Abstract
The nociceptin/orphanin FQ peptide (NOP) opioid receptors regulate neurotransmitter release via inhibition of voltage-gated Ca2+ channels (CaV2.2) in sympathetic and sensory neurons. Stimulation of NOP receptors by its endogenous agonist, nociception (Noc), leads to membrane-delimited, voltage-dependent (VD) block of CaV2.2 channel currents mediated by Gβγ protein subunits. Previously we reported that the pertussis toxin-sensitive Gαi1 and Gβ2/β4 isoforms mediate the functional coupling of NOP opioid receptors with CaV channels in rat stellate ganglion (SG) sympathetic neurons. In the present report we extended our studies by identifying the Gγ subunit that forms the heterotrimer within this signaling pathway. Small interference RNA (or siRNA) was employed to silence the expression of the natively expressed Gγ subunits. Initial PCR assays indicated that SG neurons expressed seven Gγ subunits. Silencing Gγ3 subunits did not alter signaling between NOP receptors and Ca2+ channels. However, after Gγ7 isoforms were silenced, the Noc-mediated inhibition of CaV channels was significantly decreased when compared to SG neurons transfected with scrambled siRNA. We observed that Gγ10 and Gγ11 mRNA levels increased 2.5- and 2.7-fold, respectively, after Gγ7 subunits were silenced. However, this compensatory increase in mRNA expression did not appear to fully rescue the NOP receptor coupling efficiency. Additionally, both Gγ2 and Gγ5 levels increased 50 and 75%, respectively, while Gγ3 and Gγ4 expression levels remained relatively unchanged. Taken together, our findings suggest that the Gαi1/Gβ2(β4)/Gγ7 heterotrimeric G protein complex determines the NOP receptor-mediated modulation of CaV channels in SG neurons.
Keywords: G protein subunits, opioid receptors, Ca2+ channels, G protein-coupled receptors
1. Introduction
Nociceptin/orphanin FQ peptide (NOP) opioid receptors are G protein-coupled receptors (GPCR) that are activated by the endogenous ligand, nociceptin (Noc). The NOP receptor system has been demonstrated to regulate processes including cardiovascular, pain, stress, renal function, learning and memory [1–2]. Recent studies also have shown that this opioid receptor plays an important role in sepsis and inflammatory conditions [3–4]. NOP receptor activation leads to voltage-gated Ca2+ channel (CaV) inhibition, G protein inwardly rectifying K+ (GIRK) channel activation and negative coupling to adenylyl cyclase [1–2]. CaV2.2 channels, the major Ca2+ ion carriers in SG neurons [5], have been shown to interact with NOP receptors, which influences their trafficking to the cell surface and internalization processes [6–7].
SG neurons, which innervate cardiac muscle and exert chronotropic and inotropic effects, express NOP opioid receptors [8–9]. Noc mediates its effects by coupling NOP receptors to members of the pertussis toxin (PTX)-sensitive Gαi/o family of heterotrimeric G proteins [1]. Previously, we reported that the Noc-mediated modulation of CaV currents occurred primarily via PTX-sensitive Gαi1 protein subunits in rat SG neurons [10]. Subsequently, employing small interference RNA (siRNA) approach, both Gβ2 and Gβ4 subunits were shown to mediate the coupling of NOP receptors with CaV channels [11].
Gγ subunits are crucial for efficient attachment of the Gβγ complex to the cell membrane. There are twelve known Gγ subunits [12] and recent studies have indicated that the interaction between GPCR and effector proteins can be influenced by Gγ proteins [13–16]. In the present study, we investigated whether specific Gγ subunits influenced coupling specificity of NOP opioid receptors with CaV channels in rat SG neurons.
2. Materials and Methods
2.1. Rat SG tissue isolation
The experiments performed on animals were approved by the Penn State College Medicine Institutional Animal Care and Use Committee. Male Sprague-Dawley rats were anesthetized with CO2 and then rapidly decapitated with a laboratory guillotine. After the SG tissue was removed, it was desheathed in ice-cold Hanks' balanced salt solution (Sigma-Aldrich, St. Louis, MO). Following removal of the connective tissue, multiple parallel slits (~ 1 mm apart) were made perpendicular to the long axis. The SG tissue was then placed in an RNAse- and DNAse-free 2 ml microcentrifuge tube containing optimum minimal essential medium (O-MEM, Thermo-Fisher Scientific, Grand Island, NY) supplemented with 2 mM 2,3-butanedione monoxime (BDM, Sigma-Aldrich) and kept on ice until ready for siRNA transfection.
2.2. Small intereference RNA (siRNA) transfection
SG neurons were transfected with siRNA employing both electroporation and lipofection as previously described [11]. Gγ7 subunit siRNA sequences designed in this study were chosen based on an eight point criteria with a scoring system (10 = best, 1 = worse) [17]. The sequences were obtained with a macro written by Dr. Stephen R. Ikeda (National Institutes of Health/NIAAA) on Igor Pro 6.37 (WaveMetrics, Inc., Lake Oswego, OR). The target siRNA oligonucleotide sequences for rat Gγ7, score in parenthesis, were as follows: sense, 5 –UGA UGU CAG GUA CUA ACA AUU-3 (8) corresponding to nucleotide positions 2–20. The antisense sequence was 5 –UUG UUA GUA CCU GAC AUC AUU-3. The control SG tissue was transfected with scrambled siRNA sequences (both from Thermo-Fisher Scientific).
Immediately after dissection, the SG tissues were preserved in ice-cold Opti-MEM and BDM prior to transfer to the electroporation solution. The lipofection solutions were prepared first and contained Opti-MEM, scrambled siRNA or Gγ7 siRNA (both at 1.5 μM), BDM (2 mM) and 10 μl Lipofectamine 2000 (Thermo-Fisher Scientific) in a final volume of 1 ml per group. Thereafter, each lipofection solution was transferred to a 22 mm dish (12-well plate) and stored in a 37°C humidified incubator (95% air-5% CO2) until ready for use. The electroporation solutions were prepared by mixing R solution (provided with NEON electroporator kit, Thermo-Fisher Scientific) with either scrambled siRNA (1.5 μM) or Gγ7 (1.5 μM) siRNA and BDM (2 mM). The ganglia were then transferred into their respective solution for 15–20 min at room temperature. After the incubation period in the electroporation solution, the ganglia were drawn up into a 100 μl electroporator tip and electroporated using the NEON electroporator (Thermo-Fisher Scientific), 3 pulses, each 1000 V and for 20 msec.
Immediately after electroporation the ganglia were placed in the 22 mm dish containing the appropriate lipofection solution and incubated for 5 hr in the humidified incubator. Following the 5 hr incubation, the ganglia were rinsed 3 times with MEM (minimum essential medium, Thermo Fisher Scientific) and placed back in the incubator. The transfection procedure was repeated 48 and 96 hr later.
2.3. SG tissue dispersion
Following the transfection period, the SG tissue was enzymatically dissociated as previously described [11]. Briefly, the tissue was incubated in a shaking water bath (35°C) for 60 min in Earle's balanced salt solution containing 0.6 mg/ml collagenase Type D (Roche Diagnostics, Indianapolis, IN), 0.4 mg/ml trypsin (Worthington Biochemicals, Freehold, NJ) and 0.1 mg/ml DNase (Sigma-Aldrich). Afterwards, the cells were dissociated in the flask by vigorous shaking and the neuron suspension was centrifuged twice for 6 min at 53 × g and resuspended in MEM supplemented with 10% fetal bovine serum, 1% penicillin-streptomycin and 1% glutamine. The dispersed neurons were plated onto 35 mm poly-L-lysine-coated dishes and stored in a humidified incubator.
2.4. Electrophysiology and data analysis
SG Ca2+ channel currents were recorded at room temperature employing the whole-cell variant of the patch-clamp technique. Recording pipettes were pulled on a micropipette puller (P-97, Sutter Instrument, Novato, CA) and fire polished on a microforge. Ca2 channel currents were acquired with a patch-clamp amplifier (Axopatch 200B, Molecular Devices, Sunnyvale, CA), analog filtered at 2 kHz (−3 dB; 4-pole low-pass Bessel filter) and digitized at 10 kHz with an A/D converter board (ITC-18, HEKA Instruments Inc., Bellmore, NY) employing custom-designed software (S5, Dr. Stephen R. Ikeda, NIH/NIAAA) on a PowerMacG4 computer (Apple Inc., Cupertino, CA). The series resistance and membrane capacitance were electronically compensated (80–85%). Ca2 currents were evoked with the triple-pulse voltage protocol [18]. This protocol is routinely employed to study the agonist-mediated voltage-dependent inhibition of Ca2 channel currents. The protocol consists of a test pulse to +10 mV (prepulse) followed by a large depolarizing conditioning test pulse to +80 mV, a brief return to −80 mV, and followed by a test pulse to +10 mV (postpulse). The peak Ca2 current amplitude was measured isochronally 10 msec after the initiation of the prepulse and postpulse. Data and statistical analyses were performed with Igor Pro 6.37 and Prism 6.0 (GraphPad Software, Inc., San Diego, CA) software packages with P < 0.05 considered statistically significant. Current traces and graphs were created with both Igor Pro 6.37 and Graphic (Autodesk, San Rafael, CA) software packages. The data shown are mean ± SE.
2.5. Solutions and drugs
The internal pipette solution contained (in mM): 80 N-methyl-D-glucamine, 20 tetraethylammonium hydroxide (TEA-OH), 11 EGTA, 10 HEPES, 1 CaCl2, 4 Mg-ATP, 0.3 Na2GTP, 20 CsCl, 40 CsOH and 14 tris creatine phosphate. Methanesulfonic acid was used to adjust the final pH to 7.2 and the osmolality ranged from 293–303 mOsmol/kg. The external solution consisted of (in mM): 145 TEA-OH, 140 methanesulfonic acid, 10 HEPES, 15 glucose, 10 CaCl2, and 0.0003 tetrodotoxin. The pH was adjusted to 7.4 with TEA-OH and the osmolality ranged from 316–320 mOsmol/kg. A stock solution of Noc (Tocris Cookson, Ellisville, MO) was prepared in water and diluted in the external solution to its final concentration.
2.6. Quantitative real-time-PCR (QRT-PCR)
In this set of experiments, RNA from SG tissue was isolated with the Nucleospin RNA/Protein Kit (Macherey-Nagel, Bethlehem, PA). RNA concentrations were determined with the Qubit®2.0 fluorometer (Thermo Fisher Scientific) and stored at −80°C until ready for use. For QRT-PCR assays, equal quantities of total RNA were used to synthesize cDNA using the High Capacity cDNA RT Kit (Thermo Fisher Scientific). For the QRT-PCR reactions, we employed the TaqMan Gene Expression Assay (Thermo Fisher Scientific) specific for rat Gγ and GAPDH with equal cDNA quantities for each group. The assays were then run on a QuantStudio 12K Flex Real-Time PCR system (Thermo Fisher Scientific) and analyzed using the comparative Ct method. The results were normalized to internal GAPDH mRNA controls.
3. Results
To determine which of the known Gγ subunits couple NOP receptors to CaV, we first focused on the Gγ3 isoform, which has been reported to be involved in mu opioid receptor signaling cascades associated with rewarding of high fat diets in female mice [19]. Thus, Gγ3 subunits were silenced in SG neurons. QRT-PCR assays of SG tissue were performed 72, 96 and 120 hr post-siRNA transfection. When compared to scrambled siRNA-transfected SG tissue, Gγ3 mRNA levels were 76, 92 and 91% lower 72, 96, and 120 hr post-transfection, respectively. However, when the NOP receptor-mediated inhibition of CaV was examined, the modulation of the Ca2+ currents was indistinguishable in scrambled and Gγ3 siRNA-transfected groups. For instance, exposure to Noc (1 μM) resulted in a mean Ca2+ current block of 61.1 ± 4.2% (n=9) and 58.2 ± 6.8% (n=6) in SG neurons transfected with scrambled and Gγ3 siRNA, respectively, 120 hr post-transfection.
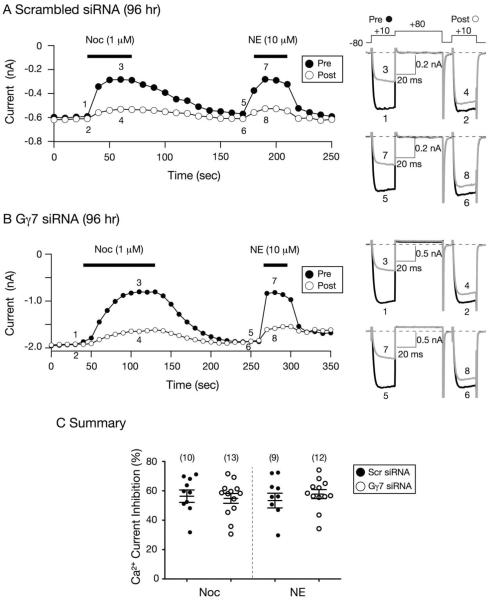
Thereafter, Gγ7 proteins were targeted and their silencing in SG neurons was optimized. Figure 1 shows time courses of Ca2+ current modulation by Noc in SG neurons 96 hr post-transfection with either scrambled siRNA (Fig. 1A) or Gγ7 siRNA (Fig. 1B). Pre- and post-pulse Ca2+ currents were evoked every 10 sec with the triple pulse protocol shown in Fig. 1A (top right) and the current traces are shown to the right. The protocol consists of two identical test pulses to +10 mV (holding potential of −80) separated by a large depolarizing conditioning pulse to +80 mV. Ca2+ current amplitude was measured isochronally 10 msec after the initiation of the prepulse. In the control neuron, it can be observed that prior to Noc (1 μM) application the prepulse current (trace 1) was fast and reached a plateau within 5 msec. During Noc application, the prepulse current was blocked maximally (~ 50%) within 20 sec. It can also be observed that the prepulse current (trace 3) exhibited kinetic `slowing', characteristic of the voltage-dependent (VD) modulation of Ca2+ currents [20]. Following a recovery period, the neuron was exposed to NE (10 μM) and the prepulse current was also blocked 50% (traces 5 and 7). This α2-adrenergic receptor agonist was employed as a positive control for stimulation of G protein signaling. The time course in Figure 1B shows the modulation of CaV currents of an SG neuron transfected with Gγ7 siRNA in the presence of Noc or NE. Similar to the scrambled siRNA-transfected neuron, application of Noc led to a 50% block of Ca2+ currents. However, it can be observed that the cell exhibited a delay (> 60 sec) in reaching maximal inhibition (trace 3) when compared to the control SG neuron. The onset of the steady-state Noc-mediated Ca2+ current inhibition was fit to a single exponential function. The mean tau values for control and Gγ7 siRNA-transfected cells were 30.4 ± 4.4 and 46.5 ± 8.4 sec (P = 0.16, NS, unpaired t-test), respectively. On the other hand, a delay was not observed following NE exposure (trace 7) when compared to the control neuron. The summary plot in Figure 1C illustrates that silencing Gγ7 did not significantly alter the coupling pathways between NOP receptors and CaV channels.
Figure 1. Effect of Gγ7 knockdown on the Noc- and NE-mediated Ca2+ current inhibition 96 hr post siRNA transfection.
A and B) Time courses of peak Ca2+ current amplitude inhibition for pre- (●) and postpulse (○) acquired from the sequential application of Noc (1 μM) and NE (10 μM) in neurons transfected with control scrambled (A) and Gγ7 (B) siRNA, respectively. Superimposed Ca2+ current traces evoked with the `triple-pulse' voltage protocol (shown on top of 1A, right) in the absence (1 and 2; 5 and 6, black) or presence (3 and 4; 7 and 8, grey) of Noc and NE, respectively, are shown to the right. Currents were evoked every 10 sec. The filled bars indicate the application of agonists. C) Summary dot plot of mean (± SE) Ca2+ current inhibition produced by application of Noc or NE in neurons transfected with control scrambled or Gγ7 siRNA. Inhibition was determined from the Ca2+ current amplitude measured isochronally at 10 msec into the prepulse (+10 mV) in the absence or presence of Noc or NE. Numbers in parenthesis indicate the number of neurons tested.
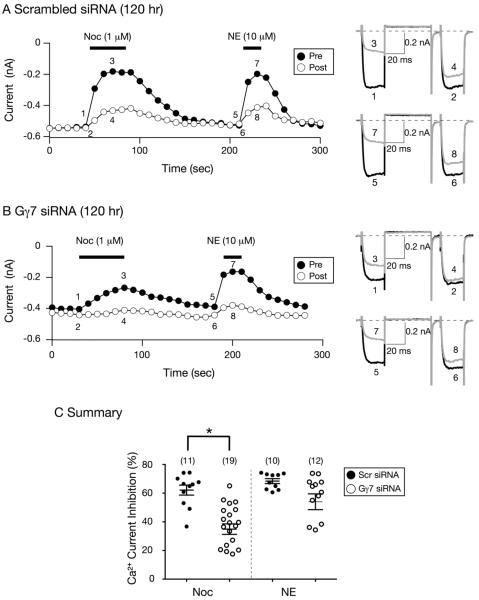
In the next set of experiments, we examined the coupling in SG neurons 120 hr post-siRNA transfection. Figures 2A and 2B are time courses of Ca2+ current inhibition for neurons transfected with scrambled and Gγ7 siRNA, respectively. Figure 2A shows that the modulation of Ca2+ currents by Noc or NE were similar to control the group described for Fig. 1A. Further, the Ca2+ currents were blocked in a VD manner. Exposure of the Gγ7 siRNA-transfected neuron to Noc (Fig. 2B), on the other hand, again resulted in a delay in reaching maximal Ca2+ current inhibition as well as an attenuation of the current block. The mean tau value for the onset of the steady-state Noc-mediated Ca2+ current inhibition in control neurons was 21.0 ± 3.1 sec, while it was 37.5 ± 9.6 sec for Gγ7 siRNA-transfected cells (P=0.14, NS, unpaired t-test). Unlike Noc, the NE-mediated modulation of CaV currents was unaffected by Gγ7 silencing. The summary dot plot in Figure 2C shows that Gγ7 siRNA transfection significantly (P < 0.05) decreased the Noc-mediated Ca2+ modulation in SG neurons. These results suggest that with this approach, a period of 120 hr was required to decrease Gγ7 expression levels and alter NOP receptor coupling.
Figure 2. Effect of Gγ7 silencing 120 hr post siRNA transfection on the Noc- and NE-mediated Ca2+ current inhibition in SG neurons.
A and B) Time courses of peak Ca2+ current amplitude inhibition for pre- (●) and postpulse (○) acquired from the sequential application of Noc (1 μM) and NE (10 μM) in neurons transfected with control scrambled (A) and Gγ7 (B) siRNA, respectively. Superimposed Ca2+ current traces (shown to the right) evoked with the `triple-pulse' voltage protocol (shown on top of 1A) in the absence (1 and 2; 5 and 6, black) or presence (3 and 4; 7 and 8, grey) of either NE or Noc. Currents were evoked every 10 sec. The filled bars indicate the application of agonists. C) Summary graph of mean (± SE) Ca2+ current inhibition produced by application of Noc or NE in neurons transfected with scrambled or Gγ7 siRNA. Inhibition was determined from the Ca2+ current amplitude measured isochronally at 10 msec into the prepulse (+10 mV) in the absence or presence of Noc or NE. Numbers in parenthesis indicate the number of neurons tested. * P < 0.05 compared to neurons transfected with scrambled siRNA, Student's t test.
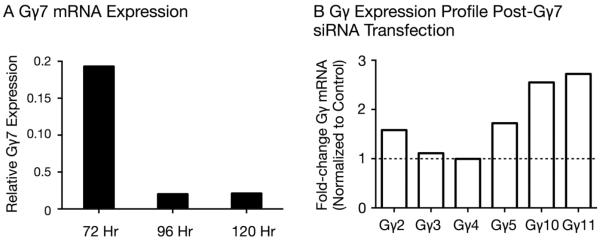
The lack of specific rat Gγ7 protein antibodies precluded us from performing Western blotting assays on siRNA-transfected tissue to measure protein levels. However, QRT-PCR assays on scrambled and Gγ7 siRNA-transfected SG tissue were performed 72, 96 and 120 hr post-siRNA transfection. Figure 3A shows that Gγ7 mRNA expression levels in SG tissue decreased 80% 72 hr post-transfection and greater than 98% 96–120 hr following siRNA transfection. In a second set of experiments, QRT-PCR assays were performed in SG tissue to examine the effect of Gγ7 siRNA transfection on mRNA expression levels of other Gγ protein subunits 120 hr post-siRNA transfection. Of the six Gγ proteins tested, Gγ10 and Gγ11 mRNA levels exhibited the highest increased changes of 2.5- and 2.7-fold, respectively. Gγ2 and Gγ5 increased by 50 to 75% while Gγ3 and Gγ4 levels remained relatively unchanged.
Figure 3. Quantitative assessment of Gγ subunit mRNA expression by QRT-PCR in SG tissue 72–120 hr post-Gγ7 siRNA transfection.
A) QRT-PCR analysis showing Gγ7 mRNA expression levels in SG tissue transfected with either scrambled siRNA or Gγ7 siRNA 72, 96 and 120 hr post-transfection. For each experimental group, 1–2 rats were employed. B) Relative Gγ mRNA expression levels 120 hr post-transfection in SG tissue following Gγ7 subunit silencing. QRT-PCR was carried out with total RNA from scrambled and Gγ7 siRNA-transfected SG tissue. The fold-differences were calculated with the Ct values for each probe and corrected for GAPDH expression levels in each sample. The corrected expression level of each Gγ subunit was then normalized to its corresponding value obtained from SG tissue transfected with scrambled siRNA.
4. Discussion
In the present study we examined whether Gγ subunits influence coupling specificity between NOP receptors and CaV channels. We identified previously that Gβ2, Gβ4 and PTX-sensitive Gα subunits play a crucial role in the Noc-mediated modulation of CaV currents in SG neurons [10–11]. The approach employed in our experiments was to silence specific Gγ subunits employing siRNA assays. Since twelve Gγ proteins have been described and few studies have explored Gγ coupling specificity, we employed a judicious approach. Gγ1 and Gγ8 subunits, for instance, exhibit restricted tissue distribution and, thus, were not studied [12, 21]. Our initial focus was the Gγ3 isoform that has been previously linked to mu opioid receptor signaling mechanisms in response to a high-fed diet [19]. Our results showed that silencing Gγ3 did not overtly alter the coupling between NOP receptors and Ca2+ channels, which suggests this isoform does not play a crucial role in this signaling pathway.
The sequence homology of Gγ7 [12, 21], coupled with its functional interaction with Gγ3 subunits [22], prompted us to next probe the role of this isoform on the NOP receptor signaling pathway. After the Gγ7 proteins were silenced, the Noc-mediated block of Ca2+ currents was significantly decreased, indicating it is an important component in this signaling process. That Gγ subunits can influence coupling has been shown with other GPCR. Robishaw and colleagues, for instance, reported that Gγ7 subunits were critical components of the G protein heterotrimer employed by adenosine A2A receptors in mice striatum [14], dopamine (D1) receptors [15] and β1 adrenergic receptors [16]. Others have found that Gγ11 subunits exhibited preferential coupling to adenosine A1 and 5-HT1A receptors [13].
Similar to our previous study [10], a 120 hr period was necessary to effectively silence Gγ7 proteins and alter coupling specificity between NOP receptors and CaV channels. On the other hand, we also reported that a 72 hr period was required to silence Gβ2 subunits to significantly uncouple NOP receptor signaling in SG neurons [11]. However, unlike Gγ7 and Gαi1, silencing Gβ2 led to an upregulation of Gβ4 mRNA and protein such that the Noc-mediated Ca2+ current inhibition was restored. In the present study, QRT-PCR results showed upregulation of some Gγ isoforms following Gγ7 siRNA transfection, but lacked a compensation of the NOP coupling pathway.
The mRNA expression profile of non-targeted Gγ subunits indicated that the Gγ7 protein levels influence the expression of Gγ10 and Gγ11 subunits, and to lesser extent that of Gγ2 and Gγ5. The upregulation of these Gγ proteins suggests that there is a compensatory mechanism for a Gγ7-dependent signaling pathway, but independent of CaV channel modulation. It is also interesting to note that we did not observe decreases of Gγ expression levels in light of a recent study which reported a series of experiments designed to silence eight Gα and Gβ isoforms in HeLa cells [23]. In that report, for instance, Gαi1 expression levels significantly increased while Gβ2 and Gβ4 levels decreased significantly following Gαs silencing. Further, when Gβ1-β4 subunits were silenced, the expression levels of all Gα subunits tested significantly decreased. The effect of silencing either Gα or Gβ isoform on Gγ subunits was not determined [23].
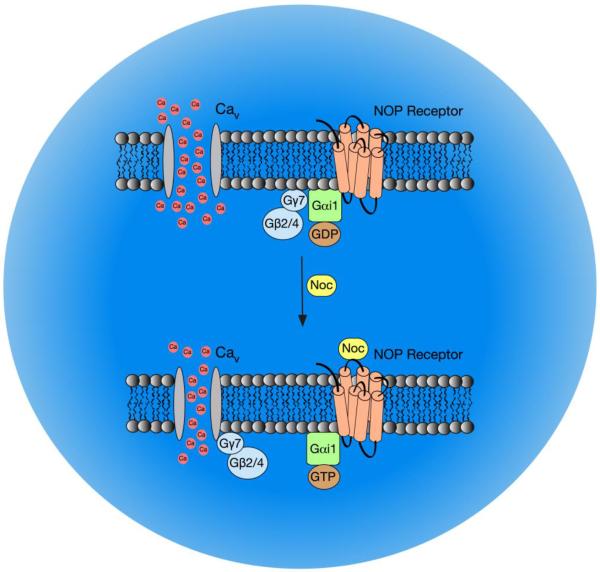
In summary, the findings of the present study show that Gγ7 subunits are crucial elements for NOP receptor coupling to CaV channels, while the Gγ3 does not appear to be involved in this signaling pathway. In addition, as Gγ7 mRNA levels decreased 96 hr post-transfection, and presumably protein, there was a slowing of the onset of the Noc-mediated Ca2+ current inhibition when compared to scrambled siRNA-transfected cells. The modulation of Ca2+ currents was significantly decreased 120 hr post-siRNA transfection when compared to the control group. Following Gγ7 silencing, the mRNA levels of other Gγ proteins (Gγ2, Gγ5, Gγ10 and Gγ11) increased, yet the coupling between CaV channels and NOP receptors was diminished. The current results, in combination with our previous reports, indicate that NOP receptors employ Gαi1, Gβ2/Gβ4, and Gγ7 proteins to modulate CaV channel function (Fig. 4). Thus, the Gαβγ heterotrimer highly impacts the NOP receptor signal transduction pathway in SG neurons. This striking specificity could be exploited therapeutically in conditions, such as sepsis, where high Noc levels are associated with increased morbidity and/or mortality (see [4] for review). Targeting these G protein isoforms would suppress the NOP signaling pathway, preserve modulation of CaV channels by other GPCR and markedly limit side effects.
Figure 4. Diagram summarizing the NOP receptor signaling pathway that mediates CaV inhibition following NOP receptor stimulation by Noc in rat SG neurons.
Prior to Noc exposure, NOP receptors are coupled to the Gαi1/β2(β4)/γ7 heterotrimer. Upon activation by Noc, free Gβ2(β4)γ7 dimers bind to CaV channels leading to VD inhibition of Ca2+ currents.
Highlights.
Examined Gγ coupling specificity of NOP receptors and CaV in rat sympathetic neurons.
Silencing Gγ7 isoform significantly decreased the Noc-mediated CaV modulation.
Gγ7 siRNA-transfected cells exhibited increased Gγ2, Gγ5, Gγ10 and Gγ11 mRNA levels.
Gαi1/Gβ2(β4)/Gγ7 heterotrimer couples mediate coupling of NOP receptors and CaV.
Acknowledgment
We thank Paul Herold for technical assistance. This work was supported by NIH grants RO1 DA025574 and RO1 AR059397 to V.R.-V.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest There is no conflict of interest to report.
References
- [1].Mogil JS, Pasternak GW. The molecular and behavioral pharmacology of the orphanin FQ/nociception peptide and receptor family. Pharmacol. Rev. 2001;53:381–415. [PubMed] [Google Scholar]
- [2].Toll L, Bruchas MR, Calo G, Cox BM, Zaveri NT. Nociceptin/orphanin FQ receptor structure, signaling ligands, functions, and interactions with opioid systems. Pharmacol. Rev. 2016;68:419–457. doi: 10.1124/pr.114.009209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Carvalho D, Petronilho F, Vuolo F, Machado RA, Constantino L, Guerrini R, Calo G, Gavioli EC, Streck EL, Dal-Pizzol F. The nociception/orphanin FQ-NOP receptor antagonist effects on an animal model of sepsis. Intensive Care Med. 2008;34:2284–2290. doi: 10.1007/s00134-008-1313-3. [DOI] [PubMed] [Google Scholar]
- [4].Gavioli EC, de Medeiros IU, Monteiro MC, Calo G, Romao PR. Nociceptin/orphanin FQ-NOP receptor system in inflammatory and immune-mediated diseases. Vitam. Horm. 2015;97:241–266. doi: 10.1016/bs.vh.2014.11.003. [DOI] [PubMed] [Google Scholar]
- [5].Fuller BC, Sumner AD, Kutzler MA, Ruiz-Velasco V. A novel approach employing ultrasound guidance for percutaneous cardiac muscle injection to retrograde label rat stellate ganglion neurons. Neurosci. Lett. 2004;363:252–256. doi: 10.1016/j.neulet.2004.03.087. [DOI] [PubMed] [Google Scholar]
- [6].Altier C, Khosravani H, Evans RM, Hameed S, Peloquin JB, Vartian BA, Chen L, Beedle AM, Ferguson SSG, Mezghrani A, Dubel SJ, Bourinet E, McRory JE, Zamponi GW. ORL1 receptor-mediated internalization of N-type calcium channels. Nat. Neurosci. 2006;9:31–40. doi: 10.1038/nn1605. [DOI] [PubMed] [Google Scholar]
- [7].Tedford HW, Zamponi GW. Direct G protein modulation of CaV2 calcium channels. Pharmacol. Rev. 2006;58:837–862. doi: 10.1124/pr.58.4.11. [DOI] [PubMed] [Google Scholar]
- [8].Wallis D, Watson AH, Mo N. Cardiac neurons of autonomic ganglia. Micros. Res. Tech. 1996;35:69–79. doi: 10.1002/(SICI)1097-0029(19960901)35:1<69::AID-JEMT6>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- [9].Ruiz-Velasco V, Puhl HL, Fuller BC, Sumner AD. Modulation of Ca2+ channels by opioid receptor-like 1 receptors natively expressed in rat stellate ganglion neurons innervating cardiac muscle. J. Pharmacol. Exp. Ther. 2005;314:987–994. doi: 10.1124/jpet.105.089284. [DOI] [PubMed] [Google Scholar]
- [10].Margas W, Sedeek K, Ruiz-Velasco V. Coupling specificity of NOP opioid receptors to pertussis-toxin-sensitive Gα proteins in adult rat stellate ganglion neurons using small interference RNA. J. Neurophysiol. 2008;100:1420–1432. doi: 10.1152/jn.90405.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Mahmoud S, Yun JK, Ruiz-Velasco V. Gβ2 and Gβ4 participate in the opioid and adrenergic receptor-mediated Ca2+ channel modulation in rat sympathetic neurons. J. Physiol. 2012;590:4673–4689. doi: 10.1113/jphysiol.2012.237644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Khan SM, Sleno R, Gora S, Zylbergold P, Laverdure JP, Labbe JC, Miller GJ, Hebert TE. The expanding roles of Gβγ subunits in G protein-coupled receptor signaling and drug action. Pharmacol. Rev. 2013;65:545–577. doi: 10.1124/pr.111.005603. [DOI] [PubMed] [Google Scholar]
- [13].Lim WK, Myung CS, Garrison JC, Neubig RR. Receptor-G protein γ specificity: γ11 shows unique potency for A1 adenosine and 5-HT1A receptors. Biochem. 2001;40:10532–10541. doi: 10.1021/bi010950c. [DOI] [PubMed] [Google Scholar]
- [14].Schwindinger WF, Murphree Mihalcik LJ, Giger KE, Betz KS, Stauffer AM, Linden J, Herve D, Robishaw JD. Adenosine A2A receptor signaling and Golf assembly show a specific requirement for the γ7 subtype in the striatum. J. Biol. Chem. 2010;285:29787–29796. doi: 10.1074/jbc.M110.142620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wang Q, Jolly JP, Surmeier JD, Mullah BM, Lidow MS, Bergson CM, Robishaw JD. Differential dependence of the D1 and D5 dopamine receptors on the G protein γ7 subunit for activation of adenylylcyclase. J. Biol. Chem. 2001;276:39386–39393. doi: 10.1074/jbc.M104981200. [DOI] [PubMed] [Google Scholar]
- [16].Wang Q, Mullah BK, Robishaw JD. Ribozyme approach identifies a functional association between the G protein β1γ7 subunits in the β-adrenergic receptor signaling pathway. J. Biol. Chem. 1999;274:17365–17371. doi: 10.1074/jbc.274.24.17365. [DOI] [PubMed] [Google Scholar]
- [17].Reynolds A, Leake D, Boese Q, Scarings S, Marshall WS, Khvorova A. Rational siRNA design for RNA interference. Nat. Biotechnol. 2004;22:326–330. doi: 10.1038/nbt936. [DOI] [PubMed] [Google Scholar]
- [18].Ikeda SR. Double-pulse calcium channel current facilitation in adult rat sympathetic neurons. J.Physiol. 1991;439:181–214. doi: 10.1113/jphysiol.1991.sp018663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Schwindinger WF, Borrell BM, Waldman LC, Robishaw JD. Mice lacking the G protein γ3-subunit show resistance to opioids and diet induced obesity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009;297:R1494–R1502. doi: 10.1152/ajpregu.00308.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ikeda SR, Dunlap K. Voltage-dependent modulation of N-type calcium channels: role of G protein subunits. Adv. Sec. Messenger Phosphoprotein Res. 1999;33:131–151. doi: 10.1016/s1040-7952(99)80008-1. [DOI] [PubMed] [Google Scholar]
- [21].Jones MB, Siderovski DP, Hooks SB. The Gβγ dimer as a novel source of selectivity in G-protein signaling: GGL-ing at convention. Mol. Interv. 2004;4:200–214. doi: 10.1124/mi.4.4.4. [DOI] [PubMed] [Google Scholar]
- [22].Schwindinger WF, Mirshahi UL, Baylor KA, Sheridan KM, Stauffer AM, Usefof S, Stecker MM, Mirshahi T, Robishaw JD. Synergistic roles for G-protein γ3 and γ7 subtypes in seizure susceptibility as revealed in double knock-out mice. J. Biol. Chem. 2012;287:7121–7133. doi: 10.1074/jbc.M111.308395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Krumins AM, Gilman AG. Targeted knockdown of G protein subunits selectively prevents receptor-mediated modulation of effectors and reveals complex changes in non-targeted signaling proteins. J. Biol. Chem. 2006;281:10250–10262. doi: 10.1074/jbc.M511551200. [DOI] [PubMed] [Google Scholar]