memory (Dumas, 2005b). As hippocampal neurons mature in culture (Barria and Malinow, 2002) and during the third postnatal week in vivo, synaptic NMDA receptors with GluN2B subunits are replaced by NMDA receptors containing GluN2A subunits (Monyer et al., 1994; Williams et al., 1993). This subunit switch alters numerous properties of NMDA receptor function including channel open probability, channel deactivation, and intracellular protein-protein signaling (Sanders et al., 2013). In a recent study, chimeric GluN2 subunits were expressed in transgenic mice to isolate changes in channel dynamics, and hence calcium conductance (amino and transmembrane regions), and intracellular protein-protein signaling (carboxy terminus) at various postnatal ages (Sanders et al., 2013) (Fig. 6). Isolated Modification to calcium conductance domains, but not intracellular protein-protein signaling domains, elicited mature levels of spontaneous alternation at P17–19 (Sanders et al., 2013), implicating calcium conductance dynamics in the final maturation of the hippocampus and the emergence of spatial navigation. Related research in genetically modified mice in which GluN2B is conditionally deleted (Gray et al., 2011; Hall et al., 2007) or replaced with GluN2A (Wang et al., 2011) suggest that the developmental NMDA receptor subunit switch promotes maturation of excitatory synaptic transmission and precedes the changes in AMPA receptor structure and function. Concerted modifications to NMDA and AMPA receptors likely underlie the late postnatal emergence of hippocampal-dependent behaviors.
Fig. 6.
Expression of chimeric GluN2 subunits allows for separation of the roles of NMDA receptor-dependent calcium-conductance and intracellular protein–protein signaling in the maturation of glutamatergic synapses and spatial navigation. (A) A shift from predominantly GluN2B (blue) to GluN2A subunits (green) is associated with mature hippocampal function in wildtype animals. In the ABc chimera, the GluN2A regulatory domains for calcium conductance (amino and transmembrane, TM) are fused to the Glun2B intracellular protein-protein signaling domains (carboxy terminus). (B) Animals expressing ABc (blue and green striped bars) display precocious spatial navigation ability at P17–19 as compared to wildtype littermates (blue bar). This suggests that incorporation of GluN2A-type calcium conductance domains (red box in subunit illustrations), more so than intracellular protein–protein signaling, permits the late postnatal emergence of hippocampus-dependent behavior (red box in bar graph). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
Adapted from Sanders et al. (2013).
4. Summary and conclusions
A blossoming of research into the neural factors that regulate hippocampal maturation is underway. As more investigators become involved, it is important to lay the groundwork for appropriate methods for experimentation and unification of research findings. It is imperative to design behavioral assays that are amenable to performance by smaller, weaker animals and that are acute so as to best define temporal aspects of behavioral modifications and minimize stress. Preliminary findings support the notion that alterations in neural network function both within the hippocampus and between the hippocampus and other late-developing, pertinent brain structures (such as prefrontal cortex) underlie late postnatal changes in spatial navigation and novel object–place–context recognition abilities. Moreover, changes in excitatory synaptic transmission and plasticity, involving structural and functional modifications to both AMPA and NMDA receptors, are primary cellular candidates that enable the network changes permitting adult-like hippocampal processing.
The time is ripe to fully understand how the hippocampus is built and, in doing so, to reveal the individual contributions of different forms of synaptic plasticity in the construction of neural networks, encoding of experiences, retrieval of learned information for goal-directed behaviors, and complex novelty recognition. Information gleaned from such investigation will undeniably lead to better treatments for memory loss due to congenital disorders, neural injury, neurodegeneration, and aging.
Acknowledgments
This project was supported by the Thomas F. and Kate Miller Jeffress Memorial Trust Fund and the Department of Defense (ONR# N00014-10-1-0198).
References
- Aadland J, Beatty WW, Maki RH. Spatial memory of children and adults assessed in the radial maze. Dev. Psychobiol. 1985;18:163–172. doi: 10.1002/dev.420180208. http://dx.doi.org/10.1002/dev.420180208. [DOI] [PubMed] [Google Scholar]
- Ainge JA, Tamosiunaite M, Woergoetter F, Dudchenko PA. Hippocampal CA1 place cells encode intended destination on a maze with multiple choice points. J. Neurosci. 2007;27:9769–9779. doi: 10.1523/JNEUROSCI.2011-07.2007. http://dx.doi.org/10.1523/JNEUROSCI.2011-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainge JA, Tamosiunaite M, Worgotter F, Dudchenko PA. Hippocampal place cells encode intended destination, and not a discriminative stimulus, in a conditional T-maze task. Hippocampus. 2012;22:534–543. doi: 10.1002/hipo.20919. http://dx.doi.org/10.1002/hipo.20919. [DOI] [PubMed] [Google Scholar]
- Arai AC, Kessler M. Pharmacology of ampakine modulators: from AMPA receptors to synapses and behavior. Curr. Drug Targets. 2007;8:583–602. doi: 10.2174/138945007780618490. http://dx.doi.org/10.2174/138945007780618490. [DOI] [PubMed] [Google Scholar]
- Barker GR, Warburton EC. When is the hippocampus involved in recognition memory? J. Neurosci. 2011;31:10721–10731. doi: 10.1523/JNEUROSCI.6413-10.2011. http://dx.doi.org/10.1523/JNEUROSCI.6413-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barria A, Malinow R. Subunit-specific NMDA receptor traffic-king to synapses. Neuron. 2002;35:345–353. doi: 10.1016/s0896-6273(02)00776-6. http://dx.doi.org/10.1016/S0896-6273(02)00776-6. [DOI] [PubMed] [Google Scholar]
- *Blair MG, *Nguyen NN-Q, Albani SH, L’Etoile MM, Andrawis MM, Owen LM, Oliveira RF, Johnson MW, Purvis DL, Sanders EM, Stoneham ET, Dumas TC. Developmental changes in structural and functional properties of hippocampal AMPA receptors parallels the emergence of deliberative spatial navigation in juvenile rats (*authors contributed equally) J. Neurosci. 2013;33:12218–12228. doi: 10.1523/JNEUROSCI.4827-12.2013. http://dx.doi.org/10.1523/JNEUROSCI.4827-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadbent NJ, Gaskin S, Squire LR, Clark RE. Object recognition memory and the rodent hippocampus. Learn. Mem. 2010;17:5–11. doi: 10.1101/lm.1650110. http://dx.doi.org/10.1101/lm.1650110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronstein PM, Spear NE. Acquisition of a spatial discrimination by rats as a function of age. J. Comp. Physiol. Psychol. 1972;78:208–212. doi: 10.1037/h0032188. http://dx.doi.org/10.1037/h0032188. [DOI] [PubMed] [Google Scholar]
- Browning PG, Easton A, Buckley MJ, Gaffan D. The role of prefrontal cortex in object-in-place learning in monkeys. Eur. J. Neurosci. 2005;22:3281–3291. doi: 10.1111/j.1460-9568.2005.04477.x. [DOI] [PubMed] [Google Scholar]
- Bulut FG, Altman J. Spatial and tactile discrimination learning in infant rats motivated by homing. Dev. Psychobiol. 1974;7:465–473. doi: 10.1002/dev.420070510. http://dx.doi.org/10.1002/dev.420070510. [DOI] [PubMed] [Google Scholar]
- Butcher SP, Davis S, Morris RG. A dose-related impairment of spatial learning by the NMDA receptor antagonist, 2-amino-5-phosphonovalerate (AP5) Eur. Neuropsychopharmacol. 1990;1:15–20. doi: 10.1016/0924-977x(90)90005-u. http://dx.doi.org/10.1016/0924-977X(90)90005-U. [DOI] [PubMed] [Google Scholar]
- Carman HM, Mactutus CF. Proximal versus distal cue utilization in spatial navigation: the role of visual acuity? Neurobiol. Learn. Mem. 2002;78:332–346. doi: 10.1006/nlme.2002.4062. http://dx.doi.org/10.1006/nlme.2002.4062. [DOI] [PubMed] [Google Scholar]
- Carman HM, Booze RM, Snow DM, Mactutus CF. Proximal versus distal cue utilization in preweanling spatial localization: the influence of cue number and location. Physiol. Behav. 2003;79:157–165. doi: 10.1016/s0031-9384(03)00089-1. http://dx.doi.org/10.1016/S0031-9384(03)00089-1. [DOI] [PubMed] [Google Scholar]
- Cohen SJ, Munchow AH, Rios LM, Zhang G, Asgeirsdóttir HN, Stackman RW., Jr The rodent hippocampus is essential for nonspatial object memory. Curr. Biol. 2013;23:1685–1690. doi: 10.1016/j.cub.2013.07.002. http://dx.doi.org/10.1016/j.cub.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge GL. Synaptic plasticity. The role of NMDA receptors in learning and memory. Nature. 1987;330:604–605. doi: 10.1038/330604a0. http://dx.doi.org/10.1038/330604a0. [DOI] [PubMed] [Google Scholar]
- Collingridge GL, Singer W. Excitatory amino acid receptors and synaptic plasticity. Trends Pharmacol. Sci. 1990;11:290–296. doi: 10.1016/0165-6147(90)90011-v. http://dx.doi.org/10.1016/0165-6147(90)90011-V. [DOI] [PubMed] [Google Scholar]
- Collingridge GL. The induction of N-methyl-d-aspartate receptor-dependent long-term potentiation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2003;358:635–641. doi: 10.1098/rstb.2002.1241. http://dx.doi.org/10.1098/rstb.2002.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad CD, Galea LA, Kuroda Y, McEwen BS. Chronic stress impairs rat spatial memory on the Y maze, and this effect is blocked by tianeptine pretreatment. Behav. Neurosci. 1996;100:1321–1334. doi: 10.1037//0735-7044.110.6.1321. http://dx.doi.org/10.1037/0735-7044.110.6.1321. [DOI] [PubMed] [Google Scholar]
- Dember WN, Fowler H. Spontaneous alternation behavior. Psychol. Bull. 1958;55:412–428. doi: 10.1037/h0045446. http://dx.doi.org/10.1037/h0045446. [DOI] [PubMed] [Google Scholar]
- Douglas RJ, Isaacson RL. Homogeneity of single trial response tendencies and spontaneous alternation in the T-maze. Psychol. Rep. 1965;16:87–92. doi: 10.2466/pr0.1965.16.1.87. [DOI] [PubMed] [Google Scholar]
- Douglas RJ. Cues for spontaneous alternation. J. Comp. Physiol. Psychol. 1966;62:171–183. doi: 10.1037/h0023668. [DOI] [PubMed] [Google Scholar]
- Douglas RJ. Pavlovian conditioning and the brain. In: Boakes RA, Halliday MS, editors. Inhibition and Learning. New York: Academic press; 1972. pp. 529–553. [Google Scholar]
- Douglas RJ, Peterson JJ, Douglas DP. The ontogeny of a hippocampus-dependent response in two rodent species. Behav. Biol. 1973;8:27–37. doi: 10.1016/s0091-6773(73)80003-3. http://dx.doi.org/10.1016/S0091-6773(73)80003-3. [DOI] [PubMed] [Google Scholar]
- Douglas RJ. The development of hippocampal function: implications for theory and therapy. In: Isaacson RL, Pribram KH, editors. The Hippocampus: Neurophysiology and Behavior. Vol. 2. New York: Plenum; 1975. pp. 327–361. [Google Scholar]
- Dumas TC. Early eyelid opening enhances spontaneous alternation and accelerates the development of perforant path synaptic strength in the hippocampus of juvenile rats. Dev. Psychobiol. 2004;45:1–9. doi: 10.1002/dev.20011. http://dx.doi.org/10.1002/dev.20011. [DOI] [PubMed] [Google Scholar]
- Dumas TC. Late postnatal maturation of excitatory synaptic transmission permits the expression of adult-like hippocampal-dependent behaviors. Hip-pocampus. 2005a;15:562–578. doi: 10.1002/hipo.20077. http://dx.doi.org/10.1002/hipo.20077. [DOI] [PubMed] [Google Scholar]
- Dumas TC. Developmental regulation of cognitive abilities: modified composition of a molecular switch turns on associative learning. Prog. Neurobiol. 2005b;76:189–211. doi: 10.1016/j.pneurobio.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Dumas TC, Rudy JW. Development of the hippocampal memory system: Creating networks and modifiable synapses. In: Blumberg MS, Freeman JH, Robinson SR, editors. Oxford Handbook of Developmental Behavioral Neuroscience. Oxford Library of Neuroscience. New York: Oxford University Press; 2010. pp. 58–606. [Google Scholar]
- Egger GJ, Livesey PJ, Dawson RG. Ontogenic aspects of central cholinergic involvement in spontaneous alternation behavior. Dev. Psychobiol. 1973;6:289–299. doi: 10.1002/dev.420060402. http://dx.doi.org/10.1002/dev.420060402. [DOI] [PubMed] [Google Scholar]
- Elias GM, Elias LA, Apostolides PF, Kriegstein AR, Nicoll RA. Differential trafficking of AMPA and NMDA receptors by SAP102 and PSD-95 underlies synapse development. Proc. Natl. Acad. Sci. U. S. A. 2008;105:20953–20958. doi: 10.1073/pnas.0811025106. http://dx.doi.org/10.1073/pnas.0811025106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagiolini M, Pizzorusso T, Berardi N, Domenici L, Maffei L. Functional postnatal development of the rat primary visual cortex and the role of visual experience: dark rearing and monocular deprivation. Vision Res. 1994;34:709–720. doi: 10.1016/0042-6989(94)90210-0. http://dx.doi.org/10.1016/0042-6989(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Ferbinteanu J, Shapiro ML. Prospective and retrospective memory coding in the hippocampus. Neuron. 2003;40:1227–1239. doi: 10.1016/s0896-6273(03)00752-9. http://dx.doi.org/10.1016/S0896-6273(03)00752-9. [DOI] [PubMed] [Google Scholar]
- Foreman N, Altaha M. The development of exploration and spontaneous alternation in hooded rat pups: effects of unusually early eyelid opening. Dev. Psychobiol. 1992;24:521–537. doi: 10.1002/dev.420240706. http://dx.doi.org/10.1002/dev.420240706. [DOI] [PubMed] [Google Scholar]
- Foster DJ, Knierim JJ. Sequence learning and the role of the hippocampus in rodent navigation. Curr. Opin. Neurobiol. 2012;22:294–300. doi: 10.1016/j.conb.2011.12.005. http://dx.doi.org/10.1016/j.conb.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göb R, Köllner U, Köllner O, Klingberg F. Early postnatal development of open field behaviour is changed by single doses of fenfluramine or p-chloroamphetamine. Biomed. Biochim. Acta. 1987;46:189–198. [PubMed] [Google Scholar]
- Gray JA, Shi Y, Usui H, During MJ, Sakimura K, Nicoli RA. Distinct modes of AMPA receptor suppression at developing synapses by GluN2A and GluN2B: single-cell NMDA receptor subunit deletion in vivo. Neuron. 2011;71:1085–1101. doi: 10.1016/j.neuron.2011.08.007. http://dx.doi.org/10.1016/j.neuron.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall BJ, Ripley B, Ghosh A. NR2B signaling regulates the development of synaptic AMPA receptor current. J. Neurosci. 2007;27:13446–13456. doi: 10.1523/JNEUROSCI.3793-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME. What is the function of hippocampal theta rhythm? Linking behavioral data to phasic properties of field potential and unit recording data. Hippocampus. 2005;15:936–949. doi: 10.1002/hipo.20116. http://dx.doi.org/10.1002/hipo.20116. [DOI] [PubMed] [Google Scholar]
- Hughes RN. The value of spontaneous alternation behavior (SAB) as a test of retention in pharmacological investigations of memory. Neurosci. Biobehav. Rev. 2004;28:497–505. doi: 10.1016/j.neubiorev.2004.06.006. http://dx.doi.org/10.1016/j.neubiorev.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Huttenlocher J. Coding location: the view from toddler studies. Am. Psychol. 2008;63:641–648. doi: 10.1037/0003-066X.63.8.641. http://dx.doi.org/10.1037/0003-066X.63.8.641. [DOI] [PubMed] [Google Scholar]
- Iivonen H, Nurminen L, Harri M, Tanila H, Puolivali J. Hypothermia in mice tested in Morris Water Maze. Behav. Brain Res. 2003;141:207–213. doi: 10.1016/s0166-4328(02)00369-8. http://dx.doi.org/10.1016/S0166-4328(02)00369-8. [DOI] [PubMed] [Google Scholar]
- Jackson AC, Nicoll RA. Stargazing from a new vantage – TARP modulation of AMPA receptor pharmacology. J. Physiol. 2011;589:5909–5910. doi: 10.1113/jphysiol.2011.223495. http://dx.doi.org/10.1113/jphysiol.2011.223495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji D, Wilson MA. Firing rate dynamics in the hippocampus induced by trajectory learning. J. Neurosci. 2008;28:4679–4689. doi: 10.1523/JNEUROSCI.4597-07.2008. http://dx.doi.org/10.1523/JNEUROSCI.4597-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A, Redish AD. Neural ensembles in CA3 transiently encode paths forward of the animal at a decision point. J. Neurosci. 2007;27:12176–12189. doi: 10.1523/JNEUROSCI.3761-07.2007. http://dx.doi.org/10.1523/JNEUROSCI.3761-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny PA, Turkewitz G. Effects of unusually early visual stimulation on the development of homing behavior in the rat pup. Dev. Psychobiol. 1986;19:57–66. doi: 10.1002/dev.420190107. http://dx.doi.org/10.1002/dev.420190107. [DOI] [PubMed] [Google Scholar]
- Kentros CG, Agnihotri NT, Streater S, Hawkins RD, Kandel ER. Increased attention to spatial context increases both place field stability and spatial memory. Neuron. 2004;42:283–295. doi: 10.1016/s0896-6273(04)00192-8. http://dx.doi.org/10.1016/S0896-6273(04)00192-8. [DOI] [PubMed] [Google Scholar]
- Kirby RJ. A maturation factor in spontaneous alternation. Nature. 1967;215:784. doi: 10.1038/215784a0. http://dx.doi.org/10.1038/215784a0. [DOI] [PubMed] [Google Scholar]
- Kraemer PJ, Randall CK. Spatial learning in preweanling rats trained in a Morris water maze. Psychobiology. 1995;23:144–152. http://dx.doi.org/10.3758/BF03327070. [Google Scholar]
- Lalonde R. The neurobiological basis of spontaneous alternation. Neurosci. Biobehav. Rev. 2002;26:91–104. doi: 10.1016/s0149-7634(01)00041-0. http://dx.doi.org/10.1016/S0149-7634(01)00041-0. [DOI] [PubMed] [Google Scholar]
- Langston RF, Ainge JA, Couey JJ, Canto CB, Bjerknes TL, Witter MP, Moser EI, Moser MB. Development of the spatial representation system in the rat. Science. 2010;328:1576–1580. doi: 10.1126/science.1188210. http://dx.doi.org/10.1126/science.1188210. [DOI] [PubMed] [Google Scholar]
- Langston RF, Wood ER. Associative recognition and the hippocampus: differential effects of hippocampal lesions on object–place, object–context and object–place–context memory. Hippocampus. 2010;20:1139–1153. doi: 10.1002/hipo.20714. http://dx.doi.org/10.1002/hipo.20714. [DOI] [PubMed] [Google Scholar]
- Liao DS, Krahe TE, Prusky GT, Medina AE, Ramoa AS. Recovery of cortical binocularity and orientation selectivity after the critical period for ocular dominance plasticity. J. Neurophysiol. 2004;92:2113–2121. doi: 10.1152/jn.00266.2004. http://dx.doi.org/10.1152/jn.00266.2004. [DOI] [PubMed] [Google Scholar]
- Means LW, Douglas RJ. Effects of hippocampal lesions on cue utilization in spatial discrimination in rats. J. Comp. Physiol. Psychol. 1970;73:254–260. doi: 10.1037/h0030205. http://dx.doi.org/10.1037/h0030205. [DOI] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. http://dx.doi.org/10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. http://dx.doi.org/10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Morris RG. Synaptic plasticity and learning: selective impairment of learning rats and blockade of long-term potentiation in vivo by the N-methyl-d-aspartate receptor antagonist AP5. J. Neurosci. 1989;9:3040–3057. doi: 10.1523/JNEUROSCI.09-09-03040.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RGM, Davis S, Butcher SP. Hippocampal synaptic plasticity and NMDA receptors: a role in information storage? Philos. Trans. R. Soc. Lond. B Biol. Sci. 1990;329:187–204. doi: 10.1098/rstb.1990.0164. http://dx.doi.org/10.1098/rstb.1990.0164. [DOI] [PubMed] [Google Scholar]
- Morris RGM, Steele RJ, Bell JE, Martin SJ. N-methyl-d-aspartate receptors, learning and memory: chronic intraventricular infusion of the NMDA receptor antagonist d-AP5 interacts directly with the neural mechanisms of spatial learning. Eur. J. Neurosci. 2013;37:700–717. doi: 10.1111/ejn.12086. http://dx.doi.org/10.1111/ejn.12086. [DOI] [PubMed] [Google Scholar]
- Moye TB, Rudy JW. Ontogenesis of trace conditioning in young rats: dissociation of associative and memory processes. Dev. Psychobiol. 1987;20:405–414. doi: 10.1002/dev.420200405. http://dx.doi.org/10.1002/dev.420200405. [DOI] [PubMed] [Google Scholar]
- Mumby DG, Gaskin S, Glenn MJ, Schramek TE, Lehmann H. Hippocampal damage and exploratory preferences in rats: memory for objects, places, and contexts. Learn. Mem. 2002;9:49–57. doi: 10.1101/lm.41302. http://dx.doi.org/10.1101/lm.41302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg L, McIntosh AR, Cabeza R, Habib R, Houle S, Tulving E. General and specific brain regions involved in encoding and retrieval of events: what, where, and when. Proc. Natl. Acad. Sci. U. S. A. 1996;93:11280–11285. doi: 10.1073/pnas.93.20.11280. http://dx.doi.org/10.1073/pnas.93.20.11280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Keefe J, Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971;3:171–175. doi: 10.1016/0006-8993(71)90358-1. http://dx.doi.org/10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Nadel L. Précis of O’Keefe and Nadel’s The hippocampus as a cognitive map. Behav. Brain Sci. 1979;2:487–494. http://dx.doi.org/10.1017/S0140525X00063949. [Google Scholar]
- Pfeiffer BE, Foster DJ. Hippocampal place-cell sequences depict future paths to remembered goals. Nature. 2013;497:74–79. doi: 10.1038/nature12112. http://dx.doi.org/10.1038/nature12112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piterkin P, Cole E, Cossette M, Gaskin S, Mumby DG. A limited role for the hippocampus in the modulation of novel-object preference by contextual cues. Learn. Mem. 2008;15:785–791. doi: 10.1101/lm.1035508. http://dx.doi.org/10.1101/lm.1035508. [DOI] [PubMed] [Google Scholar]
- Poucet B, Chapuis N, Durup M, Thinus-Blanc C. A study of exploratory behaviour as an index of spatial knowledge in hamsters. Anim. Learn. Behav. 1986;14:93–100. http://dx.doi.org/10.3758/BF03200043. [Google Scholar]
- Prusky GT, Douglas RM. Developmental plasticity of mouse visual acuity. Eur. J. Neurosci. 2003;17:167–173. doi: 10.1046/j.1460-9568.2003.02420.x. http://dx.doi.org/10.1046/j.1460-9568.2003.02420.x. [DOI] [PubMed] [Google Scholar]
- Richman CL, Dember WN, Kim P. Spontaneous alternation behavior in animals: a review. Curr. Psychol. 1986;5:358–391. http://dx.doi.org/10.1007/BF02686603. [Google Scholar]
- Rudy JW, Stadler-Morris S, Albert P. Ontogeny of spatial navigation behaviors in the rat: Dissociation of “proximal” and “distal”-cue-based behaviors. Behav. Neurosci. 1987;101:62–73. doi: 10.1037//0735-7044.101.1.62. http://dx.doi.org/10.1037//0735-7044.101.1.62. [DOI] [PubMed] [Google Scholar]
- Rudy JW. Development of learning: from elemental to configural associative networks. In: Rovee-Collier C, Lipsitt LP, Hayne H, editors. Advances in Infancy Research. Vol. 7. New York: Ablex Pub. Corp.; 1992. pp. 247–289. [Google Scholar]
- Sanders EM, Nguyen MA, Zhou KC, Hanks ME, Yusef KA, Cox DN, Dumas TC. Developmental modification of synaptic NMDAR composition and maturation of glutamatergic synapses: Matching postsynaptic slots with receptor pegs. Biol. Bull. 2013;224:1–13. doi: 10.1086/BBLv224n1p1. [DOI] [PubMed] [Google Scholar]
- Schachtele SJ, Losh J, Dailey ME, Green SH. Spine formation and maturation in the developing rat auditory cortex. J. Comp. Neurol. 2011;519:3327–3345. doi: 10.1002/cne.22728. http://dx.doi.org/10.1002/cne.22728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott RC, Richard GR, Holmes GL, Lenck-Santini P. Maturational dynamics of hippocampal place cells in immature rats. Hippocampus. 2011;21:347–353. doi: 10.1002/hipo.20789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GJ, Bogomolny A. Appetitive instrumental training in prewean-ling rats: I. Motivational determinants. Dev. Psychobiol. 1983;16:119–128. doi: 10.1002/dev.420160205. http://dx.doi.org/10.1002/dev.420160205. [DOI] [PubMed] [Google Scholar]
- Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol. Rev. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. http://dx.doi.org/10.1037/0033-295X.99.2.195. [DOI] [PubMed] [Google Scholar]
- Squire LR, Zola SM. Episodic memory, semantic memory, and amnesia. Hippocampus. 1998;8:205–211. doi: 10.1002/(SICI)1098-1063(1998)8:3<205::AID-HIPO3>3.0.CO;2-I. http://dx.doi.org/10.1002/(SICI)1098-1063(1998)8:3<205::AID-HIPO3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Stanton ME. Multiple memory systems, development and conditioning. Behav. Brain Res. 2000;110:25–37. doi: 10.1016/s0166-4328(99)00182-5. http://dx.doi.org/10.1016/S0166-4328(99)00182-5. [DOI] [PubMed] [Google Scholar]
- Vanderwolf CH. Hippocampal electrical activity and voluntary movement in the rat. Electroencephalogr. Clin. Neurophysiol. 1969;26:407–418. doi: 10.1016/0013-4694(69)90092-3. http://dx.doi.org/10.1016/0013-4694(69)90092-3. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 2006;1:848–858. doi: 10.1038/nprot.2006.116. http://dx.doi.org/10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Held RG, Chang S, Yang L, Delpire E, Ghosh A, Hall BJ. A critical role for GluN2B-containing NMDA receptors in cortical development and function. Neuron. 2011;72:789–805. doi: 10.1016/j.neuron.2011.09.023. http://dx.doi.org/10.1016/j.neuron.2011.09.023. [DOI] [PubMed] [Google Scholar]
- Williams K, Russell SL, Shen YM, Molinoff PB. Developmental switch in the expression of NMDA receptors occurs in vivo and in vitro. Neuron. 1993;10:267–278. doi: 10.1016/0896-6273(93)90317-k. [DOI] [PubMed] [Google Scholar]
- Wills TJ, Cacucci F, Burgess N, O’Keefe J. Development of the hippocampal cognitive map in preweanling rats. Science. 2010;328:1487–1488. doi: 10.1126/science.1188224. http://dx.doi.org/10.1126/science.1188224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills TJ, Muessig L, Cacucci F. The development of spatial behaviour and the hippocampal neural representation of space. Phil. Trans. R. Soc. B. 2013;369 doi: 10.1098/rstb.2013.0409. http://dx.doi.org/10.1098/rstb.2013.0409. [DOI] [PMC free article] [PubMed] [Google Scholar]