Abstract
Background
Preclinical drug vs. food choice is an emerging group of drug self-administration procedures that have shown predictive validity to clinical drug addiction. Emerging data suggest that serotonin (5-HT)2A receptors modulate mesolimbic dopamine function, such that 5-HT2A antagonists blunt the abuse-related neurochemical effects of monoamine transporter substrates, such as amphetamine or methamphetamine. Whether subchronic 5-HT2A antagonist treatment attenuates methamphetamine reinforcement in any preclinical drug self-administration procedure is unknown. The study aim was therefore to determine 7-day treatment effects with the 5-HT2A inverse agonist/antagonist pimavanserin on methamphetamine vs. food choice in monkeys.
Methods
Behavior was maintained under a concurrent schedule of food delivery (1-g pellets, fixed-ratio 100 schedule) and intravenous methamphetamine injections (0–0.32 mg/kg/injection, fixed-ratio 10 schedule) in male rhesus monkeys (n=3). Methamphetamine choice dose-effect functions were determined daily before and during 7-day repeated pimavanserin (1.0–10 mg/kg/day, intramuscular) treatment periods.
Results
Under control conditions, increasing methamphetamine doses resulted in a corresponding increase in methamphetamine vs. food choice. Repeated pimavanserin administration failed to attenuate methamphetamine choice and produce a reciprocal increase in food choice in any monkey up to doses (3.2–10 mg/kg) that suppressed rates of operant responding primarily during components where behavior was maintained by food pellets.
Conclusions
Repeated 5-HT2A receptor inverse agonist/antagonist treatment did not attenuate methamphetamine reinforcement under a concurrent schedule of intravenous methamphetamine and food presentation in nonhuman primates. Overall, these results do not support the therapeutic potential of 5-HT2A inverse agonists/antagonists as candidate medications for methamphetamine addiction.
Keywords: Methamphetamine, choice, rhesus monkey, 5-HT2A, addiction, pimavanserin
1. INTRODUCTION
Methamphetamine addiction continues to be an insidious and global public health problem for which there are no efficacious pharmacological or behavioral treatment strategies (Brensilver et al., 2013; Carson and Taylor, 2014). Specifically, the United States Drug Enforcement Agency (DEA) reported that methamphetamine was the second most nationally identified illicit substance, only behind cannabis (DEA, 2015). Moreover, methamphetamine use disorder accounted for the majority of global persons entering treatment for drug use (UNODC, 2015). In summary, these epidemiological data support the need for preclinical research to improve our understanding of the neuropharmacological mechanisms involved in methamphetamine reinforcement. This improved mechanistic understanding should facilitate the development of clinically effective strategies to treat methamphetamine addiction.
Previous studies have implicated a role of serotonin (5-HT)2A receptors in the abuse-related neurochemical and behavioral effects of amphetamine or methamphetamine. For example, pretreatment with the 5-HT2A antagonist SR46349B or M100,907 attenuated amphetamine-induced increases in extracellular dopamine (DA) levels in the striatum and nucleus accumbens of rodents (Auclair et al., 2004; Porras et al., 2002) and in the caudate nucleus of nonhuman primates (Murnane et al., 2013a), respectively. Consistent with these neurochemical results, pretreatment with the 5-HT2A/2C agonist 5-dimethyoxy-4-iodoamphetamine (DOI) enhanced methamphetamine discriminative stimulus effects (Marona-Lewicka and Nichols, 1997; Munzar et al., 2002, 1999), whereas the 5-HT2A/2C antagonist ketanserin attenuated methamphetamine discriminative stimulus effects (Munzar et al., 1999). Although these data implicate a potential role of 5-HT2A receptors in methamphetamine abuse-related effects, there are no published studies determining whether 5-HT2A receptors are necessary for methamphetamine reinforcement.
The study aim was to determine repeated 5-HT2A inverse agonist/antagonist pimavanserin treatment effects on methamphetamine reinforcement under a methamphetamine vs. food choice procedure. A preclinical drug vs. food choice procedure was utilized to investigate methamphetamine reinforcement mechanisms for the following two reasons. First, preclinical drug vs. food choice procedures have been predictive of human drug abuse and addiction (Ahmed, 2010; Banks and Negus, 2012). Second, preclinical drug vs. food choice procedures provide a dependent measure of behavioral allocation that may be less sensitive to reinforcement-independent rate-altering drug effects produced by treatment drugs that may have potential as candidate medications (Banks et al., 2015). Pimavanserin was selected because it is more selective for 5-HT2A vs. 5-HT2C receptors than M100,907 (Vanover et al., 2006) and has been recently approved by the Food and Drug Administration for Parkinson’s disease-induced psychosis treatment (Cummings et al., 2014; Walsh, 2016). If pimavanserin attenuated methamphetamine choice and produced a corresponding increase in food choice, these preclinical results would suggest 5-HT2A receptors were necessary for methamphetamine reinforcement and support further research evaluating 5-HT2A receptor inverse agonists/antagonists as candidate anti-methamphetamine addiction medications.
2. METHODS
2.1 Subjects
Studies were conducted in three adult male rhesus monkeys (Macaca mulatta) surgically implanted with a double-lumen catheter (0.76 mm ID × 2.36 mm OD, STI Flow, Morrisville, NC) inserted into a femoral or jugular vein and who had methamphetamine self-administration histories (Banks and Blough, 2015; Schwienteck and Banks, 2015). Monkeys were maintained on a diet of fresh fruit and food biscuits (Lab Diet High Protein Monkey Biscuits #5045, PMI Nutrition Inc., St. Louis, MO) delivered after the behavioral session. Water was continuously available in the housing chamber and a 12 h light-dark cycle was in effect. Monkeys had visual, auditory and olfactory contact with other monkeys throughout the study. Operant procedures and foraging toys were provided for environmental manipulation and enrichment. Videos or music was also played daily in animal housing rooms to provide additional environmental enrichment. Animal research and maintenance were conducted according to the Guide for the Care and Use of Laboratory Animals (Council, 2011). Animal facilities were licensed by the United States Department of Agriculture and accredited by the Association for Assessment and Accreditation of Laboratory Animal Care. The Institutional Animal Care and Use Committee approved both the research and environmental enrichment protocols.
2.2 Apparatus
The housing chamber served as the experimental chamber and was equipped with a custom operant panel, a pellet dispenser (Med Associates, Model ENV-203-1000, St. Albans, VT), and two syringe pumps (Model PHM-108, Med Associates). One “self-administration” pump delivered contingent methamphetamine injections through one catheter lumen. The second “treatment” pump delivered a 0.1 mL noncontingent saline infusion through the second catheter lumen at a programmed rate of every 20 minutes from 1200 each day until 1100 the following morning. The intravenous catheter was protected by a customized stainless steel tether and jacket system (Lomir Biomedical, Malone, NY) that permitted monkeys to move freely within the home chamber. Catheter patency was periodically evaluated by intravenous ketamine (5 mg/kg) administration through one lumen of the double-lumen catheter. The catheter was considered patent if intravenous ketamine administration produced muscle tone loss within 10 s.
2.3 Methamphetamine Versus Food Choice Procedure
Daily experimental sessions were conducted from 0900 to 1100 h in each monkey’s home chamber as described previously (Banks and Blough, 2015). The terminal choice procedure consisted of five 20-min components, with a different unit methamphetamine dose available during each successive component (0, 0.01, 0.032, 0.1, and 0.32 mg/kg/injection during components 1–5, respectively). Manipulating the injection volume controlled the methamphetamine dose (0, 0.03, 0.1, 0.3, and 1.0 mL/injection, respectively). Components were separated by 5-min timeout periods. During each component, the left, food-associated key was transilluminated red, and completion of the FR requirement (FR100) resulted in 1-g food pellet delivery. The right, methamphetamine-associated key was transilluminated green, and completion of the FR requirement (FR10) resulted in delivery of the intravenous unit methamphetamine dose available during that component. Stimulus lights for the methamphetamine-associated key were flashed on and off in 3s cycles, and longer flashes were associated with larger methamphetamine doses. Monkeys could complete up to a total of 10 ratio requirements on both the food- and methamphetamine-associated keys. Responding on either key reset the ratio requirement on the other key. Each ratio requirement completion initiated a 30-s timeout, during which all stimulus lights were turned off, and responding had no programmed consequences. Choice behavior was considered stable when the lowest unit methamphetamine dose maintaining greater than 80% methamphetamine vs. food choice varied by ≤0.5 log units for 3 consecutive days.
Once methamphetamine vs. food choice was stable, test sessions were conducted to determine 7-day repeated pimavanserin (1–10 mg/kg, IM) treatment effects on methamphetamine vs. food choice. Pimavanserin was administered between 0755 and 0805 h, approximately 60 min before the 0900h start of the behavioral session. Pimavanserin treatment was tested up to doses that decreased either methamphetamine choice or operant responding. The 3-day saline infusion period before each test drug treatment was used as the “baseline.” At the conclusion of each 7-day treatment period, intramuscular injections were terminated for at least 4 days and until methamphetamine vs. food choice had returned to pretreatment levels. Pimavanserin doses were counterbalanced across subjects.
2.4 Data Analysis
The primary dependent measures were (1) percent methamphetamine choice, defined as (number of ratios completed on the methamphetamine-associated key ÷ total number of ratios completed)*100 and (2) number of ratio requirements (hereafter referred to as “choices”) completed. The last 3-day mean of each experimental condition for each monkey for each dependent measure was then plotted as a function of unit methamphetamine dose during the behavioral session. Results were analyzed using a linear mixed-effects analysis with unit methamphetamine dose and pimavanserin dose as the fixed main effects and subjects as the random effect. Post-hoc comparisons against baseline conditions within a given methamphetamine dose were performed using the Dunnett’s test following a significant main effect of pimavanserin dose or methamphetamine dose × pimavanserin dose interaction. The criterion for significance was set a priori at the 95% confidence level (p < 0.05). All analyses were conducted using JMP Pro 12.2, SAS, Cary, NC.
2.5 Drugs
(+)-Methamphetamine HCl and pimavanserin L-tartrate were provided by the National Institute on Drug Abuse Drug Supply Program (Bethesda, MD). All drug doses were expressed as the salt forms listed above and all drug solutions were passed through a sterile 0.2 μm filter (Millipore, Billerica, MA) before administration.
3. RESULTS
3.1 Effects of pimavanserin on methamphetamine vs. food choice
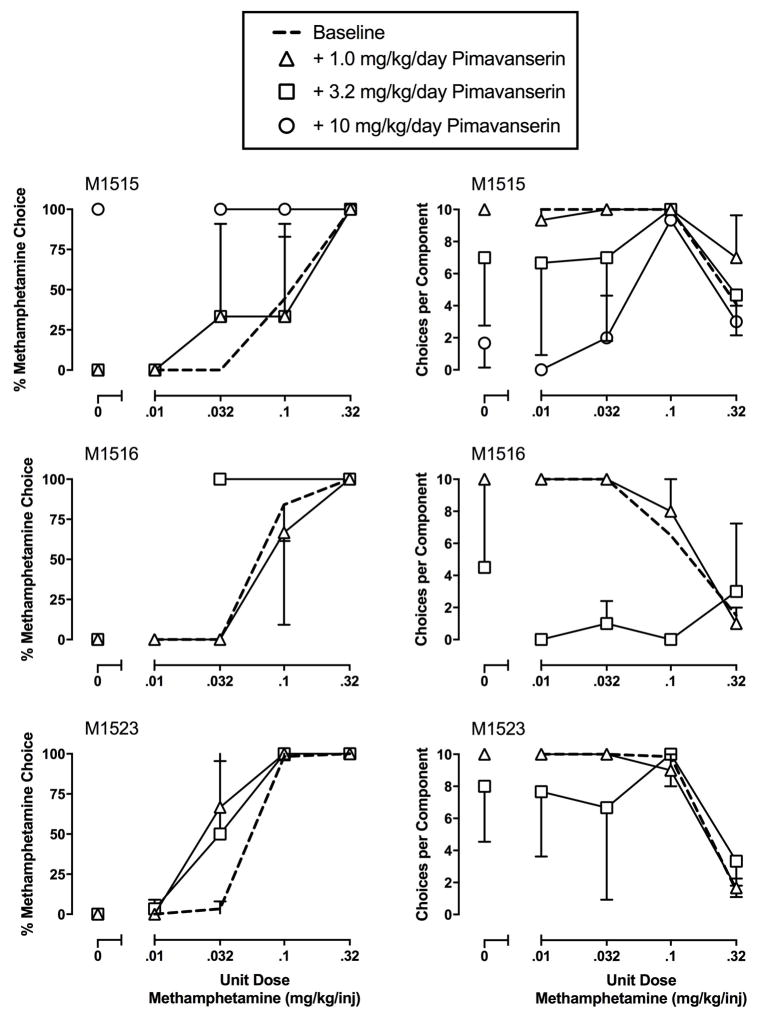
Under control conditions during which saline was continuously infused through the treatment lumen “baseline”, increasing methamphetamine doses resulted in behavioral reallocation away from the food-associated key and towards the methamphetamine-associated key (Figure 1). Repeated pimavanserin treatment failed to significantly alter either methamphetamine vs. food choice or choices completed per component (Figure 1). Due to individual subject sensitivity to pimavanserin potency to produce rate-altering effects, individual data are shown in Figure 2. In monkey M1515, repeated pimavanserin treatment had no effect up to pimavanserin doses (10 mg/kg) that decreased rates of operant responding primarily during components maintained by food. Furthermore, repeated 10 mg/kg pimavanserin treatment also decreased body weight in this monkey by more than 1 kg. In both monkey M1516 and M1523, a pimavanserin dose of 3.2 mg/kg decreased rates of operant responding to such an extent that larger pimavanserin doses were not tested.
Figure 1.
Effects of 7-day repeated pimavanserin (1.0–3.2 mg/kg, intramuscular) treatment on choice between methamphetamine and food in rhesus monkeys (n=3). Abscissae: unit dose methamphetamine in mg/kg/injection. Top ordinate: percent methamphetamine choice. Bottom ordinate: number of ratio requirements (choices) completed per choice session component. All points represent mean ± SEM obtained during days 5–7 of each 7-day treatment period. Numbers in parentheses denote the number of monkeys contributing to that data point if less than the maximal number of monkeys tested and indicate a component where one or more monkeys failed to complete at least one ratio requirement.
Figure 2.
Effects of 7-day repeated pimavanserin (1.0–3.2 mg/kg, intramuscular) treatment on choice between methamphetamine and food in individual rhesus monkeys. Abscissae: unit dose methamphetamine in mg/kg/injection. Left ordinates: percent methamphetamine choice. Right ordinates: number of ratio requirements (choices) completed per choice session component. All points represent mean ± SEM obtained during days 5–7 of each 7-day treatment period. 10 mg/kg pimavanserin treatment was only tested in M1515. Missing data points indicate that a monkey failed to complete at least one ratio requirement during that component.
4. DISCUSSION
The study aim was to determine whether repeated 5-HT2A inverse agonist/antagonist pimavanserin administration decreased methamphetamine reinforcement in monkeys. The main finding was that pimavanserin did not attenuate methamphetamine choice and produce a corresponding increase in food choice in any monkey up to doses that decreased operant response rates and produced significant weight loss. Overall, the present results do not support the potential clinical utility of 5-HT2A inverse agonists/antagonists as anti-methamphetamine addiction medications.
The present behavioral results were inconsistent with previous neurochemical (Auclair et al., 2004; Murnane et al., 2013a; Porras et al., 2002) and behavioral (Munzar et al., 1999) results demonstrating 5-HT2A antagonists attenuated amphetamine or methamphetamine abuse-related effects. There are three potential reasons for these inconsistent results. First, potential species differences between rats and nonhuman primates in either 5-HT2A or methamphetamine neuropharmacology could explain these inconsistent results. A second potential explanation could be related to differences in dosing regimens. For example, previous studies utilized acute dosing regimens whereas the present study determined 5-HT2A antagonist effects under a repeated subchronic dosing regimen. Examination of pimavanserin treatment days 1–3 did not reveal a rightward shift in the methamphetamine choice dose-effect function in any monkey (data not shown). A third potential explanation could be related to differences in experimental dependent measures. Previous studies determined 5-HT2A antagonist effects on amphetamine-induced dopamine release in either nucleus accumbens (Auclair et al., 2004; Porras et al., 2002) or caudate nucleus (Murnane et al., 2013a) and methamphetamine discriminative stimulus effects (Munzar et al., 1999); whereas the present study determined 5-HT2A antagonist effects on methamphetamine reinforcement. However, repeated pimavanserin treatment effects on methamphetamine self-administration were consistent with previous studies evaluating ketanserin and M100,907 acute pretreatments on cocaine self-administration (Fantegrossi et al., 2002; Murnane et al., 2013b). Overall, the present results and the previous literature highlight the importance of repeated pharmacological pretreatments and determination of treatment effects on multiple dependent measures.
Conceptually, 5-HT2A receptor antagonists represent an “antagonist-like” pharmacotherapeutic approach for methamphetamine addiction with the neurobiological aim of blunting methamphetamine-induced nucleus accumbens dopamine release and corresponding reinforcing effects. The present behavioral results suggest this may not be a therapeutically advantageous treatment option for methamphetamine addiction. The present results are consistent with previous methamphetamine vs. food choice studies in nonhuman primates evaluating “antagonist-like” pharmacological treatments such as dopamine antagonists PG01037, buspirone, and risperidone or the dopamine D3 partial agonist PG619 (Banks and Blough, 2015; John et al., 2015a, 2015b). Furthermore, both the dopamine partial agonist aripiprazole and the dopamine antagonist risperidone have failed to reduce methamphetamine choice in the human laboratory (Stoops et al., 2013) or methamphetamine use in clinical trials (Coffin et al., 2013; Nejtek et al., 2008; Tiihonen et al., 2007). Moreover, “antagonist-like” approaches have not been successful pharmacotherapeutic strategies to treat amphetamine-type or cocaine addictions based on a recent meta-analysis (Kishi et al., 2013). In summary, we interpret this scientific literature to suggest methamphetamine addiction medications development might benefit from a paradigm shift to novel “agonist-like” therapies that both decrease methamphetamine use and promote more adaptive behavior maintained by alternative non-drug reinforcers.
Highlights.
Methamphetamine maintained dose-dependent preference over food pellet
5HT2A antagonist treatment did not attenuate methamphetamine choice
Results do not support 5HT2A antagonists as candidate medications
Acknowledgments
Role of funding source
Research reported in this publication was supported by the National Institute on Drug Abuse of the National Institutes of Health under Award Numbers R01 DA031719. The content is solely the responsibility of the author and does not necessarily represent the official views of the National Institutes of Health.
We acknowledge the technical assistance of Jennifer Gough and Kevin Costa for writing the original version of the behavioral programs.
Footnotes
Contributors
Banks designed the study, performed the data analysis, and drafted the manuscript.
Conflicts of interest
The author has no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed SH. Validation crisis in animal models of drug addiction: beyond non-disordered drug use toward drug addiction. Neurosci Biobehav Rev. 2010;35:172–184. doi: 10.1016/j.neubiorev.2010.04.005. http://dx.doi.org/10.1016/j.neubiorev.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Auclair A, Blanc G, Glowinski J, Tassin J-P. Role of serotonin2A receptors in the d-amphetamine-induced release of dopamine: comparison with previous data on α1b-adrenergic receptors. J Neurochem. 2004;91:318–326. doi: 10.1111/j.1471-4159.2004.02714.x. [DOI] [PubMed] [Google Scholar]
- Banks ML, Blough BE. Effects of environmental maniuplations and bupropion and risperidone treatments on choice between methamphetamine and food in rhesus monkeys. Neuropsychoharmacology. 2015;40:2198–2206. doi: 10.1038/npp.2015.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Hutsell BA, Schwienteck KL, Negus SS. Use of preclinical drug vs. food choice procedures to evaluate candidate medications for cocaine addiction. Curr Treat Options Psychiatry. 2015;2:136–150. doi: 10.1007/s40501-015-0042-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Negus SS. Preclinical determinants of drug choice under concurrent schedules of drug self-administration. Adv Pharmacol Sci. 2012;2012:281768. doi: 10.1155/2012/281768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brensilver M, Heinzerling KG, Shoptaw S. Pharmacotherapy of amphetamine-type stimulant dependence: an update. Drug Alcohol Rev. 2013;32:449–460. doi: 10.1111/dar.12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson DS, Taylor ER. Commentary on Heinzerling et al. (2014): a growing methamphetamine dependence therapeutics graveyard. Addiction. 2014;109:1887–1888. doi: 10.1111/add.12709. [DOI] [PubMed] [Google Scholar]
- Coffin PO, Santos GM, Das M, Santos DM, Huffaker S, Matheson T, Gasper J, Vittinghoff E, Colfax GN. Aripiprazole for the treatment of methamphetamine dependence: a randomized, double-blind, placebo-controlled trial. Addiction. 2013;108:751–761. doi: 10.1111/add.12073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Council, National Research. Guide For The Care And Use Of Laboratory Animals. National Academies Press; Washington DC: 2011. [Google Scholar]
- Cummings J, Isaacson S, Mills R, Williams H, Chi-Burris K, Corbett A, Dhall R, Ballard C. Pimavanserin for patients with Parkinson’s disease psychosis: a randomised, placebo-controlled phase 3 trial. Lancet. 2014;383:533–540. doi: 10.1016/S0140-6736(13)62106-6. http://dx.doi.org/10.1016/S0140-6736(13)62106-6. [DOI] [PubMed] [Google Scholar]
- DEA; Department of Justice, editor. National Foreensic Laboratory Information System: 2014 Annual Report. Drug Enforcement Administration; Springfield, VA: 2015. [Google Scholar]
- Fantegrossi WE, Ullrich T, Rice KC, Woods JH, Winger G. 3,4-Methylenedioxymethamphetamine (MDMA, “ecstasy”) and its stereoisomers as reinforcers in rhesus monkeys: serotonergic involvement. Psychopharmacology. 2002;161:356–364. doi: 10.1007/s00213-002-1021-6. [DOI] [PubMed] [Google Scholar]
- John WS, Banala AK, Newman AH, Nader MA. Effects of buspirone and the dopamine D3 receptor compound PG619 on cocaine and methamphetamine self-administration in rhesus monkeys using a food-drug choice paradigm. Psychopharmacology. 2015a;232:1279–1289. doi: 10.1007/s00213-014-3760-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John WS, Newman AH, Nader MA. Differential effects of the dopamine D3 receptor antagonist PG01037 on cocaine and methamphetamine self-administration in rhesus monkeys. Neuropharmacology. 2015b;92:34–43. doi: 10.1016/j.neuropharm.2014.12.024. http://dx.doi.org/10.1016/j.neuropharm.2014.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi T, Matsuda Y, Iwata N, Correll CU. Antipsychotics for cocaine or psychostimulant dependence: systematic review and meta-analysis of randomized, placebo-controlled trials. J Clin Psychiatry. 2013;74:e1169–1180. doi: 10.4088/JCP.13r08525. [DOI] [PubMed] [Google Scholar]
- Marona-Lewicka D, Nichols DE. 5-HT2A/2C receptor agonists potentiate the discriminative cue of (+)-amphetamine in the rat. Neuropharmacology. 1997;36:1471–1475. doi: 10.1016/s0028-3908(97)00106-8. http://dx.doi.org/10.1016/S0028-3908(97)00106-8. [DOI] [PubMed] [Google Scholar]
- Munzar P, Justinova Z, Kutkat SW, Goldberg SR. Differential involvement of 5-HT2A receptors in the discriminative-stimulus effects of cocaine and methamphetamine. Eur J Pharmacol. 2002;436:75–82. doi: 10.1016/s0014-2999(01)01598-9. http://dx.doi.org/10.1016/S0014-2999(01)01598-9. [DOI] [PubMed] [Google Scholar]
- Munzar P, Laufert MD, Kutkat SW, Nováková J, Goldberg SR. Effects of various serotonin agonists, antagonists, and uptake inhibitors on the discriminative stimulus effects of methamphetamine in rats. J Pharmacol Exp Ther. 1999;291:239–250. [PubMed] [Google Scholar]
- Murnane KS, Andersen ML, Rice KC, Howell LL. Selective serotonin 2A receptor antagonism attenuates the effects of amphetamine on arousal and dopamine overflow in non-human primates. J Sleep Res. 2013a;22:581–588. doi: 10.1111/jsr.12045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murnane KS, Winschel J, Schmidt KT, Stewart LM, Rose SJ, Cheng K, Rice KC, Howell LL. Serotonin 2A receptors differentially contribute to abuse-related effects of cocaine and cocaine-induced nigrostriatal and mesolimbic dopamine overflow in nonhuman primates. J Neurosci. 2013b;33:13367–13374. doi: 10.1523/jneurosci.1437-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nejtek VA, Avila M, Chen LA, Zielinski T, Djokovic M, Podawiltz A, Kaiser K, Bae S, Rush AJ. Do atypical antipsychotics effectively treat co-occurring bipolar disorder and stimulant dependence? A randomized, double-blind trial. J Clin Psychiatry. 2008;69:1257–1266. doi: 10.4088/jcp.v69n0808. [DOI] [PubMed] [Google Scholar]
- Porras G, Di Matteo V, Fracasso C, Lucas G, De Deurwaerdere P, Caccia S, Esposito E, Spampinato U. 5-HT2A and 5-HT2C/2B receptor subtypes modulate dopamine release induced in vivo by amphetamine and morphine in both the rat nucleus accumbens and striatum. Neuropsychopharmacology. 2002;26:311–324. doi: 10.1016/S0893-133X(01)00333-5. [DOI] [PubMed] [Google Scholar]
- Schwienteck KL, Banks ML. Effects of continuous 7-day d-amphetamine, methylphenidate, and cocaine treatment on choice between methamphetamine and food in male rhesus monkeys. Drug Alcohol Depend. 2015;155:16–23. doi: 10.1016/j.drugalcdep.2015.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoops WW, Bennett JA, Lile JA, Sevak RJ, Rush CR. Influence of aripiprazole pretreatment on the reinforcing effects of methamphetamine in humans. Prog Neuropsychopharmacol Biol Psychiatry. 2013;47:111–117. doi: 10.1016/j.pnpbp.2013.08.007. http://dx.doi.org/10.1016/j.pnpbp.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiihonen J, Kuoppasalmi K, Fohr J, Tuomola P, Kuikanmaki O, Vorma H, Sokero P, Haukka J, Meririnne E. A comparison of aripiprazole, methylphenidate, and placebo for amphetamine dependence. Am J Psychiatry. 2007;164:160–162. doi: 10.1176/appi.ajp.164.1.160. [DOI] [PubMed] [Google Scholar]
- UNODC. World Drug Report 2015. United Nations Office on Drugs and Crime; Vienna: 2015. [Google Scholar]
- Vanover KE, Weiner DM, Makhay M, Veinbergs I, Gardell LR, Lameh J, Del Tredici AL, Piu F, Schiffer HH, Ott TR, Burstein ES, Uldam AK, Thygesen MB, Schlienger N, Andersson CM, Son TY, Harvey SC, Powell SB, Geyer MA, Tolf B-R, Brann MR, Davis RE. Pharmacological and behavioral profile of N-(4-Fluorophenylmethyl)-N-(1-methylpiperidin-4-yl)-N′-(4-(2-methylpropyloxy)phenylmethyl) carbamide (2R,3R)-dihydroxybutanedioate (2:1) (ACP-103), a novel 5-hydroxytryptamine2A receptor inverse agonist. J Pharmacol Exp Ther. 2006;317:910–918. doi: 10.1124/jpet.105.097006. [DOI] [PubMed] [Google Scholar]
- Walsh S. [accessed on 05/06/2016];FDA approves first drug to treat hallucinations and delusions associated with Parkinson’s disease. 2016 http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm498442.htm.