Abstract
Background
Progesterone-derived neuroactive steroids have shown promise clinically (e.g., anti-seizure medications) but, as with other GABAA receptor modulators (e.g., benzodiazepines), may have the potential for abuse.
Methods
We evaluated the reinforcing effects of progesterone, a steroid precursor of endogenous neuroactive steroids, with and without pretreatments with the neuroactive steroid synthesis inhibitor, finasteride, in rhesus monkeys trained under a progressive-ratio (PR) schedule of i.v. midazolam injection. We also assessed reinforcing effects of the short-acting neuroactive steroid alphaxolone and the long-acting neuroactive steroid ganaxolone in comparison with the short-acting benzodiazepine triazolam and the long-acting benzodiazepine clonazepam.
Results
At least one dose of progesterone, alphaxolone, and ganaxolone was self-administered significantly above vehicle levels in all monkeys tested (n=4 for progesterone, n=3 for alphaxolone and ganaxolone). The 5α-reductase inhibitor finasteride attenuated progesterone self-administration, consistent with the reinforcing effects of progesterone being mediated by the in vivo synthesis of neuroactive steroids. The comparison drugs, triazolam and clonazepam, were self-administered significantly above vehicle by all monkeys. Although the maximum number of injections/session maintained by the neuroactive steroids were below those maintained by the midazolam training dose, analysis of break points (i.e., highest response requirement achieved) suggested modest differences in relative reinforcing effectiveness for neuroactive steroids compared with benzodiazepines.
Conclusions
Our results are consistent with endogenous and synthetic neuroactive steroids having reinforcing effects similar to that of benzodiazepines, with reinforcing effectiveness possibly lower for the neuroactive steroids compared with benzodiazepines based on some measures.
Keywords: Neuroactive steroid, benzodiazepine, GABAA receptor, self-administration, reinforcement, rhesus monkey (Macaca mulatta)
1. INTRODUCTION
Neuroactive steroids are cholesterol-based compounds that can act as positive allosteric modulators of γ-aminobutyric acid (GABA) type A receptors (Lambert et al., 2003; Carver and Reddy, 2013). Many neuroactive steroids are synthesized endogenously from the progestogen steroid progesterone, which is typically identified as a female reproductive hormone but exists in both females and males. Both exogenous and endogenous progesterone have been evaluated as mediators of the addictive effects of drugs (e.g., Evans and Foltin, 2010; Babalonis et al., 2011); however, the extent to which progesterone has abuse-related effects itself is unknown.
Progesterone is converted in the CNS via 5α-reductase to neuroactive steroids such as pregnanolone, which we have shown previously to be self-administered by male rhesus monkeys (Fischer and Rowlett, 2011). In the present study, we evaluated the reinforcing effects of progesterone, based on the hypothesis that it might be self-administered due to conversion to neuroactive steroids. Because only pregnanolone has been evaluated in our self-administration procedure, we investigated the potential reinforcing effects of two synthetic neuroactive steroids alphaxalone and ganaxolone in order to confirm the generality of neuroactive steroid self-administration. For comparison purposes, we additionally included tests with the relatively short-acting benzodiazepine triazolam and the relatively long-acting benzodiazepine clonazepam.
2. MATERIAL AND METHODS
2.1. Animals
Four experimentally-naïve, adult male rhesus monkeys (Macaca mulatta), weighing 8–9 kg, were housed individually and maintained on a 12-h lights-on/12-h lights-off cycle (lights on at 7:00 AM). Temperature and humidity were controlled automatically and water was available continuously. Monkeys received Teklad monkey diet, supplemented with fruits and vegetables, at least 2 h prior to the daily sessions. One monkey, Mm-68-08, was brought in to provide data for progesterone only and then was switched to another previously-planned study. Animals were maintained in accordance with the guidelines of the Committee on Animals of Harvard Medical School and the Guide for Care and Use of Laboratory Animals (8th edition, 2011). Research protocols were approved by the Harvard Medical School Institutional Animal Care and Use Committee. Monkeys were prepared with a chronic indwelling venous catheter according to previously described procedures (Platt et al., 2011).
2.2. Drugs
Alphaxalone, progesterone, ganaxolone and finasteride (Tocris Biosciences, Bristol, UK) were dissolved in 45% (w/v) 2-hydroxypropyl-β-cyclodextrin and then diluted in sterile water. Midazolam (5.0 mg/ml; Henry Schein, Dublin, OH) was diluted in sterile saline. Clonazepam and triazolam (both from Sigma-Aldrich, St. Louis, MO) were dissolved in 100% propylene glycol and diluted to a 50/50% propylene glycol/water mixture. Doses were based on our previous research (Fischer and Rowlett, 2011) or dose-ranging pilot studies.
2.3. Self-Administration Procedure
Monkeys were trained to self-administer (in the home cage) the benzodiazepine midazolam (0.03 or 0.056 mg/kg/infusion) under a PR schedule of i.v. drug injection (for details, see Fischer and Rowlett, 2011). Briefly, sessions consisted of 5 components made up of 4 trials each. Each trial consisted of a response requirement signaled by white stimulus lights available for 30 min or until the response requirement was completed. Each trial was separated by a 30-min timeout. The response requirement remained constant for each of the 4 trials within a component and doubled during each successive component in the following series: 40, 80, 160, 320, and 640 responses per injection. The session ended when a maximum of 20 injections were delivered or when the response requirement was not completed for two consecutive trials. Midazolam or saline was made available on alternating days until self-administration was stable (>11 midazolam injections/session and <5 saline injections/session). Test sessions (T) were added to the alternating sequence of midazolam (M) and saline (S) sessions according to the following sequence: MTSMTSTMST. Drugs were tested in the order of presentation shown below, but doses within a drug were counterbalanced across monkeys.
After dose-response functions were obtained for all drugs, we conducted a study with progesterone using the 5α-reductase inhibitor, finasteride, which blocks the rate-limiting conversion of progesterone to neuroactive steroids (Finn et al., 2006). Finasteride was given via the i.v. catheter 5 min before a test session with a peak reinforcing dose of progesterone (individually determined).
2.5. Data Analysis
Test sessions with progesterone resulted in a notable degree of variability, although orderly data were obtained for individual monkeys. To adjust for variance in the progesterone tests, we evaluated progesterone and all other drugs in this study computing a 95% confidence interval based on (a) the last three sessions of midazolam availability and (b) three vehicle tests for each monkey. These data were graphed for individual monkeys in panels for each drug. Any individual subject’s data point above the upper level of the 95% confidence interval (represented as error bars) of each monkey’s vehicle control was considered to represent significant reinforcing effects. The 95% confidence intervals constructed for midazolam training conditions also were plotted. For analyses of group data, separate one-way repeated-measures analyses of variance (ANOVA) were used to evaluate the injection per session data for each drug. Differences from vehicle were determined by Bonferroni t-tests (p≤ 0.05).
To more accurately compare the relative reinforcing effectiveness of the neuroactive steroids with the benzodiazepines triazolam and clonazepam, we calculated BPmax values, which are the highest break points (i.e., last response requirement completed) obtained irrespective of dose for a given drug. The BPmax measure provides an index of reinforcing effectiveness that takes into account individual differences in peak BP values. These data were analyzed with the nonparametric Friedman’s ANOVA on ranks (p≤ 0.05).
3. RESULTS
3.1. Self-administration of progesterone and neuroactive steroids
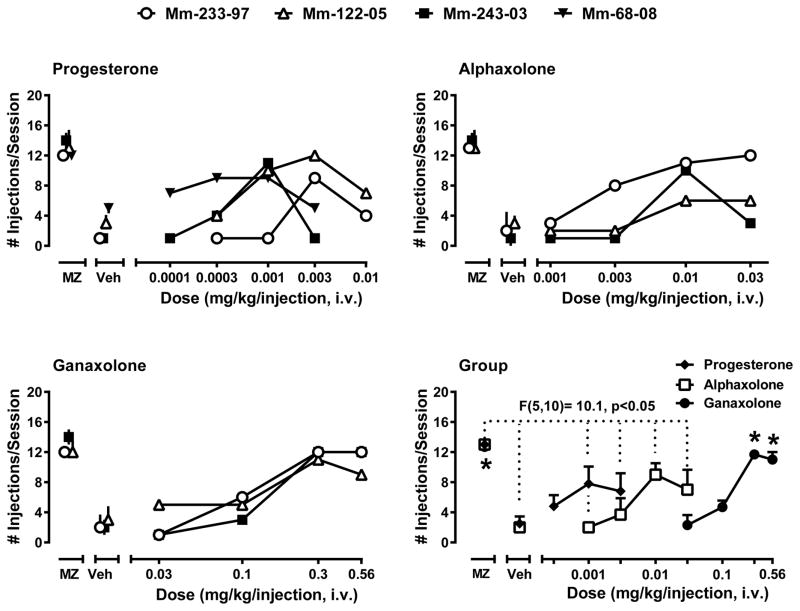
The data for individual monkeys for progesterone and the two neuroactive steroids are shown in separate panels in Figure 1 with group data summarized in the bottom right panel. Analysis of individual-subject data with progesterone (Figure 1, top left panel; note that symbols obscured the error bars in some instances) revealed evidence for significant self-administration with different peak doses evident across monkeys (e.g., compare Mm-68-08 with Mm-243-03). For all monkeys, self-administration dose-response functions were biphasic (i.e., approximated a characteristic inverted U-shaped function) with at least one dose self-administered above vehicle levels and peak doses in most cases significantly lower than the midazolam training dose. Due to the variability observed with the progesterone data, the dose range was extended to 0.0001 and 0.01 mg/kg/injection for 2 monkeys in order to obtain complete dose-response functions with ascending and descending “limbs” of the curves.
Figure 1.
Self-administration of reproductive hormone progesterone and the synthetic neuroactive steroids alphaxolone and ganaxolone by male rhesus monkeys trained under a progressive-ratio schedule of i.v. midazolam injection. The top two and lower left panels represent the number of injections/session self-administered by individual subjects (N=3–4 monkeys). Error bars represent 95% confidence intervals for 3 determinations of either the midazolam training dose (0.03 mg/kg/injection) or vehicle. Note that symbols obscure error bars in some instances. The lower right panel shows the group data, represented as mean number of injections/session + SEM. Asterisks indicate significance from vehicle for group data (Bonferroni t-tests, p<0.05). The F value and dotted lines represent results from a repeated measures ANOVA showing a main effect of alphaxolone dose (no multiple comparisons were significant).
We repeated self-administration tests in monkeys Mm-243-03 and Mm-122-05 using their peak progesterone dose (0.001 and 0.003 mg/kg/injection, respectively), after 5-min pre-treatments with finasteride (data not shown). Pretreatment with finasteride dose-dependently decreased the number of progesterone injections/session to the levels maintained under conditions of vehicle availability. For Mm-243-03, 0.001 mg/kg/injection progesterone maintained 10 injections/session that was reduced to 6 and 2 injections/session by 0.01 and 0.03 mg/kg, i.v., of finasteride, respectively. Similarly, for Mm-122-05, 0.003 mg/kg/injection progesterone maintained 12 injections/session, that was reduced to 8 and 1 injections/session by 0.01 and 0.03 mg/kg, i.v., respectively.
For the neuroactive steroid alphaxolone, the individual-subject analysis using the 95% confidence intervals did reveal that the majority of monkeys self-administered alphaxolone above vehicle levels for at least 0.003 to 0.03 mg/kg/injection based on individual monkey’s 95% confidence intervals (Figure 1, top right panel). Except for Mm-233-97, peak doses of alphaxolone were below individual 95% confidence intervals vs. midazolam. Analysis of the individual-subject data for ganaxolone (Figue 1, bottom left panel) demonstrated that all monkeys self-administered both 0.3 and 0.56 mg/kg/injection of ganaxolone above vehicle levels based on individual vehicle determinations. Similar to alphaxolone, the peak number of injections/session were below the midazolam training dose for each monkey except Mm-233-97.
Repeated measures ANOVA was computed for the progesterone grouped data using the “middle” doses of 0.0003 – 0.03 mg/kg/injection which was determined in all subjects. This analysis did not reveal a statistically significant effect [F(3,9)= 1.60, p>0.05]. Presumably the variance in potencies resulted in the relatively flat dose-response function for the group mean and consequent lack of statistical significance. For alphaxolone, there was a main effect of dose [F(4,8)= 36.6, p< 0.05]; however, Bonferroni t-tests comparing the mean number of injections/session for individual doses to vehicle levels did not reveal significant effects. Ganaxolone showed an effect of dose [F(4,8)= 65.83, p< 0.05], with Bonferroni t-tests revealing that the mean number of injections/session maintained by 0.3 mg/kg/injection was significantly higher than injections/session maintained by vehicle.
3.2. Self-administration of benzodiazepines
For triazolam, the individual-subjects analysis using the 95% confidence interval upper limit (Figure 2, top panel; as with other figures, note that symbols obscured error bars in some instances) showed that the majority of monkeys self-administered triazolam above the upper limit for all doses (individual subjects’ comparisons). Analysis of the individual-subject data for clonazepam (Figure 2, middle panel) demonstrated that 0.003 and 0.01 mg/kg/injection were self-administered above vehicle levels. The peak doses for both triazolam and clonazepam maintained injections/session that were statistically no different from the midazolam training dose (comparison of 95% confidence intervals).
Figure 2.
Self-administration of benzodiazepines triazolam and clonazepam under a progressive-ratio schedule of reinforcement in rhesus monkeys (N=3). Data are from individual animals (top and middle panel) and the group monkeys (bottom panel). Other applicable details as in Figure 1.
For triazolam group data, a repeated-measures ANOVA showed an effect of dose [F(4,8)= 67.07, p< 0.05], and Bonferroni t-tests demonstrated that the mean number of injections/session maintained by 0.003 mg/kg/injection was significantly higher than the mean number of injections/session maintained by vehicle (Figure 2, bottom panel). Clonazepam also was self-administered above vehicle levels (effect of dose [F(4,8)= 55.83, p< 0.05]) and Bonferroni t-tests revealed that the mean number of injections/session maintained by 0.003 mg/kg/injection of clonazepam was significantly higher than that maintained by the clonazepam vehicle.
3.3. Reinforcing Effectiveness
For BPmax values, a Friedman’s ANOVA on ranks showed an effect that approached but did not achieve statistical significance (Friedman’s Statistic= 9.4, p=0.06). The median values (range) were: triazolam= 320 (320-320); clonazepam= 160 (160-160); progesterone= 160 (160–320); alphaxolone= 160 (80–160); ganaxolone= 160 (160-160).
4. DISCUSSION
Although progesterone is typically thought of as primarily a female reproductive hormone, exogenous administration of this hormone has been reported to engender acute behavioral effects similar to GABAergic drugs in general (e.g., increased self-reported measures of sedation; de Wit et al., 2001; Söderpalm et al., 2004; Babalonis et al., 2011). These behavioral effects engendered by progesterone, and characteristic of neuroactive steroids, are consistent with the idea that this hormone is metabolized to neuroactive steroids that act as positive allosteric modulators of GABAA receptors (cf. Babalonis et al., 2011). Moreover, in the present study at least one dose of progesterone maintained injections/session above vehicle levels in every animal tested, an expected finding if progesterone was converted to GABAergic neuroactive steroids. To evaluate this hypothesis, peak doses of progesterone were reevaluated in the presence of finasteride, which inhibits 5α-reductase, thereby blocking neuroactive steroid formation. Finasteride attenuated progesterone self-administration in a dose-dependent manner, supporting the hypothesis that progesterone functioned as a reinforcer in male monkeys via neuroactive steroid formation.
An alternative explanation for the reinforcing effects of progesterone is that this hormone directly stimulates dopamine release in the nucleus accumbens, perhaps via a glutamatergic mechanism of action (for review, see Zheng, 2009). However, there also is evidence that progesterone has an inhibitory effect on dopamine neurotransmission (Shimizu and Bray, 1993), and multiple studies in both human and non-human subjects indicate that progesterone can attenuate the effects of monoamine transport blockers such as cocaine (for reviews, see Evans and Foltin, 2010; Anker and Carroll, 2010). Clearly more research is needed to uncover the mechanisms by which progesterone may serve as a reinforcer under certain circumstances.
Most of the drugs tested here are currently in clinical use, with ganaxolone in clinical trials as adjunctive therapy for seizures (Bialer et al., 2015). Although the potential for therapeutic use of neuroactive steroids is promising, the potential for abuse of these drugs is not well understood. Here we report that both alphaxolone and ganaxolone functioned as reinforcers in benzodiazepine-experienced subjects in a manner similar to the representative benzodiazepines triazolam and clonazepam; and irrespective of duration of action. Conclusions regarding the reinforcing effectiveness of neuroactive steroids (and progesterone) are somewhat equivocal: Based on differences from vehicle and/or differences from the midazolam training dose, the neuroactive steroids appeared to have lower reinforcing efficacy compared with the benzodiazepines. However, the BPmax values were similar to that observed with the benzodiazepines. Regardless, these findings raise the possibility that neuroactive steroids can be abused drugs, although other factors (e.g., availability, solubility, other side effects) may limit the actual illicit diversion and misuse of these drugs.
Highlights.
Reinforcing effects of progesterone and neuroactive steroids were assessed
For comparison, we evaluated self-administration of benzodiazepines
Progesterone and neuroactive steroids were self-administered by all monkeys
Benzodiazepines were slightly more effective than steroids as reinforcers
Acknowledgments
Role of Funding Source: The research described in this report was supported financially by grants awarded by the National Institutes of Health, United States Department of Health and Human Services. The grants provided financial support for the conduct of the research and the preparation of the article. The National Institutes of Health played no direct role in the study design; collection, analysis and interpretation of data; writing of the report; and decision to submit the article for publication.
The authors thank Melissa Szafir, Laura Teixeira, and Donna Reed for assistance with this study. We also thank Drs Sally Huskinson and Donna Platt for comments on earlier versions of this manuscript. This study was supported by NIH grants: DA011792, DA033795 and OD011103.
Footnotes
Contributors
Zhiqiang Meng supervised the experiments, performed data analysis, and co-wrote the manuscript with James K. Rowlett. Both authors approved the final version of the manuscript.
Contributors: All authors have contributed to the article, and each individual contribution is declared in the separate “Contributors” section provided to the journal. All authors materially participated in the research and/or article preparation. All authors have approved the final version of the manuscript as submitted.
Conflict of Interest: The authors have no Conflicts of Interest to declare concerning the data presented in this report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anker JJ, Carroll ME. The role of progestins in the behavioral effects of cocaine and other drugs of abuse: human and animal research. Neurosci Biobehav Rev. 2010;35:315–333. doi: 10.1016/j.neubiorev.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babalonis S, Lile JA, Martin CA, Kelly TH. Physiological doses of progesterone potentiate the effects of triazolam in healthy, premenopausal women. Psychopharmacology. 2011;215:429–439. doi: 10.1007/s00213-011-2206-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialer M, Johannessen SI, Levy RH, Perucca E, Tomson T, White HS. Progress report on new antiepileptic drugs: a summary of the Twelfth Eilat Conference (EILAT XII) Epilepsy Res. 2015;111:85–141. doi: 10.1016/j.eplepsyres.2015.01.001. [DOI] [PubMed] [Google Scholar]
- Carver CM, Reddy DS. Neurosteroid interactions with synaptic and extrasynaptic GABAA receptors: regulation of subunit plasticity, phasic and tonic inhibition, and neuronal network excitability. Psychopharmacology. 2013;230:151–188. doi: 10.1007/s00213-013-3276-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit H, Schmitt L, Purdy R, Hauger R. Effects of acute progesterone administration in healthy postmenopausal women and normally-cycling women. Psychoneuroindocrinology. 2001;26:697–710. doi: 10.1016/s0306-4530(01)00024-5. [DOI] [PubMed] [Google Scholar]
- Evans SM, Foltin RW. Does the response to cocaine differ as a function of sex or hormonal status in human and non-human primates? Hormones Behav. 2011;58:13–21. doi: 10.1016/j.yhbeh.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn DA, Beadles-Bohling AS, Beckley EH, Ford MM, Gililland KR, Gorin-Meyer RE, Wiren KM. A new look at the 5α-reductase inhibitor finasteride. CNS Drug Rev. 2006;12:53–76. doi: 10.1111/j.1527-3458.2006.00053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer BD, Rowlett JK. Anticonflict and reinforcing effects of triazolam + pregnanolone combinations in rhesus monkeys. J Pharmacol Exp Ther. 2011;337:805–811. doi: 10.1124/jpet.111.180422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert JJ, Cooper MA, Simmons RD, Weir CJ, Belelli D. Neurosteroids: endogenous allosteric modulators of GABAA receptors. Psychoneuroendocrinology. 2009;34(Suppl 1):S48–S58. doi: 10.1016/j.psyneuen.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Platt DM, Carey GJ, Spealman RD. Intravenous self-administration techniques in monkeys. In: Enna S, Williams M, Ferkany J, Kenakin T, Porsolt R, Sullivam J, editors. Current Protocols in Neuroscience. Units 9–21. Wiley; New York: 2011. [DOI] [PubMed] [Google Scholar]
- Rowlett JK, Winger G, Carter RB, Wood PL, Woods JH, Woolverton WL. Reinforcing and discriminative stimulus effects of the neuroactive steroids pregnanolone and Co 8-7071 in rhesus monkeys. Psychopharmacology. 1999;145:205–212. doi: 10.1007/s002130051050. [DOI] [PubMed] [Google Scholar]
- Shimzu H, Bray GA. Effects of castration, estrogen replacement and estrus cycle on monoamine metabolism in the nucleus accumbens, measured by microdialysis. Brain Res. 1993;621:200–206. doi: 10.1016/0006-8993(93)90107-x. [DOI] [PubMed] [Google Scholar]
- Söderpalm AH, Lindsey S, Purdy RH, Hauger R, de Wit H. Administration of progesterone produces mild sedative-like effects in men and women. Psychoneuroendocrinology. 2004;29:339–354. doi: 10.1016/s0306-4530(03)00033-7. [DOI] [PubMed] [Google Scholar]
- Zheng P. Neuroactive steroid regulation of neurotransmitter release in the CNS: action, mechansims and possible significance. Prog Neurobiol. 2009;89:134–152. doi: 10.1016/j.pneurobio.2009.07.001. [DOI] [PubMed] [Google Scholar]