Abstract
Angiogenesis is an important adaptation for recovery from peripheral ischemia. Here, we determined whether 20-hydroxyeicosatetraenoic acid (20-HETE) contributes to ischemia-induced angiogenesis and assessed its underlying molecular and cellular mechanisms using a mouse hindlimb-ischemia angiogenesis model. Hindlimb blood flow was measured by laser doppler perfusion imaging and microvessel density was determined by CD31 and Tomato Lectin staining. We found that systemic and local administration of a 20-HETE synthesis inhibitor, DDMS, or a 20-HETE antagonist, 6,15-20-HEDGE significantly reduced blood flow recovery and microvessel formation in response to ischemia. 20-HETE production, measured by LC/MS/MS, was markedly increased in ischemic muscles (91 ± 11 vs 8 ± 2 pg/mg in controls), which was associated with prominent upregulation of the 20-HETE synthase, CYP4A12. Immunofluorescence co-localized increased CYP4A12 expression in response to ischemia to CD31-positive EC in the ischemic hindlimb microvessels. We further showed that ischemia increased HIF-1α, VEGF, and VEGFR2 expression in gracilis muscles and that these increases were negated by DDMS and 6, 15-20-HEDGE. Lastly, we showed that ERK1/2 of MAPK is a component of 20-HETE regulated ischemic angiogenesis. Taken together, these data indicate that 20-HETE is a critical contributor of ischemia-induced angiogenesis in vivo.
Graphical abstract

1. Introduction
Angiogenesis within the context of ischemia-induced compensatory neovascularization minimizes tissue damage caused by atherosclerotic diseases, including peripheral arteriopathy [1,2]. However, ischemic angiogenesis, like arteriogenesis, in patients with peripheral artery disease is often insufficient to maintain adequate tissue perfusion resulting in critical limb ischemia and amputation [3]. Thus, discovering novel factors which increase angiogenesis in response to ischemia in animal models of hindlimb ischemia is of potential clinical relevance.
20-hydroxyeicosatetraenoic acid (20-HETE) is a vasoactive arachidonic acid (AA) metabolite produced by cytochrome P450 ω-hydroxylases (most notably of the CYP4A and CYP4F gene subfamilies in humans [4–7]). It is primarily found in the microcirculation [8,9]. Accumulating evidence shows that 20-HETE plays an important role in angiogenic processes [10–16]. 20-HETE has been shown to activate vascular endothelial cells (EC) and vascular smooth muscle cells (VSMC) by inducing their proliferation, migration, survival and tube formation as well as secretion of pro-angiogenic growth factors, such as vascular endothelial growth factor (VEGF) by EC and its upstream transcriptional factor hypoxia inducible factor 1α (HIF-1α) [11,12,17,18]. The HIF-1α/VEGF signaling pathway is essential for angiogenesis [19,20]. We recently reported that 20-HETE regulates angiogenic function of endothelial progenitor cells (EPC) in vitro and EPC-mediated angiogenesis in vivo [13,21]. Amaral and colleagues first reported that CYP4A, and by extension 20-HETE, play a critical role in angiogenesis induced by chronic electrical stimulation of skeletal muscle [15]. Jiang et al. have shown that CYP4A1 overexpression in smooth muscle promotes endothelial sprouting in renal arterial microvessels [22]. Furthermore, 20-HETE has been shown to induce angiogenic responses in rat cornea and HET0016 (N-hydroxy-N′-(4-butyl-2-methylphenol) formamidine), a selective 20-HETE synthase inhibitor, markedly reduced epithelial growth factor (EGF)-, VEGF-, and fibroblast growth factor (FGF)-induced angiogenic responses in the cornea [16]. These studies indicate that 20-HETE stimulates a variety of pro-angiogenic responses in cultured cells.
However, it is unknown whether 20-HETE plays a role in ischemia-induced angiogenesis in vivo. Furthermore, the cellular and molecular mechanism(s) by which it may do so are completely unexplored. The present study was designed to investigate whether 20-HETE plays a central role in angiogenesis in the ischemic hindlimb and by which mechanism it does so.
2. Material and Methods
2.1. Cell Culture
Human microvascular endothelial cells (HMVEC) were purchased from Promo Cells (Heidelberg, Germany) and cultured according to the manufacturer’s recommendations and maintained at 37°C in a humidified incubator containing 5% CO2 as previously described [11]. Cultures from two separate batches with passage numbers 3–4 were used. Prior to the experiments, cells were plated at 60% density in EC growth media (Promo Cells) and allowed to grow overnight before incubating under either nomoxia (20% O2) or hypoxia (3% O2; GCA Precision hypoxic Incubator) for 16 hr.
2.2. Mouse Hindlimb Ischemia Angiogenesis Model
The mouse hindlimb ischemia model is a well-established in vivo angiogenesis model [23,24], in which angiogenesis occurs in the gracilis muscle, while arteriogenesis occurs proximal to the ligation, in the adductor muscle [25–30]. Twelve-week-old Balb/c mice were purchased from the Jackson Laboratory (Bar Harbor, MA). All animal experiments were performed according to the NIH guidelines using animal care and experimental protocols that were approved by the Institutional Animal Care and Use Committee at New York Medical College. Briefly, mice were anesthetized with ketamine (80 mg/kg, i.p.) and xylazine (10 mg/kg i.p.) as described previously [31] prior to unilateral (right) femoral artery ligation. The femoral artery was exposed through a 1 cm incision in the right inguinal region. Then, the artery was separated from the vein and nerve and ligated with a 4-0 suture proximal to the common femoral artery and distal to the bifurcation of the profunda and excised in between. The incision was closed after the wound was irrigated with sterile saline and topical antibiotic was applied after wound closure. The arteries on the left side were not ligated and served as control. At the end of each experimental time point, mice were sacrificed by CO2 inhalation prior to tissue harvest for further analysis. An overall experimental protocol and design based on the mouse ischemia hindlimb angiogenesis assay used in this study is depicted in Supplemental Fig. S1.
2.3. Pharmacological 20-HETE Interventions
20-HETE synthesis inhibitor, dibromo-dodecenyl-methylsulfimide (DDMS) and 20-HETE antagonist, N-(20-hydroxyeicosa-6(Z), 15(Z)-dienoyl) glycine (6, 15-20-HEDGE) were synthesized by Dr. Falck and used to interfere with the synthesis or action of 20-HETE in the mouse ischemic hindlimb angiogenesis model. The concentrations of DDMS and 6, 15-20-HEDGE we chose were based on several previous in vivo studies from our groups and the others. For systemic administration, mice were treated with vehicle (DMSO), DDMS (10 mg/kg/day; i.p.), or 6, 15-20-HEDGE (10 mg/kg/day; i.p.) for 2 days prior to the unilateral femoral ligation. Treatment continued daily for the next 21 days. For local administration of DDMS and 6,15-20-HEDGE to hindlimb gracilis muscle, an Alzet mini-osmotic pump (model 2002 or 2004; Durect Corporation, Cupertino, CA) was filled with the drugs (5 mg/kg/day) and pre-conditioned in sterile 0.9% saline at 37°C overnight and then was attached with polyethylene catheter tubing (an inside diameter (I.D) 0.8mm). Mice were anesthetized the next day and a small incision was made in the skin between the scapulae. Using a hemostat, a small pocket was formed by spreading the subcutaneous connective tissues apart. The pump was inserted into the pocket and the open end of the catheter was directed and anchored onto the left gracilis muscles by suturing 2 days prior to femoral artery ligation and drugs were continuously delivered for the entire duration of the experiment. For the 20-HETE rescuing experiment, animals were treated with DDMS (10 mg/kg/day; i.p.) to inhibit systemic 20-HETE synthesis for 2 days prior to the unilateral femoral ligation. DMSO was used as vehicle control. Synthetic 20-HETE (5 mg/kg/d) (Cayman Chemical, Ann Arbor, MI) was delivered to the ischemic gracilis muscle in the presence and absence of DDMS via osmotic pump on the day of ligation.
2.4. Assessment of Blood Flow
All mice were anesthetized with 1% isoflurane and placed on a heat pad at 37°C. Excess hindlimb hair was removed by depilatory cream before analysis. Blood flow in both limbs was measured using a Laser Doppler Perfusion Imaging (LDPI) scanner, PeriscanPIM3 (PeriMed, Jarfalla, Sweden) [31] before surgery (day 0) and then 1 day after the surgery to confirm the surgical outcome of limb ischemia. Then, LDPI was continued in DMSO-, DDMS-, 6,15-20-HEDGE-, and/or 20-HETE treated mice on days 1, 7, 14, and 21 post-ligation to quantify hindlimb blood flow in both legs. A region of interest (ROI) was created around each limb relative to the anatomical marker (mid-inguinal point-between iliac crest and symphysis pubis) and the ratio of blood perfusion in the ischemic limb vs. the non-ischemic limb was calculated.
2.5. Measurement of Blood Pressure
Systolic blood pressure measurements were taken via the CODA tail-cuff system (Kent Scientific), which utilizes volume pressure recording sensor technology. Separate groups of vehicle-, DDMS- and 6,15-20-HEDGE treated mice were acclimated to the machine for 1 week prior to day 0 and blood pressure was monitored throughout the length of the experiment, at days 1, 7, 12, 14, 21, 26, 28 and 32 post ligation as previously described [32]. Twenty individual readings were taken and recorded. Values within ±10% of their mean blood pressure measurements were obtained.
2.6. Histology and Immunofluorescent (IF) Microscopy
For microvessel density (MVD) analysis, hindlimb gracilis muscles from control and experimental groups were surgically excised at the end of experiments (day 21), frozen sectioned (6 μm). Microvessels were identified and labeled by double IF staining against two different endothelial markers, CD31 and Tomato Lectin. Briefly, sections were washed with 0.1%Tween-20/PBS 3X and non-specific reactions were blocked with 5% BSA/0.3M Glycine/0.1% Tween-20/PBS (blocking buffer) at room temperature (RT) for 2 hr. After blocking, the sections were incubated with the primary antibody rat anti-mouse CD31 (1:100) (eBioscience, San Diego, CA) at 4°C overnight followed by anti-rat Cy3-conjugated secondary antibodies (1:500) (Jackson Immunoresearch, West Grove, PA) for 3 hr at RT. The samples were thoroughly washed and then treated with 10 mg/ml Tomato Lectin-FITC (Vector Laboratory, Burlingame, CA) at RT for 30 min, followed by 0.1 mg/ml DAPI (Sigma-aldrich, St Louis, MO, USA) counterstain for 10 min. Images were acquired using the Zeiss AXIO Imager.M1 fluorescence microscope. Six-eight fields were chosen randomly from various sections to ensure objectivity. CD31 and tomato lectin double positive microvessels were counted. The number of muscle fibers was also counted within the same field on bright field images. MVD data is shown as the microvessel to muscle fiber ratio in each field.
For analysis of arterioles, rat anti-α-smooth muscle actin (1:250; VSMC markers; eBioscience) was used to stain for VSMCs in combination with anti-rat FITC-conjugated secondary antibodies (1:1000). IF images from 6–8 randomly chosen fields were captured using the Zeiss AXIO Imager.M1 fluorescence microscope. α-SMA positive arterioles and the number of muscle fibers were counted. Data was shown as the ratio of arterioles to muscle fibers in each field.
For 20-HETE synthase CYP4A12 analysis, ischemic hindlimb gracilis muscles were harvested at 3 days post ligation in a separate experiment and frozen-sectioned as described above. Sections were incubated with rat anti-CYP4A12 (mouse 20-HETE synthase) (1:500; 4°C overnight) (kindly gifted by Dr. Wolf-Hagen Schunck Max Delbrueck Center for Molecular Medicine, Berlin, Germany) followed by anti-rat FITC-conjugated secondary antibodies (1:1000) (Jackson Immunoresearch) for 3 hr at RT. Rat anti-mouse CD31 (endothelial markers) (1:100) (eBioscience) was also used followed by anti-rat Cy3-conjugated secondary antibodies (1:500) (Jackson Immunoresearch) for 3 hr at RT.
2.7. LC/MS/MS Analysis of 20-HETE
For animal experiments, gracilis muscles from both non-ischemic and ischemic hindlimb were surgically removed on days 3 and 21 post-ligation and homogenized in oxygenated Kreb’s buffer with glass homogenizer on ice followed by centrifugation. For HMVEC experiments, cell media and cell lysate (1 mg/ml) were both collected for 20-HETE analysis. Muscle and cell lysate samples were then incubated with 1 mM NADPH (Calbiochem, San Diego, CA) for 1 hr at 37°C [21,33]. The reaction was stopped by acidification to pH 4.0 using 10% acetic acid. In brief, samples were loaded onto preconditioned solid phase extraction Phenomenex Strata C18-E columns (Phenomenex, Torrance, CA) with d6-20-HETE as an internal standard (0.5 ng) (Cayman Chemical, Ann Arbor, MI). Columns were then washed with 10% methanol and eluted with 2 ml of 100% methanol. Samples were concentrated under nitrogen and stored at −80°C until LC/MS/MS analysis. 20-HETE production was quantified with a Shimadzu UFMS Triple Quadrupole Mass Spectrometer LCMS-8050 combined with a Nexera UHPLC using negative ionization MRM mode. This ultra-sensitive method achieves 1 pg 20-HETE as the limit of quantitation. The details of analytical conditions are given in Supplemental Table I.
2.8. Western Blot Analysis
Non-ischemic control and ischemic gracilis muscles were extracted from vehicle-, DDMS-, and 6,15-20-HEDGE-treated animals 3 and 21 days post-ligation and homogenized using RIPA buffer. Western blot analysis was performed as previously described [34]. Equal amounts of protein (20–40 μg) were separated on a 10% Tris-glycine gel, transferred to a PVDF membrane, and incubated with anti-HIF-1α (1:1000) (Abcam, Cambridge, UK), anti-VEGF (1:1000) (Cell Signaling Technology, Beverly, MA), and anti-VEGFR2 antibody (1:1000) (Cell Signaling Technology, Beverly, MA) at 4°C overnight. Then the membrane were incubated with anti-rabbit IRDye®800 CW secondary antibody (LI-COR, Lincoln, NE) at 1:10,000 at RT for 2 hr and scanned with the LI-COR Odyssey Infrared Imaging system. Mouse β-tubulin (1:100,000) (Abcam, Cambridge, UK) was used as a loading control. MAPK and PI-3 kinase cell signaling pathways were also assessed using anti-phospho-ERK (1:1000; Santa Cruz Biotechnology, Inc., Dallas, TX) and anti-phospho-Akt antibody (1:250; Santa Cruz Biotechnology) in these samples. Total ERK and Akt were used as corresponding controls.
2.9. Statistical Analysis
Data are expressed as Mean ± SEM in all experiments. Significance of difference in mean values was determined using t test and 2-way ANOVA, followed by the Newman–Keul post hoc test. p<0.05 was considered to be significant. Two-way ANOVA was performed to assess time- vs. treatment-dependent effects on blood pressure.
3. Results
3.1. Systemic Pharmacological 20-HETE Inhibition Decreases Ischemic Angiogenesis
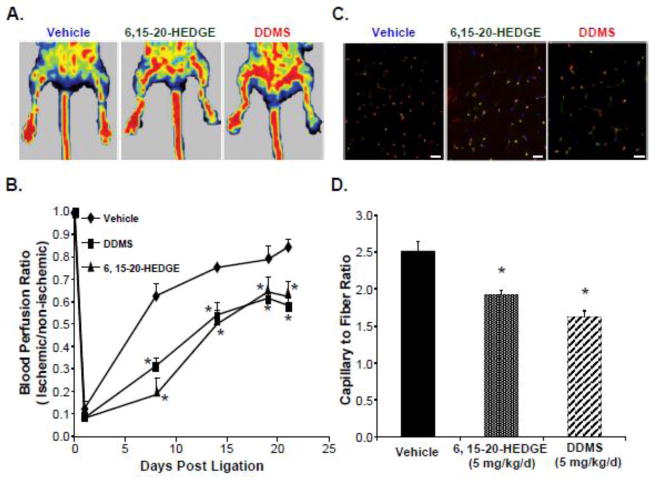
Ischemic angiogenesis in mice subjected to unilateral femoral artery ligation was compared in groups receiving i.p. pretreatment with 20-HETE synthase inhibitor, DDMS (10 mg/kg/day), or 20-HETE antagonist, 6,15-20-HEDGE (10 mg/kg/day), relative to vehicle (DMSO)-treated control. Fig. 1A shows the representative Doppler images taken on day 21. Blood flow in the ischemic hindlimb was similar to that in the non-ischemic, control hindlimb in vehicle-treated mice, whereas the blood flow in the ischemic hindlimb was significantly impaired in DDMS- and 6,15-20-HEDGE-treated animals compared to their corresponding non-ischemic control hindlimb. Fig. 1B illustrates that femoral artery ligation led to precipitous decreases in perfusion in all treatment groups one day post ischemia. Perfusion recovery subsequently took place, as expected, as the result of a normal course of compensatory neovascularization over the next 20-day period in vehicle-treated animals, but not in 6,15-20-HEDGE- or DDMS-treated animals (Fig 1A).
Figure 1. Systemic inhibition of the cyp4a-20-HETE system inhibits ischemia-induced compensatory angiogenesis in a mouse hindlimb.
Mice were injected 2 days prior to surgery with vehicle (DMSO), DDMS (10 mg/kg/d, i.p.) or 20-HEDGE (10 mg/kg/d, i.p.), which treatments continued for an additional 21 days after unilateral femoral artery ligation. Compensatory angiogenesis was measured (n=8). (A) Representative blood perfusion scans using Laser Doppler Perfusion Imaging at the end of the experiment. Left: non-ischemic hindlimb; Right: Ischemic hindlimb. (B) Quantification of blood perfusion rates (Means ± SEM; *,* p<0.05 vs control). (C) Representative merged immunofluorescence images stained with CD31 (Red), Tomato Lectin-FITC (Green) and DAPI (Blue) were shown. Scale bar=50 μm. (D) Assessment of microvessel density by counting CD31 and tomato lectin double positive stains from hindlimb gracilis muscle sections, which then were normalized to the number of muscle fibers in the field, and expressed as capillary to fiber ratio. Grey bars represent capillary to fiber ratio counts in the non-ischemic control muscles of each treatment group (Means ± SD; *p<0.05 vs vehicle controls). (E) Assessment of arterioles density by counting α-SMA positive vessels from hindlimb gracilis muscle sections, which then were normalized to the number of muscle fibers in the field, and expressed as arterioles to fiber ratio. Grey bars represent arterioles to fiber ratio counts in the non-ischemic control muscles of each corresponding treatment group (n=8; Means ± SD; *p<0.05 vs vehicle controls).
Likewise, microvascular density, identified as immunofluorescence staining for EC markers CD31 and tomato lectin per muscle fiber, was significantly lower in the gracilis muscles from ischemic hindlimbs from DDMS (46±7.5% vs. vehicle; p<0.05) and 6,15-20-HEDGE-treated (34±6% vs. vehicle; p<0.05) mice than from ischemic gracilis muscles from vehicle-treated animals (Fig. 1C and 1D) or from their non-ischemic control limbs (data not shown).
We further assessed the effects of pharmacological 20-HETE interference on arteriogenesis in the gracilis muscles by performing IF staining of α-SMA positive arterioles in ischemic gracilis muscle. Fig. 1D shows that both DDMS and 6,15-20-HEDGE did inhibit the formation of arterioles in the ischemic gracilis muscles, by 14±4.7% and 10±5.1% vs. vehicle (p<0.05), respectively; however, this effect is significantly less than that on angiogenesis. No significant differences in both capillary and arterioles density were found in non-ischemic control muscles in any treatment group (Fig. 1D). It is important to note that both DDMS and 6,15-20-HEDGE at the dose of 10 mg/kg/day had no significant effects on blood pressure of the animals during the entire course of the treatment (BP: 120±9 mmHg before and 120±5 mmHg after DDMS and 114±2 mmHg after 6,15-20-HEDGE treatment, respectively; supplemental Fig. S2).
3.2 Ischemia Increases 20-HETE Production in Gracilis Muscle
Next, we assessed the production of 20-HETE in gracilis muscles from the ischemic and the non-ischemic hindlimbs at 3 and 21 days post-ligation using LC/MS/MS analysis. Muscles from non-surgical animals were also included as additional controls. The data presented in Fig. 2 demonstrates that the ischemic hindlimb muscle produces high levels of 20-HETE 3 days post ischemic ligation, whereas non-ischemic, control muscle produces negligible amounts of 20-HETE (91±11 vs. 8±2 pg/mg of protein; p<0.05). Interestingly, 20-HETE production was found to be negligible at day 21 in all groups (data not shown). In addition, we confirmed that the 20-HETE synthesis inhibitor, DDMS attenuated the ischemia-induced 20-HETE production by ~70% (p<0.05), whereas the 20-HETE antagonist, 6,15-20-HEDGE, as expected, had no effects on the increase in 20-HETE post ischemia (Fig. 2).
Figure 2. Comparison of 20-HETE levels in non-ischemic vs ischemic gracilis muscles using LC/MS/MS analysis.
Gracilis muscles from non-ischemic and ischemic hindlimbs were collected from animals treated with vehicle, DDMS and 6,15-20-HEDGE at day 3 post-ligation and lipid-extracted for 20-HETE measurement. 20-HETE levels were measured using LC/MS/MS. 20-HETE levels from all groups were quantified and normalized to total protein (pg/mg protein) (n=6; Means ± SEM; *p<0.05 vs non-ischemic muscles and #p<0.05 vs ischemic muscle + vehicle).
3.3 Local 20-HETE Inhibition Attenuates Ischemic Angiogenesis
To determine whether systemic of local production of 20-HETE plays a major role in angiogenesis in response to ischemia, we delivered DDMS and 6,15-20-HEDGE specifically to ischemic gracilis muscle via osmotic mini-pumps in a different group of mice undergoing unilateral femoral artery ligation. As shown in the Fig. 3, local administration of DDMS or 6,15-20-HEDGE also significantly impaired blood flow recovery post ischemia in the ischemic limb (Fig. 3A and 3B) and inhibited MVD in the ischemic gracilis muscles by 35.5±5% and 23.5±4% vs. vehicle (p<0.05), respectively (Fig. 3C and 3D).
Figure 3. Local inhibition of the cyp4a-20-HETE also significantly reduces ischemic angiogenesis.
Animals were pre-treated 2 days prior to surgery with DDMS (5 mg/kg/d, s.c. osmotic pump) or 6,15-20-HEDGE (5 mg/kg/d, s.c. osmotic pump) and then treatment continued for 21 days after femoral artery ligation. DMSO was used as vehicle control. Compensatory angiogenesis was measured using Laser Doppler Perfusion Imaging and microvascular density analysis. (A) Representative blood perfusion scans using Laser Doppler Perfusion Imaging at the end of the experiment. Left: non-ischemic hindlimb; Right: Ischemic hindlimb. (B) Quantification of perfusion rates (n=6; Means ± SEM; *p<0.05 vs control). Gracilis muscles from each group were again dissected, frozen-sectioned and stained for CD31 and tomato lectin. (C) Representative merged immunofluorescence images stained with CD31 (Red), Tomato Lectin-FITC (Green) and DAPI (Blue) were shown. Scale bar=50 μm. (D) Assessment of microvessel density by counting CD31 and tomato lectin double positive stains from muscle sections in 6–7 random fields, which then were normalized to the number of muscle fibers in the field, and expressed as capillary to fiber ratio. (n=6; Means ± SD; *p<0.05 vs control).
3.4. 20-HETE Rescues Ischemic Angiogenesis Decreased by 20-HETE Inhibition
To confirm that 20-HETE deficiency due to pharmacological interference with 20-HETE production and action was responsible for impaired ischemic angiogenesis, we delivered synthetic 20-HETE (5 mg/kg/day) locally to the ischemic muscle via osmotic pump in another group of mice, which were pre-treated with DDMS (10 mg/k/d; i.p.) for 2 days, on the day of unilateral femoral artery ligation. LDPI and MVD analysis showed that adding back the 20-HETE partially (~68%; p<0.05) rescued impaired angiogenesis in the ischemic gracilis muscles (Fig. 4A and 4B). Local infusion of 20-HETE alone also increased compensatory neovascularization in response to ischemia.
Figure 4. 20-HETE rescues ischemic angiogenesis decreased by 20-HETE Inhibition.
Animals were pre-treated 2 days prior to surgery with 20-HETE synthesis inhibitor DDMS (10 mg/kg/d, i.p.) and then treatment continued daily for 21 days after femoral artery ligation. DMSO was used as vehicle control. On the day of ligation, 20-HETE (5 mg/kg/d; s.c. osmotic pump) was administered directly to the ischemic gracilis muscle in both control and DDMS–treated groups. Compensatory angiogenesis was measured using Laser Doppler Perfusion Imaging and microvascular density analysis. (A) Quantification of perfusion rates over a 21 day period (n=6; Means ± SEM; *p<0.05 vs control and #p<0.05 vs DDMS-treated groups). (B) MVD analysis was performed in gracilis muscles from each group by counting CD31 and tomato lectin positive stains from 8 random fields, which then were normalized to the number of muscle fibers in the field, and expressed as capillary to fiber ratio. (n=6; Means ± SD; *, #p<0.05 vs control).
3.5. Ischemia-induced 20-HETE May Be Endothelial in Origin
In mice, highly homologous members of the human CYP4A/4F subfamily have been identified, including cyp4a10, cyp4a12, and cyp4a14, among which CYP4A12 is the most prominent 20-HETE-producing enzyme [35,36]. Because local 20-HETE inhibition was similarly effective as systemic inhibition in inhibiting ischemic angiogenesis, we wondered whether ischemia regulated CYP4A12 expression. Therefore, we examined the expression level of CYP4A12 in the vasculature of the gracilis muscles in the ischemic vs. control, non-ischemic hindlimb. Fig. 5A shows that while CYP4A12 was not expressed in non-ischemic muscles, it is highly induced in ischemic muscle. Moreover, CYP4A12 expression co-localized perfectly the EC-specific marker, with CD31 (Fig. 5A). In cell culture, we also found that human microvascular EC grown under hypoxia synthesized significantly higher levels of 20-HETE compared to cells grown under normoxia, which produce little 20-HETE (Fig. 5B).
Figure 5. Ischemia-induced 20-HETE increases may be endothelium in origin.
(A) Gracilis muscles from non-ischemic and ischemic hindlimb were surgically extracted and frozen-sectioned 3 days post-ischemia. Muscle samples were incubated with anti-cyp4a12-FITC (Green) and anti-CD31 (Red) antibodies. Immunofluorescent microscopy was carried out and representative images were shown (n=3). Scale bar=100 μm. (B) Total 20-HETE synthesis (cell media and cell lysate) in normoxic vs. hypoxic HMVEC were measured using LC/MS/MS. *p<0.05 compared to normoxia (n=3 in triplicates; Means ± SD; *p<0.05 vs normoxia).
3.6. 20-HETE Mediates Ischemia-induced Angiogenesis via HIF-1α/VEGF and ERK Induction
Since induction of HIF-1α/VEGF signaling is a fundamental component of ischemic angiogenesis [19,20] and 20-HETE has been shown to be capable of inducing VEGF in vitro [11,13,37], we explored the possibility that 20-HETE-mediated angiogenesis in response to hindlimb ischemia in vivo through HIF-1α/VEGF signaling. Non-ischemic and ischemic gracilis muscles were harvested 3 and 21 days post unilateral ligation. Fig. 6A shows that on day 3 post-ligation, the time point at which 20-HETE levels were maximally induced in the ischemic gracilis muscle, HIF-1α, VEGF, and VEGFR2 expression were likewise very significantly increased in the ischemic vs. non-ischemic muscle (up to ~9 fold; p<0.05). No differences were observed in HIF-1α/VEGF signaling between non-ischemic and ischemic hindlimbs at 21 days post-ligation, correlating with lack of difference in 20-HETE levels. Importantly, administration of DDMS and/or 6,15-20-HEDGE markedly blunted the observed increased expression of HIF-1α/VEGF/VEGFR2 in ischemic muscle harvested from mice 3 days post-ligation (Fig. 6B). DDMS and/or 6,15-20-HEDGE had no significant effects on basal HIF-1α/VEGF/VEGFR2 expression in the non-ischemic control muscle (data not shown).
Figure 6. Effects of 20-HETE inhibition on VEGF angiogenic signaling pathways in gracilis muscle post-ischemic ligation.
Mice were pre-treated 2-days prior to surgery with DDMS (10 mg/kg/d, i.p.) or 6,15-20-HEDGE (10 mg/kg/d, i.p.). Hindlimb gracilis muscles were harvested 3 and 21 days after femoral artery ligation and homogenized for Western blot analysis. Non-ischemic muscle was used as control and DMSO was also used as vehicle control. (A) HIF-1α, VEGF, and VEGFR2 expression in non-ischemic limb vs. ischemic limb at 3 and 21 days post-ligation and the corresponding densitometry analysis. (B) HIF-1α, VEGF, and VEGFR2 expression in the presence/absence of 20-HETE synthesis inhibitor, DDMS and 20-HETE antagonist, 6,15-20-HEDGE in ischemic gracilis muscles at 3 days after ligation and the corresponding densitometry analysis. Representative blots were shown from n=6 (Means ± SD; *p<0.5 vs corresponding controls). (C) Expression of p-ERK and p-Akt in the presence/absence of 20-HETE synthesis inhibitor, DDMS and 20-HETE antagonist, 6,15-20-HEDGE in ischemic gracilis muscles at 3 days after ligation and the corresponding densitometry analysis. Non-ischemic muscle samples were used as controls. Representative blots were shown from n=6 (Means ± SD; *p<0.5 vs non-ischemic controls and #p<0.5 vs Ischemic vehicle control). C: non-ischemic control; V: Ischemic vehicle control; D: DDMS; and H: 6,15-20-HEDGE.
To elucidate a mechanism by which 20-HETE may regulate HIF-1α/VEGF, we also studied whether pharmacological 20-HETE interference alters ERK1/2 MAPK and Akt signaling pathways in ischemic muscle. Fig. 6C demonstrates that ischemic ligation activates both MAPK and PI-3 kinase pathway by inducing the expression of phospho-ERK1/2 (p-ERK1/2) and phospho-Akt (p-Akt) by 4.8±0.4 and 1.9±0.3 folds (p<0.05), respectively. Both DDMS and 6,15-20-HEDGE significantly inhibited the ischemia-induced p-ERK1/2, expression whereas had no significant effects on the ischemia-induced p-Akt expression, indicating the contribution of 20-HETE to ischemic angiogenesis involves an ERK1/2 MAPK component.
4. Discussion
Although 20-HETE has been studied extensively in its regulation in hypertension [4, 38, 39], it was recently recognized as an initiator of angiogenesis in various vascular beds. Whether 20-HETE is a contributing component during ischemia-induced angiogenesis is completely unknown. The current study demonstrates that the ischemia-induced angiogenic response to unilateral femoral artery ligation relies on a mechanism involving 20-HETE-mediated activation of the HIF-1α/VEGF and ERK-dependent signaling pathways. This conclusion is based on three major findings.
The first major finding of our studies is that systemic pharmacological interferences in the synthesis or the actions of 20-HETE attenuate the time-dependent recovery of blood perfusion and the accompanying increases in the number of micro-vessels (an index of angiogenesis) in the mouse ischemic hindlimb. The impaired functional blood flow recovery in response to DDMS and 6, 15-20-HEDGE suggested that 20-HETE may not only involve in angiogenesis but also in arteriogenesis. In this regard, we also found that DDMS and 6,15-20-HEDGE both suppressed the numbers of arteriole present (an index of arteriogenesis) in ischemic gracilis muscles. The level of inhibition of arteriogenesis by 20-HETE inhibition was much less compared to its effects on angiogenesis. However, we cannot exclude an effect of 20-HETE on arteriogenesis since we did not assess arteriogenesis in the adductor muscle, where it is the predominant form of revascularization in response to femoral artery occlusion, where angiogenesis predominates in the gracilis muscle.
Local delivery of 20-HETE inhibitors to the ischemic hindlimb gracilis muscle also yielded similar decreases in perfusion and microvessel density as seen with the systemic treatment. This finding implies that 20-HETE manufactured locally in the ischemic tissue may be responsible for promoting the angiogenic response. However, we cannot exclude the possibility that locally delivered DDMS and 6, 15-20-HEDGE may act on systemic 20-HETE by entering the systemic micro-circulation. Furthermore, adding back 20-HETE to the local ischemic gracilis muscle in mice treated with DDMS markedly restored at least in part the angiogenesis, which further suggests that 20-HETE deficiency was primarily responsible for defects in ischemic angiogenesis. This observed partial rescue may be due to a) a potential involvement of additional systemic 20-HETE component; or b) an insufficient bioavailable 20-HETE within all ischemic tissues. Bearing on this conclusion, previous reports documented that 20-HETE has pro-angiogenic activity in vitro [10–16,21,22] and promotes corneal neovascularization [16] and skeletal muscle angiogenesis [15] in vivo. Interestingly, 20-HETE has been shown to have both assenting and opposing actions on nitric oxide (NO), an important regulator of ischemia-induced angiogenesis [40], in different vascular beds [39,41]. Therefore, potential interactions between increased 20-HETE and NO within the context of ischemia-induced angiogenesis remain to be studied.
The second major finding of our studies is that the expression of the major murine 20-HETE synthesizing enzyme, CYP4A12 [36], and the synthesis of its product, 20-HETE are both elevated in ischemic gracilis muscles compared to non-ischemic controls. Furthermore, immunofluorescent microscopy revealed that the increased CYP4A12 expression was co-localized with CD31, an EC marker, in the ischemic hindlimb. To this end, LC/MS/MS analysis showed that the hindlimb gracilis muscles harvested 3 days post-ligation produced high levels of 20-HETE whereas in non-ischemic muscle controls 20-HETE levels were below the detection limit. It is interesting that 20-HETE production fell below detectable levels at the end of 21 days, indicating an early increase in 20-HETE that potentially triggers the angiogenic response. As the blood flow in the ischemic hindlimb gradually normalizes, 20-HETE production fades. It is possible that this early increase of 20-HETE may be either the result of an inflammatory or bone marrow responses to the initial injury and ischemia-induced angiogenesis is secondary to such increase in 20-HETE and its subsequent interaction with additional cell signaling pathways such as VEGF, NO, etc. Identification of increases in CYP4A12 and 20-HETE in the ischemic gracilis muscle suggested that a local 20-HETE production may be one of the major regulators of ischemia-induced angiogenesis at the target sites.
It is important to note that a precise identification and characterization of the cellular origin of 20-HETE in gracilis muscle post ischemic injury is challenging. There are several possible cellular sources from which 20-HETE can be derived in ischemic muscle: the microcirculation (vascular wall), circulating cells [7,9,14,17,42–46], skeletal muscle [15], and/or connective tissue [45,46]. The blockade of ischemic angiogenesis by local DDMS and 6,15-20-HEDGE administration implied that the ischemic tissues may be the source of 20-HETE during the angiogenic response. Although EC in culture and vascular EC in most circulatory beds had little or no 20-HETE synthase activity under normoxic conditions [42], and vascular synthesis and release of 20-HETE have been shown to occur primarily from VSMC [4,9,47,48], we recently reported that hypoxia up-regulates 20-HETE synthase CYP4A11 expression and 20-HETE production in human EPC in culture [21]. In this study, we show that ischemia increases cyp4a12-20-HETE in EC in vitro and in vivo. These findings strengthen the possibility that 20-HETE may be produced by EC under ischemic conditions. On the other hand, our recent findings that human EPC express high levels of 20-HETE synthase CYP4A11 and produce 20-HETE [13,21] also opens the possibility that the observed local increases in 20-HETE in the ischemic hindlimb could also be derived from bone marrow (BM)-mobilized EPC which homed to sites of neovascularization. In addition, inflammatory cells such as macrophages [49] and neutrophils [50] can also be a potential source of 20-HETE since both produce 20-HETE and have been shown to play a role in angiogenesis. The use of pharmacological inhibitors only partially addresses the potential cellular origin of 20-HETE; the ultimate approach will be specifically targeting the cyp4a-20-HETE system in each cell using cell-specific delivery of shRNA constructs in adenoviral and lentiviral vectors.
The third major finding of our studies is that 20-HETE regulates the ischemic angiogenesis via activation of the HIF-1α/VEGF and ERK-dependent signaling pathways. Previous studies by our group and others suggested the existence of an interaction between 20-HETE and HIF-1α/VEGF pathways, one of the most fundamental angiogenic pathways [19,20], in vitro and in vivo. Amaral et al. first reported that VEGF and 20-HETE are important mediators of skeletal muscle angiogenesis under electric shock stimulation and that VEGF-neutralizing antibody blocked the increases in 20-HETE formation [15]. Moreover, the selective 20-HETE synthesis inhibitor, HET0016, blocks VEGF-induced EPC proliferation and migration [13] and VEGF-induced corneal neovascularization [16]. This data are consistent with 20-HETE being downstream of the VEGF pathway. Conversely, we reported that 20-HETE can stimulate the HIF-1α/VEGF signaling in human EPC and EC in vitro [11–13,15,16]. These later reports suggested that 20-HETE can also function upstream of the HIF-1α/VEGF pathway. The current study revealed that 1) ischemia increases HIF-1α/VEGF/VEGFR2 expression in hindlimb gracilis muscle and 2) pharmacological blockade of 20-HETE synthesis or action markedly blunted these increases. These results support the notion that 20-HETE is an upstream regulator of HIF1α/VEGF signaling during ischemic angiogenesis. We did not find any differences between HIF-1α/VEGF/VEGF2 expressions between non-ischemic vs. ischemic hindlimb at 21 days post-ligation which is consistent with our earlier observations that negligible amounts of 20-HETE were found at day 21. It is possible that angiogenesis is accelerated as a result of a further positive feedback interaction between these two pathways, the detailed mechanism of which requires additional studies. Chen et al reported that increased 20-HETE at the site of neovascularization can further amplify its signaling through interaction with additional angiogenic growth factor secretion [10].
Pharmacological 20-HETE interference also markedly reduced ischemia-induced ERK1/2 MAPK activation, while the ischemia-mediated increase in Akt activation was not significantly altered. Consistent with previous work indicating that ERK1/2 signaling is a major pathway in 20-HETE-mediated cellular actions [11,42,51]; the current findings indicate a crucial role for ERK1/2 MAPK in ischemia-induced angiogenesis involving 20-HETE.
In summary, our findings provide strong evidence that 20-HETE is a critical contributor to the ischemia-induced angiogenesis. Thus, 20-HETE may be a therapeutic target in correcting impaired angiogenesis underlying the ischemic diseases.
Supplementary Material
Acknowledgments
We would like to thank Dr. Wolf-Hagen Schunck and Dr. Ramona Zummach for gifting the mouse CYP4A12 antibody. This study would not have been possible without their seminal contributions.
Funding
This work was supported by the American Heart Association [Grant 11SDG6870004] (A.M.G.); the National Institutes of Health [Grants HL34300] (M.L.S.), [Grant DK38226] (J.R.F.); the Robert A. Welch Foundation [GL625910] (J.R.F.).
Abbreviations
- AA
arachidonic acid
- 20-HETE
20-hydroxyeicosatetraenoic acid
- EC
endothelial cells
- HMVEC
human microvascular endothelial cells
- VEGF
vascular endothelial growth factor
- VEGFR2
vascular endothelial growth factor receptor 2
- HIF-1α
hypoxia-inducible factor-1-alpha
- HET0016
N-hydroxy-N′-(4-n-butyl-2-methylphenyl) Formamidine
- DDMS
Dibromo-dodecenyl-methylsulfimide
- 6,15-20-HEDGE
N-(20-hydroxyeicosa-6(Z),15(Z)-dienoyl) glycine
Footnotes
Conflict of interest
None declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Howangyin KY, Silvestre JS. Diabetes mellitus and ischemic diseases: molecular mechanisms of vascular repair dysfunction. Arterioscler Thromb Vasc Biol. 2014;34(6):1126–1135. doi: 10.1161/ATVBAHA.114.303090. [DOI] [PubMed] [Google Scholar]
- 2.Vouillarmet J, Bourron O, Gaudric J, Lermusiaux P, Millon A, Hartemann A. Lower-extremity arterial revascularization: Is there any evidence for diabetic foot ulcer-healing? Diabetes Metab. 2015;42(1):4–15. doi: 10.1016/j.diabet.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 3.Idris NM, Haider HK, Goh MW, Sim EK. Therapeutic angiogenesis for treatment of peripheral vascular disease. Growth Factors. 2004;22(4):269–279. doi: 10.1080/08977190412331284344. [DOI] [PubMed] [Google Scholar]
- 4.Williams JM, Murphy S, Burke M, Roman RJ. 20-Hydroxyeicosatetraeonic Acid: a New Target for the Treatment of Hypertension. J Cardiovasc Pharmacol. 2010;56(4):336–344. doi: 10.1097/FJC.0b013e3181f04b1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nguyen X, Wang MH, Reddy KM, Falck JR, Schwartzman ML. Kinetic profile of the rat CYP4A isoforms: arachidonic acid metabolism and isoform-specific inhibitors. Am J Physiol. 1999;276(6 Pt 2):R1691–700. doi: 10.1152/ajpregu.1999.276.6.R1691. [DOI] [PubMed] [Google Scholar]
- 6.Lasker JM, Chen WB, Wolf I, Bloswick BP, Wilson PD, Powell PK. Formation of 20-hydroxyeicosatetraenoic acid, a vasoactive and natriuretic eicosanoid, in human kidney. Role of Cyp4F2 and Cyp4A11. J Biol Chem. 2000;275(6):4118–4126. doi: 10.1074/jbc.275.6.4118. [DOI] [PubMed] [Google Scholar]
- 7.Fleming I. Cytochrome p450 and vascular homeostasis. Circ Res. 2001;89(9):753–762. doi: 10.1161/hh2101.099268. [DOI] [PubMed] [Google Scholar]
- 8.Carroll MA, Balazy M, Huang DD, Rybalova S, Falck JR, McGiff JC. Cytochrome P450-derived renal HETEs: storage and release. Kidney Int. 1997;51(6):1696–1702. doi: 10.1038/ki.1997.234. [DOI] [PubMed] [Google Scholar]
- 9.Miyata N, Roman RJ. Role of 20-hydroxyeicosatetraenoic acid (20-HETE) in vascular system. J Smooth Muscle Res. 2005;41(4):175–193. doi: 10.1540/jsmr.41.175. [DOI] [PubMed] [Google Scholar]
- 10.Chen L, Ackerman R, Guo AM. 20-HETE in neovascularization. Prostaglandins Other Lipid Mediat. 2012;98(3–4):63–68. doi: 10.1016/j.prostaglandins.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 11.Guo AM, Arbab AS, Falck JR, Chen P, Edwards PA, Roman RJ, Scicli AG. Activation of vascular endothelial growth factor through reactive oxygen species mediates 20-hydroxyeicosatetraenoic acid-induced endothelial cell proliferation. J Pharmacol Exp Ther. 2007;321(1):18–27. doi: 10.1124/jpet.106.115360. [DOI] [PubMed] [Google Scholar]
- 12.Guo AM, Scicli G, Sheng J, Falck JC, Edwards PA, Scicli AG. 20-HETE can act as a nonhypoxic regulator of HIF-1alpha in human microvascular endothelial cells. Am J Physiol Heart Circ Physiol. 2009;297(2):H602–13. doi: 10.1152/ajpheart.00874.2008. [DOI] [PubMed] [Google Scholar]
- 13.Guo AM, Janic B, Sheng J, Falck JR, Roman RJ, Edwards PA, Arbab AS, Scicli AG. The cytochrome P450 4A/F-20-hydroxyeicosatetraenoic acid system: a regulator of endothelial precursor cells derived from human umbilical cord blood. J Pharmacol Exp Ther. 2011;338(2):421–429. doi: 10.1124/jpet.111.179036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stec DE, Gannon KP, Beaird JS, Drummond HA. 20-Hydroxyeicosatetraenoic acid (20-HETE) stimulates migration of vascular smooth muscle cells. Cell Physiol Biochem. 2007;19(1–4):121–128. doi: 10.1159/000099200. [DOI] [PubMed] [Google Scholar]
- 15.Amaral SL, Maier KG, Schippers DN, Roman RJ, Greene AS. CYP4A metabolites of arachidonic acid and VEGF are mediators of skeletal muscle angiogenesis. Am J Physiol Heart Circ Physiol. 2003;284(5):H1528–35. doi: 10.1152/ajpheart.00406.2002. [DOI] [PubMed] [Google Scholar]
- 16.Chen P, Guo M, Wygle D, Edwards PA, Falck JR, Roman RJ, Scicli AG. Inhibitors of cytochrome P450 4A suppress angiogenic responses. Am J Pathol. 2005;166(2):615–624. doi: 10.1016/S0002-9440(10)62282-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishizuka T, Cheng J, Singh H, Vitto MD, Manthati VL, Falck JR, Laniado-Schwartzman M. 20-Hydroxyeicosatetraenoic acid stimulates nuclear factor-kappaB activation and the production of inflammatory cytokines in human endothelial cells. J Pharmacol Exp Ther. 2008;324(1):103–110. doi: 10.1124/jpet.107.130336. [DOI] [PubMed] [Google Scholar]
- 18.Dhanasekaran A, Bodiga S, Gruenloh S, Gao Y, Dunn L, Falck JR, Buonaccorsi JN, Medhora M, Jacobs ER. 20-HETE increases survival and decreases apoptosis in pulmonary arteries and pulmonary artery endothelial cells. Am J Physiol Heart Circ Physiol. 2009;296(3):H777–86. doi: 10.1152/ajpheart.01087.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamakawa M, Liu LX, Date T, Belanger AJ, Vincent KA, Akita GY, Kuriyama T, Cheng SH, Gregory RJ, Jiang C. Hypoxia-inducible factor-1 mediates activation of cultured vascular endothelial cells by inducing multiple angiogenic factors. Circ Res. 2003;93(7):664–673. doi: 10.1161/01.RES.0000093984.48643.D7. [DOI] [PubMed] [Google Scholar]
- 20.Uchida C, Haas TL. Evolving strategies in manipulating VEGF/VEGFR signaling for the promotion of angiogenesis in ischemic muscle. Curr Pharm Des. 2009;15(4):411–421. doi: 10.2174/138161209787315800. [DOI] [PubMed] [Google Scholar]
- 21.Chen L, Ackerman R, Saleh M, Gotlinger KH, Kessler M, Mendelowitz LG, Falck JR, Arbab AS, Scicli AG, Schwartzman ML, Yang J, Guo AM. 20-HETE Regulates the Angiogenic Functions of Human Endothelial Progenitor Cells and Contributes to Angiogenesis In Vivo. J Pharmacol Exp Ther. 2014;348(3):442–451. doi: 10.1124/jpet.113.210120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang M, Mezentsev A, Kemp R, Byun K, Falck JR, Miano JM, Nasjletti A, Abraham NG, Laniado-Schwartzman M. Smooth muscle--specific expression of CYP4A1 induces endothelial sprouting in renal arterial microvessels. Circ Res. 2004;94(2):167–174. doi: 10.1161/01.RES.0000111523.12842.FC. [DOI] [PubMed] [Google Scholar]
- 23.Carmeliet P, Moons L, Collen D. Mouse models of angiogenesis, arterial stenosis, atherosclerosis and hemostasis. Cardiovasc Res. 1998;39(1):8–33. doi: 10.1016/s0008-6363(98)00108-4. [DOI] [PubMed] [Google Scholar]
- 24.Couffinhal T, Dufourcq P, Barandon L, Leroux L, Duplaa C. Mouse models to study angiogenesis in the context of cardiovascular diseases. Front Biosci (Landmark Ed) 2009;14:3310–3325. doi: 10.2741/3454. [DOI] [PubMed] [Google Scholar]
- 25.Wahlberg E. Angiogenesis and arteriogenesis in limb ischemia. J Vasc Surg. 2003;38(1):198–203. doi: 10.1016/s0741-5214(03)00151-4. [DOI] [PubMed] [Google Scholar]
- 26.Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, Magner M, Isner JM, Asahara T. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5(4):434–438. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- 27.Niiyama H, Huang NF, Rollins MD, Cooke JP. Murine model of hindlimb ischemia. J Vis Exp. 2009;(23) doi: 10.3791/1035. pii: 1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Falco E, Porcelli D, Torella AR, Straino S, Iachininoto MG, Orlandi A, Truffa S, Biglioli P, Napolitano M, Capogrossi MC, Pesce M. SDF-1 involvement in endothelial phenotype and ischemia-induced recruitment of bone marrow progenitor cells. Blood. 2004;104(12):3472–3482. doi: 10.1182/blood-2003-12-4423. [DOI] [PubMed] [Google Scholar]
- 29.Ceradini DJ, Gurtner GC. Homing to hypoxia: HIF-1 as a mediator of progenitor cell recruitment to injured tissue. Trends Cardiovasc Med. 2005;15(2):57–63. doi: 10.1016/j.tcm.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 30.Galasso G, De Rosa R, Ciccarelli M, Sorriento D, Del Giudice C, Strisciuglio T, De Biase C, Luciano R, Piccolo R, Pierri A, Di Gioia G, Prevete N, Trimarco B, Piscione F, Iaccarino G. beta2-Adrenergic Receptor Stimulation Improves Endothelial Progenitor Cells Mediated Ischemic Neoangiogenesis. Circ Res. 2013;112(7):1026–1034. doi: 10.1161/CIRCRESAHA.111.300152. [DOI] [PubMed] [Google Scholar]
- 31.Limbourg A, Korff T, Napp LC, Schaper W, Drexler H, Limbourg FP. Evaluation of postnatal arteriogenesis and angiogenesis in a mouse model of hind-limb ischemia. Nat Protoc. 2009;4(12):1737–1746. doi: 10.1038/nprot.2009.185. [DOI] [PubMed] [Google Scholar]
- 32.Wu CC, Cheng J, Zhang FF, Gotlinger KH, Kelkar M, Zhang Y, Jat JL, Falck JR, Schwartzman ML. Androgen-dependent hypertension is mediated by 20-hydroxy-5,8,11,14-eicosatetraenoic acid-induced vascular dysfunction: role of inhibitor of kappaB Kinase. Hypertension. 2011;57(4):788–794. doi: 10.1161/HYPERTENSIONAHA.110.161570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Inoue K, Sodhi K, Puri N, Gotlinger KH, Cao J, Rezzani R, Falck JR, Abraham NG, Laniado-Schwartzman M. Endothelial-specific CYP4A2 overexpression leads to renal injury and hypertension via increased production of 20-HETE. Am J Physiol Renal Physiol. 2009;297(4):F875–84. doi: 10.1152/ajprenal.00364.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guo M, Roman RJ, Falck JR, Edwards PA, Scicli AG. Human U251 glioma cell proliferation is suppressed by HET0016 [N-hydroxy-N′-(4-butyl-2-methylphenyl)formamidine], a selective inhibitor of CYP4A. J Pharmacol Exp Ther. 2005;315(2):526–533. doi: 10.1124/jpet.105.088567. [DOI] [PubMed] [Google Scholar]
- 35.Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev. 2002;82(1):131–185. doi: 10.1152/physrev.00021.2001. [DOI] [PubMed] [Google Scholar]
- 36.Muller DN, Schmidt C, Barbosa-Sicard E, Wellner M, Gross V, Hercule H, Markovic M, Honeck H, Luft FC, Schunck WH. Mouse Cyp4a isoforms: enzymatic properties, gender- and strain-specific expression, and role in renal 20-hydroxyeicosatetraenoic acid formation. Biochem J. 2007;403(1):109–118. doi: 10.1042/BJ20061328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu W, Chen L, Yang YQ, Falck JR, Guo AM, Li Y, Yang J. Cytochrome P450 omega-hydroxylase promotes angiogenesis and metastasis by upregulation of VEGF and MMP-9 in non-small cell lung cancer. Cancer Chemother Pharmacol. 2011;68(3):619–629. doi: 10.1007/s00280-010-1521-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sacerdoti D, Pesce P, Di Pascoli M, Brocco S, Cecchetto L, Bolognesi M. Arachidonic acid metabolites and endothelial dysfunction of portal hypertension. Prostaglandins Other Lipid Mediat. 2015;120:80–90. doi: 10.1016/j.prostaglandins.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 39.Wu CC, Gupta T, Garcia V, Ding Y, Schwartzman ML. 20-HETE and blood pressure regulation: clinical implications. Cardiol Rev. 2014;22(1):1–12. doi: 10.1097/CRD.0b013e3182961659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mendoza MG, Robles HV, Romo E, Rios A, Escalante B. Nitric oxide-dependent neovascularization role in the lower extremity disease. Curr Pharm Des. 2007;13(35):3591–3596. doi: 10.2174/138161207782794103. [DOI] [PubMed] [Google Scholar]
- 41.Jacobs ER, Zhu D, Gruenloh S, Lopez B, Medhora M. VEGF-induced relaxation of pulmonary arteries is mediated by endothelial cytochrome P-450 hydroxylase. Am J Physiol Lung Cell Mol Physiol. 2006;291(3):L369–77. doi: 10.1152/ajplung.00265.2004. [DOI] [PubMed] [Google Scholar]
- 42.Cheng J, Ou JS, Singh H, Falck JR, Narsimhaswamy D, Pritchard KA, Jr, Schwartzman ML. 20-hydroxyeicosatetraenoic acid causes endothelial dysfunction via eNOS uncoupling. Am J Physiol Heart Circ Physiol. 2008;294(2):H1018–26. doi: 10.1152/ajpheart.01172.2007. [DOI] [PubMed] [Google Scholar]
- 43.Hill E, Murphy RC. Quantitation of 20-hydroxy-5,8,11,14-eicosatetraenoic acid (20-HETE) produced by human polymorphonuclear leukocytes using electron capture ionization gas chromatography/mass spectrometry. Biol Mass Spectrom. 1992;21(5):249–253. doi: 10.1002/bms.1200210505. [DOI] [PubMed] [Google Scholar]
- 44.Zhu D, Zhang C, Medhora M, Jacobs ER. CYP4A mRNA, protein, and product in rat lungs: novel localization in vascular endothelium. J Appl Physiol. 2002;93(1):330–337. doi: 10.1152/japplphysiol.01159.2001. [DOI] [PubMed] [Google Scholar]
- 45.Harder DR, Lange AR, Gebremedhin D, Birks EK, Roman RJ. Cytochrome P450 metabolites of arachidonic acid as intracellular signaling molecules in vascular tissue. J Vasc Res. 1997;34(3):237–243. doi: 10.1159/000159228. [DOI] [PubMed] [Google Scholar]
- 46.Kalyankrishna S, Malik KU. Norepinephrine-induced stimulation of p38 mitogen-activated protein kinase is mediated by arachidonic acid metabolites generated by activation of cytosolic phospholipase A(2) in vascular smooth muscle cells. J Pharmacol Exp Ther. 2003;304(2):761–772. doi: 10.1124/jpet.102.040949. [DOI] [PubMed] [Google Scholar]
- 47.Parmentier JH, Lavrentyev EN, Falck JR, Capdevila JH, Malik KU. Evaluation of cytochrome P450 4 family as mediator of phospholipase D activation in aortic vascular smooth muscle cells. Life Sci. 2005;77(9):1015–1029. doi: 10.1016/j.lfs.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 48.Hoopes SL, Garcia V, Edin ML, Schwartzman ML, Zeldin DC. Vascular actions of 20-HETE. Prostaglandins Other Lipid Mediat. 2015;120:9–16. doi: 10.1016/j.prostaglandins.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu D, Effros RM, Harder DR, Roman RJ, Jacobs ER. Tissue sources of cytochrome P450 4A and 20-HETE synthesis in rabbit lungs. Am J Respir Cell Mol Biol. 1998;19(1):121–128. doi: 10.1165/ajrcmb.19.1.3145. [DOI] [PubMed] [Google Scholar]
- 50.Tsai IJ, Croft KD, Puddey IB, Beilin LJ, Barden A. 20-Hydroxyeicosatetraenoic acid synthesis is increased in human neutrophils and platelets by angiotensin II and endothelin-1. Am J Physiol Heart Circ Physiol. 2011;300(4):H1194–200. doi: 10.1152/ajpheart.00733.2010. [DOI] [PubMed] [Google Scholar]
- 51.Akbulut T, Regner KR, Roman RJ, Avner ED, Falck JR, Park F. 20-HETE activates the Raf/MEK/ERK pathway in renal epithelial cells through an EGFR- and c-Src-dependent mechanism. Am J Physiol Renal Physiol. 2009;297(3):F662–70. doi: 10.1152/ajprenal.00146.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.