Abstract
Background
Food allergy prevalence appears to have recently risen, with larger increases among non-Hispanic blacks. However, it is unclear whether these trends represent shifts in recognition of food allergy or in sensitization.
Objective
To determine whether sensitization to common food allergens increased in U.S. children from 1988–1994 to 2005–2006 and whether these trends differed by race/ethnicity.
Methods
Food-specific IgE (to peanut, milk, egg and shrimp) was measured by ImmunoCap in stored sera from subjects aged 6–19 in the National Health and Nutrition Examination Survey (NHANES) III (1988–1994) and was compared to NHANES 2005–2006. Sensitization to foods was defined as overall (IgE ≥0.35 kU/L), moderate-level (IgE ≥2 kU/L) and high-level (IgE ≥ commonly used 95% predictive values). Sensitization to individual and combined foods was compared between surveys, with analyses further stratified by race/ethnicity.
Results
7,896 subjects (NHANES III: n=4995, NHANES 2005–2006: n=2,901) were included. In NHANES III, prevalence of food sensitization was 24.3% (95%CI:22.1–26.5) compared to 21.6% (95% CI:19.5–23.7) in NHANES 2005–2006. There were no significant changes in the prevalence of any level of milk, egg or peanut sensitization, but shrimp sensitization at all levels decreased markedly; overall sensitization NHANES III: 11.2% (95%CI:10.0–12.5) versus NHANES 2005–2006: 6.1% (95%CI:4.5–7.7). There was a trend towards increased prevalence of moderate- and high-level sensitization to the combination of milk, egg and peanut among non-Hispanic blacks but not other groups.
Conclusion
In contrast to our expectations, sensitization to common food allergens did not increase between the late-1980s/early-1990s and mid-2000s among U.S. 6–19 year olds, and in fact decreased to shrimp.
Keywords: Food allergy, NHANES, Food sensitization, Epidemiology
Introduction
Food allergy is a common childhood condition that appears to have increased rapidly in prevalence over the past few decades.(1–4) This increase has led to substantial efforts to understand the causes of the “food allergy epidemic,” such as the timing of food introduction,(5) changes in diet and nutrition,(6, 7) and microbial exposures.(8) The data demonstrating an increase in prevalence, however, are based largely on self-reported food allergy and health care utilization for manifestations of food allergy, but there are currently no data on national time trends in laboratory correlates of food allergy. Thus, it is not known whether this increased prevalence observed in questionnaires and health care utilization is accompanied by increases in serologic biomarkers of food allergy.(9)
In addition, current evidence points to large and widening disparities in the prevalence of food allergy by race/ethnicity. In a recent systematic review, the increase in prevalence of self-reported food allergy was significantly higher in children of non-Hispanic Black race/ethnicity compared to Non-Hispanic White and Hispanic children over the past two decades.(2) It is not currently known, however, whether this rapid increase in self-reported food allergy in Non-Hispanic Blacks is correlated with changes in allergic sensitization.
Prior to this study, only one national survey in the U.S., The National Health and Nutrition Examination Survey (NHANES) 2005–2006, measured food-specific IgE. Here we measured food-specific IgE to milk, egg, peanut, and shrimp, in stored sera from NHANES III, which was conducted between 1988 and 1994. Our objective was to directly compare food-specific IgE levels to common food allergens in representative samples of the United States population over a span of approximately 15 years, in order to determine whether the increase observed in self-reported and diagnosed food allergy during this time period has been associated with changes in allergic sensitization.
Methods
The National Health and Nutrition Examination Surveys (NHANES) are a series of cross-sectional studies conducted by the National Center for Health Statistics (NCHS) of the Centers for Disease Control (CDC). NHANES III was conducted from 1988–1994, and since 1999, NHANES has been conducted continuously in 2-year blocks.(10) Sampling is designed to be representative of the overall non-institutionalized U.S. population, with over-sampling of certain groups to ensure power for sub-group analyses. Interviews are initially conducted in subject’s homes, followed by physical exams in mobile centers. Response rate for the examination portion of the survey was 78% for NHANES III and 77% for NHANES 2005–2006. The NHANES and use of stored sera were approved by the IRB of the NCHS and all subjects provided informed consent. Children 12 and older for NHANES III and 7 and older for NHANES 2005–6 provided informed assent.
Participants aged 6–19 with frozen stored surplus sera in NHANES III or with food-specific measurements in NHANES 2005–2006 were included. These cut-offs were chosen because stored sera was not available in children younger than age 6 in NHANES III and the NCHS preferred to use 19 as a cut-off for analyses of stored sera. The sera were collected using similar standardized protocols in both NHANES III and NHANES 2005–2006 and were stored at −70C.
Definitions
Food-specific IgE to cow’s milk (“milk”), hen’s egg white (“egg”), peanut, and shrimp was measured at the same clinical laboratory (Elmhurt Reference Laboratory, Elmhurst, Illinois) using standard techniques (ImmunoCap, Phadia. Upsalla, Sweden) for both surveys.(1) Food-specific IgE from NHANES III was analyzed between 2014 and 2015. Data are reported as the concentration of allergen specific units (kU/L), and the lower limit of detection was 0.35 kU/L.
Food-specific IgE levels for each food were used to define three a priori overlapping categories: overall, moderate-level, and high-level sensitization. Overall sensitization was defined as food-specific IgE ≥ 0.35 kU/L for all foods, moderate-level sensitization as food-specific IgE ≥ 2 kU/L for all foods, and high-level sensitization as food-specific IgE at or above values that have previously been considered 95% predictive probability cut-offs (15 kU/L for milk, 7 kU/L for egg, and 14 kU/L for peanut).(11, 12) As there is not a well-established cut-off for shrimp, a value of 5 kU/L was chosen to define high-level sensitization, consistent with previous studies using NHANES.(1)
When considering the foods together, individuals were grouped into overall, moderate-level or high-level sensitization to “Milk, Egg, or Peanut” if they had at least one food-specific IgE in the cut-off range to these foods. As sensitization patterns for shrimp demonstrated a different trend than those seen with milk, egg, and peanut, we treated sensitization to this food separately.
Race/ethnicity was obtained by self-report, and was categorized as “Non-Hispanic White,” “Non-Hispanic Black,” “Mexican-American,” and “Other” in NHANES III. In NHANES 2005–2006, the same categories were used with the addition of “Other Hispanic” (n=349), a population that had been included in “Other” in NHANES III. For the purpose of these analyses, “Non-Hispanic White,” “Non-Hispanic Black,” and “Mexican-American,” categories were used. Because there were so few subjects in the “Other” category, and because the composition of this category likely changed between surveys, stratified analyses in this category are not shown.
In order to define the allergic profile of the populations at both time points, prevalence of self-reported asthma and allergic rhinitis are presented. Asthma was defined as a positive response to the question, “Has a doctor (or other health professional) ever told you that you have asthma?” Similarly, allergic rhinitis was defined as a positive response to the question, “Has a doctor (or other health professional) ever told you that you had hay fever?”
Statistical Methods
To account for oversampling, complex sampling methods, and non-response, we used cluster sampling units, pseudo-strata and sampling weights provided by the CDC for all analyses using the “SVY” commands in STATA 12.1. As the sampling units and pseudo-strata could not be combined between these two surveys, the proportion of children with overall, moderate, and high-level sensitization to each food, as well as these proportions by race/ethnicity and age, were independently calculated for each survey. Risk difference estimates were then calculated for the proportions across surveys, with p-values and confidence intervals calculated with the delta equation using the computed estimates and standard errors.(13) Sensitivity analyses were done to evaluate the effect of changing demographics between the studies by direct standardization of NHANES III to match the racial/ethnic distribution of NHANES 2005–2006, using the techniques suggested in the NHANES documentation (http://www.cdc.gov/nchs/tutorials/NHANES/NHANESAnalyses/agestandardization/age_standardization_intro.htm).(14, 15) Two-tailed p values < 0.05 were considered statistically significant. All analyses were performed manually or using STATA 12.1 (College Station, TX).
Results
Study Population
A total of 7,896 children between the ages of 6 and 19 were included in this study (4,995 from NHANES III and 2,901 from NHANES 2005–2006). The two populations were similar in terms of age distribution and sex, but the percentage of Mexican-American individuals was higher in 2005–2006 (Table 1). The percentage of children with asthma was higher in 2005–2006, whereas the percentage with allergic rhinitis was lower (Table 1).
Table 1.
Demographic Characteristics of Study Population in NHANES III and NHANES 2005–2006
| Characteristic | NHANES III Percentage (95% CI) (n = 4995) |
NHANES 2005–2006 Percentage (95% CI) (n = 2901) |
p value† |
|---|---|---|---|
| Age (y) | |||
| 6–10 | 33 (30 – 35) | 34 (30 – 38) | 0.52 |
| 11–15 | 38 (36 – 41) | 36 (34 – 39) | 0.95 |
| 16–19 | 29 (26 – 32) | 30 (26 – 33) | 0.98 |
| Sex | |||
| Female | 49 (47 – 52) | 49 (46 – 51) | 0.75 |
| Race/Ethnicity | |||
| Non-Hispanic White | 66 (62 – 70) | 64 (57 – 71) | 0.55 |
| Non-Hispanic Black | 15 (13 – 17) | 15 (9 – 21) | 0.94 |
| Mexican-American | 9 (7 – 11) | 13 (10 – 17) | 0.01 |
| Other | 10 (7 – 13) | 8 (4 – 11) | 0.26 |
| Poverty Income Ratio (PIR) | |||
| ≤1.0 | 23 (20 – 26) | 19 (16 – 23) | 0.09 |
| Allergic Outcomes | |||
| Asthma | 11 (9 – 13) | 17 (16 – 19) | <0.001 |
| Allergic Rhinitis | 8 (7 – 10) | 5 (3 – 6) | 0.001 |
Values are reported as percentages (95% CIs)
P value for the risk difference estimate
Changes in Food-Specific IgE Over Time
Overall, 24.3% (95% CI 22.1–26.5) of children were sensitized (IgE≥0.35 kU/L) to milk, egg, peanut, or shrimp in NHANES III, whereas 21.6% (95% CI 19.5–23.7) of children were sensitized in NHANES 2005–2006 (p = 0.07), as shown in Table 2 (unweighted numbers are given in Table 3). There was no change in the prevalence of moderate-level food-specific sensitization (≥ 2 kU/L) to any food, and the percentage of those with high-level food sensitization actually decreased from 2.8% (95% CI 2.0–3.6) to 1.5% (95% CI 1.0–2.1, p = 0.01) between NHANES III and NHANES 2005–2006.
Table 2.
Prevalence of food sensitization in NHANES III and NHANES 2005–2006
| Food Sensitization* | NHANES III Percentage (95% CI) (n = 4995**) |
NHANES 2005–2006 Percentage (95% CI) (n = 2901) |
p value† |
|---|---|---|---|
| Milk | |||
| Overall | 8.3 (7.0 – 9.8) | 8.1 (6.1 – 10.2) | 0.90 |
| Moderate-level | 0.4 (0.1 – 0.7) | 0.5 (0.1 – 0.9) | 0.70 |
| High-level | 0 | 0.008 (−0.01 – 0.03) | 0.33 |
| Egg | |||
| Overall | 3.2 (2.5 – 4.0) | 4.0 (2.9 – 5.1) | 0.2 |
| Moderate-level | 0.04 (0.007 – 0.08) | 0.2 (−0.004 – 0.5) | 0.09 |
| High-level | 0 | 0.05 (−0.01 – 0.1) | 0.10 |
| Peanut | |||
| Overall | 11.2 (9.5 – 13.1) | 10.5 (8.4 – 12.7) | 0.62 |
| Moderate-level | 3.7 (2.7 – 4.6) | 4.5 (3.3 – 5.7) | 0.26 |
| High-level | 0.7 (0.4 – 1.2) | 0.9 (0.4 – 1.4) | 0.39 |
| Shrimp | |||
| Overall | 11.2 (10.0 – 12.5) | 6.1 (4.5 – 7.7) | <0.001 |
| Moderate-level | 4.1 (3.3 – 4.9) | 1.7 (1.2 – 2.2) | <0.001 |
| High-level | 2.2 (1.6 – 2.9) | 0.6 (0.4 – 0.9) | <0.001 |
| Milk, Egg, or Peanut | |||
| Overall | 18.2 (15.7 – 20.6) | 18.2 (15.4 – 20.9) | >0.99 |
| Moderate-level | 4.0 (2.9 – 5.1) | 4.9 (3.7 – 6.1) | 0.23 |
| High-level | 0.6 (0.3 – 1.1) | 0.9 (0.5 – 1.4) | 0.35 |
| All Foods | |||
| Overall | 24.3 (22.1 – 26.5) | 21.6 (19.5 – 23.7) | 0.07 |
| Moderate-level | 7.2 (6.0 – 8.5) | 6.2 (4.8 – 7.5) | 0.23 |
| High-level | 2.8 (2.0 – 3.6) | 1.5 (1.0 – 2.1) | 0.01 |
Values are reported as percentages (95% CIs)
P value for the risk difference estimate
Overall sensitization is defined as IgE ≥ 0.35 kU/L; moderate sensitization is defined as IgE ≥ 2 kU/L; high sensitization is defined as ≥15 kU/L for milk, ≥7 kU/L for egg, ≥14 kU/L for peanut, and ≥5 kU/L for shrimp.
n=4991 for milk, 4994 for shrimp, and 4995 for egg and peanut.
Table 3.
Number‡ of children with food sensitization in NHANES III and NHANES 2005–2006
| Food Sensitization* | NHANES III (n = 4995**) |
NHANES 2005–2006 (n = 2901) |
|---|---|---|
| Milk | ||
| Overall | 526 | 254 |
| Moderate-level | 34 | 14 |
| High-level | 0 | 1 |
| Egg | ||
| Overall | 186 | 100 |
| Moderate-level | 7 | 7 |
| High-level | 0 | 3 |
| Peanut | ||
| Overall | 568 | 347 |
| Moderate-level | 201 | 153 |
| High-level | 29 | 35 |
| Shrimp | ||
| Overall | 712 | 257 |
| Moderate-level | 303 | 89 |
| High-level | 159 | 47 |
| Milk, Egg, or Peanut | ||
| Overall | 1034 | 565 |
| Moderate-level | 485 | 231 |
| High-level | 29 | 37 |
| All Foods | ||
| Overall | 1431 | 707 |
| Moderate-level | 485 | 231 |
| High-level | 182 | 80 |
Unweighted
Overall sensitization is defined as IgE ≥ 0.35 kU/L; moderate sensitization is defined as IgE ≥ 2 kU/L; high sensitization is defined as ≥15 kU/L for milk, ≥7 kU/L for egg, ≥14 kU/L for peanut, and ≥5 kU/L for shrimp.
n=4991 for milk, 4994 for shrimp, and 4995 for egg and peanut.
For the individual foods, there was no significant change in the prevalence of overall, moderate, or high-level sensitization to milk, egg, or peanut (Table 2). There was, however, a significant decrease in the prevalence of shrimp sensitization for overall (11.2; 95% CI 10.0 – 12.5 versus 6.1%; 95% CI 4.5 – 7.7; p = <0.001), moderate (4.1%; 95% CI 3.3 – 4.9 versus 1.7%; 95% CI 1.2 – 2.2; p < 0.001) and high-level sensitization (2.2%; 95% CI 1.6 – 2.9 versus 0.6%; 95% CI 0.4 – 0.9; p < 0.001).
To examine age-specific trends, we examined three age categories: 6–9 years, 10–15 years, and 16–19 years (Table 4). Overall shrimp sensitization decreased significantly between NHANES III and NHANES 2005–2006 in all age groups, whereas the decrease in moderate-level and high-level sensitization was only significant in children older than 10 years of age. In adolescents ages 16–19, a significant increase in high-level sensitization to the combination of milk, egg, or peanut was observed (0.1%; 95% CI 0 – 0.2 versus 0.7%; 95% CI 0.1 – 1.2; p = 0.02), which was due to an increase in high-level peanut sensitization (data not shown).
Table 4.
Changes in individual food sensitization by age
| Food Sensitization | NHANES III | NHANES 2005–2006 | p value† |
|---|---|---|---|
| 6–9 Years Old | |||
| Milk, Peanut, or Egg | |||
| Overall | 20.6 (17.0 – 24.3) | 21.4 (16.5 – 26.2) | 0.79 |
| Moderate-level | 3.9 (2.3 – 5.6) | 4.3 (2.6 – 5.9) | 0.76 |
| High-level | 0.9 (0.2 – 1.6) | 0.9 (0.1 – 1.7) | 0.97 |
| Shrimp | |||
| Overall | 8.3 (6.7 – 9.9) | 3.2 (1.7 – 4.6) | <0.00001 |
| Moderate-level | 2.3 (1.4 – 3.2) | 1.6 (0.5 – 2.6) | 0.31 |
| High-level | 0.7 (0.4 – 1.1) | 0.4 (0.0 – 0.8) | 0.26 |
| 10 – 15 Years Old | |||
| Milk, Peanut, or Egg | |||
| Overall | 19.7 (15.5 – 23.9) | 17.9 (14.5 – 21.4) | 0.51 |
| Moderate-level | 4.5 (2.8 – 6.2) | 6.1 (4.0 – 8.3) | 0.21 |
| High-level | 0.9 (0.1– 1.7) | 1.2 (0.3 – 2.2) | 0.56 |
| Shrimp | |||
| Overall | 11.3 (9.0 – 13.5) | 6.8 (4.8 – 8.7) | <0.01 |
| Moderate-level | 4.4 (2.9 – 5.8) | 1.1 (0.6 – 1.5) | <0.00001 |
| High-level | 2.6 (1.5 – 3.7) | 0.6 (0.3 – 1.0) | <0.001 |
| 16–19 Years Old | |||
| Milk, Peanut, or Egg | |||
| Overall | 13.4 (10.1 – 16.7) | 14.8 (9.8 – 19.8) | 0.62 |
| Moderate-level | 3.5 (2.0 – 4.9) | 4.2 (1.8 – 6.7) | 0.57 |
| High-level | 0.1 (0.0 – 0.2) | 0.7 (0.1 – 1.2) | 0.02 |
| Shrimp | |||
| Overall | 14.4 (11.5 – 17.3) | 8.7 (5.6 – 11.8) | 0.005 |
| Moderate-level | 5.9 (4.2 – 7.6) | 2.6 (1.5 – 3.7) | 0.001 |
| High-level | 3.4 (2.1 – 4.7) | 0.9 (0.4 – 1.4) | 0.0002 |
Values are % (95% CI)
p value for the calculated risk difference between NHANES III and NHANES 2005–2006
Standardization of NHANES III to match the racial/ethnic distribution that existed at the time of NHANES 2005–2006 did not affect the results (Table 5).
Table 5.
Prevalence of food sensitization in NHANES III and NHANES 2005–2006 with race/ethnicity standardization of NHANES III to NHANES 2005–6.
| Food Sensitization* | NHANES III Percentage (95% CI) (n = 4995**) |
NHANES 2005–2006 Percentage (95% CI) (n = 2901) |
p value† |
|---|---|---|---|
| Milk | |||
| Overall | 8.2 (7.1 – 9.3) | 8.1 (6.1 – 10.2) | 0.94 |
| Moderate-level | 0.4 (0.2 – 0.6) | 0.5 (0.1 – 0.9) | 0.58 |
| High-level | 0 | 0.008 (−0.01 – 0.02) | 0.33 |
| Egg | |||
| Overall | 3.2 (2.5 – 4.0) | 4.0 (2.9 – 5.1) | 0.22 |
| Moderate-level | 0.05 (0.01 – 0.08) | 0.2 (−0.004 – 0.5) | 0.10 |
| High-level | 0 | 0.05 (−0.01 – 0.1) | 0.10 |
| Peanut | |||
| Overall | 11.1 (9.8 – 12.5) | 10.5 (8.4 – 12.7) | 0.60 |
| Moderate-level | 3.6 (2.9 – 4.4) | 4.5 (3.3 – 5.7) | 0.22 |
| High-level | 0.6 (0.3 – 1.0) | 0.9 (0.4 – 1.4) | 0.37 |
| Shrimp | |||
| Overall | 11.1 (10.0 – 12.3) | 6.1 (4.5 – 7.7) | <0.001 |
| Moderate-level | 4.0 (3.3 – 4.8) | 1.7 (1.2 – 2.2) | <0.001 |
| High-level | 2.1 (1.6 – 2.7) | 0.6 (0.4 – 0.9) | <0.001 |
| Milk, Egg, or Peanut | |||
| Overall | 18.1 (16.5 – 19.7) | 18.2 (15.4 – 20.0) | 0.96 |
| Moderate-level | 4.0 (3.2– 4.8) | 4.9 (3.7 – 6.1) | 0.17 |
| High-level | 0.6 (0.3 – 1.0) | 0.9 (0.5 – 1.4) | 0.32 |
| All Foods | |||
| Overall | 24.1 (22.3 – 25.9) | 21.6 (19.5 – 23.7) | 0.056 |
| Moderate-level | 7.2 (6.2 – 8.1) | 6.2 (4.8 – 7.5) | 0.23 |
| High-level | 2.7 (2.0 – 3.3) | 1.5 (1.0 – 2.1) | 0.005 |
Values are reported as percentages (95% CIs)
p value for the calculated risk difference between NHANES III and NHANES 2005–2006
Overall sensitization is defined as IgE ≥ 0.35 kU/L; moderate sensitization is defined as IgE ≥ 2 kU/L; high sensitization is defined as ≥15 kU/L for milk, ≥7 kU/L for egg, ≥14 kU/L for peanut, and ≥5 kU/L for shrimp.
n=4991 for milk, 4994 for shrimp, and 4995 for egg and peanut.
Ethnicity-specific Trends Over Time
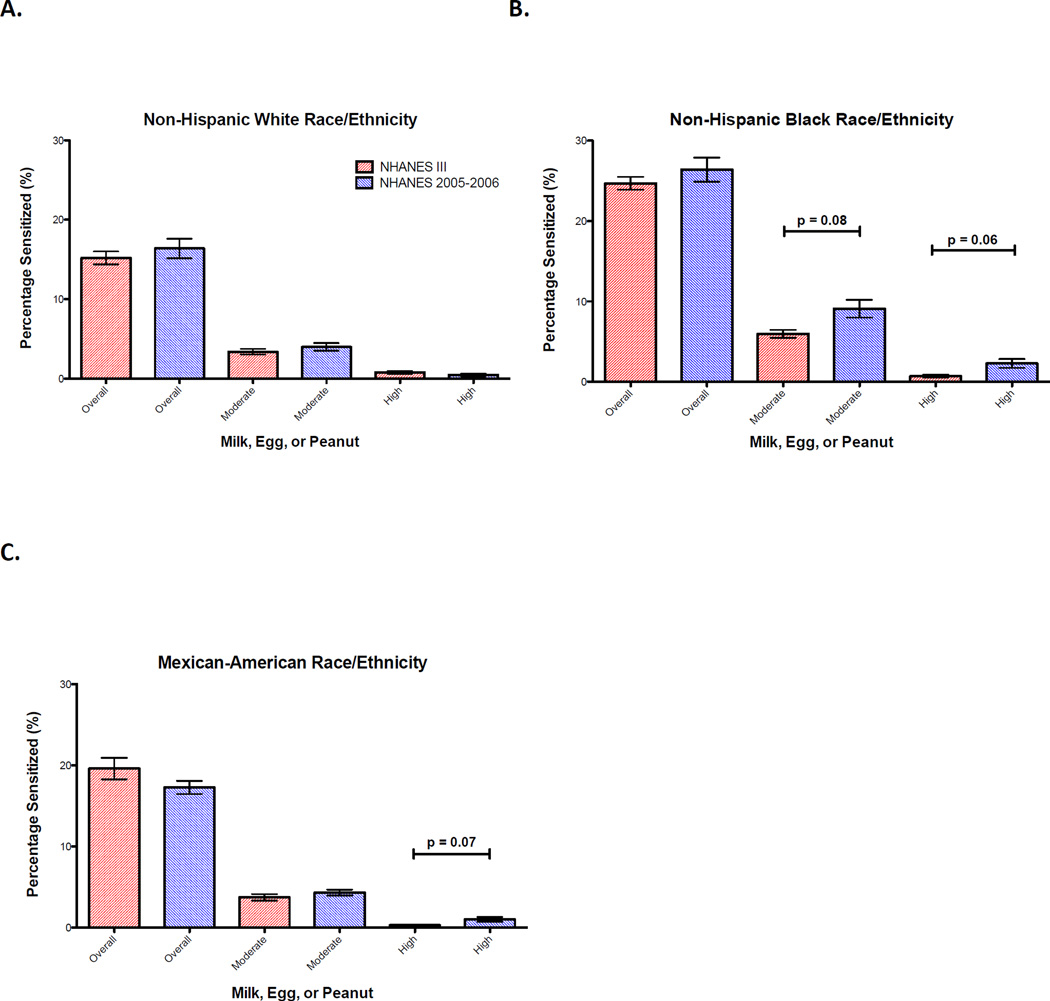
Overall, non-Hispanic Black children had higher levels of sensitization to milk, egg, peanut, and shrimp than Mexican-American and non-Hispanic White children in both surveys (Figure 1 and Table 6). While a decrease in overall and moderate shrimp sensitization was observed in all race/ethnicities, there was a trend towards an increase in moderate-level (6.0%; 95% CI 4.4 – 7.5 versus 9.1%; 95% CI 5.6 – 12.6; p = 0.08) and high-level sensitization (0.7%; 95% CI 0.2 – 1.2 versus 2.3%; 95% CI 0.5 – 4.0; p = 0.06) to the combination of milk, egg, or peanut in non-Hispanic Blacks (Figure 1). This was largely due to an increase in prevalence of moderate and high-level sensitization to peanut (Table 6). Among non-Hispanic White and Mexican-American children, there was no significant change in the prevalence of overall or moderate-level food sensitization to milk, egg, or peanut over time (Figure 1).
Figure 1. Trends in food sensitization over time by race/ethnicity.
Proportion of children ages 6–19 with overall, moderate-level, and high-level food-specific IgE levels to milk, egg, or peanut in NHANES III (red) and NHANES 2005–2006 (blue) by race/ethnicity. (A) Non-Hispanic White; (B) Non-Hispanic Black; (C) Mexican-American. Bars and whiskers represent 95% confidence intervals (CIs).
Table 6.
Prevalence of food sensitization by survey and race/ethnicity
| Food Sensitization | NHANES III | NHANES 2005–2006 | p value† |
|---|---|---|---|
| Non-Hispanic White | |||
| Milk | |||
| IgE ≥ 0.35 | 5.7 (4.0 – 7.4) | 7.3 (4.5 – 10.1) | 0.32 |
| IgE ≥2 | 0.03 (−0.03 – 0.09) | 0.5 (−0.1 – 1.0) | 0.11 |
| IgE≥15 | 0 | 0 | 1.0 |
| Egg | |||
| IgE ≥ 0.35 | 3.3 (2.1 – 4.4) | 4.4 (2.8 – 5.9) | 0.22 |
| IgE≥2 | 0 | 0.2 (−0.2 – 0.5) | 0.33 |
| IgE ≥ 7 | 0 | 0 | >0.99 |
| Peanut | |||
| IgE ≥ 0.35 | 10.7 (8.7 – 12.7) | 8.9 (5.8 – 11.9) | 0.30 |
| IgE ≥ 2 | 3.3 (2.4 – 4.2) | 3.5 (2.1 – 5.0) | 0.85 |
| IgE ≥ 14 | 0.8 (0.2 – 1.4) | 0.5 (−0.08 – 1.0) | 0.38 |
| Shrimp | |||
| IgE ≥ 0.35 | 7.1 (5.9 – 8.3) | 4.2 (2.5 – 5.9) | 0.004 |
| IgE≥2 | 1.9 (1.3 – 2.4) | 0.9 (0.2 – 1.5) | 0.01 |
| IgE ≥ 5 | 0.9 (0.4 – 1.3) | 0 | <0.001 |
| Non-Hispanic Black | |||
| Milk | |||
| IgE ≥0.35 | 11.7 (10.1 – 13.3) | 11.7 (8.7 – 14.6) | 0.97 |
| IgE ≥ 2 | 0.9 (0.4 – 1.3) | 0.9 (0.3 – 1.5) | 0.96 |
| IgE ≥ 15 | 0 | 0.06 (−0.06 – 0.2) | 0.30 |
| Egg | |||
| IgE ≥ 0.35 | 4.4 (3.3 – 5.6) | 4.8 (3.2 – 6.3) | 0.72 |
| IgE ≥ 2 | 0.2 (−0.0009 – 0.4) | 0.4 (−0.1 – 1.0) | 0.43 |
| IgE ≥ 7 | 0 | 0.2 (−0.01 – 0.5) | 0.19 |
| Peanut | |||
| IgE≥0.35 | 14.0 (11.6 – 16.5) | 17.0 (12.2 – 21.7) | 0.25 |
| IgE ≥2 | 5.1 (3.6 – 6.7) | 8.6 (5.1 – 12.0) | 0.06 |
| IgE ≥ 14 | 0.7 (0.2 – 1.2) | 2.2 (0.5– 3.9) | 0.07 |
| Shrimp | |||
| IgE ≥ 0.35 | 21.1 (18.9 – 23.3) | 12.5 (9.1 – 15.8) | <0.001 |
| IgE ≥ 2 | 11.3 (9.7 – 12.8) | 5.8 (3.6 – 8.1) | <0.001 |
| IgE ≥ 5 | 6.7 (5.3 – 8.1) | 3.5 (2.1 – 4.9) | <0.001 |
| Mexican-American | |||
| Milk | |||
| IgE≥0.35 | 10.7 (7.8 – 13.6) | 9.0 (6.6 – 11.5) | 0.36 |
| IgE ≥ 2 | 0.4 (0.1 – 0.7) | 0.3 (−0.2 – 0.8) | 0.57 |
| IgE ≥ 15 | 0 | 0 | >0.99 |
| Egg | |||
| IgE ≥ 0.35 | 2.7 (1.8 – 3.7) | 2.8 (1.6 – 4.1) | 0.92 |
| IgE ≥ 2 | 0.5 (0.02 – 1.0) | 0.1 (−0.2 – 0.4) | 0.98 |
| IgE ≥ 7 | 0 | 0.1 (−0.2 – 0.4) | 0.32 |
| Peanut | |||
| IgE ≥ 0.35 | 10.2 (7.9 – 12.6) | 10.1 (7.7 – 12.6) | 0.98 |
| IgE ≥2 | 3.3 (2.4 – 4.2) | 3.9 (2.9 – 4.8) | 0.36 |
| IgE ≥14 | 0.3 (0.004 – 0.5) | 0.9 (0.05 – 1.7) | 0.14 |
| Shrimp | |||
| IgE ≥0.35 | 12.0 (8.8 – 15.1) | 7.8 (5.1 – 10.5) | 0.04 |
| IgE ≥ 2 | 3.6 (2.1 – 5.2) | 1.9 (1.0 – 2.8) | 0.054 |
| IgE ≥5 | 1.1 (0.6 – 1.7) | 0.8 (0.2 – 1.5) | 0.43 |
Values are % (95% CI)
p value for the calculated risk difference between NHANES III and NHANES 2005–2006
Discussion
In this comparison of two nationally-representative surveys conducted in a similar fashion approximately 15 years apart, we found that sensitization to milk, peanut, and egg, as measured by food-specific IgE, remained constant, and sensitization to shrimp decreased. These findings are contrary to our hypothesis that sensitization to common food allergens increased in recent years, and stand in contrast with a body of literature showing increased prevalence of self-reported food allergy and increased health-care utilization for its manifestations.
Evidence supporting an increasing prevalence over the past several decades of food allergy in general, and peanut allergy in particular, comes from a variety of sources, but has not included biomarkers of food allergy. Within the National Health Interview Survey, parental-reported food allergy among children increased from 3.4% in 1997–1999 to 5.1% in 2009–2011(4), while Sicherer et al demonstrated that the proportion of children with self-reported peanut allergy increased from 0.4% in 1997 to 0.8% in 2002 to 1.2% in 2008 in a series of telephone surveys.(1) Similar trends have been seen in manifestations of food allergy, including emergency room visits,(16) hospital discharges,(17) and anaphylaxis.(18, 19) Although some of these studies, such as the National Health Interview Survey, were not designed to specifically elicit symptoms consistent with IgE-mediated allergy, Sicherer et al’s tailored questionnaires about peanut allergy and data on food-related anaphylaxis suggest increases in prevalence of IgE mediated allergy in general, and peanut allergy in particular, in the U.S. Because IgE sensitization is a necessary component of IgE-mediated food allergy, it would be reasonable to expect that sensitization to common food allergens would have increased over the time period when self and parental report of and manifestations of food allergy appear to have increased. Our surprising finding that sensitization did not increase, if true, suggests that changes in IgE sensitization to common food allergens is not the cause of the apparent increase in self-reported and diagnosed food allergy. Instead, we propose two possible hypotheses to reconcile our results with the large body of literature suggesting increases in self-reported and diagnosed food allergy: 1) it is the relationship between sensitization and IgE-mediated food allergy that has changed over time or 2) the apparent increase in food allergy prevalence is the result of increased recognition and diagnosis rather than a true increase in IgE-mediated food allergy.
The recent LEAP trial of early peanut introduction provided persuasive evidence that peanut avoidance in infancy may increase the rate of clinical reactivity, but there was no difference in rates of IgE sensitization to peanut or mean peanut-specific IgE levels between those children who consumed or avoided the food.(5) Thus our findings could be consistent with the idea that delayed introduction to peanut and other allergens contributed to higher rates of clinical food allergy over the years studied, despite similar rates of sensitization. Besides delayed introduction, it is also possible that other changing environmental exposures may have affected the relationship between sensitization and clinical allergy during this time. Although it is well known that only a minority of those sensitized to foods have clinical manifestations of food allergy(20), little research has been done on the factors that may promote or protect against the development of clinical disease among those sensitized to foods. Screening data from the LEAP study suggested that specific IgE may have a weaker relationship to clinical disease among non-White populations for unclear reasons(21). For allergic rhinitis, there is some evidence from the International Study of Asthma and Allergies in Childhood that the relationship between sensitization to aeroallergens and clinical rhinitis and asthma is stronger in affluent than non-affluent countries(22, 23). One hypothesis for these findings is differences in infectious exposures, although geographic differences between sensitization and clinical disease are not fully understood. Further research into the environmental factors that may affect the relationship between sensitization and clinical allergy could identify modifiable targets for prevention of food allergy and other allergic diseases.
Alternatively, these findings could be consistent with changing recognition of food allergy. It is well known that over-perception of food allergy is common, and it is possible that perception of food allergy has changed over time as awareness has grown. When based on self-report, the prevalence of food allergy ranges from 3 to 35%, whereas when based on more objective measures, such as oral food challenges, the prevalence decreases to 1–11%.(9) Previous studies have further shown that among children with parental-reported food allergy, 30% were not diagnosed by a physician, and only 20% of those who were seen by a physician underwent an oral food challenge.(24) Physician diagnosis may also be unreliable, as food allergy is commonly over-diagnosed(25) and recognition and management of anaphylaxis can be quite variable.(26–28) Thus, it is possible that the apparent increase in food allergy seen in previous reports based on self-report and diagnosis codes is at least in part a result of changing recognition, rather than changing clinical disease.
While levels of overall sensitization to milk, egg, and peanut did not change between NHANES III and NHANES 2005–2006, there was a trend towards increased moderate and high-level sensitization to the combination of milk, egg, and peanut among children of Non-Hispanic Black race/ethnicity. Thus, we cannot fully exclude increased sensitization as an explanation for the trends towards increasing racial/ethnic disparities in self-reported food allergy.(2, 29) Whether these trends are due to gene-environment interactions, differences in the distribution of environmental risk factors or changes in recognition remains unclear and warrants further study.
Finally, we found that sensitization to shrimp decreased significantly between NHANES III and NHANES 2005–2006 across all racial/ethnic groups and ages. The major shrimp allergen, tropomysin, is cross-reactive with cockroach, dust mite and nematodes(30). It has been suggested that cockroach and/or dust mite exposure may drive shrimp sensitization, and it is possible that changes in exposure and sensitization to these cross-reactive allergens could account for these findings.(31, 32) As there is little longitudinal data on either shellfish or cockroach allergy in the United States, this is an area that clearly requires further research.
This study is limited by the fact that food sensitization was only assessed in children 6 and older because stored sera was not available in younger children in NHANES III. While the prevalence of food allergy to milk and egg is greatest in infancy, and different trends may have been seen in this age group, the group studied represents sensitization trends observed in the clinically important category of persistent food allergy. However, we cannot exclude the possibility that food sensitization increased among those born after the year 2000, or alternatively prior to NHANES III. Our study is further limited by the lack of clinical data to support or refute a diagnosis of true food allergy in these surveys, and we cannot give an estimate of the true rate of clinical reactivity to foods based on this data. We used previously defined levels of IgE to indicate moderate- and high- level IgE, but it is not clear how these IgE levels relate to risk of clinical allergy.2,20 Similarly, we could not directly compare changes in self-reported food allergy over time to changes in sensitization in these surveys, so we cannot exclude the possibility that self-reported food allergy did not differ between these two study populations, although changes in self-reported food allergy have been shown in many other studies. Food-specific IgE was measured in stored sera, which may have resulted in degradation of IgE, potentially resulting in measurement bias. However, given the similar sera collection, handling, and storage techniques, as well as the known stability of IgE in the setting of repeated freeze/thaw cycles, this measurement error is likely to be minimal. (33, 34) In addition, we only assessed IgE to milk, egg, peanut, and shrimp, so cannot exclude the possibility that other food allergens showed different sensitization trends. Finally, we did not measure allergen component-specific IgE (such as to Arah2), which might have provided further information on children more likely to be clinically reactive to certain foods.(35–37)
However, these weaknesses are balanced by several strengths: (1) NHANES are large cross-sectional surveys of the US population of children and are hence generalizable, (2) the same laboratory methods were performed by the same laboratory for both surveys, and (3) this is the only population-based comparison of food sensitization that has been performed to date in the U.S.
In conclusion, in this comparison of two nationally representative surveys conducted in a similar fashion approximately 15 years apart, we found that prevalence of positive food-specific IgE to milk, peanut, and egg remained constant among 6–19 year olds, whereas shrimp-specific IgE levels decreased. This finding is in sharp contrast to other studies that have reported a recent rise in self-report of food allergy and in health care utilization and suggests either changes in the relationship between sensitization and clinical allergy or changes in the recognition and diagnosis of food allergy.
Highlights.
What is already known about this topic?
Many studies of self-reported food allergy and health care utilization for food allergy have shown increased prevalence in the past three decades, but there is no data about changes in biomarkers of food allergy.
What does this article add to our knowledge?
In this study of stored sera from NHANES III compared to analyses from NHANES 2005–6, we found that prevalence of IgE sensitization to peanut, milk, egg and shrimp did not increase from the late 1980s/early 1990s to the mid-2000s.
How does this study impact current management guidelines?
These surprising results suggest that changes in IgE sensitization to common food allergens is not the cause of the apparent increase in self-reported and diagnosed food allergy in the U.S. over the past several decades.
Acknowledgments
This work was funded by the NIH through the following grants: (NIAID) 1K23AI103187, (NCRR) 1KL2TR001077, and (NIAID) R21AI107085 and by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences (Z01 ES025041). The funders had no role in the design, analysis, or interpretation of this work.
Abbreviations used
- NHANES
National Health and Nutrition Examination Survey
- NCHS
National Center for Health Statistics
- CDC
Centers for Disease Control
- PIR
Poverty Income Ratio
- IgE
Immunoglobulin E
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no relevant conflicts to disclose.
Contributor Information
Emily C. McGowan, Email: emcgowa4@jhmi.edu, Johns Hopkins University School of Medicine, Division of Allergy and Clinical Immunology, and Graduate Student, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD.
Roger Peng, Email: rpeng@jhsph.edu, Johns Hopkins Bloomberg School of Public Health, Department of Biostatistics, Baltimore, MD.
Päivi M. Salo, Email: salo1@niehs.nih.gov, The Division of Intramural Research, National Institute of Environmental Health Sciences, National Institutes of Health, Research Triangle Park, NC.
Darryl C. Zeldin, Email: zeldin@niehs.nih.gov, The Division of Intramural Research, National Institute of Environmental Health Sciences, National Institutes of Health, Research Triangle Park, NC.
Corinne A. Keet, Email: ckeet1@jhmi.edu, Johns Hopkins University School of Medicine, Division of Pediatric Allergy and Immunology.
References
- 1.Liu AH, Jaramillo R, Sicherer SH, Wood RA, Bock SA, Burks AW, et al. National prevalence and risk factors for food allergy and relationship to asthma: results from the National Health and Nutrition Examination Survey 2005–2006. The Journal of allergy and clinical immunology. 2010 Oct;126(4):798–806. e13. doi: 10.1016/j.jaci.2010.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keet CA, Savage JH, Seopaul S, Peng RD, Wood RA, Matsui EC. Temporal trends and racial/ethnic disparity in self-reported pediatric food allergy in the United States. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. 2014 Mar;112(3):222–229. e3. doi: 10.1016/j.anai.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Venter C, Hasan Arshad S, Grundy J, Pereira B, Bernie Clayton C, Voigt K, et al. Time trends in the prevalence of peanut allergy: three cohorts of children from the same geographical location in the UK. Allergy. 2010 Jan;65(1):103–108. doi: 10.1111/j.1398-9995.2009.02176.x. [DOI] [PubMed] [Google Scholar]
- 4.Jackson KD, Howie LD, Akinbami LJ. Trends in allergic conditions among children: United States, 1997–2011. NCHS data brief. 2013 May;(121):1–8. [PubMed] [Google Scholar]
- 5.Fleischer DM, Sicherer S, Greenhawt M, Campbell D, Chan E, Muraro A, et al. Consensus Communication on Early Peanut Introduction and Prevention of Peanut Allergy in High-Risk Infants. Pediatric dermatology. 2015 Sep 10; [Google Scholar]
- 6.Sausenthaler S, Heinrich J, Koletzko S. Early diet and the risk of allergy: what can we learn from the prospective birth cohort studies GINIplus and LISAplus? The American journal of clinical nutrition. 2011 Dec;94(6 Suppl):2012S–2017S. doi: 10.3945/ajcn.110.001180. [DOI] [PubMed] [Google Scholar]
- 7.Keet CA, Matsui EC, Savage JH, Neuman-Sunshine DL, Skripak J, Peng RD, et al. Potential mechanisms for the association between fall birth and food allergy. Allergy. 2012 Jun;67(6):775–782. doi: 10.1111/j.1398-9995.2012.02823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Azad MB, Konya T, Guttman DS, Field CJ, Sears MR, HayGlass KT, et al. Infant gut microbiota and food sensitization: associations in the first year of life. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2015 Mar;45(3):632–643. doi: 10.1111/cea.12487. [DOI] [PubMed] [Google Scholar]
- 9.Rona RJ, Keil T, Summers C, Gislason D, Zuidmeer L, Sodergren E, et al. The prevalence of food allergy: a meta-analysis. The Journal of allergy and clinical immunology. 2007 Sep;120(3):638–646. doi: 10.1016/j.jaci.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention (CDC) National Center for Health Statistics (NCHS) National Health and Nutrition Examination Survey Data. Hyattsville, MD: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2005–2006. 1988–1994. [Google Scholar]
- 11.Sampson HA, Ho DG. Relationship between food-specific IgE concentrations and the risk of positive food challenges in children and adolescents. The Journal of allergy and clinical immunology. 1997 Oct;100(4):444–451. doi: 10.1016/s0091-6749(97)70133-7. [DOI] [PubMed] [Google Scholar]
- 12.Sampson HA. Utility of food-specific IgE concentrations in predicting symptomatic food allergy. The Journal of allergy and clinical immunology. 2001 May;107(5):891–896. doi: 10.1067/mai.2001.114708. [DOI] [PubMed] [Google Scholar]
- 13.Lehmann ECG. Theory of Point Estimation. 2nd. New York, New York: Springer; 1998. [Google Scholar]
- 14.Rosner B. Fundamentals of Biostatistics. Boston, MA: Brooks/Cole, Cengage Learning; 2011. [Google Scholar]
- 15.Age Standardizaiton and Population Counts. [cited 2015 9/9/2015];National Center for Health Statistics. 2014 [Google Scholar]
- 16.Rudders SA, Banerji A, Vassallo MF, Clark S, Camargo CA., Jr Trends in pediatric emergency department visits for food-induced anaphylaxis. The Journal of allergy and clinical immunology. 2010 Aug;126(2):385–388. doi: 10.1016/j.jaci.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 17.Branum AM, Lukacs SL. Food allergy among U.S. children: trends in prevalence and hospitalizations. NCHS data brief. 2008 Oct;(10):1–8. [PubMed] [Google Scholar]
- 18.Rudders SA, Arias SA, Camargo CA., Jr Trends in hospitalizations for food-induced anaphylaxis in US children, 2000–2009. The Journal of allergy and clinical immunology. 2014 Oct;134(4):960–962. e3. doi: 10.1016/j.jaci.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 19.Lin RY, Anderson AS, Shah SN, Nurruzzaman F. Increasing anaphylaxis hospitalizations in the first 2 decades of life: New York State, 1990–2006. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. 2008 Oct;101(4):387–393. doi: 10.1016/S1081-1206(10)60315-8. [DOI] [PubMed] [Google Scholar]
- 20.Keet CA, Wood RA, Matsui EC. Limitations of reliance on specific IgE for epidemiologic surveillance of food allergy. The Journal of allergy and clinical immunology. 2012 Nov;130(5):1207–1209. e10. doi: 10.1016/j.jaci.2012.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Du Toit G, Roberts G, Sayre PH, Plaut M, Bahnson HT, Mitchell H, et al. Identifying infants at high risk of peanut allergy: the Learning Early About Peanut Allergy (LEAP) screening study. The Journal of allergy and clinical immunology. 2013 Jan;131(1):135–143. e1–e12. doi: 10.1016/j.jaci.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 22.Yemaneberhan H, Bekele Z, Venn A, Lewis S, Parry E, Britton J. Prevalence of wheeze and asthma and relation to atopy in urban and rural Ethiopia. Lancet. 1997 Jul 12;350(9071):85–90. doi: 10.1016/S0140-6736(97)01151-3. [Comparative Study Research Support, Non-U.S. Gov't] [DOI] [PubMed] [Google Scholar]
- 23.Kaur B, Anderson HR, Austin J, Burr M, Harkins LS, Strachan DP, et al. Prevalence of asthma symptoms, diagnosis, and treatment in 12–14 year old children across Great Britain (international study of asthma and allergies in childhood, ISAAC UK) Bmj. 1998 Jan 10;316(7125):118–124. doi: 10.1136/bmj.316.7125.118. [Research Support, Non-U.S. Gov't] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gupta RS, Springston EE, Smith B, Pongracic J, Holl JL, Warrier MR. Parent report of physician diagnosis in pediatric food allergy. The Journal of allergy and clinical immunology. 2013 Jan;131(1):150–156. doi: 10.1016/j.jaci.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 25.Fleischer DM, Bock SA, Spears GC, Wilson CG, Miyazawa NK, Gleason MC, et al. Oral food challenges in children with a diagnosis of food allergy. The Journal of pediatrics. 2011 Apr;158(4):578–583. e1. doi: 10.1016/j.jpeds.2010.09.027. [DOI] [PubMed] [Google Scholar]
- 26.Ross MP, Ferguson M, Street D, Klontz K, Schroeder T, Luccioli S. Analysis of food-allergic and anaphylactic events in the National Electronic Injury Surveillance System. The Journal of allergy and clinical immunology. 2008 Jan;121(1):166–171. doi: 10.1016/j.jaci.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 27.Campbell RL, Hagan JB, Manivannan V, Decker WW, Kanthala AR, Bellolio MF, et al. Evaluation of national institute of allergy and infectious diseases/food allergy and anaphylaxis network criteria for the diagnosis of anaphylaxis in emergency department patients. The Journal of allergy and clinical immunology. 2012 Mar;129(3):748–752. doi: 10.1016/j.jaci.2011.09.030. [DOI] [PubMed] [Google Scholar]
- 28.Klein JS, Yocum MW. Underreporting of anaphylaxis in a community emergency room. The Journal of allergy and clinical immunology. 1995 Feb;95(2):637–638. doi: 10.1016/s0091-6749(95)70329-2. [DOI] [PubMed] [Google Scholar]
- 29.Sicherer SH, Munoz-Furlong A, Sampson HA. Prevalence of peanut and tree nut allergy in the United States determined by means of a random digit dial telephone survey: a 5-year follow-up study. The Journal of allergy and clinical immunology. 2003 Dec;112(6):1203–1207. doi: 10.1016/s0091-6749(03)02026-8. [DOI] [PubMed] [Google Scholar]
- 30.Reese G, Ayuso R, Lehrer SB. Tropomyosin: an invertebrate pan-allergen. Int Arch Allergy Immunol. 1999 Aug;119(4):247–258. doi: 10.1159/000024201. [DOI] [PubMed] [Google Scholar]
- 31.Fernandes J, Reshef A, Patton L, Ayuso R, Reese G, Lehrer SB. Immunoglobulin E antibody reactivity to the major shrimp allergen, tropomyosin, in unexposed Orthodox Jews. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2003 Jul;33(7):956–961. doi: 10.1046/j.1365-2222.2003.01722.x. [DOI] [PubMed] [Google Scholar]
- 32.Wang J, Calatroni A, Visness CM, Sampson HA. Correlation of specific IgE to shrimp with cockroach and dust mite exposure and sensitization in an inner-city population. The Journal of allergy and clinical immunology. 2011 Oct;128(4):834–837. doi: 10.1016/j.jaci.2011.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hill PB, DeBoer DJ. Quantification of serum total IgE concentration in dogs by use of an enzyme-linked immunosorbent assay containing monoclonal murine anti-canine IgE. American journal of veterinary research. 1994 Jul;55(7):944–948. [PubMed] [Google Scholar]
- 34.Henderson CE, Ownby D, Klebanoff M, Levine RJ. Stability of immunoglobulin E (IgE) in stored obstetric sera. Journal of immunological methods. 1998 Apr 1;213(1):99–101. doi: 10.1016/s0022-1759(98)00014-3. [DOI] [PubMed] [Google Scholar]
- 35.Asarnoj A, Moverare R, Ostblom E, Poorafshar M, Lilja G, Hedlin G, et al. IgE to peanut allergen components: relation to peanut symptoms and pollen sensitization in 8-year-olds. Allergy. 2010 Sep;65(9):1189–1195. doi: 10.1111/j.1398-9995.2010.02334.x. [DOI] [PubMed] [Google Scholar]
- 36.Koppelman SJ, Wensing M, Ertmann M, Knulst AC, Knol EF. Relevance of Ara h1, Ara h2 and Ara h3 in peanut-allergic patients, as determined by immunoglobulin E Western blotting, basophil-histamine release and intracutaneous testing: Ara h2 is the most important peanut allergen. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2004 Apr;34(4):583–590. doi: 10.1111/j.1365-2222.2004.1923.x. [DOI] [PubMed] [Google Scholar]
- 37.Nicolaou N, Poorafshar M, Murray C, Simpson A, Winell H, Kerry G, et al. Allergy or tolerance in children sensitized to peanut: prevalence and differentiation using component-resolved diagnostics. The Journal of allergy and clinical immunology. 2010 Jan;125(1):191–197. e1–e13. doi: 10.1016/j.jaci.2009.10.008. [DOI] [PubMed] [Google Scholar]