Abstract
This study identified trajectories of morningness/eveningness and physical activity when chronological (i.e., time since birth) versus gynecological (i.e., time since menarche) age is used to indicate maturation. Piecewise models were fit for girls (N = 262, ages 11–19) using chronological or gynecological age as the time metric. Girls stayed up later (i.e., eveningness) as they approach menarche. After menarche no change in morningness/eveningness was observed. In contrast, no change in morningness/eveningness was detected with chronological age. No change in physical activity was observed before menarche and physical activity declined after menarche. With chronological age, physical activity declined as girls got older. Gynecological age may be more appropriate than chronological age as a metric for understanding changes in morningness/eveningness and physical activity.
Keywords: adolescence, physical activity, morningness/eveningness, menarche
Many biological and psychosocial changes occur during childhood and adolescence; assessment of change inherently requires the identification of time, that is, a time metric. Change may be seen across days, months or years (e.g., years of schooling; Wang, Hill, & Hofkens, 2014), in response to specific events (e.g., educational intervention; Chittleborough, Mittinty, Lawlor, & Lynch, 2014), or with biological maturation (e.g., auditory and visual development; Steinbrink, Zimmer, Lachmann, Dirichs, & Kammer, 2014). The variability in what drives changes in development makes identifying the appropriate time metric challenging, particularly when there is nonlinearity in the phenomenon of interest (Graber & Brooks-Gunn, 1996). Time metrics must therefore be based on theory and hypotheses about the change process, constructs being examined, and mechanisms underlying change, as well as information derived from model estimation itself (Grimm, Ram, & Hamagami, 2011). This is important because modeling change ultimately impacts findings in our developmental studies.
There are many instances in which the mechanisms of change for developmental phenomenon – and therefore the appropriate time metric – are either multifaceted or unknown. For example, morningness/eveningness (e.g., a characteristic of humans reflecting the time of day when sleep or awakefullness is preferred; Adan et al., 2012) shifts during adolescence and may coincide with increased vulnerability to risky behaviors (e.g., substance use, antisocial behavior; Negriff, Dorn, Pabst, & Susman, 2011; Susman et al., 2007). This is also true for physical activity, where girls’ participation declines across adolescence, leaving them more vulnerable to obesity and adult cardiovascular risks (Williams, 2002). While such changes in morningness/eveningness (M/E) and physical activity are well documented (Bradley, McRitchie, Houts, Nader, & O’Brien, 2011; Petta & Carskadon, 1984), less is known about what drives these changes.
There are several competing hypotheses about why changes in M/E and physical activity occur during adolescence. M/E and physical activity may change because of shifts in psychosocial norms, social roles and values (Kimm et al., 2006; Ohayon, Carskadon, Guilleminault, & Vitiello, 2004). For example, Gradisar and colleagues (2011) found in their review of sleep across adolescence that teens get less sleep because shifts in their chronotype lead them to stay awake later and desire to sleep later, a need that is often not accommodated on school days. Further, Roenneberg and colleagues (2004) reported that eveningness becomes increasingly common into the 20s, followed by a move back to morningness. Similar patterns have been observed with physical activity. Across adolescence, attitudes about physical activity (e.g., consistency with gender norms, lack of time or interest) predict being sedentary above and beyond other adolescent behaviors (e.g., television-watching; Kimm et al., 2006). Drawing from this literature, one might anticipate that the trajectory for physical activity would follow a curvilinear pattern with age, gradually moving toward lower levels of physical activity across adolescence based on chronological age, until eventually reaching a plateau.
Alternatively, it may be that changes in M/E toward eveningness or declines in physical activity occur because of hormonal and biological changes related to puberty (Baker, Birch, Trost, & Davison, 2007; Carskadon, Vieira, & Acebo, 1993; Davison, Werder, Trost, Baker, & Birch, 2007). There is some evidence of a shift toward eveningness for girls showing more advanced pubertal maturation that is not seen in girls with no or few signs of pubertal changes (Carskadon et al., 1993). This shift in M/E has consistently been found to occur around the time of menarche (Frey, Balu, Greusing, Rothen, & Cajochen, 2009). The literature addressing declines in physical activity during adolescence has recently pointed to puberty as a mechanism, with evidence that girls with advanced pubertal status engage in less physical activity than their same-aged peers (Baker et al., 2007). These findings indicate that a model comparing changes in both M/E and physical activity using gynecological age, or time since menarche, as the time metric may be ideal for identifying influences of these developmental changes.
Specifying appropriate time metrics in longitudinal studies is also important because, as mentioned previously, how time is measured can impact our ability to detect effects between covariates and outcomes (Grimm et al., 2011). For example, there have been relatively consistent findings in the associations between depressive symptoms and both physical activity (Paluska & Schwenk, 2000; Sallis, Prochaska, & Taylor, 2000) and M/E (Gau et al., 2007; Kim et al., 2010); similar to M/E and physical activity, depressive symptoms also change during adolescence. Whether change in depressive symptoms is associated with physical maturation (i.e., puberty; Angold, Costello, Erkanli, & Worthman, 1999; Angold, Costello, & Worthman, 1998) or other processes is not clear. How time is specified (i.e., as pre-post menarche, chronological age, time since baseline) in models estimating associations among M/E, depressive symptoms and physical activity contribute to variability in findings and our conclusions about how depressive symptoms relate to M/E or physical activity.
This study sought to examine competing hypotheses regarding whether chronological or gynecological age was more informative for describing sleep preferences and physical activity trajectories. This was accomplished by identifying (a) changes in trajectories of M/E and physical activity among adolescent girls, (b) how trajectories differ depending on chronological age (i.e. time since birth) compared to gynecological age (i.e. time since menarche), and (c) whether the index of development (chronological or gynecological age) influences associations between depressive symptoms and M/E or physical activity. Clarifying whether there are differences in trajectories of M/E or physical activity when chronological and gynecological age are used may assist in understanding the conflicting literature regarding which mechanism of development (chronological age versus gynecological age) drives observed patterns of M/E and physical activity and guide future research and prevention efforts about when to target at-risk girls around issues of sleep habits and physical activity.
Method
Participants
Data were derived from a cross-sequential longitudinal study of healthy adolescent girls (n = 262) designed to examine the impact of substance use and depressive symptoms on reproductive and bone health (Dorn et al., 2013). Girls were enrolled by age cohort (11, 13, 15, and 17 years) and were primarily White (61%) or African American (31%). Socioeconomic status was reported by parents using the Hollingshead Four-Factor Index (Hollingshead, 1975), which can range from 8 to 66 (higher scores indicate higher SES). The sample mean was 37.32 (SD = 13.67), indicating that households of the girls in this study were, on average, headed by caregivers with education and employment levels of skilled craftsmen, clerical, and sales employees. Girls were recruited from the greater Cincinnati area, a large Midwest city comprised of a concentrated urban core surrounded by suburban communities. Retention rates for this longitudinal study were high (90% present for at least two time-points). Of the girls participating, 66% were present at all times of measurement. Between 210 and 262 girls provided data at any given timepoint. Analyses indicated no significant differences in M/E or physical activity at baseline for girls who were present versus those missing at any subsequent time points (p > .05). Maximum likelihood estimation was used for all analyses, resulting in the analytic sample size of 262.
Procedures
Healthy girls were recruited from a teen clinic in a large children’s hospital and its surrounding community. The study was approved by the Institutional Review Board of the hospital. Parents provided consent and girls provided assent to participate. Annual interviews were conducted at a Clinical Translational Research Center (Time [T]1, T2, and T3) followed by phone interviews three, six, and nine months later, resulting in nine waves of data collection. The first wave of measurement occurred between December 2003 and October 2007 and the final wave of data collection was completed in December 2010. Exclusion criteria at baseline were (1) pregnancy or breastfeeding within 6 months of enrollment, (2) primary (> age 16) or secondary (< 6 cycles per year) amenorrhea, (3) body mass index at or below the first percentile or a weight above 300 pounds, (4) medication or illness that would influence bone density (a focus of the initial study), or (5) psychological disorders that would limit comprehension or compliance.
Measures
Physical activity was assessed using the stem “Have you done the following activities in the past 7 days?” for 25 activities (e.g., basketball, playing outside). Responses ranged from 1 (Did not do) to 5 (≥7 times in past 7 days) and were averaged, such that higher scores indicated more activity (Crocker, Bailey, Faulkner, Kowalski, & McGrath, 1997). M/E was assessed using the Morningness/Eveningness Scale for Children, a 10 item questionnaire with responses ranging from 1 (minimal morning preference) to a max of 5 (maximum morning preference), such that higher scores indicated a stronger morning preference, with scores ranging from 10 to 43 (Carskadon et al., 1993). The Physical Activities Questionnaire and Morningness/Eveningness Scale for Children were administered every 3 months for 36 months and a total of 9 timepoints. Age of menarche was reported by girls during clinician interviews. Mean age of menarche was 12.41 years (SD = 1.25) and 20% of the sample was pre-menarcheal at baseline. Gynecological age was computed by subtracting age at menarche from the age at the study visit. Body mass index was measured annually using clinician-assessed height and weight with a standardized scale and stadiometer, and percent body fat was obtained from dual energy x-ray absorptiometry annually for 3 years. Depressive symptoms were measured using the Children’s Depression Inventory (CDI), a well-established self-report measure of depressive symptoms in children ages 8–17 (Kovacs et al., 1992). The CDI was administered at all annual and 6 month timepoints (i.e., T1, T1.6, T2, T2.6, and T3). Responses to each item on the CDI range from 0 to 2, and participants are asked to consider their experiences in the past 2 weeks. Internal consistency of the CDI ranges from .71 to .89. These analyses used t-scores based on published norms from a non-clinical sample. Using these norms, a score of 50 is considered average, and a score above 65 (1.5 SD above the mean) is considered at-risk for clinical levels of symptoms. In this sample, 5 participants had t-scores above 65 at 1 or more timepoints. Across years of measurement, t-scores in our study had an alpha of .94. Race was coded as two dichotomous variables: a) African-Americans versus European Americans, and b) other minorities and European Americans.
Analytic Plan
A linear growth model was initially fit using chronological age as the timing metric. In these models, age was centered at 12 years, the mean age for menarche. The linear slope was scaled in years (e.g., ytn = b1n + b2n · (agetn − 12) + etn). Additionally, a dichotomous variable was created at each assessment for each individual indicating whether the observation occurred before or after menarche. This variable was included as a time-varying covariate to examine whether the onset of menarche had an effect on the age-based change trajectory. To examine trajectories by gynecological age, a piecewise model was fit, which allows for estimation of a linear slope prior to menarche and a second linear slope after menarche, such that ytn = b1n + b2n · min(agetn − menarchen, 0) + b3n · max(agetn − menarchen, 0)+ etn where b1n is the intercept centered at the age of menarche (menarchen), b2n is the linear slope prior to menarche, b3n is the linear slope after menarche, and etn is the time-dependent residual. This piecewise model was compared to a linear growth model with gynecological age as the timing metric to determine whether allowing two slopes significantly improved model fit. Unconditional models were initially fit and followed by models that included means of body mass index, percent body fat, and depressive symptoms, as well as race.
Results
Chronological age-based and gynecological age-based models were estimated for physical activity and M/E. Scores for physical activity ranged from 1 to 5, while scores for M/E ranged from 11 to 43, with lower scores indicating eveningness chronotype.
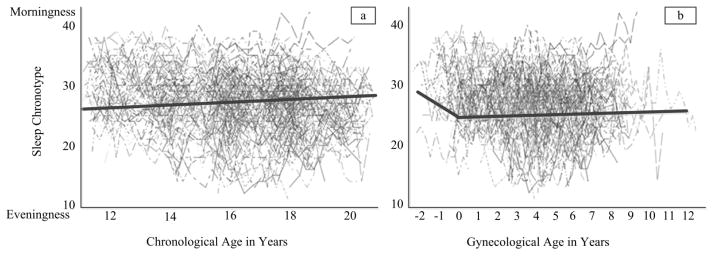
Estimated trajectories for M/E differed by metric of time used. Specifically, the linear growth model using chronological age indicated that M/E did not significantly change, on average, from age 11 to age 20 years. Menarche did not significantly alter the individual age-based trajectory. In the unconditional age-based model, the mean intercept (centered at age 12) was estimated at 26.68 (p < .01) indicating M/E did not strongly lean toward either a morning or evening chronotype and there was a non-significant mean slope of 0.03 (p = .73) suggesting no mean changes in M/E across adolescence (Figure 1a).
Figure 1.
Trajectories of Morningness-Eveningness (M/E) when chronological age (i.e., years since birth, left) and gynecological age (i.e., years since age at menarche, right) are used. Higher values for M/E represent a morningness orientation. Negative values for gynecological age represent time prior to menarche.
For the gynecological age-based models, the piecewise model fit significantly better than the linear model suggesting that the individual rate of change was altered as a result of menarche. Specifically, prior to menarche there was a negative decline in M/E, with girls staying up later and sleeping later (i.e. eveningness) as menarche approached. After menarche, no change in M/E was observed. For this model, the mean intercept (centered at the age of menarche) was estimated at 26.56 (p < .01). Before menarche, girls experienced a significant decline in M/E (B = −0.76, p < .05) indicating a move from morningness to eveningness as girls approach menarche, on average. After menarche the change in M/E was non-significant (B = 0.06, p = .45), on average (Figure 1b).
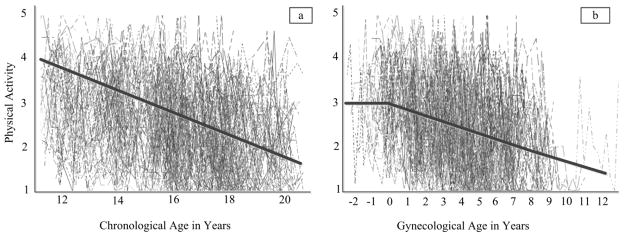
The models for physical activity showed a somewhat different pattern. In chronological age-based models, there was a significant negative average trend indicating that physical activity decreased from 11 to 20 years of age. In this model, the mean intercept was estimated at 3.25 (p < .01) indicating a moderate activity level and the mean slope was estimated to be −.20 (p < .01). Accounting for the timing of menarche in the chronological age-based model did not significantly improve model fit suggesting the onset of menarche did not alter the within-person trajectory for the chronological age-based linear growth model (Figure 2a).
Figure 2.
Trajectories of physical activity (i.e., bouts of moderate to vigorous activity per week) when chronological age (i.e., years since birth, left; a) and gynecological age (i.e., pre or post-menarche, right; b) are used. Negative values for gynecological age represent time prior to menarche.
For the gynecological age-based models of physical activity, the piecewise model fit significantly better than the linear model suggesting that the individual rate of change was altered as a result of menarche. Specifically, prior to menarche there was no significant change in physical activity; however, after menarche, a significant negative mean slope was observed suggesting a decrease in physical activity post menarche. For this model, the mean intercept (centered at the age of menarche) was estimated at 3.11 (p < .01). Before menarche, there was no mean change in physical activity (B = −0.05, p = .52). After menarche, the mean change in physical activity was negative and significantly different from zero (B = −0.19, p < .01; Figure 2b).
To further examine potential differences in models based on time metrics, variables known to co-vary with M/E and physical activity were also included in these models (see tables 1 and 2). This included race, age at menarche (for chronological age only), percent body fat, body mass index, and depressive symptoms. When those covariates were included in these models, there were few associations to note. Higher depressive symptoms were significantly associated with less physical activity and not associated with M/E regardless of time metric used. African-American girls showed more negative changes in physical activities when chronological age was the time metric and also showed more negative changes in physical activity after menarche when the piecewise model was fit. There was also evidence of a stronger shift toward eveningness across time for African-American girls when chronological and gynecological age was used.
Table 1.
Relations among Covariates, Chronological and Gynecological Age on Morningness/Eveningness
| Effect | Chronological Age | Gynecological Age | ||||
|---|---|---|---|---|---|---|
| Estimate | Error | 95% CI | Estimate | Error | 95% CI | |
| Intercept | 35.49** | 5.63 | 24.40 – 46.58 | 30.31** | 1.72 | 26.92 – 33.70 |
| Chronological Age | −1.00 | 1.02 | −3.01 – 1.01 | --- | --- | --- |
| Menarche | −0.37 | 0.39 | −1.13 – 0.40 | --- | --- | --- |
| Pre-menarche | --- | --- | --- | −0.83** | 0.27 | −1.36 – −0.30 |
| Post-menarche | --- | --- | --- | −0.40 | 0.34 | −1.07 – 0.27 |
| Black (Race/ethnicity) | 0.81 | 1.08 | −1.32 – 2.94 | 0.90 | 0.96 | −0.99 – 2.79 |
| Other (Race/ethnicity) | 1.61 | 1.81 | −1.96 – 5.17 | 1.13 | 1.65 | −2.12 – 4.38 |
| Time* Menarche | 0.08 | 0.12 | −0.16 – 0.31 | 0.05 | 0.11 | −0.17 – 0.27 |
| Time* Black | −0.34** | 0.08 | −0.50 – −0.18 | −0.30** | 0.08 | 0.14 – 0.46 |
| Time* Other | −0.20 | 0.15 | −0.50 – 0.10 | −0.15 | 0.14 | −0.43 – 0.13 |
| Between Person Percent Body Fat | 0.03 | 0.07 | −0.11 – 0.17 | 0.05 | 0.11 | −0.17 – 0.27 |
| Between Person Depressive Symptoms | 0.16 | 0.20 | −0.23 – 0.55 | 0.14 | 0.19 | −0.23 – 0.51 |
| Time* Between Person Percent Body Fat | −0.24 | 0.34 | −0.91 – 0.43 | −0.16 | 0.34 | −0.83 – 0.51 |
| Time* Between Person Depressive Symptoms | −0.01 | 0.02 | −0.05 – 0.03 | 0.00 | 0.02 | −0.04 – 0.04 |
| Within Person Percent Body Fat | 0.00 | 0.02 | −0.04 – 0.04 | −0.01 | 0.02 | −0.05 – 0.03 |
| Within Person Depressive Symptoms | 0.03 | 0.03 | −0.03 – 0.08 | 0.02 | 0.03 | −0.04 – 0.08 |
p < .05;
p < .01
Table 2.
Impact of Covariates, Chronological and Gynecological Age on Physical Activity
| Effect | Chronological Age | Gynecological Age | ||||
|---|---|---|---|---|---|---|
| Estimate | Error | 95% CI | Estimate | Error | 95% CI | |
| Intercept | 3.92** | 1.03 | 1.89 – 5.95 | 3.28** | 0.30 | 2.69 – 3.87 |
| Age | −0.26 | 0.20 | −0.65 – 0.13 | --- | --- | --- |
| Menarche | −0.03 | 0.07 | −1.17 – 0.11 | --- | --- | --- |
| Pre-menarche | --- | --- | --- | −0.06 | 0.08 | −.22 – 0.10 |
| Post-menarche | --- | --- | --- | −0.21** | 0.06 | −0.33 – −0.09 |
| Black (Race/ethnicity) | 0.19 | 0.19 | −0.18 – 0.56 | 0.27 | 0.17 | −0.06 – 0.60 |
| Other (Race/ethnicity) | −0.02 | 0.31 | −0.63 – 0.59 | −0.13 | 0.28 | −0.68 – 0.42 |
| Age* Menarche | −0.02 | 0.02 | −0.06 – 0.02 | −0.01 | 0.02 | −0.05 – 0.03 |
| Age* Black | −0.04** | 0.01 | −0.06 – −0.02 | −0.03* | 0.01 | −0.05 – −0.01 |
| Age* Other | 0.02 | 0.03 | −0.04 – 0.08 | 0.01 | 0.02 | −0.03 – 0.05 |
| Between Person % Body Fat | 0.01 | 0.01 | −0.01 – 0.02 | −0.01 | 0.02 | −0.05 – .03 |
| Between Person Depressive Symptoms | −0.09** | 0.04 | −0.17 – −0.01 | −0.09** | 0.04 | −0.17 – −0.01 |
| Age* Between Person % Body Fat | −0.09 | 0.07 | −0.23 – 0.05 | −0.06 | 0.06 | −0.18 – 0.06 |
| Age* Between Person Depressive Symptoms | 0.00 | 0.00 | −0.00 – 0.00 | 0.00 | 0.00 | −0.00 – 0.00 |
| Within Person Percent Body Fat | 0.00 | 0.00 | −0.00 – 0.00 | 0.00 | 0.00 | −0.00 – 0.00 |
| Within Person Depressive Symptoms | 0.00 | 0.01 | −0.02 – 0.02 | 0.00 | 0.01 | −0.02 – 0.02 |
p < .05;
p < .01
Discussion
Taken together, findings indicate that the trajectories for physical activity and M/E vary depending on whether gynecological or chronological age is used as the time metric. It is not appropriate to statistically compare the fit of the growth models when using different time metrics; however, from a substantive point of view, it appears that the models fit using gynecological age may be more consistent with the developmental mechanisms driving change. When slopes were estimated separately before and after onset of menarche, there was evidence of a move toward eveningness orientation (which was not evident when chronological age is used) and no evidence of change in physical activity prior to menarche (which appeared to be decreasing in the model using chronological age as the time metric). The slopes for chronological age mirrored the post menarche slopes, which is likely a result of data density (most observations occurred after menarche).
These findings have substantive importance for M/E in adolescence. Results suggest that, when chronological age is used, there is no change in M/E. However, with gynecological age, there appears to be clear movement toward eveningness only prior to the onset of menses, with no significant change after menarche. This indicates that variability in M/E may be attributable, at least in part, to menarcheal changes not accounted for in many previous studies, which most often considered the role of puberty, as opposed to the use of menarche to determine gynecological age in the current instance, in shaping M/E and sleep patterns (Baker et al., 2007; Carskadon et al., 1993; Davison et al., 2007; Frey et al., 2009). Of note, the current findings are consistent with Carskadon’s previous work, which suggested movement toward eveningness when girls were showing signs of pubertal changes and the strongest evening preference among girls with the greatest pubertal changes – all of whom had also experienced onset of menses. While the underlying cause of pre- versus post-menarcheal shifts in M/E needs to be examined, it is possible that estrogen plays an activational role in altering M/E (Manber & Armitage, 1999). Recent work by Igarashi and colleagues (2015) suggests that estradiol influences sleep/wake cycles and patterns of sleep behavior in female rats, consistent with the current findings. Likewise, Schwartz and Mong (2013) reported evidence that estradiol acts as a mechanism to regulate sleep in rats.
Our M/E findings may also extend to research suggesting that sleep and wake times shift on week-nights and week-ends, such that teens get less sleep on week-nights (Gradisar et al., 2011), and that there is a strong shift toward eveningness across ages 11–20, followed by a peak and shift toward morningness across the remainder of the lifespan (Roenneberg et al., 2004). Inconsistencies between our findings and Roenneberg’s work, which suggested declines in sleep across adolescence (in contrast to our findings that declines in M/E occur only before menarche), likely result from two sources: First, our data represents within-person, rather than between-person shifts, using repeated measures; thus, our slope reflects how individuals, rather than populations, are changing. Second, Carskadon and colleague’s (1993) measure, which was used in the current study, is designed specifically for teens and indicates preferences, and not actual daily experiences, as Roennenberg’s (2004) measure reflects. It is worth noting that while we detected no change in M/E post-menarche, the estimates at those timepoints favored evening orientation. Further, it may be that many of these teens were experiencing a mis-alignment of M/E and the social demands of the world teens live in (particularly during the school-year), which would alter when they preferred to sleep and number of hours slept. The M/E measure used in this study does not allow us to examine mechanisms; future studies are needed to further explain the mechanism causing these findings.
With regard to physical activity, our findings again suggest that menarcheal changes are important to consider when examining trajectories, extending previous research on the impact of puberty on physical activity (Baker et al., 2007). Our findings indicated a decline in physical activity with age, and that the onset of decline appears to coincide with menarche. While our study was not designed to account for the impact of estradiol on physical activity, there appear to be some similarities between the pattern we found with respect to menarche and the separate literature examining hormones and physical activity. Specifically, estradiol is known to increase throughout puberty in girls with the highest estradiol levels observed in the later stages of puberty (although in a cyclical fashion; Garcia-Mayor et al., 1997); menarche often coincides with later stages of puberty (Martí-Henneberg & Vizmanos, 1997). In non-human primates, physical activity increases in the luteal phase (when estradiol levels are lower) compared to the periovulatory phase (when estradiol levels are the highest; Nyakudya, Fuller, Meyer, Maloney, & Mitchell, 2012). Likewise, in their review of the literature Balzer and colleagues (2015) concluded that a rise in estradiol during adolescence is associated with altered behavior. More research to understand these endocrine mechanisms as they relate to physical activity is warranted.
Our finding may also offer insight as researchers grapple with how to assess metrics of change in their research, potentially beyond application to physical activity or M/E. In this study we used gynecological age, rather than other measures of puberty (e.g., Tanner stage, pubertal timing) – how to best include measures of puberty in future studies, including when and how various measures of puberty are most meaningful for phenomenon of interest, is clearly an important consideration (Dorn, 2006). Of note, menarche is a part of the pubertal process but puberty and menarche are not synonyms. Therefore, thoughtful consideration of these findings and comparisons with other studies using different measures of puberty is essential, as other various indices of puberty capture different aspects of reproductive development.
Replication of this study should be conducted with larger sample sizes, along with inclusion of additional markers of menarche (e.g., phase of the menstrual cycle, hormone levels) or other markers of puberty (e.g., Tanner staging) and adolescents from other socio-cultural backgrounds in order to enhance generalization. Further, it was not clear whether these findings would replicate with a larger sample of premenarcheal girls or with models estimated starting earlier than age 11. Although we included available control variables when they were theoretically relevant, it may be that an unmeasured confound is contributing to these findings. Finally, our study only included girls; the inclusion of boys is an important area for future research, particularly because of challenges in assessing pubertal development with males (Coleman & Coleman, 2002); particularly with a measure comparable to menarche. Better understanding of the manifestation of these trajectories in boys could be highly informative given the known sex differences identified in both sleep (Roenneberg et al., 2004) and physical activity (Sallis, Zakarian, Hovell, & Hofstetter, 1996).
In spite of these limitations, our findings reiterate the importance of specifying time metrics accurately in longitudinal studies examining change. Time metrics chosen should reflect the underlying mechanisms hypothesized to drive changes in the phenomenon of interest. In the case of physical activity and M/E, it appears that gynecological age, or time oriented to menarche, is a more appropriate metric than using chronological age, or time oriented to date of birth. Future studies examining potential alternative time metrics in longitudinal studies, beyond the use of chronological age or time since the first wave of data collection, should replicate and expand on our finding.
Acknowledgments
This research was supported in part by Grant Number R01 DA 16402, National Institute of Drug Abuse, NIH, PI: Lorah D. Dorn, PhD and by USPHS Grant # TR000077-04 from the National Center for Research Resources, NIH and by funds from the Bureau of Health Professions (BHPr), Health Resources and Services Administration (HRSA), Department of Health and Human Services (DHHS), under Grant # T32HP10027. This paper was presented as a poster at the Society for Research in Child Development (2013, March).
References
- Adan A, Archer SN, Hidalgo MP, Di Milia L, Natale V, Randler C. Circadian typology: a comprehensive review. Chronobiology International. 2012;29(9):1153–1175. doi: 10.3109/07420528.2012.719971. [DOI] [PubMed] [Google Scholar]
- Angold A, Costello EJ, Erkanli A, Worthman CM. Pubertal changes in hormone levels and depression in girls. Psychological Medicine. 1999;29(5):1043–1053. doi: 10.1017/S0033291799008946. [DOI] [PubMed] [Google Scholar]
- Angold A, Costello EJ, Worthman CM. Puberty and depression: The roles of age, pubertal status and pubertal timing. Psychological Medicine. 1998;28(1):51–61. doi: 10.1017/S003329179700593X. [DOI] [PubMed] [Google Scholar]
- Baker BL, Birch LL, Trost SG, Davison KK. Advanced pubertal status at age 11 and lower physical activity in adolescent girls. The Journal of pediatrics. 2007;151(5):488–493. doi: 10.1016/j.jpeds.2007.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balzer BW, Duke SA, Hawke CI, Steinbeck KS. The effects of estradiol on mood and behavior in human female adolescents: a systematic review. European Journal of Pediatrics. 2015;174(3):289–298. doi: 10.1007/s00431-014-2475-3. [DOI] [PubMed] [Google Scholar]
- Bradley RH, McRitchie S, Houts RM, Nader P, O’Brien M. Parenting and the decline of physical activity from age 9 to 15. The international journal of behavioral nutrition and physical activity. 2011;8:33. doi: 10.1186/1479-5868-8-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carskadon MA, Vieira C, Acebo C. Associations between puberty and delayed phase preference. Sleep. 1993;16(3):258–262. doi: 10.1093/sleep/16.3.258. [DOI] [PubMed] [Google Scholar]
- Chittleborough CR, Mittinty MN, Lawlor DA, Lynch JW. Effects of Simulated Interventions to Improve School Entry Academic Skills on Socioeconomic Inequalities in Educational Achievement. Child development. 2014;85(6):2247–2262. doi: 10.1111/cdev.12309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman L, Coleman J. The measurement of puberty: a review. J Adolesc. 2002;25(5):535–550. doi: 10.1006/jado.2002.0494. [DOI] [PubMed] [Google Scholar]
- Crocker PR, Bailey DA, Faulkner RA, Kowalski KC, McGrath R. Measuring general levels of physical activity: Preliminary evidence for the Physical Activity Questionnaire for Older Children. Medicine and Science in Sports and Exercise. 1997;29(10):1344–1349. doi: 10.1097/00005768-199710000-00011. [DOI] [PubMed] [Google Scholar]
- Davison KK, Werder JL, Trost SG, Baker BL, Birch LL. Why are early maturing girls less active? Links between pubertal development, psychological well-being, and physical activity among girls at ages 11 and 13. Social Science & Medicine. 2007;64(12):2391–2404. doi: 10.1016/j.socscimed.2007.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn LD. Measuring puberty. Journal of Adolescent Health. 2006;39(5):625–626. doi: 10.1016/j.jadohealth.2006.05.014. [DOI] [PubMed] [Google Scholar]
- Dorn LD, Beal SJ, Kalkwarf HJ, Pabst S, Noll JG, Susman EJ. Longitudinal impact of substance use and depressive symptoms on bone accrual among girls aged 11–19 years. J Adolesc Health. 2013;52(4):393–399. doi: 10.1016/j.jadohealth.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey S, Balu S, Greusing S, Rothen N, Cajochen C. Consequences of the timing of menarche on female adolescent sleep phase preference. PLoS One. 2009;4(4):e5217. doi: 10.1371/journal.pone.0005217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Mayor RV, Andrade MA, Rios M, Lage M, Dieguez C, Casanueva FF. Serum leptin levels in normal children: relationship to age, gender, body mass index, pituitary-gonadal hormones, and pubertal stage 1. The Journal of Clinical Endocrinology & Metabolism. 1997;82(9):2849–2855. doi: 10.1210/jcem.82.9.4235. [DOI] [PubMed] [Google Scholar]
- Gau SSF, Shang CY, Merikangas KR, Chiu YN, Soong WT, Cheng ATA. Association between morningness-eveningness and behavioral/emotional problems among adolescents. Journal of Biological Rhythms. 2007;22(3):268–274. doi: 10.1177/0748730406298447. [DOI] [PubMed] [Google Scholar]
- Graber JA, Brooks-Gunn J. Transitions and turning points: Navigating the passage from childhood through adolescence. Dev Psychol. 1996;32(4):768. [Google Scholar]
- Gradisar M, Gardner G, Dohnt H. Recent worldwide sleep patterns and problems during adolescence: a review and meta-analysis of age, region, and sleep. Sleep medicine. 2011;12(2):110–118. doi: 10.1016/j.sleep.2010.11.008. [DOI] [PubMed] [Google Scholar]
- Grimm KJ, Ram N, Hamagami F. Nonlinear growth curves in developmental research. Child development. 2011;82(5):1357–1371. doi: 10.1111/j.1467-8624.2011.01630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead AB. A Four-Factor Classification of Social Status. New Haven, CT: Yale University CT Press; 1975. [Google Scholar]
- Igarashi A, Omura N, Miura M, Mima N, Nishimura Y, Mabuchi K, Takamata A. Effect of Estradiol Replacement on Diurnal Sleep/Wake Pattern in Ovariectomized Rats Measured with a Subcutaneously Implanted Acceleration Sensor. The FASEB Journal. 2015;29(1 Supplement):676–671. [Google Scholar]
- Kim SJ, Lee YJ, Kim H, Cho IH, Lee JY, Cho SJ. Age as a moderator of the association between depressive symptoms and morningness–eveningness. Journal of Psychosomatic Research. 2010;68(2):159–164. doi: 10.1016/j.jpsychores.2009.06.010. [DOI] [PubMed] [Google Scholar]
- Kimm SY, Glynn NW, McMahon RP, Voorhees CC, Striegel-Moore RH, Daniels SR. Self-perceived barriers to activity participation among sedentary adolescent girls. Medicine and Science in Sports and Exercise. 2006;38(3):534–540. doi: 10.1249/01.mss.0000189316.71784.dc. [DOI] [PubMed] [Google Scholar]
- Kovacs M, Beck AT, Dadds MR, Sanders MR, Morrison M, Rebgetz M. Child Depression Inventory. Childhood depression and conduct disorder: II An analysis of family interaction patterns in the home. 1992;101:505–513. [PubMed] [Google Scholar]
- Martí-Henneberg C, Vizmanos B. The duration of puberty in girls is related to the timing of its onset. The Journal of pediatrics. 1997;131(4):618–621. doi: 10.1016/s0022-3476(97)70073-8. [DOI] [PubMed] [Google Scholar]
- Negriff S, Dorn LD, Pabst SR, Susman EJ. Morningness/eveningness, pubertal timing, and substance use in adolescent girls. Psychiatry Research. 2011;185(3):408–413. doi: 10.1016/j.psychres.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyakudya TT, Fuller A, Meyer LC, Maloney SK, Mitchell D. Body temperature and physical activity correlates of the menstrual cycle in chacma baboons (Papio hamadryas ursinus) American Journal of Primatology. 2012;74(12):1143–1153. doi: 10.1002/ajp.22073. [DOI] [PubMed] [Google Scholar]
- Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27(7):1255–1273. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- Paluska SA, Schwenk TL. Physical activity and mental health. Sports Medicine. 2000;29(3):167–180. doi: 10.2165/00007256-200029030-00003. [DOI] [PubMed] [Google Scholar]
- Petta D, Carskadon MA. Sleep habits in children aged 7–13 years. Sleep Research. 1984;13:86. [Google Scholar]
- Roenneberg T, Kuehnle T, Pramstaller PP, Ricken J, Havel M, Guth A, Merrow M. A marker for the end of adolescence. Current Biology. 2004;14(24):R1038–R1039. doi: 10.1016/j.cub.2004.11.039. [DOI] [PubMed] [Google Scholar]
- Sallis JF, Prochaska JJ, Taylor WC. A review of correlates of physical activity of children and adolescents. Medicine and Science in Sports and Exercise. 2000;32(5):963–975. doi: 10.1097/00005768-200005000-00014. [DOI] [PubMed] [Google Scholar]
- Sallis JF, Zakarian JM, Hovell MF, Hofstetter CR. Ethnic, socioeconomic, and sex differences in physical activity among adolescents. J Clin Epidemiol. 1996;49(2):125–134. doi: 10.1016/0895-4356(95)00514-5. [DOI] [PubMed] [Google Scholar]
- Schwartz MD, Mong JA. Estradiol modulates recovery of REM sleep in a time-of-day-dependent manner. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2013;305(3):R271–R280. doi: 10.1152/ajpregu.00474.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbrink C, Zimmer K, Lachmann T, Dirichs M, Kammer T. Development of Rapid Temporal Processing and Its Impact on Literacy Skills in Primary School Children. Child development. 2014;85(4):1711–1726. doi: 10.1111/cdev.12208. [DOI] [PubMed] [Google Scholar]
- Susman EJ, Dockray S, Schiefelbein VL, Herwehe S, Heaton JA, Dorn LD. Morningness/eveningness, morning-to-afternoon cortisol ratio, and antisocial behavior problems during puberty. Dev Psychol. 2007;43(4):811–822. doi: 10.1037/0012-1649.43.4.811. [DOI] [PubMed] [Google Scholar]
- Wang MT, Hill NE, Hofkens T. Parental Involvement and African American and European American Adolescents’ Academic, Behavioral, and Emotional Development in Secondary School. Child development. 2014;85(6):2151–2168. doi: 10.1111/cdev.12284. [DOI] [PubMed] [Google Scholar]
- Williams CL. Cardiovascular health in childhood: A statement for health professionals from the Committee on Atherosclerosis, Hypertension, and Obesity in the Young (AHOY) of the Council on Cardiovascular Disease in the Young, American Heart Association. Circulation. 2002;106(1):143–160. doi: 10.1161/01.cir.0000019555.61092.9e. [DOI] [PubMed] [Google Scholar]