Abstract
Cannabinoid receptor agonists such as delta-9-tetrahydrocannabinol (Δ9-THC) enhance some (antinociceptive) but not other (positive reinforcing) effects of mu opioid receptor agonists, suggesting that cannabinoids might be combined with opioids to treat pain without increasing, and possibly decreasing, abuse. The degree to which cannabinoids enhance antinociceptive effects of opioids varies across drugs insofar as Δ9-THC and the synthetic cannabinoid receptor agonist CP55940 increase the potency of some mu opioid receptor agonists (e.g., fentanyl) more than others (e.g., nalbuphine). It is not known whether interactions between cannabinoids and opioids vary similarly for other (abuse-related) effects. This study examined whether Δ9-THC and CP55940 differentially impact the discriminative stimulus effects of fentanyl and nalbuphine in monkeys (n=4) discriminating 0.01 mg/kg of fentanyl (s.c.) from saline. Fentanyl (0.00178–0.0178 mg/kg) and nalbuphine (0.01–0.32 mg/kg) dose-dependently increased drug-lever responding. Neither Δ9-THC (0.032–1.0 mg/kg) nor CP55940 (0.0032–0.032 mg/kg) enhanced the discriminative stimulus effects of fentanyl or nalbuphine; however, doses of Δ9-THC and CP55940 that shifted the nalbuphine dose-effect curve markedly to the right and/or down were less effective or ineffective in shifting the fentanyl dose-effect curve. The mu opioid receptor antagonist naltrexone (0.032 mg/kg) attenuated the discriminative stimulus effects of fentanyl and nalbuphine similarly. These data indicate that the discriminative stimulus effects of nalbuphine are more sensitive to attenuation by cannabinoids than those of fentanyl. That the discriminative stimulus effects of some opioids are more susceptible to modification by drugs from other classes has implications for developing maximally effective therapeutic drug mixtures with reduced abuse liability.
Keywords: opioid, cannabinoid, drug-drug interactions, drug discrimination, monkeys
1. Introduction
Pain remains a significant clinical problem (e.g., Institute of Medicine, 2011; Gaskin and Richard, 2012) and although mu opioid receptor agonists are used to treat pain, their utility is limited by unwanted effects (e.g., Benyamin et al., 2008). Drugs can be used in combination to treat pain (e.g., Gilron et al., 2013; Raffa, 2001), to increase effectiveness or to reduce the doses needed. Opioid agonists have been combined with non-opioid drugs such as nonsteroidal anti-inflammatory drugs to treat pain (e.g., Wideman et al. 1999; Sunshine et al. 1997; Raffa et al., 2010) although currently available mixtures retain many of the adverse effects of opioids alone and contribute to abuse and overdose (e.g., Atluri et al., 2014; Centers for Disease Control and Prevention, 2012; Edlund et al., 2014; Jones et al., 2013).
Cannabinoid receptor agonists such as delta-9-tetrahydrocannabinol (Δ9-THC) are used to treat pain (e.g., Hosking and Zajicek, 2008; Ware et al., 2010; Lynch and Campbell, 2011), although alone their effectiveness is limited (e.g., Kraft, 2012). Cannabinoids augment the analgesic effects of opioids in patients (e.g., Narang et al., 2008) and enhance the antinociceptive effects of opioids in nonhumans (e.g., Cichewicz et al., 1999; Cox et al., 2007; Li et al., 2008; Welch, 2009; Maguire et al., 2013; Maguire and France, 2014). While cannabinoids increase the potency of opioids for antinociception, the magnitude of enhancement varies among agonists (e.g., Cichewicz et al., 1999; Maguire et al., 2014), increasing the potency of some agonists (fentanyl) to a greater extent than others (morphine; Maguire and France, 2014). Fentanyl has greater efficacy than morphine (e.g., Saeki and Yaksh 1993; Gerak et al., 1994; Traynor and Nahorski 1995; Emmerson et al. 1996; Morgan et al. 1999) suggesting that efficacy impacts cannabinoid enhancement of opioid antinociceptive effects. It is unclear whether interactions between cannabinoids and opioids also vary for other measures. Characterizing such differences will aid in evaluating the benefits and risks of opioid/cannabinoid mixtures for treating pain.
Cannabinoids enhance antinociceptive but not some other effects of opioids in rhesus monkeys. In fact, cannabinoids attenuate the discriminative stimulus and positive reinforcing effects of some opioids (e.g., Li et al., 2008; Li et al., 2012; Maguire et al., 2013; Maguire and France, 2015). That cannabinoids enhance some but not other effects of opioids supports the view that cannabinoids could be combined with opioids to treat pain without increasing adverse effects. It is not known whether interactions between cannabinoids and the discriminative stimulus effects of opioids also depend on the drugs in the mixture. This study examined Δ9-THC and CP55940 for their ability to modify the discriminative stimulus effects of fentanyl and nalbuphine in monkeys discriminating fentanyl. Δ9-THC and CP55940 were studied because they attenuate the discriminative stimulus effects of morphine (Li et al., 2008; Maguire et al., 2013). Fentanyl and nalbuphine were studied because they share discriminative stimulus effects with morphine, and because their antinociceptive effects were the most (fentanyl) and the least (nalbuphine) sensitive to enhancement by cannabinoids (Maguire and France, 2014).
2. Materials and Methods
2.1 Subjects
Four adult female rhesus monkeys (AM, CI, HA, MA; Macaca mulatta) were previously trained to discriminate morphine from saline and participated in earlier studies (e.g., Li et al. 2008; Li et al., 2011; Maguire et al. 2013). Body weight (range: 5 to 9 kg) was maintained via post-session feeding of chow (Harlan Teklad, High Protein Monkey Diet, Madison, WI, USA), fresh fruit, and peanuts; water was continuously available in the home cage. Subjects were housed individually in a colony room maintained under a 14/10-h light/dark cycle (lights on at 0600 h) in an AAALAC accredited facility. Monkeys used in these studies were maintained in accordance with the Institutional Animal Care and Use Committee of The University of Texas Health Science Center at San Antonio, and the 2011 Guide for the Care and Use of Laboratory Animals (8th edition; Institute of Laboratory Animals Resources on Life Sciences, National Research Council, National Academy of Sciences).
2.2 Apparatus
Monkeys were seated in primate chairs (Model R001, Primate Products, Miami, FL, USA) and positioned in sound-attenuating operant conditioning chambers. Each chamber contained a custom-made response panel with two horizontally aligned response levers located 32 cm apart. Lights that could be illuminated red were located above each lever. Feet were placed in shoes containing brass electrodes to which a brief (250 ms, 3 mA) electric stimulus could be delivered from a remote current generator (H13–15, Coulbourn Instruments, Allentown, PA, USA). Extraneous sounds were masked by white noise and an exhaust fan provided ventilation. Experimental events were arranged and data were collected by an interface (Med Associates, Inc., Georgia, VT, USA) connected to a PC computer operating with Med-PC IV software (Med Associates).
2.3 Behavioral Procedure
Monkeys were previously trained to discriminate morphine from saline (Li et al., 2008); however, one monkey (MA) developed a reaction to morphine injections and the training stimulus for all monkeys was changed to fentanyl (0.01 mg/kg). This dose of fentanyl was chosen because it produced high levels of morphine-appropriate responding in all monkeys and maintained reliable discrimination performance during training. Sessions comprised 2 to 8 cycles, with each cycle consisting of a 10-min timeout period followed by a 5-min response period. During the timeout all lights were off and responding had no programmed consequence; injections occurred during the first min of each timeout. The beginning of the response period was signaled by illumination of both side key lights red. When the red lights were on, a brief electric stimulus was scheduled to be delivered every 15 s. Ten consecutive responses on the correct lever turned off the red lights and suspended the schedule of stimulus presentation for a 30-s timeout (reinforcer). After the timeout, the red key lights were turned on and the stimulus presentation schedule was restarted. Response periods ended after 10 reinforcer presentations, 4 stimulus presentations, or after 5 min elapsed, whichever occurred first.
For training, levers were designated as correct and incorrect for each cycle based on the injection given during the first min of the cycle. For monkeys CI and HA, the left lever was correct following saline injections (saline lever) and the right lever was correct following injections of 0.01 mg/kg of fentanyl (drug lever); for monkeys AM and MA, the contingencies were reversed. Responses on the incorrect lever reset the response requirement on the correct lever and were counted but otherwise had no programmed consequence. During saline training sessions, saline or sham injections were administered prior to 2 to 6 cycles, and no drug was administered. During fentanyl training sessions, 0.01 mg/kg of fentanyl was administered at the beginning of one cycle that was preceded by 0 to 5 saline or sham training cycles; the number of saline or sham cycles preceding the fentanyl training cycle varied quasi-randomly across sessions.
Test sessions occurred when the following criteria were satisfied for at least two consecutive sessions, consisting of at least one fentanyl training session and at least one saline training session: 1) at least 80% of responses in all cycles were on the correct (injection-appropriate) lever; and 2) fewer than 10 responses were on the incorrect lever prior to the first reinforcer presentation. Test sessions were identical to training sessions except that completion of 10 consecutive responses on either lever was reinforced.
Dose-effect curves for fentanyl and nalbuphine were determined under test conditions using a cumulative dosing method. Saline or naltrexone (see below) was administered during the first cycle of the session and increasing doses of drug were administered across successive cycles in 0.25-log unit increments until at least 80% of responses in a cycle were on the drug lever or when 8 cycles were completed, whichever occurred first. Based on previous studies (Li et al., 2008; Maguire et al., 2013), Δ9-THC and CP55940 were administered 60 min prior to the start of the first cycle when tested in combination with fentanyl or nalbuphine. Naltrexone was administered at the beginning of the first cycle instead of saline. In all monkeys, 0.01 mg/kg of CP55940, 0.32 mg/kg of Δ9-THC, and 0.032 mg/kg of naltrexone were tested in combination with both fentanyl and nalbuphine. Tests with additional doses of each cannabinoid were selected individually for each monkey in order to assess differential sensitivity to cannabinoids between opioids. Tests were conducted in an irregular order across subjects, and tests with cannabinoids were separated by at least 6 days.
2.4 Data Analyses
For each cycle, the number of responses on the drug lever was divided by the total number of responses on either lever and multiplied by 100 to yield the percentage of drug-lever responding. Response rate was calculated for each cycle by dividing the total number of responses on either lever by the duration of the response period (i.e., the total time that the red lights were illuminated). Percent drug-lever responding and response rate were calculated for each dose of fentanyl and nalbuphine alone and following pretreatment with a cannabinoid or naltrexone and plotted as a function of dose. Control dose-effect curves for fentanyl and nalbuphine represent the average of three tests, conducted at the beginning, middle, and end of the experiment, with one exception: the dose-effect curve for nalbuphine administered alone was determined twice for monkey AM, at the beginning and at the end of the experiment. Tests with each dose of Δ9-THC, CP55940, and naltrexone were determined once.
The potency of fentanyl and nalbuphine to increase drug-lever responding was determined for individual monkeys by estimating the dose required to produce 50% drug-lever responding (ED50) using linear regression. Only data comprising the linear portion of the dose-effect curve were used, ranging from the largest dose that produced less than 25% drug-lever responding to the smallest dose that produced more than 75% drug-lever responding. In cases where responding did not exceed 75%, it was assumed that the next largest dose of fentanyl or nalbuphine that would have been tested, on a 0.25-log unit scale, would produce 100% drug-lever responding, providing the most conservative estimate of rightward shifts in the dose-effect curve. Shifts in the fentanyl and nalbuphine dose-effect curves after pretreatment with Δ9-THC, CP55940, and naltrexone were quantified by calculating a potency ratio as follows: test ED50 value divided by control ED50 value. A ratio greater than 1 indicated a rightward shift in the dose-effect curve and a reduction in the potency of the opioid to occasion drug-lever responding. All curve fitting and data analyses were conducted using GraphPad Prism (5.0, GraphPad Software, Inc., San Diego, CA, USA).
2.5 Drugs
Fentanyl, naltrexone, and delta-9-tetrahydrocannabinol (Δ9-THC; 100 mg/ml in absolute ethanol) were provided by the National Institute on Drug Abuse Drug Supply Program (Bethesda, MD, USA). Nalbuphine (Mallinckrodt, Inc., St. Louis, MO, USA) and CP55940 (Sigma-Aldrich, St. Louis, MO, USA) were purchased from commercial sources. Fentanyl, nalbuphine, and naltrexone were dissolved in sterile water. CP55940 was dissolved in a 1:1:18 mixture of absolute ethanol, Emulphor-620 (Rhone-Poulenc Inc., Princeton, NJ, USA), and 0.9% sterile saline. Δ9-THC was stored in absolute ethanol and was further diluted in a mixture of ethanol, Emulphor-620, and saline ensuring it was dissolved in the 1:1:18 vehicle. All drugs were administered s.c. in the back in a volume of 0.2 to 0.8 ml.
3. Results
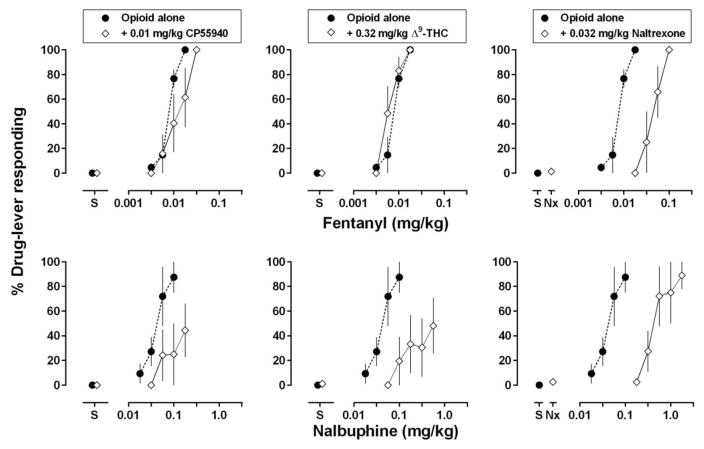
When administered alone, saline occasioned exclusive responding on the saline lever (Fig. 1, circles above “S”), and response rates averaged 2.7 responses per second (range: 2.4 to 3.1). Fentanyl dose-dependently increased drug-lever responding (Fig. 1, top row, circles) with an average ED50 value of 0.008 mg/kg (Table 1). Nalbuphine also increased drug-lever responding (Fig. 1, bottom row, circles), being slightly less effective and less potent than fentanyl, with an average ED50 value of 0.04 mg/kg (Table 1).
Fig 1.
Effects of the cannabinoid receptor agonists CP55940 and Δ9-THC as well as the opioid receptor antagonist naltrexone on the discriminative stimulus effects of fentanyl (top) and nalbuphine (bottom). Filled circles indicate group mean dose-effect curves for the opioid agonists administered alone using cumulative dosing with 15-min inter-injection intervals. In the left panels, diamonds indicate dose-effect curves for the opioid agonists determined 60 min after the administration of 0.01 mg/kg of CP55940. In the center panels, diamonds indicate group mean dose-effect curves for the opioid agonists determined 60 min after the administration of 0.32 mg/kg of Δ9-THC. In the right panels, diamonds indicate the effect of 0.032 mg/kg of naltrexone administered alone (data above “Nx”) and in combination with cumulative doses of each agonist. Abscissae: dose in milligrams per kilogram body weight. “S” indicates data that were collected when saline was administered during the first test cycle. Ordinate: group mean percentage of the drug-lever responding ± 1 standard error of the mean (S.E.M.).
Table 1.
ED50 values and potency ratios with 95% confidence intervals for the discriminative stimulus effects of fentanyl and nalbuphine alone and combination with selected doses of CP55940, Δ9-THC, and naltrexone in monkeys discriminating fentanyl.
| ED50 (mg/kg)
|
Potency ratiob
|
|||
|---|---|---|---|---|
| Mean | 95%CIa | Mean | 95%CI | |
| Fentanyl | 0.008 | 0.006–0.01 | ||
| + 0.01 mg/kg CP55940 | 0.012 | 0.006–0.023 | 1.7 | 0.9–2.4 |
| + 0.32 mg/kg Δ9-THC | 0.006 | 0.004–0.009 | 0.8 | 0.6–1.0 |
| + 0.032 mg/kg Naltrexone | 0.045 | 0.028–0.071 | 6.1 | 3.8–8.4c |
| Nalbuphine | 0.04 | 0.02–0.08 | ||
| + 0.01 mg/kg CP55940 | 0.22 | 0.06–0.88 | 6.9 | 1.9–11.9c |
| + 0.32 mg/kg Δ9-THC | 0.39 | 0.15–1.05 | 13.3 | 1.6–25.1c |
| + 0.032 mg/kg Naltrexone | 0.52 | 0.23–1.16 | 12.3 | 8.4–16.2c |
95% confidence intervals.
Potency ratio is calculated by dividing the test ED50 by the control ED50.
Significant shift in the opioid dose-effect curve following pretreatment.
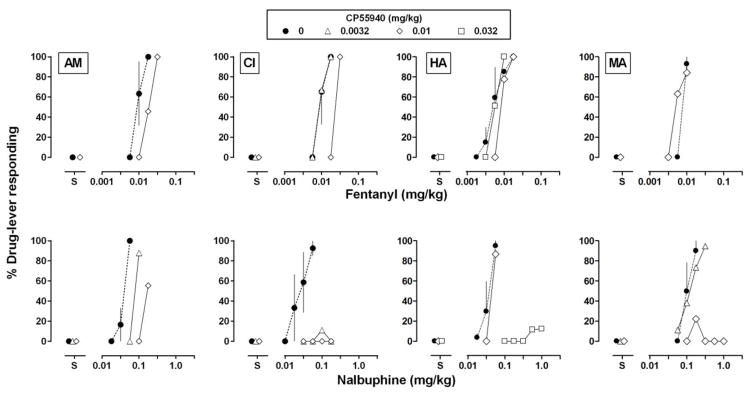
Pretreatment with 0.01 mg/kg of CP55940 significantly attenuated the discriminative stimulus effects of nalbuphine, shifting the dose-effect curve rightward and downward (Fig. 1, bottom left panel; Table 1); the same dose of CP55940 did not significantly impact the discriminative stimulus effects of fentanyl (Fig. 1, top left panel; Table 1). Because monkeys differed in sensitivity to the effects of CP55940, other doses were tested in individual monkeys; nevertheless, effects in individual monkeys reflected those observed at the group level (Fig. 2). For example, in monkey CI a dose of 0.056 mg/kg of nalbuphine administered alone occasioned more than 90% drug-lever responding. Following administration of 0.0032 mg/kg of CP55940, doses of nalbuphine up to 0.178 mg/kg failed to increase drug-lever responding; the same dose of CP55940 did not impact the discriminative stimulus effects of fentanyl. Similarly, for monkeys HA and MA, doses of CP55940 (0.032 mg/kg for HA and 0.01 mg/kg for MA) that markedly attenuated the discriminative stimulus effects of nalbuphine and shifted the nalbuphine dose-effect curve downward (Table 2), did not markedly impact the fentanyl dose-effect curve. Although for MA, CP55940 modestly shifted the fentanyl dose-effect curve 1.4-fold to the left. For AM, 0.01 mg/kg of CP55940 shifted the nalbuphine dose-effect curve farther to the right (4.4 fold) than the fentanyl dose-effect curve (1.9 fold; Table 2).
Fig 2.
Effects of the cannabinoid receptor agonist CP55940 on the discriminative stimulus effects of the mu opioid receptor agonists fentanyl (top) and nalbuphine (bottom) in individual monkeys (AM, CI, HA, and MA; shown across columns). Filled circles indicate dose-effect curves for the opioid agonists administered alone, and open symbols indicate dose-effect curves determined beginning 60 min after the administration of 0.0032 (triangles), 0.01 (diamonds), or 0.032 (squares) mg/kg of CP55940. Abscissae: dose in milligrams per kilogram body weight. “S” indicates data that were collected when saline was administered during the first test cycle. Ordinate: mean percentage of the drug-lever responding ± 1 standard error of the mean (S.E.M.).
Table 2.
ED50 values for fentanyl and nalbuphine administered alone and following pretreatment with CP55940, Δ9-THC, or naltrexone in individual monkeys. Potency ratios for tests following pretreatments are also indicated.
| Monkey
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AM
|
CI
|
HA
|
MA
|
|||||||
| Fentanyl | Nalbuphine | Fentanyl | Nalbuphine | Fentanyl | Nalbuphine | Fentanyl | Nalbuphine | |||
|
| ||||||||||
| Controla | ED50b | 0.009 | 0.04 | 0.009 | 0.03 | 0.005 | 0.03 | 0.008 | 0.10 | |
| 95% CIc | 0.005–0.03 | 0.03–0.19 | 0.005–0.03 | 0.02–0.14 | 0.004–0.027 | 0.02–0.12 | 0.001–0.016 | 0.07–0.39 | ||
|
| ||||||||||
| CP55940 (mg/kg) | 0.0032 | ED50 | 0.08 | 0.009 | ≥ 0.24e,f | 0.12 | ||||
| Ratiod | 2.0 | 1.0 | ≥ 9.3 | 1.2 | ||||||
| 0.01 | ED50 | 0.018 | 0.17 | 0.025 | ≥ 0.24e | 0.008 | 0.04 | 0.006 | ≥ 1.33e | |
| Ratio | 1.9 | 4.4 | 2.5 | ≥ 9.3 | 1.5 | 1.3 | 0.7 | ≥ 12.8 | ||
| 0.032 | ED50 | 0.006 | ≥ 1.28e | |||||||
| Ratio | 1.1 | ≥ 37.0 | ||||||||
|
| ||||||||||
| Δ9-THC (mg/kg) | 0.032 | ED50 | 0.04 | |||||||
| Ratio | 1.7 | |||||||||
| 0.1 | ED50 | 0.24e | 0.03 | 0.14 | ||||||
| Ratio | 9.31 | 0.79 | 1.34 | |||||||
| 0.32 | ED50 | 0.01 | 0.09 | 0.007 | ≥ 0.75e | 0.004 | 0.52e | 0.004 | ≥ 0.69e | |
| Ratio | 1.1 | 2.3 | 0.8 | ≥ 29.4 | 0.8 | 15.0 | 0.6 | ≥ 6.6 | ||
| 1 | ED50 | ≥ 0.13e | ≥0.13e | |||||||
| Ratio | ≥ 25.00 | ≥17.40 | ||||||||
|
| ||||||||||
| Naltrexone (mg/kg) | 0.032 | ED50 | 0.55e | 0.28e | 0.042e | 0.34e | 0.024 | 0.42e | 0.72e | 1.74e |
| Ratio | 5.8 | 7.1 | 4.5 | 13.4 | 4.5 | 12.2 | 9.4 | 16.7 | ||
Control data are the mean of multiple determinations of the fentanyl and nalbuphine dose-effect curves determined alone.
ED50 is expressed as mg/kg.
95% confidence intervals of the control dose-effect curve.
Potency ratio is calculated by dividing the test ED50 by the control ED50.
ED50 value for the test is considered significantly different from control if it falls outside of the 95% confidence intervals of control.
≥ indicates a test during which drug-lever responding did not exceed 75%; ED50 was estimated as described in Data Analyses.
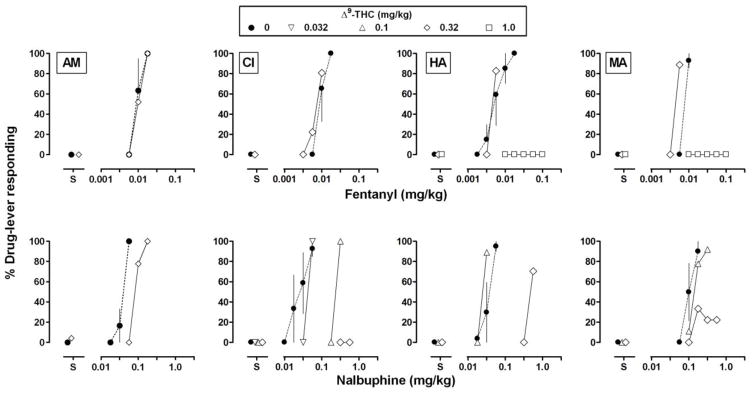
Similar to the results obtained with CP55940, pretreatment with 0.32 mg/kg of Δ9-THC significantly attenuated the discriminative stimulus effects of nalbuphine, shifting the dose-effect curve rightward and downward (Fig. 1, bottom center panel; Table 1), but did not impact the discriminative stimulus effects of fentanyl (Fig. 1, top center panel; Table 1). For individual monkeys, the same doses of Δ9-THC that significantly impacted the nalbuphine dose-effect curve had smaller effects or no effect on the fentanyl dose-effect curve (Fig. 3, top row). For example, in 3 monkeys (CI, HA, and MA) a dose of 0.32 mg/kg of Δ9-THC significantly reduced the discriminative stimulus effects of nalbuphine, resulting in rightward or downward shifts in the dose-effect curve (at least 29.4-, 15.0-, and 6.6- fold, respectively; Table 2). The same dose of Δ9-THC had little effect on the dose-effect curve of fentanyl in the same monkeys. For monkeys HA and MA, 1.0 mg/kg of Δ9-THC reduced the discriminative stimulus effects of fentanyl up to a dose (0.1 mg/kg) that was 10-fold larger than the training stimulus. For AM, the difference in effect of Δ9-THC between nalbuphine and fentanyl was smaller in comparison with the other monkeys; however, consistent with the other monkeys, the effect of 0.32 mg/kg of Δ9-THC with nalbuphine (2.3-fold shift) exceeded the effect of the same dose of Δ9-THC with fentanyl (i.e., no shift; Table 2).
Fig 3.
Effects of the cannabinoid receptor agonist Δ9-THC on the discriminative stimulus effects of fentanyl (top) and nalbuphine (bottom). Filled circles indicate dose-effect curves for the opioid agonists administered alone, and open symbols indicate dose-effect curves determined beginning 60 min after the administration of 0.032 (inverted triangles), 0.1 (upright triangles), 0.32 (diamonds), or 1.0 (squares) mg/kg of Δ9-THC. Other details are the same as in Fig. 2.
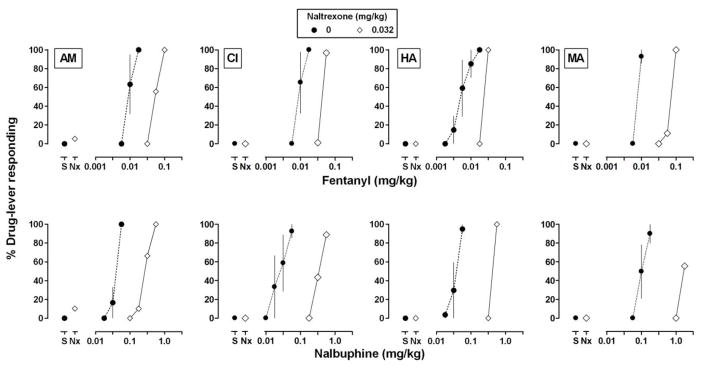
In contrast to the results obtained with CP55940 and Δ9-THC , pretreatment with 0.032 mg/kg of naltrexone significantly attenuated the discriminative stimulus effects of nalbuphine and fentanyl, shifting dose-effects curves to the right on average 6.1 and 12.3 fold, respectively (Fig. 1, right panels; Table 1). For individual monkeys (Fig. 4), shifts in the fentanyl curve, ranging from 4.5 to 9.4 fold across monkeys, were similar to although slightly smaller than shifts in the nalbuphine curve, ranging from 7.1 to 16.7 fold (Table 2).
Fig 4.
Effects of the mu opioid receptor antagonist naltrexone on the discriminative stimulus effects of fentanyl (top) and nalbuphine (bottom). Filled circles indicate dose-effect curves for the opioid agonists administered alone, and diamonds indicate the effect of 0.032 mg/kg of naltrexone administered alone (data above “Nx”) and in combination with cumulative doses of each agonist. Other details are the same as in Fig. 2.
When administered alone, neither fentanyl nor nalbuphine decreased response rates below 70% of control rates (Fig. S1). For some monkeys, CP55940 modestly decreased response rate, particularly when combined with large doses of nalbuphine; however, response rates never fell below 40% of control (Fig. S1). With one exception, pretreatment with Δ9-THC did not substantially impact response rates (Fig. S2). For monkey MA, 1.0 mg/kg of Δ9-THC decreased response rates to 68% of control during the first (saline) cycle and response rates decreased further with increasing doses of fentanyl; however, response rates never fell below 40% of control. In some cases after naltrexone pretreatment, response rates decreased following administration of larger doses of fentanyl and nalbuphine; however, rates never fell below 50% of control (Fig. S3).
4. Discussion
Cannabinoid receptor agonists enhance the antinociceptive effects of mu opioid receptor agonists although the magnitude of enhancement depends on the properties of the drugs in the mixture (e.g., Cichewicz et al., 1999; Maguire and France, 2014). For antinociception in rhesus monkeys, the cannabinoid receptor agonists Δ9-THC and CP55940 increase the potency of some mu opioid receptor agonists (e.g., fentanyl) more than equi-effective doses of other mu opioid receptor agonists (e.g., nalbuphine); these results suggested that efficacy might be a factor in these interactions (Maguire and France, 2014). Characterizing drug interactions across multiple measures is necessary for understanding the therapeutic potential as well as adverse effects of drug mixtures. This study examined whether cannabinoid receptor agonists impact the discriminative stimulus effects of mu opioid receptor agonists differentially in order to determine whether interactions for discriminative stimulus effects also depend on the agonists in the mixture.
Fentanyl and nalbuphine dose dependently increased drug-lever responding to greater than 80% in monkeys discriminating fentanyl from saline. The relative potency of fentanyl and nalbuphine (fentanyl > nalbuphine) was consistent with other studies in which both drugs were tested in subjects discriminating a mu opioid receptor agonist (e.g., Walker and Young, 1993; Picker et al., 1993; Gerak et al., 1996; Zhang et al., 2000). In previous studies, the cannabinoid receptor agonists Δ9-THC, CP55940, and WIN55212 attenuated the discriminative stimulus effects of morphine (Li et al., 2008; Maguire et al., 2013). The current results extend previous studies by showing that Δ9-THC and CP55940 also attenuate the discriminative stimulus effects of nalbuphine and, in some cases, fentanyl. These data build upon earlier results showing that even though cannabinoid receptor agonists enhance the antinociceptive effects of mu opioid receptor agonists (Maguire and France 2014), they either do not impact or attenuate their discriminative stimulus effects. To the extent that the discriminative stimulus effects of opioids are related to their subjective effects (e.g., Preston and Bigelow, 1991), these data also suggest that cannabinoids might attenuate the subjective effects of opioids. In the current study, doses of Δ9-THC and CP55940 that shifted the nalbuphine dose-effect curve rightward or downward were less effective (or not effective) in shifting the fentanyl dose-effect curve, indicating that interactions between cannabinoids and opioids with regard to the discriminative stimulus effects of opioids depend on the agonist.
It is unclear why cannabinoid receptor agonists attenuate the discriminative stimulus effects of nalbuphine more than the discriminative stimulus effects of fentanyl. It is unlikely that different populations of receptors mediate the discriminative stimulus effects of the two opioids (e.g., those mediating the effects of nalbuphine being more susceptible to modulation by cannabinoid receptor activation) because naltrexone attenuated the effects of fentanyl and nalbuphine similarly - consistent with previous studies showing that the discriminative stimulus effects of fentanyl and nalbuphine are modified similarly by opioid receptor antagonists and, therefore, are likely mediated by the same mu opioid receptors (Picker and Dykstra, 1989; Gerak et al., 1996). Although naltrexone shifted the nalbuphine dose-effect curve slightly further to the right than it shifted the fentanyl dose-effect curve (4.5–9.4 fold versus 7.1–16.7 fold; less than a 2-fold difference between agonists), this difference for naltrexone across agonists is much smaller than the difference for cannabinoids (Δ9-THC [0.32 mg/kg] and CP55940 [0.01 mg/kg] shifted the nalbuphine dose-effect curve more than 17- and 6-fold, respectively, farther to the right than the fentanyl dose-effect curve).
Opioid and cannabinoid receptor systems interact at multiple cellular and molecular targets (e.g., Vigano et al., 2005; Bushlin et al., 2010) and it has been suggested that cannabinoids alter opioid receptor signaling by modulating opioid receptor function or changing post-receptor signaling (e.g., Rios et al. 2006; Canals and Milligan 2008). Disruption of opioid receptor signaling could result in the need for higher concentrations of an agonist to produce a particular level of effect. Nalbuphine has lower efficacy than fentanyl at mu opioid receptors (e.g., Gerak et al., 1994; Emmerson et al. 1996; Morgan et al. 1999). As such, nalbuphine requires higher opioid receptor occupancy than fentanyl to produce the same effect. Although nalbuphine has effects that are equivalent to those of fentanyl under some conditions (e.g. Walker and Young 2002; current experiment), changing the pool of opioid receptors or otherwise disrupting opioid receptor signaling might attenuate the effects of nalbuphine without impacting, or impacting less, the effects of fentanyl (e.g., Morgan and Picker 1998). Thus, a decrease in opioid receptor signaling (e.g., Rios et al. 2006) could account for Δ9-THC and CP55940 having a greater effect on the discriminative stimulus effects of the low efficacy agonist nalbuphine, compared with fentanyl. However, such an interpretation would be difficult to reconcile with studies showing that cannabinoid receptor agonists enhance the antinociceptive effects of opioid receptor agonists (e.g., Li et al. 2008; Maguire et al. 2013; Maguire and France 2014) across the same range of doses that attenuate their discriminative stimulus effects. Presumably, any decrease in mu opioid receptor signaling by cannabinoid receptor agonists would impact all effects of mu opioid receptor agonists similarly, including antinociceptive and discriminative stimulus effects (Bowen et al. 2002).
Alternatively, cannabinoids might attenuate the discriminative stimulus effects of opioids through perceptual processes. Drug discrimination is a highly pharmacologically selective procedure insofar as drugs with similar actions (e.g., agonism) at the same receptor(s) typically share discriminative stimulus effects whereas drugs with different actions at that receptor (e.g., antagonism) or with actions at different receptors usually do not share discriminative stimulus effects. Indeed, cannabinoid receptor agonists do not occasion drug-appropriate responding in subjects trained to discriminate an opioid receptor agonist (e.g., Li et al., 2008; Maguire et al., 2013; current study) and opioid receptor agonists do not occasion drug-appropriate responding in subjects trained to discriminate a cannabinoid receptor agonist (e.g., Wiley et al., 1995; McMahon 2006; Li et al. 2008; Lile et al. 2008). However, the discriminative stimulus effects of some drugs, including opioids, can be altered by drugs acting through presumably unrelated receptors. For example, dopaminergic drugs (e.g., amphetamine) can attenuate or enhance the discriminative stimulus effects of morphine (e.g., Gauvin and Young, 1989). Such interactions raise the possibility that other non-pharmacological (i.e., perceptual) mechanisms play a role, particularly in cases when the discriminative stimulus effects of a drug are attenuated while other effects mediated by the same receptors are not. In rhesus monkeys, serotonin receptor agonists such as DOM [1-(2,5-dimethoxy-4-methylphenyl)-2-aminopropane] attenuate the discriminative stimulus effects of morphine; however, the same dose of serotonin receptor agonists markedly enhance the antinociceptive effects of morphine (Li et al. 2011). Given that both the discriminative stimulus and the antinociceptive effects of morphine are mediated by mu receptors (e.g., Bowen et al. 2002), it seems unlikely that the effect of a serotonin receptor agonist on the morphine discriminative stimulus is due to reduced signaling through mu opioid receptors per se. Rather, it might be the case that serotonin receptor agonists, as well as cannabinoid receptor agonists, block or perceptually mask the discriminative stimulus effects of morphine and other mu receptor agonists.
Results of this study as well as other studies with cannabinoids (Li et al., 2008; Maguire et al. 2013; Maguire and France 2014) are similar to results of studies with serotonin receptor agonists (Li et al. 2011) insofar as cannabinoids enhance the antinociceptive effects of some mu opioid receptor agonists while attenuating their discriminative stimulus effects. To the extent that perceptual mechanisms play a role, the intensity of the discriminative stimulus (e.g., mu opioid receptor activity) as well as the intensity of the disrupting stimulus (e.g., cannabinoid receptor activity) might interact to determine the effectiveness of the opioid to increase drug-lever responding, with a lower intensity discriminative stimulus being more susceptible to disruption than a higher intensity stimulus.
In summary, previous studies demonstrated that cannabinoid receptor agonists enhance the antinociceptive effects of mu opioid receptor agonists with the magnitude of enhancement varying depending on the properties of the agonist in the mixture (e.g., Maguire and France 2014). The current study determined whether Δ9-THC and CP55940 differentially modify the discriminative stimulus effects of the mu opioid receptor agonists fentanyl and nalbuphine. Fentanyl and nalbuphine increased drug-lever responding similarly in monkeys trained to discriminate fentanyl. Δ9-THC and CP55940 did not enhance and in many cases attenuated the discriminative stimulus effects of both opioids. Moreover, the discriminative stimulus effects of nalbuphine were more sensitive than the discriminative stimulus effects of fentanyl to modulation by cannabinoids. These data indicate that interactions between cannabinoids and opioids with regard to discriminative stimulus effects also depend upon the constituents of the drug mixture, and taken together with earlier studies, provide evidence that the qualitative nature of interactions between cannabinoids and opioids (i.e., whether cannabinoids enhance or attenuate the effects of opioids) varies across different measures of drug action.
Supplementary Material
Acknowledgments
The authors thank Marlisa Burton, Nicole Garcia, Hillary Halderman, and Jeff Pressley, for excellent technical assistance. This work was supported by the National Institutes of Health National Institute on Drug Abuse [Grants R01DA005018, F32DA035605, T32DA031115, K05DA17918]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the National Institute on Drug Abuse. Portions of these data were presented at the annual meeting of the American Society for Pharmacology and Experimental Therapeutics in 2014, and an abstract has been archived in the FASEB journal (Maguire and France, 2014, Volume 28, Issue 1 Supplement, Page 658.4).
Footnotes
Disclosure/Conflict of Interest: The authors have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Atluri S, Sudarshan G, Manchikanti L. Assessment of the trends in medical use and misuse of opioid analgesics from 2004 to 2011. Pain physician. 2014;17:E119–E128. [PubMed] [Google Scholar]
- Benyamin R, Trescot AM, Datta S, Buenaventura R, Adlaka R, Sehgal N, Glaser SE, Vallejo R. Opioid complications and side effects. Pain Physician. 2008;11:S105–S120. [PubMed] [Google Scholar]
- Bowen CA, Fischer BD, Mello NK, Negus SS. Antagonism of the antinociceptive and discriminative stimulus effects of heroin and morphine by 3-methoxynaltrexone and naltrexone in rhesus monkeys. J Pharmacol Exp Ther. 2002;302:264–273. doi: 10.1124/jpet.302.1.264. [DOI] [PubMed] [Google Scholar]
- Bushlin I, Rozenfeld R, Devi LA. Cannabinoid-opioid interactions during neuropathic pain and analgesia. Curr Opin Pharmacol. 2010;10:80–86. doi: 10.1016/j.coph.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canals M, Milligan G. Constitutive activity of the cannabinoid CB1 receptor regulates the function of co-expressed Mu opioid receptors. J Biol Chem. 2008;283:11424–11434. doi: 10.1074/jbc.M710300200. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. CDC Grand Rounds: Prescription Drug Overdoses — a U.S. Epidemic. [Accessed 31 August 2015];MMWR Morb Mortal Wkly Rep. 2012 61:10–13. at http://www.cdc.gov/mmwr/pdf/wk/mm6101.pdf. [PubMed] [Google Scholar]
- Cichewicz DL. Synergistic interactions between cannabinoid and opioid analgesics. Life Sci. 2004;74:1317–1324. doi: 10.1016/j.lfs.2003.09.038. [DOI] [PubMed] [Google Scholar]
- Cox ML, Haller VL, Welch SP. Synergy between Δ 9-tetrahydrocannabinol and morphine in the arthritic rat. EurJ Pharmacol. 2007;567:125–130. doi: 10.1016/j.ejphar.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Edlund MJ, Martin BC, Russo JE, DeVries A, Braden JB, Sullivan MD. The role of opioid prescription in incident opioid abuse and dependence among individuals with chronic noncancer pain: the role of opioid prescription. Clin J Pain. 2014;30:557–564. doi: 10.1097/AJP.0000000000000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmerson PJ, Clark MJ, Mansour A, Akil H, Woods JH, Medzihradsky Characterization of opioid agonist efficacy in a C6 glioma cell line expressing the mu opioid receptor. J Pharmacol Exp Ther. 1996;278:1121–1127. [PubMed] [Google Scholar]
- Gaskin DJ, Richard P. The economic costs of pain in the United States. J Pain. 2012;13:715–724. doi: 10.1016/j.jpain.2012.03.009. [DOI] [PubMed] [Google Scholar]
- Gauvin DV, Young AM. Evidence for perceptual masking of the discriminative morphine stimulus. Psychopharmacology. 1989;98:212–221. doi: 10.1007/BF00444694. [DOI] [PubMed] [Google Scholar]
- Gerak LR, Butelman ER, Woods JH, France CP. Antinociceptive and respiratory effects of nalbuphine in rhesus monkeys. J Pharm Exp Ther. 1994;271:993–999. [PubMed] [Google Scholar]
- Gerak LR, France CP. Discriminative stimulus effects of nalbuphine in rhesus monkeys. J Pharm Exp Ther. 1996;276:523–531. [PubMed] [Google Scholar]
- Gilron I, Jensen TS, Dickenson AH. Combination pharmacotherapy for management of chronic pain: from bench to bedside. Lancet Neurol. 2013;12:1084–1095. doi: 10.1016/S1474-4422(13)70193-5. [DOI] [PubMed] [Google Scholar]
- Hosking RD, Zajicek JP. Therapeutic potential of cannabis in pain medicine. Br J Anaesth. 2008;101:59–68. doi: 10.1093/bja/aen119. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine. Relieving pain in America: a blueprint for transforming prevention, care, education, and research. Washington, DC: The National Academies Press; 2011. [Accessed 31 August 2015]. at http://iom.nationalacademies.org/reports/2011/relieving-pain-in-america-a-blueprint-for-transforming-prevention-care-education-research.aspx. [PubMed] [Google Scholar]
- Jones CM, Mack KA, Paulozzi LJ. Pharmaceutical overdose deaths, United States, 2010. JAMA. 2013;309:657–659. doi: 10.1001/jama.2013.272. [DOI] [PubMed] [Google Scholar]
- Kraft B. Is there any clinically relevant cannabinoid-induced analgesia? Pharmacol. 2012;89:237–246. doi: 10.1159/000337376. [DOI] [PubMed] [Google Scholar]
- Li J-X, Koek W, France CP. Interactions between Δ9-tetrahydrocannabinol and heroin: self-administration in rhesus monkeys. Behav Pharmacol. 2012;23:754–761. doi: 10.1097/FBP.0b013e32835a3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J-X, Koek W, Rice KC, France CP. Effects of direct-and indirect-acting serotonin receptor agonists on the antinociceptive and discriminative stimulus effects of morphine in rhesus monkeys. Neuropsychopharmacol. 2011;36:940–949. doi: 10.1038/npp.2010.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J-X, McMahon LR, Gerak LR, Becker GL, France CP. Interactions between Δ9-tetrahydrocannabinol and mu opioid receptor agonists in rhesus monkeys: discrimination and antinociception. Psychopharmacology. 2008;199:199–208. doi: 10.1007/s00213-008-1157-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lile JA, Kelly TH, Pinsky DJ, Hays LR. Substitution profile of Δ9- tetrahydrocannabinol, triazolam, hydromorphone, and methylphenidate in humans discriminating Δ9-tetrahydrocannabinol. Psychopharmacology. 2009;203:241–250. doi: 10.1007/s00213-008-1393-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch MA, Campbell F. Cannabinoids for treatment of chronic non-cancer pain; a systematic review of randomized trials. Brit J Clin Pharmaco. 2011;72:735–744. doi: 10.1111/j.1365-2125.2011.03970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire DR, France CP. Impact of efficacy at the μ-opioid receptor on antinociceptive effects of combinations of μ-opioid receptor agonists and cannabinoid receptor agonists. J Pharmacol Exp Ther. 2014;351:383–389. doi: 10.1124/jpet.114.216648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire DR, France CP. Effects of daily delta-9-tetrahydrocannabinol treatment on heroin self-administration in rhesus monkeys. Behav Pharmacol. 2015 doi: 10.1097/FBP.0000000000000192. Published ahead of print on 21 September 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire DR, Yang W, France CP. Interactions between μ-opioid receptor agonists and cannabinoid receptor agonists in rhesus monkeys: antinociception, drug discrimination, and drug self-administration. J Pharmacol Exp Ther. 2013;345:354–362. doi: 10.1124/jpet.113.204099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon LR. Characterization of cannabinoid agonists and apparent pA2 analysis of cannabinoid antagonists in rhesus monkeys discriminating Δ9-tetrahydrocannabinol. J Pharmacol Exp Ther. 2006;319:1211–1218. doi: 10.1124/jpet.106.107110. [DOI] [PubMed] [Google Scholar]
- Morgan D, Cook CD, Smith MA, Picker MJ. An examination of the interactions between the antinociceptive effects of morphine and various mu-opioids: the role of intrinsic efficacy and stimulus intensity. Anesth Analg. 1999;88:407–413. doi: 10.1213/00000539-199902000-00035. [DOI] [PubMed] [Google Scholar]
- Morgan D, Picker MJ. The μ opioid irreversible antagonist beta-funaltrexamine differentiates the discriminative stimulus effects of opioids with high and low efficacy at the μ opioid receptor. Psychopharmacology. 1998;140:20–28. doi: 10.1007/s002130050734. [DOI] [PubMed] [Google Scholar]
- Narang S, Gibson D, Wasan AD, Ross EL, Michna E, Nedeljkovic SS, Jamison RN. Efficacy of dronabinol as an adjuvant treatment for chronic pain patients on opioid therapy. J Pain. 2008;9:254–264. doi: 10.1016/j.jpain.2007.10.018. [DOI] [PubMed] [Google Scholar]
- Picker MJ, Dykstra LA. Discriminative stimulus effects of mu and kappa opioids in the pigeon: analysis of the effects of full and partial mu and kappa agonists. J Pharmacol Exp Ther. 1989;249:557–566. [PubMed] [Google Scholar]
- Picker MJ, Yarbrough J, Hughes CE, Smith MA, Morgan D, Dykstra LA. Agonist and antagonist effects of mixed action opioids in the pigeon drug discrimination procedure: influence of training dose, intrinsic efficacy and interanimal differences. J Pharmacol Exp Ther. 1993;266:756–767. [PubMed] [Google Scholar]
- Preston KL, Bigelow GE. Subjective and discriminative effects of drugs. Behav Pharmacol. 1991;2:293–314. [PubMed] [Google Scholar]
- Raffa RB. Pharmacology of oral combination analgesics: rational therapy for pain. J Clin Pharm Ther. 2001;26:257–264. doi: 10.1046/j.1365-2710.2001.00355.x. [DOI] [PubMed] [Google Scholar]
- Raffa RB, Pergolizzi JV, Segarnick DJ, Tallarida RJ. Oxycodone combinations for pain relief. [Accessed on 31 Aug 2015];Drug Today (Barc) 2010 46:379–398. doi: 10.1358/dot.2010.46.6.1470106. at http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4046166/pdf/nihms573237.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios C, Gomes I, Devi LA. μ opioid and CB1 cannabinoid receptor interactions: reciprocal inhibition of receptor signaling and neuritogenesis. Brit J Pharmacol. 2006;148:387–395. doi: 10.1038/sj.bjp.0706757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeki S, Yaksh TL. Suppression of nociceptive responses by spinal mu opioid agonists: effects of stimulus intensity and agonist efficacy. Anesth Analg. 1993;77:265–274. doi: 10.1213/00000539-199308000-00010. [DOI] [PubMed] [Google Scholar]
- Sunshine A, Olson NZ, O'Neill E, Ramos I, Doyle R. Analgesic efficacy of a hydrocodone with ibuprofen combination compared with ibuprofen alone for the treatment of acute postoperative pain. J Clin Pharmacol. 1997;37:908–915. doi: 10.1002/j.1552-4604.1997.tb04265.x. [DOI] [PubMed] [Google Scholar]
- Traynor JR, Nahorski SR. Modulation by mu-opioid agonists of guanosine-5'-O-(3-[35S]thio)triphosphate binding to membranes from human neuroblastoma SH-SY5Y cells. Mol Pharmacol. 1995;47:848–854. doi: 10.1016/S0026-895X(25)08634-1. [DOI] [PubMed] [Google Scholar]
- Vigano D, Rubino T, Parolaro D. Molecular and cellular basis of cannabinoid and opioid interactions. Pharmacol Biochem Behav. 2005;81:360–368. doi: 10.1016/j.pbb.2005.01.021. [DOI] [PubMed] [Google Scholar]
- Walker EA, Young AM. Discriminative-stimulus effects of the low efficacy mu agonist nalbuphine. J Pharmacol Exp Ther. 1993;267:322–330. [PubMed] [Google Scholar]
- Welch SP. Interaction of the cannabinoid and opioid systems in the modulation of nociception. Int Rev Psychiatry. 2009;21:143–151. doi: 10.1080/09540260902782794. [DOI] [PubMed] [Google Scholar]
- Wideman GL, Keffer M, Morris E, Doyle RT, Jiang JG, Beaver WT. Analgesic efficacy of a combination of hydrocodone with ibuprofen in postoperative pain. Clin Pharmacol Ther. 1999;65:66–76. doi: 10.1016/S0009-9236(99)70123-2. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Huffman JW, Balster RL, Martin BR. Pharmacological specificity of the discriminative stimulus effects of Δ9-tetrahydrocannabinol in rhesus monkeys. Drug Alcohol Depend. 1995;40:81–86. doi: 10.1016/0376-8716(95)01193-5. [DOI] [PubMed] [Google Scholar]
- Zhang L, Walker EA, Sutherland J, 2nd, Young AM. Discriminative stimulus effects of two doses of fentanyl in rats: pharmacological selectivity and effect of training dose on agonist and antagonist effects of mu opioids. Psychopharmacology. 2000;148:136–145. doi: 10.1007/s002130050035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.