Abstract
Despite advancements in surgery and radiotherapy, the aggressive forms of brain tumors, such as gliomas, are still uniformly lethal with current therapies offering only palliation complicated by significant toxicities. Gliomas are characteristically diffuse with infiltrating edges, resistant to drugs and nearly inaccessible to systemic therapies due to the brain-tumor barrier. Currently, aggressive efforts are underway to further understand brain-tumor’s microenvironment and identify brain tumor cell-specific regulators amenable to pharmacologic interventions. While new potent agents are continuously becoming available, efficient drug delivery to brain tumors remains a limiting factor. To tackle the drug delivery issues, a multicomponent chain-like nanoparticle has been developed. These nanochains are comprised of iron oxide nanospheres and a drug-loaded liposome chemically linked into a 100-nm linear, chain-like assembly with high precision. The nanochain possesses a unique ability to scavenge the tumor endothelium. By utilizing effective vascular targeting, the nanochains achieve rapid deposition on the vascular bed of glioma sites establishing well-distributed drug reservoirs on the endothelium of brain tumors. After reaching the target sites, an on-command, external low-power radiofrequency field can remotely trigger rapid drug release, due to mechanical disruption of the liposome, facilitating widespread and effective drug delivery into regions harboring brain tumor cells. Integration of the nanochain delivery system with the appropriate combination of complementary drugs has the potential to unfold the field and allow significant expansion of therapies for the disease where success is currently very limited.
Introduction
The invasive forms of brain tumors, such as glioblastoma multiforme (GBM) are recognized as one of the deadliest cancer types with current therapies offering only palliation complicated by significant toxicities. Despite the extremely poor prognosis, no new effective protocols have been developed in more than 30 years. Current approaches for the treatment of gliomas are limited in their effectiveness, because GBMs are characteristically diffuse, highly invasive, non-localized, and drug penetration across the blood-tumor barrier (BTB) is poor for most drugs.1–3 For example, median survival is merely prolonged to 12 months following the combination of surgical resection and focal radiotherapy, which are incapable of completely removing deeply penetrating, diffuse brain tumors.2, 3 As a result, the most common brain tumors in adults (i.e., GBMs) and children (i.e., medulloblastomas) cause disproportional mortality among cancer patients. At the same time, even though highly potent cytotoxic drugs are available to oncologists, systemic therapy is not the primary treatment of choice for malignant brain tumors due to the presence of the BTB. Notably, nanoparticles have shown promise, because they can “smuggle” cytotoxic drugs into intracranial tumors due to tumor’s leaky blood vessels and the enhanced permeation and retention (EPR) effect.4 However, even though the BTB compromises the impermeable nature of the blood-brain barrier (BBB), blood vessels are not nearly as leaky as the angiogenic vessels observed in other cancer types.5 Thus, nanoparticles exhibit low penetration into gliomas with a patchy, near-perivascular distribution resulting in failure to deliver drugs particularly to the invasive sites of brain tumors.6 In addition to limited drug delivery, brain tumor cells tend to be particularly resistant to drugs, especially after tumor recurrence.
A new class of multicomponent chain-like nanoparticles, termed nanochains, seeks to tackle these challenges associated with the treatment of brain tumors.7–12 The nanochains can be comprised of different constituent nanospheres (e.g., iron oxide, gold, liposome) chemically linked into a linear assembly with high precision. Due to enhanced site-specific targeting and radiofrequency-triggered drug release, the nanochains facilitate effective delivery of drugs into hard-to-treat tumors. In this review, we concisely and briefly state the main obstacles associated with treating brain tumors, but we primarily focus on the potential and challenges of the nanochain particle. To successfully overcome the obstacles of drug delivery and drug resistance, the nanochains have to integrate their drug delivery capabilities with the appropriate combination of complementary drugs to enable effective treatment of invasive brain tumors.
TREATMENT CHALLENGES
While the presence of brain tumors compromises the integrity of BBB, the brain-tumor barrier is still a major obstacle to effective drug delivery into brain tumors.13–16 Brain vasculature consists of endothelial cells, astrocytes and pericytes, which form the BBB. BBB makes the brain vasculature highly specialized by selectively controlling the transport of molecules from systemic circulation into the brain parenchyma. For example, due to the tight junctions between endothelial cells, the brain is inaccessible to any hydrophilic molecule via passive transport. Furthermore, the brain vasculature contains various transmembrane transporter proteins (e.g., the P-glycoprotein efflux pump) that prohibit systemic therapies from reaching the brain parenchyma. However, some believe that the BTB barrier does not present significant limitations to drug delivery.17 This controversy is partially supported by the enhancement of GBMs in contrast-enhanced CT and MR imaging. However, many studies have shown that while the BBB is partially breached18 resulting in increased retention of molecules into brain tumors compared to normal brain parenchyma, the BTB barrier of GBMs consists of blood vessels that are not nearly as leaky as the angiogenic vessels observed in other cancer types.19–22 This results in the failure of drugs to reach the majority of the primary tumor mass and especially its invasive sites.
Nanoparticles have shown promising results by exploiting the enhanced permeation and retention (EPR) effect. For instance, doxorubicin, a highly potent chemotherapeutic agent, is nearly inaccessible to brain tumor cells due to insignificant penetration into GBMs.23–25 However, it was shown in GBM patients that doxorubicin loaded into liposomal nanocarriers can efficiently be deposited in gliomas.4 Unfortunately, the BTB barrier has profoundly negative effects on the delivery of nanoparticles, resulting in their low penetration into the brain tumor interstitium with a patchy, near-perivascular distribution.6 Furthermore, while the primary regions of GBMs may be relatively favorable to an EPR-driven deposition of nanoparticles, this effect is significantly attenuated at the invasive sites of brain tumors with dispersing cancer cells. This stems from the fact that invasive sites display a BBB barrier that has a very high likelihood to maintain its integrity and remain intact.16 This raises significant concerns because the infiltrating edges of GBMs are primarily responsible for recurrences.26 Most importantly, it is quite common to find dispersing brain tumor cells as far away as 4 cm from the primary site.27
In addition to the diffuse growth and brain-tumor barrier, brain tumors tend to be particularly resistant to drugs. While treatment failure can be attributed to a diverse set of underlying causes, gliomas and medulloblastomas display remarkable cellular heterogeneity and hierarchies. At the apex, subpopulations of self-renewing tumor initiating cells with stem-like properties have been identified that contribute to their high rates of therapeutic resistance and rapid recurrence.28–34 This subset of cells within GBMs is often called glioma stem cells (GSCs). It has been shown that GSCs interact with the microenvironment in vivo, including areas such as the perivascular and hypoxia niches, to promote tumor angiogenesis, cancer invasion, and immune evasion.35–37 Most importantly, many studies have identified GSC-specific regulators amenable to pharmacologic interventions, resulting in significant inhibition of disease progression and tumor recurrence.35, 36, 38–42
While the list of potent new drugs against brain tumors continues to grow, efficient drug delivery to brain tumors remains a limiting factor. Until approaches to deliver adequate concentration of drugs in brain tumor cells is achieved, we may see very little progress in the clinical deployment of contemporary targeted medicines. To address both challenges of drug delivery and resistance, integration of advanced drug delivery systems with the appropriate combination of complementary drugs is necessary to develop an effective treatment for the “general population” of glioma cells as well as the small fraction of glioma cells that are resistant.
Recent efforts using nanotechnology
Nanotherapeutics for brain tumors have been developed for passive and active targeting. Passive targeting relies on the ‘leakiness’ of blood brain barrier (BBB), and on the enhanced permeation and retention (EPR) phenomenon for extravasation and accumulation of nanoparticles in brain tumors.43 Clinical studies using PEGylated liposomal doxorubicin in patients with brain metastasis have demonstrated upto 10-fold higher deposition of drugs in the primary and metastatic brain tumors compared to normal brain tissue.44 The universality of EPR phenomenon45, 46 and the patency of BBB in primary and metastatic tumors17, 19, 47, 48 has been a topic of on-going investigations for several decades. While passive delivery of drugs using non-targeted nanocarriers may be feasible in advanced primary tumors that exhibit some degree of ‘leakiness’, growing evidence suggest that metastatic brain tumor as well as the invasive and diffuse fronts of brain tumors most likely require active targeting of drug-loaded nanocarriers.49, 50
Several approaches have been considered for active targeting. Transient opening of the BBB, using chemical modifiers such as mannitol and other high osmotic agents,51, 52 focused ultrasound,53 and hyperthermia54 have been investigated to increase delivery of nanoparticle-based therapeutics. Convention-enhanced delivery has also been extensively investigated, both pre-clinically and clinically, for increasing drug concentration and distribution using a variety of nanotherapeutics.55, 56 Due to the challenges of getting past the BTB, vascular targeting of brain tumors has become a growing area of pre-clinical research. Vascular-targeted nanotherapeutics employ receptor-mediated endocytosis for entering the endothelial cells and thereby passaging into the primary layer of BTB. Transferrin,57, 58 insulin,59 integrin,60, 61 neuropilin,62 and lipoprotein receptor-related protein61, 63 are among the most extensively studied receptors for the development of targeted nanoparticles for bypassing the BTB. Pre-clinical rodent efficacy studies using nanocarriers, including targeted liposomes,64 gold nanoparticles65 and polymeric nanoparticles have demonstrated substantial improvement in the delivery of drugs to orthotopic brain tumors. Polymeric nanocarriers decorated with polysorbate-80 starch have also been demonstrated to deliver doxorubicin in a mouse model of metastatic brain lesions.66 Although their study did not use animal survival as the efficacy end point, significant tumor inhibition rates (upto 9-fold) were observed compared to free doxorubicin. Dual-targeted nanoparticles have also been investigated for enhancing drug delivery to brain tumor cells.62, 67 The rationale for dual ligands stems from using one of the ligands to penetrate the BTB and the second ligand to seek out tumor cells. The readers are directed to several excellent review articles on various nanoparticle platforms investigated for brain tumor treatment.49, 68–70 Additionally, strategies to improve the tissue penetration of nanoparticles are even more important in brain tumors due to the high interstitial pressure and the presence of dense extracellular matrix compared to extracranial tumors.71 While the arsenal of nanotherapies for brain tumors is increasing, it is difficult to compare various nanocarrier platforms due to lack of standardization surrounding animal models, study designs and efficacy end-points. Nevertheless, these studies support the potential and development of the nanochain platform. Most importantly, the ability of nanochains to spatially and temporally release the therapeutic payload at or near the target site makes them attractive for going after the diffuse and invasive forms of brain tumor.
Multifunctional iron oxide nanoparticles have been also extensively investigated for use as imaging and theranostic agents in brain tumors.72 Iron oxide nanoparticles presenting single domain antibody against insulin-like growth factor has been investigated for imaging of brain tumor vasculature.73 An F3-targeted polymeric nanoparticle formulation encapsulating iron oxide has been evaluated for MR imaging and treatment of brain tumors.74 Theranostic iron oxide nanoworms decorated with tumor vascular homing peptides and proapoptotic peptides have also been evaluated in rodent models of GBM tumors.75 Cetuximab-conjugated iron oxide nanoparticles (IONP) have been investigated for targeting of brain tumor and glioma stem cells.76, 77 Multifunctional iron oxide nanoparticles decorated with a double emulsion nanocapsule and labeled with lactoferrin have been evaluated for co-delivering doxorubicin and curcumin in brain tumors.78 The unique characteristic of iron oxide nanoparticles to produce heat has been exploited for thermotherapy of tumors.54, 79
Despite the pre-clinical and regulatory hurdles, some of the nanotherapeutics are moving beyond early efficacy testing. In Europe, the NanoTherm® magnetic hyperthermia therapy using aminosilane-coated iron oxide nanoparticles has been approved for GBM treatment. Glutathione-targeted liposomal doxorubicin has completed Phase 1/2a clinical trials in brain tumor patients (ClinicalTrials.gov identifier: NCT01386580). Liposomal-vincistrine (Marquibo®), a clinically approved agent, has also completed a Phase 1 trial in brain tumor patients (ClinicalTrials.gov identifier: NCT01222780). Liposomal irinotecan (MM-398) currently undergoes Phase 1 clinical evaluation in brain tumor patients (ClinicalTrials.gov identifiers: NCT01770353 and NCT02022644).
THE NANOCHAIN PLATFORM
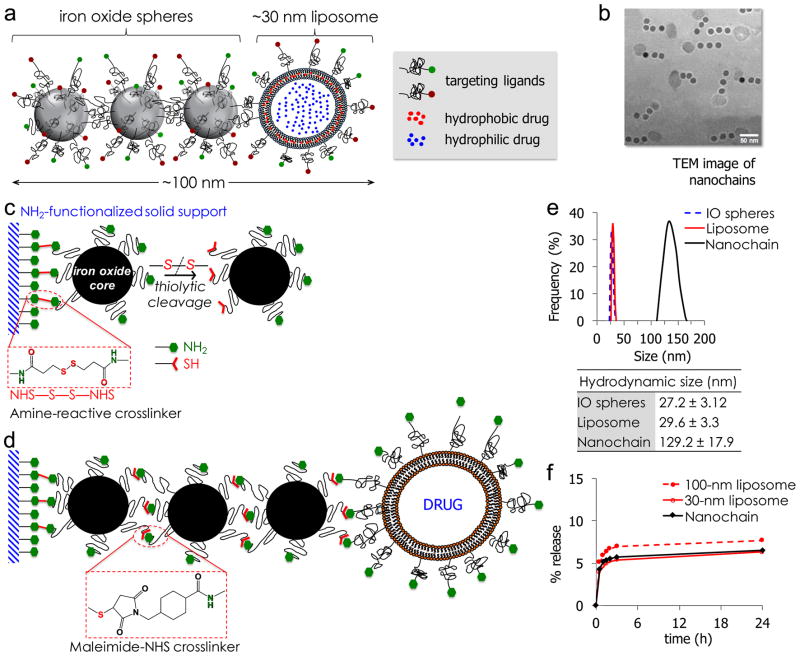
To circumvent the limitations of today’s drugs in treating invasive brain tumors, we developed a flexible chain-like nanoparticle. The multicomponent nanoparticle, termed nanochain, is comprised of three iron oxide (IO) nanospheres and one drug-loaded liposome, all chemically linked into a linear, chain-like assembly (Fig. 1a,b).7, 8
Figure 1.
(a) Schematic of a linear nanochain particle composed of three IO nanosphere and one drug-loaded liposome. (b) TEM image of nanochain particles (reprinted with permission after partial modifications from ref. 11). (c) Reaction scheme of the controlled assembly of nanochains using solid-phase chemistry. In the first step, chemical bifunctionality on the surface of parent IO nanospheres is topologically controlled resulting in nanospheres with two faces, one displaying only amines and the other only thiols. (d) In the second step, the two unique faces on the parent nanosphere serve as fittings to chemically assemble them into nanochains. (e) Size distribution of nanochain particles and their parent nanospheres obtained by DLS (data presented as mean ± s.d.). (f) Comparison of in vitro blood plasma stability of nanochains to 30-nm and 100-nm liposomal DOX. In a typical leakage procedure, 1 mL of formulation was placed in dialysis tubing with 100k MWCO and dialyzed against blood plasma at 37°C. (reprinted with permission after partial modifications from ref. 12).
Fabrication of nanochains
A stepwise solid-phase chemistry approach is utilized for fabrication of the multicomponent nanochains.7–12 In the first step, amine-PEG functionalized iron oxide nanospheres (hydrodynamic diameter of ~30 nm) are conjugated onto amine-functionalized resin. A homobifunctional crosslinker, reactive towards primary amines containing a disulphide bridge, is used for the conjugation of iron oxide nanospheres onto the resin (Fig. 1c). Once the nanospheres bind to the solid support, they are cleaved off using a reducing agent, generating iron oxide nanosphere’s with two sets of functional groups (thiol and amine), resembling a Janus particle. The asymmetric bifunctionality of these nanoparticles facilitates their chemical linkage in a linear orientation with a high degree of uniformity (Fig. 1d). The second step employs the modified nanospheres, which are introduced into the resin in a sequential manner using a heterobifunctional crosslinker for conjugation between primary amine and sulfhydryl groups. In the final step, an amine functionalized drug-loaded liposome (hydrodynamic diameter of ~35 nm) is added. Multiple washing cycles are employed in each step to remove any unbound nanospheres and excess reagents from the nanoparticle-resin complex. Finally, the nanochain particles are cleaved off the resin and recovered. It should be noted that the 35-nm liposomal component of the nanochain is prefabricated using bulk processing techniques that involve a combination of extrusion and sonication8. Furthermore, it should be emphasized that a variety of hydrophilic and hydrophobic compounds can be stably encapsulated into liposomes, using well-established passive and remote loading methods.80–82 In vitro release assay test, conduced in plasma, demonstrated the high stability of drug encapsulation within the nanochains (Fig. 1f).12
Overall, the nanochain fabrication method offers great flexibility and control in generating nanochains of various dimensions and aspect ratios with a high degree of uniformity.7 In previous studies,8, 11 analysis of nanochains using transmission electron microscopy (TEM) demonstrated that the majority of the nanochains (>85%) are comprised of three iron oxide spheres and one liposome. Furthermore, the hydrodynamic size of the nanochain particle and each constituent nanosphere, as measured by dynamic light scattering (DLS), corroborate with the TEM findings (Figure 1e). The design of nanochains also allows the incorporation of different types of constituent nanoparticles with varying functionalities. While other strategies have resulted in well-defined nanostructures,83–87 they are typically limited only to one material and/or one size (and aspect ratio). The simplified purification procedure and ease of handling of reaction vessels, using the solid-phase-based synthesis approach, enables production of large batches of nanochains with a high degree of consistency.
Organ distribution of nanochains
Overall, the biodistribution of nanochains is comparable to the behaviour of a 100-nm liposome.8, 11 As expected, nanochain particles are primarily cleared by the organs of the reticuloendothelial system i.e., liver and spleen. Analysis of nanochain distribution in non-target organs, at 24 h after administration, demonstrated accumulation in the heart, lungs, brain and kidney at ~ 3.5, 3, 2 and 4% of the injected dose, respectively.
THE NANOCHAIN STRATEGY TO DELIVER DRUGS TO BRAIN TUMORS
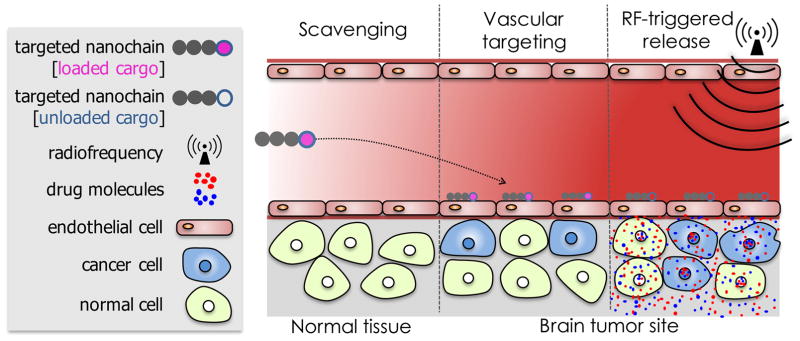
The multicomponent nature and shape of the nanochain particle result in two unique characteristics (Fig. 2) that facilitate effective treatment of difficult-to-treat brain tumors using a relatively low dose of conventional cytotoxic drugs: 1) enhanced site-specific targeting of cancer sites and 2) on-command RF-triggered drug release for increased tumor dose painting throughout the target site.8–10 First, contrary to small molecules or spherical nanoparticles, the high aspect ratio and flexibility of nanochains significantly increase vascular targeting efficiency.9 The nanochain utilizes ligands to target receptors, which are highly overexpressed on the brain tumor’s vascular bed. The amplified targeting avidity of nanoparticles, (i.e., latching on vascular target) due to geometrically enhanced multivalent attachment on the endothelial surface, results in their highly specific deposition on vascular bed of the primary brain tumor and its invasive sites.13 Second, even after successful deposition at the target site, drug molecules must reach the cancer cells resulting in widespread anticancer activity. The nanochains can be remotely triggered to release the drug molecules by mechanically induced defects in the liposomal membrane, which is the result of the oscillation of the iron oxide portion of the nanochain in the presence of an RF field.8 Thus, a few hours after the nanochains slip from the blood stream and dock on the vascular bed of brain tumors, a low-power radiofrequency (RF) field (10 kHz frequency, 5 mT amplitude) is applied covering the target region within the body. The RF field causes the nanochain to vibrate, breaking open the drug-loaded liposome and releasing the encapsulated drug molecules to facilitate their spread over the entire volume of glioma sites.8, 11
Figure 2.
Illustration of the nanochain therapeutic concept to successful deliver drugs to invasive brain tumors via vascular targeting and RF-triggered drug release.
The enhanced specificity of nanochains combined with their ability to deliver high concentration of drugs at the target site improves the drug’s therapeutic index and therefore permits a lower drug dose for administration. This could potentially result in attenuating drug-induced toxicities that are otherwise the bane of anti-cancer compounds. Furthermore, the versatile nature of the drug carrier component of the nanochain (i.e., liposome) can enable simultaneous delivery and spread of a synergistic combination of drugs in hard-to-reach target sites. Considering the highly resistant and heterogenous nature of brain tumors, the simultaneous delivery of combination chemotherapy or two or more small molecule inhibitors may lead to a significant improvement in patient outcomes.
Vascular targeting versus deep tissue targeting of tumors
To date, applications of nanoparticles have mainly focused on exploiting the leaky vasculature of tumors to enhance the intratumoral delivery via the ‘Enhanced Permeability and Retention’ (EPR) effect.88 Recent publications, however, indicate that the impact of the EPR effect is inconsistent.89 This stems from the fact that nanoparticles typically exhibit a patchy, near-perivascular accumulation in tumors.90, 91 In this context, vascular targeting may be more effective than deep tissue targeting in many occasions, since deep tissue targeting requires the EPR effect as a prerequisite. For example, several prior studies have shown that while active targeting of cancer cells (i.e., deep tissue targeting) typically enhances the intracellular transport of the nanoparticles, there is no gain in the total deposition of nanoparticles in tumors when compared to non-targeted nanoparticles.92–95
Additionally, the size and the multivalent avidity, due to formation of multiple receptor-ligand bonds, makes nanoparticles ideal for targeting of vascular-associated pathologies. This has been demonstrated using several nanoparticle platforms including our own recent work wherein vascular targeting of a gold nanoparticle radiotracer resulted in a 5.2-fold higher deposition in lung metastases than the equivalent small molecule analogue.96 Further, the nanochains were specifically developed to further enhance vascular targeting of diseased endothelium. For example, in a rodent model of metastatic breast cancer, the nanochains outperformed equivalent spherical nanoparticles in vascular targeting by exhibiting a 7.5-fold greater deposition on the endothelium that was associated with the metastatic sites.11
Site-specific targeting of nanochains to gliomas via vascular targeting
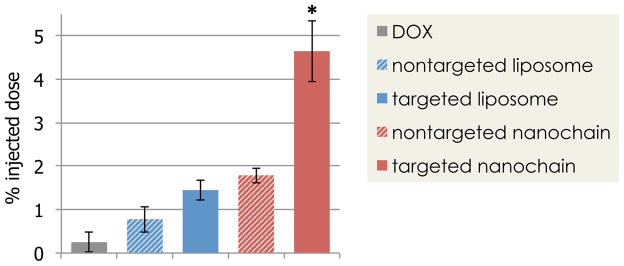
The rationale and benefits of vascular targeting become even more critical in the case of brain tumors due to their invasive nature and the presence of the BTB barrier. Although the BTB displays characteristics of a compromised BBB, the vasculature of brain tumors is relatively less permeable compared to other cancer types.5 The nanochain utilized a cyclic RGD peptide as a ligand to target the overexpressed αvβ3 integrin receptor on the vascular bed of brain tumors. In a mouse model of glioma (1 mm in size), vascular targeting using the RGD-targeted nanochain resulted in the deposition of 4.7% of the injected dose when analysed at 24 hours after drug administration (Fig. 3). This represented a 18.6-fold higher dose when compared to a conventional small molecule therapeutic.12 The drug levels were predominantly measured in the tumor mass at the primary site, due to the challenges related to identification of the margins of invasive sites with dispersive glioma cells. At the same time, the drug was nearly undetectable in the normal brain parenchyma. Despite the high drug accumulation at the target site, the efficacy of the nanochain treatment is marginal at best. This stems from the fact that the drug molecules, due to their encapsulation within liposomes, do not spread throughout the tumor volume to reach the distant cancer cells at cytotoxic levels. While free drug in its molecular form quickly spreads within the tumor interstitium,97–99 the release of drug from nanoparticles, deposited in the tumor interstitium, occurs at a relatively slow rate.
Figure 3.
Evaluation of the ability of nanochains to target invasive brain tumors in mice. At 8 days after orthotopic inoculation of CNS-1 cells in mice, the animals were injected with various formulations of doxorubicin (DOX) at a dose 0.5 mg/kg DOX. At 24 h after injection, animals were euthanized, brain tumors were excised and DOX content was measured. (reprinted with permission after partial modifications from ref. 12)
Use of appropriate animal models is essential to evaluate therapeutics targeting GBM dispersal. The most valuable models display characteristics that mimic elements of human GBM pathology. For example, a recent study characterized how commonly used human (Gli36Δ5, U-87 MG, LN-229) and rodent (CNS-1) glioma cell lines disperse in the mouse brain.100 It was shown that Gli36Δ5 and U-87 MG cells are not particularly invasive, whereas LN-229 and CNS-1 cells migrate significantly along blood vessels and white matter tracts. Thus, despite their popularity as a GBM tumor model in vitro, the U-87 MG or 9L glioma cells may not be the best in vivo models to test site-specific targeting of nanoparticles to invasive GMBs. For this reason, the nanochains were primarily evaluated in the highly invasive CNS-1 animal model. It was shown that αvβ3 integrin-mediated vascular targeting facilitated highly effective deposition of nanochains at invasive sites.12 However, a single animal model cannot capture the complexity of human disease, especially in the case of developing targeting strategies towards dispersing glioma cells. It is known that dispersive glioma cells select “permissive” pathways that have an abundance of cell adhesion molecules.101 Since perivascular growth is the most common form of glioma dispersal, it is not surprising the glioma cells upregulate integrins, which enables them to effectively interact with these molecules of the extracellular matrix found in close association with the brain vasculature. A number of molecules have been found to promote migration of dispersive glioma cells. For example, stromal cell-derived factor 1α (SDF-1α) and its receptor CXCR4,102 Na+/H+ exchanger regulatory factor 1 (NHERF-1),103 ephrin B2, ephrin B3 and the EphB2 receptor104, 105 exhibit elevated levels in dispersive glioma cells compared to the primary glioma site. In conclusion, identification of viable targetable vascular sites is highly important to effectively direct nanoparticles to infiltrating glioma cells.
RF-triggered release and widespread drug delivery to invasive brain tumors
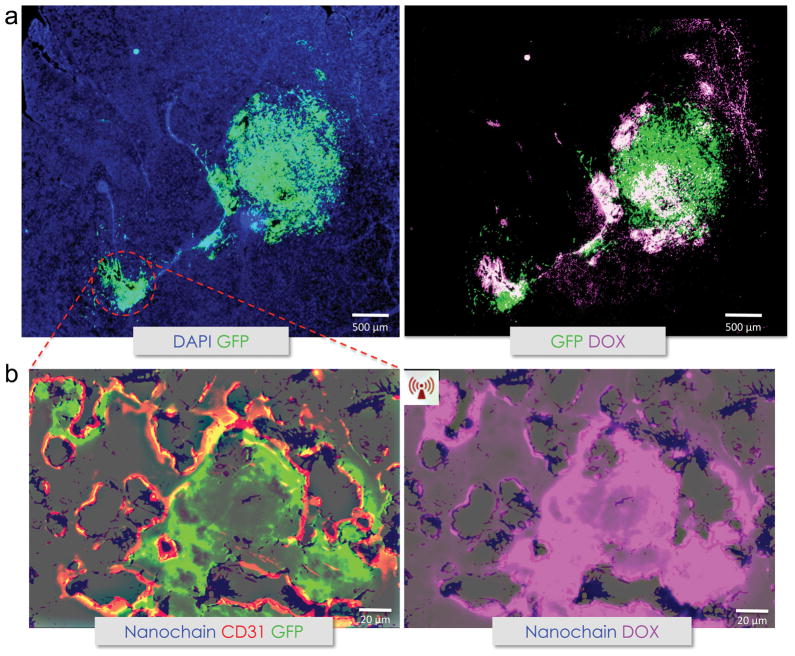
The iron oxide component of the nanochain particle, through their interaction with magnetic fields at the selected frequency of 10 kHz, efficiently converts magnetic energy to mechanical vibration resulting in “mechanical” disruption of the liposomal membrane. The mechanical disruption-based drug release phenomenon is markedly different from the more commonly used heat-induced drug release achieved by other nanoparticle designs (e.g., thermosensitive nanoparticles incorporating iron oxide or gold nanoparticles).106 Furthermore, the mechanical disruption mechanism of the nanochain particles results in rapid and efficient drug release even for very low concentration of nanoparticles. In an in vivo efficacy study in a rodent model of CNS-1 brain tumor, a low-power radiofrequency (RF) field (10 kHz frequency, 5 mT amplitude) was externally applied two hours after administration of the targeted nanochains, giving sufficient time for their deposition on the vascular bed of brain tumors. Detailed histological analysis demonstrated the localization of nanochain in the invasive brain tumor sites. More importantly the degree and topology of drug delivery critically depended on the application of the RF field (Fig. 4). Doxorubicin (DOX), one of the most commonly used anticancer drugs, was used as a model drug in this study. Although no widespread intra-tumoral delivery of DOX was observed in the case of nanochain-treated animals that were not exposed to RF (fluorescence signal of intraliposomal DOX is quenched), application of the RF field facilitated their release from the liposomal component of nanochains and spread at both the primary and invasive sites of brain tumor, resulting in targeting of distant cancer cells located away from the site of nanochain deposition.
Figure 4.
Histologic evaluation of the nanochain treatment. A, histological evaluation of the anticancer effect of nanochains was performed in the orthotopic CNS-1 model in mice [magnification, x5; green, CNS-1 glioma cells (GFP); blue, nuclear stain (DAPI); violet, doxorubicin]. Fluorescence imaging of an entire histologic section of the brain shows the primary tumor and its invasive sites (left). Fluorescence imaging of the same histologic section shows the widespread distribution of doxorubicin molecules after a 60-min application of RF (right). B, higher magnification imaging (x20) of an invasive site shows the location of nanochains (blue) with respect to the location of endothelial cells (green, CD 31) and brain tumor cells (left), and the RF-triggered release of doxorubicin (right) in the same histologic section. Nanochains were visualized by staining iron with Prussian blue. The distribution of doxorubicin molecules is shown with (C) or without (D) RF with respect to the location of cancer cells. DOX, doxorubicin. (reprinted with permission after partial modifications from ref. 12)
Survival studies using the targeted nanochains have been conducted in two different clinically relevant orthotopic brain tumor models. While free DOX had negligible therapeutic benefits in the CNS-1 model, the nanochain-treated animals followed by RF application exhibited a 2.5-fold increase in survival compared to nanochain-treated animals in the absence of the RF application. This represents a significant improvement considering the highly aggressive and invasive nature of CNS-1 tumor model. The therapeutic efficacy of nanochains was also evaluated in the aggressive but less invasive 9L glioma model. While 100% of the untreated and DOX-treated mice died within 28 days, 40% of the nanochain-treated group followed by RF was still alive after 77 days. Furthermore, the enhanced deposition of targeted nanochains at brain tumors followed by the release and spread of drug across the BTB and over the complete tumor volume enabled acceptable therapeutic outcome using an ~15-fold lower dose than the typical clinical regimens of liposomal DOX.
We have observed similar therapeutic benefits using the nanochain treatment in other hard-to-treat cancers. For example, nanochains facilitated effective treatment and ultimately eradication of micrometastatic disease using a low dose of a cytotoxic drug in animal models of triple-negative breast cancer, which is an extremely aggressive and lethal subtype of the disease.11
Mechanism of triggered drug release using RF
Our previous studies have shown that the RF-triggered drug release mechanism of nanochains is fundamentally different from heat-induced mechanism that is commonly employed by other metallic-based nanostructures.8, 12 The magnetization response of iron oxide nanoparticles, when subjected to an external and oscillating magnetic field, is governed by the Brownian and Néel relaxation mechanisms. Brownian relaxation is the more dominant relaxation mechanism for nanoparticles larger than about 25 nm in diameter. In the case of Néel relaxation, the nanoparticle must overcome an energy barrier to reorient its magnetic moment with an applied field, resulting in dissipation of excess heat. This mechanism of magnetic nanoparticles has been exploited for hyperthermia applications. For example, iron oxide nanoparticles have been approved by the European Medicines Agency (EMA) for hyperthermia treatment of recurrent malignant gliomas with alternating magnetic fields. In the case of nanochains, the chemical bond between constituent iron oxide nanospheres restricts Brownian relaxation. As a result, rather than true rotational motion, Brownian motion translates into mechanical “vibration” of the chain. Furthermore, mechanistic studies also showed that the application of 10 kHz RF field did not result in local heating around the nanochain, thereby excluding the role of Néel relaxation phenomenon in RF-triggered drug release.
The primary findings of a series of in vitro studies is summarized below:
The drug release rate from the nanochains can be triggered in a controlled manner by adjusting the parameters of the RF field at a very low concentration of nanochains expected to deposit in tumor tissues during in vivo applications.8
Similar drug release rate per nanochain particle was observed from suspensions of dramatically different concentrations of nanochains when subjected to the same RF field.8
No temperature increase occurred in the nanoparticle suspension or locally around the nanoparticles under the RF field. Using animal models of invasive brain tumors, in vivo studies verified the in vitro results.8 Furthermore, no temperature increase of the brain tissue was observed due to the RF field, indicating that the triggered release mechanism of drug from the nanochains is not based on hyperthermia.12
Nanochains were compared to liposomes encapsulating iron oxide nanospheres. While application of RF induced rapid drug release from nanochains at very low particle concentration, negligible drug release was observed from liposomes encapsulating drug and iron oxide nanospheres using the RF system at the selected frequency.8
The RF-triggered drug release from nanochains demonstrated comparable cancer cell cytotoxicity as that to free drug.
While in vitro studies have provided basic understanding of the mechanism of drug release, the in vivo applications of the nanochains involve additional complexity. For example, in addition to extracellular localization of nanochains, histological analysis showed that nanochains are also found internalized in cells, primarily in endosomes. Further, preliminary cell culture studies indicate that RF triggers drug release from intracellular nanochains resulting in liberation of drug molecules from the endosome and eventually the cell. Ongoing studies continue to investigate the release mechanisms and release patterns of different drugs (particularly in terms of hydrophobicity) from intracellular nanochains.
The RF system
Given the relatively low RF frequencies used with the nanochains compared to e.g. ultra-high field MRI (>300 MHz), the fields are well understood. Thus, the design, cost and clinical deployment of such system present a low degree of translational challenge. Because the drug release mechanism depends on the RF field, one practical challenge is obtaining uniform release across a tumor site, especially one at depth. This stems from the fact that the RF field generally decays away from the RF source. This can be controlled through the design of the coil or antenna used to deliver the RF energy, as well as the power provided to these elements. Thus, we will construct an optimized RF delivery system. The key component of this will be the design and control of the RF hardware.
FUTURE CONDSIDERATIONS
Selection of vascular targets
Identification of the appropriate cell-surface markers that are overexpressed on the endothelium of brain tumors is essential for vascular targeting strategies. Similar to other cancer types, brain tumors exhibit noticeable angiogenesis with endothelial proliferation resulting in highly tortuous, disorganized and abnormal blood vessels.13 It is well established that vascular endothelial growth factor A (known as VEGF) is a major pro-angiogenic factor and highly expressed in brain tumors. The main mediator of the effects of VEGF is its receptor, VEGFR2 (also known as KDR), which is highly overexpressed on blood vessels of brain tumors. Importantly, many small molecules have been developed for anti-angiogenic agents that target VEGFR2 with high affinity.13 In addition, increased levels of VEGF directly dictate the overexpression of adhesion molecules on the endothelium of tumors. For example, VEGF upregulates αvβ5, αvβ3 and α5β1 integrins, which are cell-matrix receptors that mediate the spreading and migration of endothelial cells. The upregulation of αvβ3 integrins on brain tumor’s vascular bed74, 107–118 led to the development of multiple αvβ3 inhibitors that have been evaluated in GBM patients.114, 115, 118 Furthermore, while upregulation of these markers is typical in the brain tumor-associated blood vessels, minimal expression is found on normal blood vessels.119, 120 Thus, various targeting ligands can be explored to direct the nanochains to upregulated specific cell-surface molecules on the vasculature of brain tumors. While traditional single-ligand nanoparticles have achieved effective targeting of brain tumors, dual-ligand strategies have shown increased targeting accuracy to tumor sites that overexpress two targetable receptors.121–125 Brain tumors cannot be regarded monolithic, since their microenvironment is highly dynamic and heterogeneous, resulting in continuous spatiotemporal changes of the expression of targetable cell-surface receptors. By using multi-ligand nanoparticles, vascular targeting could be capable of capturing such spatiotemporal alterations resulting in effective targeting of different tumor sites within the same patient that overexpress only one receptor or the entire set of targeted receptors. For example, a recent study showed that vascular targeting of a dual-ligand nanoparticle effectively targeted all the tumor sites in animal models of metastatic disease that would be otherwise missed by single-ligand strategies.126 In this context, a successful multi-ligand vascular targeting strategy may utilize more that one types of receptors to establish well-distributed drug reservoirs on the endothelium of brain tumors, which can then liberate free drug molecules in the brain tumor parenchyma using an RF field as an on-command external trigger.
Image-guided therapies
In addition to the site-specific delivery of drugs, nanochains are equipped with imaging functionality, which can facilitate simultaneous treatment and diagnosis, image-guided therapy, or monitoring of the outcome of the therapy. In previous studies,9 targeted nanochains enabled accurate detection of small lesions at an early stage of development using MRI. Thus, nanochains can be used a theranostic agent. However, a theranostic agent is often a compromise between the imaging and the therapeutic function,127, 128 since different concentrations of the two components are required to perform their corresponding functions. Thus, careful evaluation is needed to demonstrate the added value and potential clinical benefits of the additional imaging functionality.
Selection of drug candidates
Current therapies are typically ineffective after tumor recurrence due to therapeutic resistance. Numerous studies have shown that glioma stem cells (GSCs), a highly plastic cellular subpopulation functionally defined by self-renewal, multipotency, and tumor propagation, which contribute to their high rates of therapeutic resistance and rapid recurrence.28–34 In addition to standard chemotherapeutic agents, contemporary small molecule inhibitors can also be considered as part of the nanochain’s drug cargo, especially since the nanochain provides the flexibility to carry different types of molecules. Currently, aggressive efforts are underway to identify GSC-specific regulators amenable to pharmacologic targeting. For example, it was recently identified that the non-receptor tyrosine kinase, BMX (Bone Marrow X-linked), and inducible nitric oxide synthase (iNOS) are unique signal regulators in GSCs.129, 130 Identifying the optimal drug candidates for nanochain-mediated treatment of brain tumors requires a judicious understanding of the drug’s physicochemical properties, since drug molecules in nanochains are loaded within a liposome, which is the drug cargo compartment of the nanochain. Liposomes have been used as delivery vehicles for a variety of hydrophilic and hydrophobic drug molecules. Depending on their partition coefficient (log P), drug molecules can be passively or actively loaded into liposomes. Generally, hydrophilic drugs are passively encapsulated within the aqueous core of the liposomes. Hydrophobic drugs can be passively incorporated into the lipid bilayer, or actively encapsulated within the liposomal core. Several active loading techniques have been investigated for loading poorly water-soluble drugs in liposomes.131 Recently, Norvaisas studied the effect of drug hydrophobicity on liposomal nanocarriers.132 Their study analyzed the partition coefficient of drugs in DrugBank, a freely available database containing ~6700 compounds, categorized them based on their anatomical therapeutic chemical (ATC) classification levels, and assessed their compatibility of loading in drug delivery systems that are currently approved for clinical use or in clinical trials. The use of such computational approaches can greatly aid in in silico screening of drug candidates for use with nanochains. In addition to optimal physicochemical properties, the loaded drugs should also demonstrate optimal release profiles when the nanochains are triggered via external radiofrequency fields. Since the drug cargo compartment of the nanochain is the liposome. Typically, small molecules are expected to be more efficiently loaded into liposomes and exhibit satisfactory release patterns. As of now, effective RF-triggered release from the nanochains has been demonstrated for therapeutics with low molecular weights (i.e., <1,000 Da).
Challenges Ahead
Although several nanotechnologies have been developed and pre-clinically investigated, clinical translation has not kept pace with pre-clinical developments. Unlike small molecule therapeutics, clinical translation of nano-therapeutics faces unique regulatory challenges. In 2006, the Food and Drug Administration (FDA) formed the FDA Nanotechnology Task Force with the goal of determining and advocating approaches that would aid in the development of safe and effective FDA-regulated nanotechnology products. The Nanotechnology Alliance at the National Cancer Institute (NCI), including the Nanotechnology Characterization Laboratory (NCL), is a useful resource setting for moving the pre-clinical development of promising nanotechnologies beyond early efficacy investigations. The group has published important review articles on the prospects and translational challenges of nanotechnology in cancer applications.133, 134 In addition, the readers are directed to several other perspective and review articles published on important considerations surrounding the pre-clinical evaluation and clinical translation of nanotechnologies.135–138
We envision the following challenges, and therefore future areas of investigation, which the nanochains will need to surpass in order to see a successful path into clinical translation.
Efficacy testing
Effective systemic treatment of refractory high-grade brain tumors using the nanochains will most likely require delivery of more than one drug. In addition to the bulk of differentiated tumor cells, glioma stem cells, tumor-associated macrophages and tumor-associated endothelial cells are key players when screening drugs with complimentary and synergistic effects. The loading efficiencies of drugs in nanochains, their radiofrequency-triggered spatiotemporal release profiles, in vitro and in vivo stability are a few of the key parameters that will have to be optimized in pre-clinical product development. The selection of drug candidates will also require an understanding of drug’s intra-tumoral transport characteristics so that once triggered-released from the nanochains, they can diffuse and deeply penetrate in order to maximize tumor dose painting. Identifying the optimal radio-frequency (RF) triggered-release parameters (frequency, power, duration) will be an important factor in improving the therapeutic efficacy of nanochains. Optimizing ligand density on nanochains, for maximizing tumor vessel docking, will require an understanding of its impact on pharmacokinetics, vascular targeting and cellular internalization.
Given the lack of efficacy translation from the pre-clinical to the clinical domain, a thorough understanding of the efficacy and limitations of nanochains in a variety of clinically-relevant rodent brain tumors models (both adults and pediatric models) will be an important aspect of pre-clinical development. The pediatric preclinical testing program (PPTP) could provide an excellent resource for pursuing research in this direction.139 Further testing of nanochains in companion dogs with natural spontaneously occurring cancers, which share several human characteristics of cancer initiation and progression,140,141 can greatly bolster the pre-clinical efficacy package. Efficacy testing in companion dogs will, however, require pilot-scale production of nanochains and a scale-up version of the RF hardware.
Scale-up and Manufacturing
The current processes for laboratory-scale fabrication of nanochains involve solid-state chemistry (linking individual iron oxide particles) and bulk nanoparticle production (preparation of liposomal doxorubicin).8 While iron oxide and liposomal nanoparticles have been clinically approved, the chemical bridging of two nanoparticle platforms to generate the multi-component nanochains will present unique manufacturing challenges. Successful scale-up of nanochains will require a detailed understand of each of the components, process parameters and their impact on scalability and reproducibility. Some of the critical aspects of nanochain manufacturing will revolve around their physicochemical properties (structure, nanoparticle sizes and distribution, drug loading efficiency, free drug, number of targeting ligands and their efficacy, stability, sterility, drug triggered-release properties, impurity profiles etc.). The design of experiments (DOE) approach and quality by design (QbD), commonly used in the biopharma industry, could greatly aid in pre-clinical identification of critical parameters and optimization of chemistry and in-process controls that result in reproducibility of final product characteristics.142 Analytical techniques that can accurately and reproducibly characterize nanochain formulations will need to be developed and validated. Sterilization and sterility testing of nanochains will require careful attention due to the challenges with nanoparticles in general.143 While not critical at the early stage of product development, attention will need to be given to the raw materials and their suppliers to ensure availability of bulk and good manufacturing process (GMP)-grade material for use in pivotal safety-toxicology and clinical studies. Finally, scalability and regulatory considerations surrounding the RF hardware, which is an integral component of nanochain efficacy, will need to be understood and addressed.
Safety and Toxicology Testing
The multi-component nature coupled with the targeting mechanism will require a thorough understanding of the pharmacokinetics and biodistribution of nanochains. Pilot pharmacokinetic studies using the targeted nanochains have demonstrated a relatively short-half life compared to non-targeted nanochains. This is likely not to pose concerns from an efficacy perspective since their primary target is the readily accessible tumor vasculature. In fact, the short half-life may potentially diminish the nanochain’s propensity to accumulate at non-target regions, other than liver and spleen. Careful attention will also be required to investigate and address potential nanotechnology-related safety signals, specifically in the area of immunotoxicity studies.133, 144, 145
Ultimately, interdisciplinary collaborations with brain tumor biologists, oncologist, neurosurgeons, engineers and regulatory experts will be a critical factor for nanochain’s successful clinical translation.
Conclusion
Despite decades of pre-clinical and clinical investigations, invasive brain tumors represent the Achilles heel of cancer therapy. However, the last few years have seen unparalleled advances in the field of brain tumor biology and cancer ‘omics’. These advances have opened new doors for nano-scientists to push the field of cancer nanomedicine. Nanochain is a new class of engineered nanoparticles that are being developed for treatment of brain tumors. The nanochains can (1) seek out and rapidly accumulate on the surface of tumor endothelial, via active targeting, and (2) deliver high concentration of therapeutic compounds past the blood tumor barrier and into distant, hard-to-reach tumor cells, by releasing the therapeutic cargo via an externally triggered-release mechanism. Pre-clinical efficacy studies using the nanochains have shown promising results in a variety of aggressive brain tumors and metastatic tumor models, warranting their continued development. By enabling the development of highly effective systemic therapies, the nanochain platform could bring about a paradigm shift in brain cancer treatment.
Acknowledgments
This work was partially supported by grants from the National Cancer Institute (U01CA198892, R01CA177716) and Prayers from Maria Children’s Glioma Foundation.
Contributor Information
Efstathios Karathanasis, Email: stathis@case.edu, Department of Biomedical Engineering and Department of Radiology, Case Comprehensive Cancer Center, Case Western Reserve University, Cleveland, Ohio, USA.
Ketan B. Ghaghada, Email: kbghagha@texaschildrens.org, Edward B. Singleton Department of Pediatric Radiology, Texas Children’s Hospital, Department of Radiology, Baylor College of Medicine, Houston, Texas, USA
References
- 1.Black KL, Pikul BK. Gliomas--past, present, and future. Clin Neurosurg. 1999;45:160–163. [PubMed] [Google Scholar]
- 2.Juratli TA, Schackert G, Krex D. Current status of local therapy in malignant gliomas--a clinical review of three selected approaches. Pharmacol Ther. 2013;139:341–358. doi: 10.1016/j.pharmthera.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 3.Adamson C, Kanu OO, Mehta AI, Di C, Lin N, Mattox AK, Bigner DD. Glioblastoma multiforme: a review of where we have been and where we are going. Expert Opin Investig Drugs. 2009;18:1061–1083. doi: 10.1517/13543780903052764. [DOI] [PubMed] [Google Scholar]
- 4.Koukourakis MI, Koukouraki SIF, Kelekis N, Kyrias GSA, Karkavitsas N. High intratumoural accumulation of stealth liposomal doxorubicin (Caelyx) in glioblastomas and in metastatic brain tumours. Br J Cancer. 2000;83:1281–1286. doi: 10.1054/bjoc.2000.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hobbs SK, Monsky WL, Yuan F, Roberts WG, Griffith L, Torchilin VP, Jain RK. Regulation of transport pathways in tumor vessels: role of tumor type and microenvironment. Proc Natl Acad Sci U S A. 1998;95:4607–4612. doi: 10.1073/pnas.95.8.4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baumann BC, Kao GD, Mahmud A, Harada T, Swift J, Chapman C, Xu X, Discher DE, Dorsey JF. Enhancing the efficacy of drug-loaded nanocarriers against brain tumors by targeted radiation therapy. Oncotarget. 2013;4:64–79. doi: 10.18632/oncotarget.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peiris PM, Schmidt E, Calabrese M, Karathanasis E. Assembly of linear nano-chains from iron oxide nanospheres with asymmetric surface chemistry. PLoS One. 2011;6:e15927. doi: 10.1371/journal.pone.0015927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peiris PM, Bauer L, Toy R, Tran E, Pansky J, Doolittle E, Schmidt E, Hayden E, Mayer A, Keri RA, et al. Enhanced Delivery of Chemotherapy to Tumors Using a Multicomponent Nanochain with Radio-Frequency-Tunable Drug Release. ACS Nano. 2012;6:4157–4168. doi: 10.1021/nn300652p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peiris PM, Toy R, Doolittle E, Pansky J, Abramowski A, Tam M, Vicente P, Tran E, Hayden E, Camann A, et al. Imaging metastasis using an integrin-targeting chain-shaped nanoparticle. ACS Nano. 2012;6:8783–8795. doi: 10.1021/nn303833p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peiris PM, Tam M, Vicente P, Abramowski A, Toy R, Bauer L, Mayer A, Pansky J, Doolittle E, Tucci S, et al. On-command drug release from nanochains inhibits growth of breast tumors. Pharm Res. 2014;31:1460–1468. doi: 10.1007/s11095-013-1102-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peiris PM, Toy R, Abramowski A, Vicente P, Tucci S, Bauer L, Mayer A, Tam M, Doolittle E, Pansky J, et al. Treatment of cancer micrometastasis using a multicomponent chain-like nanoparticle. J Control Release. 2014;173:51–58. doi: 10.1016/j.jconrel.2013.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peiris PM, Abramowski A, Mcginnity J, Doolittle E, Toy R, Gopalakrishnan R, Shah S, Bauer L, Ghaghada KB, Hoimes C, et al. Treatment of invasive brain tumors using a chain-like nanoparticle. Cancer Research. 2015;75:1356–1365. doi: 10.1158/0008-5472.CAN-14-1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jain RK, di Tomaso E, Duda DG, Loeffler JS, Sorensen AG, Batchelor TT. Angiogenesis in brain tumours. Nat Rev Neurosci. 2007;8:610–622. doi: 10.1038/nrn2175. [DOI] [PubMed] [Google Scholar]
- 14.Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 15.Gilbertson RJ, Rich JN. Making a tumour’s bed: glioblastoma stem cells and the vascular niche. Nat Rev Cancer. 2007;7:733–736. doi: 10.1038/nrc2246. [DOI] [PubMed] [Google Scholar]
- 16.Huse JT, Holland EC. Targeting brain cancer: advances in the molecular pathology of malignant glioma and medulloblastoma. Nat Rev Cancer. 2010;10:319–331. doi: 10.1038/nrc2818. [DOI] [PubMed] [Google Scholar]
- 17.Fortin D. The blood-brain barrier: its influence in the treatment of brain tumors metastases. Curr Cancer Drug Targets. 2012;12:247–259. doi: 10.2174/156800912799277511. [DOI] [PubMed] [Google Scholar]
- 18.Liebner S, Fischmann A, Rascher G, Duffner F, Grote EH, Kalbacher H, Wolburg H. Claudin-1 and claudin-5 expression and tight junction morphology are altered in blood vessels of human glioblastoma multiforme. Acta Neuropathol. 2000;100:323–331. doi: 10.1007/s004010000180. [DOI] [PubMed] [Google Scholar]
- 19.Lockman PR, Mittapalli RK, Taskar KS, Rudraraju V, Gril B, Bohn KA, Adkins CE, Roberts A, Thorsheim HR, Gaasch JA, et al. Heterogeneous blood-tumor barrier permeability determines drug efficacy in experimental brain metastases of breast cancer. Clin Cancer Res. 2010;16:5664–5678. doi: 10.1158/1078-0432.CCR-10-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boyle FM, Eller SL, Grossman SA. Penetration of intra-arterially administered vincristine in experimental brain tumor. Neuro Oncol. 2004;6:300–305. doi: 10.1215/S1152851703000516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blanchette M, Fortin D. Blood-brain barrier disruption in the treatment of brain tumors. Methods Mol Biol. 2011;686:447–463. doi: 10.1007/978-1-60761-938-3_23. [DOI] [PubMed] [Google Scholar]
- 22.Sato S, Kawase T, Harada S, Takayama H, Suga S. Effect of hyperosmotic solutions on human brain tumour vasculature. Acta Neurochir (Wien) 1998;140:1135–1141. doi: 10.1007/s007010050227. disc 1141–1132. [DOI] [PubMed] [Google Scholar]
- 23.Ohnishi T, Tamai I, Sakanaka K, Sakata A, Yamashima T, Yamashita J, Tsuji A. In vivo and in vitro evidence for ATP-dependency of P-glycoprotein-mediated efflux of doxorubicin at the blood-brain barrier. Biochem Pharmacol. 1995;49:1541–1544. doi: 10.1016/0006-2952(95)00082-b. [DOI] [PubMed] [Google Scholar]
- 24.Mankhetkorn S, Dubru F, Hesschenbrouck J, Fiallo M, Garnier-Suillerot A. Relation among the resistance factor, kinetics of uptake, and kinetics of the P-glycoprotein-mediated efflux of doxorubicin, daunorubicin, 8-(S)-fluoroidarubicin, and idarubicin in multidrug-resistant K562 cells. Mol Pharmacol. 1996;49:532–539. [PubMed] [Google Scholar]
- 25.Rousselle C, Clair P, Lefauconnier JM, Kaczorek M, Scherrmann JM, Temsamani J. New advances in the transport of doxorubicin through the blood-brain barrier by a peptide vector-mediated strategy. Mol Pharmacol. 2000;57:679–686. doi: 10.1124/mol.57.4.679. [DOI] [PubMed] [Google Scholar]
- 26.de Vries NA, Beijnen JH, Boogerd W, van Tellingen O. Blood-brain barrier and chemotherapeutic treatment of brain tumors. Expert Rev Neurother. 2006;6:1199–1209. doi: 10.1586/14737175.6.8.1199. [DOI] [PubMed] [Google Scholar]
- 27.Iacob G, Dinca EB. Current data and strategy in glioblastoma multiforme. J Med Life. 2009;2:386–393. [PMC free article] [PubMed] [Google Scholar]
- 28.Hemmati HD, Nakano I, Lazareff JA, Masterman-Smith M, Geschwind DH, Bronner-Fraser M, Kornblum HI. Cancerous stem cells can arise from pediatric brain tumors. Proc Natl Acad Sci U S A. 2003;100:15178–15183. doi: 10.1073/pnas.2036535100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–5828. [PubMed] [Google Scholar]
- 30.Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 31.Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 32.Cheng L, Wu Q, Huang Z, Guryanova OA, Huang Q, Shou W, Rich JN, Bao S. L1CAM regulates DNA damage checkpoint response of glioblastoma stem cells through NBS1. EMBO J. 2011;30:800–813. doi: 10.1038/emboj.2011.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheng L, Bao S, Rich JN. Potential therapeutic implications of cancer stem cells in glioblastoma. Biochem Pharmacol. 2010;80:654–665. doi: 10.1016/j.bcp.2010.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu G, Yuan X, Zeng Z, Tunici P, Ng H, Abdulkadir IR, Lu L, Irvin D, Black KL, Yu JS. Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol Cancer. 2006;5:67. doi: 10.1186/1476-4598-5-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, Oh EY, Gaber MW, Finklestein D, Allen M, et al. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11:69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 36.Li Z, Bao S, Wu Q, Wang H, Eyler C, Sathornsumetee S, Shi Q, Cao Y, Lathia J, McLendon RE, et al. Hypoxia-inducible factors regulate tumorigenic capacity of glioma stem cells. Cancer Cell. 2009;15:501–513. doi: 10.1016/j.ccr.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Christensen K, Schroder HD, Kristensen BW. CD133+ niches and single cells in glioblastoma have different phenotypes. J Neurooncol. 2011;104:129–143. doi: 10.1007/s11060-010-0488-y. [DOI] [PubMed] [Google Scholar]
- 38.Bao S, Wu Q, Sathornsumetee S, Hao Y, Li Z, Hjelmeland AB, Shi Q, McLendon RE, Bigner DD, Rich JN. Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res. 2006;66:7843–7848. doi: 10.1158/0008-5472.CAN-06-1010. [DOI] [PubMed] [Google Scholar]
- 39.Heddleston JM, Li Z, McLendon RE, Hjelmeland AB, Rich JN. The hypoxic microenvironment maintains glioblastoma stem cells and promotes reprogramming towards a cancer stem cell phenotype. Cell Cycle. 2009;8:3274–3284. doi: 10.4161/cc.8.20.9701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Soeda A, Park M, Lee D, Mintz A, Androutsellis-Theotokis A, McKay RD, Engh J, Iwama T, Kunisada T, Kassam AB, et al. Hypoxia promotes expansion of the CD133-positive glioma stem cells through activation of HIF-1alpha. Oncogene. 2009;28:3949–3959. doi: 10.1038/onc.2009.252. [DOI] [PubMed] [Google Scholar]
- 41.Lathia JD, Gallagher J, Heddleston JM, Wang J, Eyler CE, Macswords J, Wu Q, Vasanji A, McLendon RE, Hjelmeland AB, et al. Integrin alpha 6 regulates glioblastoma stem cells. Cell Stem Cell. 2010;6:421–432. doi: 10.1016/j.stem.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gangemi RM, Griffero F, Marubbi D, Perera M, Capra MC, Malatesta P, Ravetti GL, Zona GL, Daga A, Corte G. SOX2 silencing in glioblastoma tumor-initiating cells causes stop of proliferation and loss of tumorigenicity. Stem Cells. 2009;27:40–48. doi: 10.1634/stemcells.2008-0493. [DOI] [PubMed] [Google Scholar]
- 43.Maeda H. Toward a full understanding of the EPR effect in primary and metastatic tumors as well as issues related to its heterogeneity. Adv Drug Deliv Rev. 2015;91:3–6. doi: 10.1016/j.addr.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 44.Koukourakis MI, Koukouraki S, Fezoulidis I, Kelekis N, Kyrias G, Archimandritis S, Karkavitsas N. High intratumoural accumulation of stealth liposomal doxorubicin (Caelyx) in glioblastomas and in metastatic brain tumours. Br J Cancer. 2000;83:1281–1286. doi: 10.1054/bjoc.2000.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bertrand N, Wu J, Xu X, Kamaly N, Farokhzad OC. Cancer nanotechnology: the impact of passive and active targeting in the era of modern cancer biology. Adv Drug Deliv Rev. 2014;66:2–25. doi: 10.1016/j.addr.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prabhakar U, Maeda H, Jain RK, Sevick-Muraca EM, Zamboni W, Farokhzad OC, Barry ST, Gabizon A, Grodzinski P, Blakey DC. Challenges and key considerations of the enhanced permeability and retention effect for nanomedicine drug delivery in oncology. Cancer Res. 2013;73:2412–2417. doi: 10.1158/0008-5472.CAN-12-4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fidler IJ. The Biology of Brain Metastasis: Challenges for Therapy. Cancer J. 2015;21:284–293. doi: 10.1097/PPO.0000000000000126. [DOI] [PubMed] [Google Scholar]
- 48.Gerstner ER, Fine RL. Increased permeability of the blood-brain barrier to chemotherapy in metastatic brain tumors: establishing a treatment paradigm. J Clin Oncol. 2007;25:2306–2312. doi: 10.1200/JCO.2006.10.0677. [DOI] [PubMed] [Google Scholar]
- 49.Kim SS, Harford JB, Pirollo KF, Chang EH. Effective treatment of glioblastoma requires crossing the blood-brain barrier and targeting tumors including cancer stem cells: The promise of nanomedicine. Biochem Biophys Res Commun. 2015 doi: 10.1016/j.bbrc.2015.06.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Claes A, Idema AJ, Wesseling P. Diffuse glioma growth: a guerilla war. Acta Neuropathol. 2007;114:443–458. doi: 10.1007/s00401-007-0293-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bellavance MA, Blanchette M, Fortin D. Recent advances in blood-brain barrier disruption as a CNS delivery strategy. AAPS J. 2008;10:166–177. doi: 10.1208/s12248-008-9018-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Doolittle ND, Muldoon LL, Culp AY, Neuwelt EA. Delivery of chemotherapeutics across the blood-brain barrier: challenges and advances. Adv Pharmacol. 2014;71:203–243. doi: 10.1016/bs.apha.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aryal M, Arvanitis CD, Alexander PM, McDannold N. Ultrasound-mediated blood-brain barrier disruption for targeted drug delivery in the central nervous system. Adv Drug Deliv Rev. 2014;72:94–109. doi: 10.1016/j.addr.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Verma J, Lal S, Van Noorden CJ. Nanoparticles for hyperthermic therapy: synthesis strategies and applications in glioblastoma. Int J Nanomedicine. 2014;9:2863–2877. doi: 10.2147/IJN.S57501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Allard E, Passirani C, Benoit JP. Convection-enhanced delivery of nanocarriers for the treatment of brain tumors. Biomaterials. 2009;30:2302–2318. doi: 10.1016/j.biomaterials.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 56.Chen PY, Ozawa T, Drummond DC, Kalra A, Fitzgerald JB, Kirpotin DB, Wei KC, Butowski N, Prados MD, Berger MS, et al. Comparing routes of delivery for nanoliposomal irinotecan shows superior anti-tumor activity of local administration in treating intracranial glioblastoma xenografts. Neuro Oncol. 2013;15:189–197. doi: 10.1093/neuonc/nos305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tortorella S, Karagiannis TC. The significance of transferrin receptors in oncology: the development of functional nano-based drug delivery systems. Curr Drug Deliv. 2014;11:427–443. doi: 10.2174/1567201810666140106115436. [DOI] [PubMed] [Google Scholar]
- 58.Wiley DT, Webster P, Gale A, Davis ME. Transcytosis and brain uptake of transferrin-containing nanoparticles by tuning avidity to transferrin receptor. Proc Natl Acad Sci U S A. 2013;110:8662–8667. doi: 10.1073/pnas.1307152110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ulbrich K, Knobloch T, Kreuter J. Targeting the insulin receptor: nanoparticles for drug delivery across the blood-brain barrier (BBB) J Drug Target. 2011;19:125–132. doi: 10.3109/10611861003734001. [DOI] [PubMed] [Google Scholar]
- 60.Gu G, Gao X, Hu Q, Kang T, Liu Z, Jiang M, Miao D, Song Q, Yao L, Tu Y, et al. The influence of the penetrating peptide iRGD on the effect of paclitaxel-loaded MT1-AF7p-conjugated nanoparticles on glioma cells. Biomaterials. 2013;34:5138–5148. doi: 10.1016/j.biomaterials.2013.03.036. [DOI] [PubMed] [Google Scholar]
- 61.Yan H, Wang L, Wang J, Weng X, Lei H, Wang X, Jiang L, Zhu J, Lu W, Wei X, et al. Two-order targeted brain tumor imaging by using an optical/paramagnetic nanoprobe across the blood brain barrier. ACS Nano. 2012;6:410–420. doi: 10.1021/nn203749v. [DOI] [PubMed] [Google Scholar]
- 62.Yang ZZ, Li JQ, Wang ZZ, Dong DW, Qi XR. Tumor-targeting dual peptides-modified cationic liposomes for delivery of siRNA and docetaxel to gliomas. Biomaterials. 2014;35:5226–5239. doi: 10.1016/j.biomaterials.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 63.Gao X, Qian J, Zheng S, Xiong Y, Man J, Cao B, Wang L, Ju S, Li C. Up-regulating blood brain barrier permeability of nanoparticles via multivalent effect. Pharm Res. 2013;30:2538–2548. doi: 10.1007/s11095-013-1004-9. [DOI] [PubMed] [Google Scholar]
- 64.Kim SS, Rait A, Kim E, DeMarco J, Pirollo KF, Chang EH. Encapsulation of temozolomide in a tumor-targeting nanocomplex enhances anti-cancer efficacy and reduces toxicity in a mouse model of glioblastoma. Cancer Lett. 2015;369:250–258. doi: 10.1016/j.canlet.2015.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cheng Y, Dai Q, Morshed RA, Fan X, Wegscheid ML, Wainwright DA, Han Y, Zhang L, Auffinger B, Tobias AL, et al. Blood-brain barrier permeable gold nanoparticles: an efficient delivery platform for enhanced malignant glioma therapy and imaging. Small. 2014;10:5137–5150. doi: 10.1002/smll.201400654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li J, Cai P, Shalviri A, Henderson JT, He C, Foltz WD, Prasad P, Brodersen PM, Chen Y, DaCosta R, et al. A multifunctional polymeric nanotheranostic system delivers doxorubicin and imaging agents across the blood-brain barrier targeting brain metastases of breast cancer. ACS Nano. 2014;8:9925–9940. doi: 10.1021/nn501069c. [DOI] [PubMed] [Google Scholar]
- 67.Gao H, Yang Z, Cao S, Xiong Y, Zhang S, Pang Z, Jiang X. Tumor cells and neovasculature dual targeting delivery for glioblastoma treatment. Biomaterials. 2014;35:2374–2382. doi: 10.1016/j.biomaterials.2013.11.076. [DOI] [PubMed] [Google Scholar]
- 68.Beduneau A, Saulnier P, Benoit JP. Active targeting of brain tumors using nanocarriers. Biomaterials. 2007;28:4947–4967. doi: 10.1016/j.biomaterials.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 69.Cheng Y, Morshed RA, Auffinger B, Tobias AL, Lesniak MS. Multifunctional nanoparticles for brain tumor imaging and therapy. Adv Drug Deliv Rev. 2014;66:42–57. doi: 10.1016/j.addr.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Meyers JD, Doane T, Burda C, Basilion JP. Nanoparticles for imaging and treating brain cancer. Nanomedicine (Lond) 2013;8:123–143. doi: 10.2217/nnm.12.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nance EA, Woodworth GF, Sailor KA, Shih TY, Xu Q, Swaminathan G, Xiang D, Eberhart C, Hanes J. A dense poly(ethylene glycol) coating improves penetration of large polymeric nanoparticles within brain tissue. Sci Transl Med. 2012;4:149ra119. doi: 10.1126/scitranslmed.3003594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wankhede M, Bouras A, Kaluzova M, Hadjipanayis CG. Magnetic nanoparticles: an emerging technology for malignant brain tumor imaging and therapy. Expert Rev Clin Pharmacol. 2012;5:173–186. doi: 10.1586/ecp.12.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tomanek B, Iqbal U, Blasiak B, Abulrob A, Albaghdadi H, Matyas JR, Ponjevic D, Sutherland GR. Evaluation of brain tumor vessels specific contrast agents for glioblastoma imaging. Neuro Oncol. 2012;14:53–63. doi: 10.1093/neuonc/nor183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Reddy GR, Bhojani MS, McConville P, Moody J, Moffat BA, Hall DE, Kim G, Koo YE, Woolliscroft MJ, Sugai JV, et al. Vascular targeted nanoparticles for imaging and treatment of brain tumors. Clin Cancer Res. 2006;12:6677–6686. doi: 10.1158/1078-0432.CCR-06-0946. [DOI] [PubMed] [Google Scholar]
- 75.Agemy L, Kotamraju VR, Friedmann-Morvinski D, Sharma S, Sugahara KN, Ruoslahti E. Proapoptotic peptide-mediated cancer therapy targeted to cell surface p32. Mol Ther. 2013;21:2195–2204. doi: 10.1038/mt.2013.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hadjipanayis CG, Machaidze R, Kaluzova M, Wang L, Schuette AJ, Chen H, Wu X, Mao H. EGFRvIII antibody-conjugated iron oxide nanoparticles for magnetic resonance imaging-guided convection-enhanced delivery and targeted therapy of glioblastoma. Cancer Res. 2010;70:6303–6312. doi: 10.1158/0008-5472.CAN-10-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kaluzova M, Bouras A, Machaidze R, Hadjipanayis CG. Targeted therapy of glioblastoma stem-like cells and tumor non-stem cells using cetuximab-conjugated iron-oxide nanoparticles. Oncotarget. 2015;6:8788–8806. doi: 10.18632/oncotarget.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fang JH, Lai YH, Chiu TL, Chen YY, Hu SH, Chen SY. Magnetic core-shell nanocapsules with dual-targeting capabilities and co-delivery of multiple drugs to treat brain gliomas. Adv Healthc Mater. 2014;3:1250–1260. doi: 10.1002/adhm.201300598. [DOI] [PubMed] [Google Scholar]
- 79.Thiesen B, Jordan A. Clinical applications of magnetic nanoparticles for hyperthermia. Int J Hyperthermia. 2008;24:467–474. doi: 10.1080/02656730802104757. [DOI] [PubMed] [Google Scholar]
- 80.Drummond DC, Noble CO, Guo Z, Hong K, Park JW, Kirpotin DB. Development of a highly active nanoliposomal irinotecan using a novel intraliposomal stabilization strategy. Cancer Res. 2006;66:3271–3277. doi: 10.1158/0008-5472.CAN-05-4007. [DOI] [PubMed] [Google Scholar]
- 81.Krauze MT, Noble CO, Kawaguchi T, Drummond D, Kirpotin DB, Yamashita Y, Kullberg E, Forsayeth J, Park JW, Bankiewicz KS. Convection-enhanced delivery of nanoliposomal CPT-11 (irinotecan) and PEGylated liposomal doxorubicin (Doxil) in rodent intracranial brain tumor xenografts. Neuro Oncol. 2007;9:393–403. doi: 10.1215/15228517-2007-019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Noble CO, Krauze MT, Drummond DC, Yamashita Y, Saito R, Berger MS, Kirpotin DB, Bankiewicz KS, Park JW. Novel nanoliposomal CPT-11 infused by convection-enhanced delivery in intracranial tumors: pharmacology and efficacy. Cancer Res. 2006;66:2801–2806. doi: 10.1158/0008-5472.CAN-05-3535. [DOI] [PubMed] [Google Scholar]
- 83.Huang X, El-Sayed IH, Qian W, El-Sayed MA. Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods. JACS. 2006;128:2215–2120. doi: 10.1021/ja057254a. [DOI] [PubMed] [Google Scholar]
- 84.Sardar R, Shumaker-Parry JS. Asymmetrically functionalized gold nanoparticles organized in one-dimensional chains. Nano Letters. 2008;8:731–736. doi: 10.1021/nl073154m. [DOI] [PubMed] [Google Scholar]
- 85.Park JH, von Maltzahn G, Zhang L, Derfus AM, Simberg D, Harris TJ, Ruoslahti E, Bhatia SN, Sailor MJ. Systematic surface engineering of magnetic nanoworms for in vivo tumor targeting. Small. 2009;5:694–700. doi: 10.1002/smll.200801789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Park JH, von Maltzahn G, Zhang LL, Schwartz MP, Ruoslahti E, Bhatia SN, Sailor MJ. Magnetic iron oxide nanoworms for tumor targeting and imaging. Advanced Materials. 2008;20:1630–1635. doi: 10.1002/adma.200800004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dai Q, Worden JG, Trullinger J, Huo Q. A “nanonecklace” synthesized from monofunctionalized gold nanoparticles. J Am Chem Soc. 2005;127:8008–8009. doi: 10.1021/ja042610v. [DOI] [PubMed] [Google Scholar]
- 88.Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release. 2000;65:271–284. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 89.Park K. Questions on the role of the EPR effect in tumor targeting. J Control Release. 2013;172:391. doi: 10.1016/j.jconrel.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 90.Dreher MR, Liu W, Michelich CR, Dewhirst MW, Yuan F, Chilkoti A. Tumor vascular permeability, accumulation, and penetration of macromolecular drug carriers. Journal of the National Cancer Institute. 2006;98:335–344. doi: 10.1093/jnci/djj070. [DOI] [PubMed] [Google Scholar]
- 91.Pluen A, Boucher Y, Ramanujan S, McKee TD, Gohongi T, di Tomaso E, Brown EB, Izumi Y, Campbell RB, Berk DA, et al. Role of tumor-host interactions in interstitial diffusion of macromolecules: cranial vs. subcutaneous tumors. Proc Natl Acad Sci U S A. 2001;98:4628–4633. doi: 10.1073/pnas.081626898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gabizon A, Horowitz AT, Goren D, Tzemach D, Shmeeda H, Zalipsky S. In vivo fate of folate-targeted polyethylene-glycol liposomes in tumor-bearing mice. Clinical Cancer Research. 2003;9:6551–6559. [PubMed] [Google Scholar]
- 93.Huang X, Peng X, Wang Y, Shin DM, El-Sayed MA, Nie S. A reexamination of active and passive tumor targeting by using rod-shaped gold nanocrystals and covalently conjugated peptide ligands. ACS Nano. 2010;4:5887–5896. doi: 10.1021/nn102055s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gabizon A, Shmeeda H, Horowitz AT, Zalipsky S. Tumor cell targeting of liposome-entrapped drugs with phospholipid-anchored folic acid-PEG conjugates. Adv Drug Deliv Rev. 2004;56:1177–1192. doi: 10.1016/j.addr.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 95.Park JW, Kirpotin DB, Hong K, Shalaby R, Shao Y, Nielsen UB, Marks JD, Papahadjopoulos D, Benz CC. Tumor targeting using anti-her2 immunoliposomes. J Control Release. 2001;74:95–113. doi: 10.1016/s0168-3659(01)00315-7. [DOI] [PubMed] [Google Scholar]
- 96.Peiris PM, Deb P, Doolittle E, Doron G, Goldberg A, Govender P, Shah S, Rao S, Carbone S, Cotey T, et al. Vascular Targeting of a Gold Nanoparticle to Breast Cancer Metastasis. J Pharm Sci. 2015;104:2600–2610. doi: 10.1002/jps.24518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Terasaki T, Iga T, Sugiyama Y, Sawada Y, Hanano M. Nuclear binding as a determinant of tissue distribution of adriamycin, daunomycin, adriamycinol, daunorubicinol and actinomycin D. J Pharmacobiodyn. 1984;7:269–277. doi: 10.1248/bpb1978.7.269. [DOI] [PubMed] [Google Scholar]
- 98.Marafino BJ, Jr, Giri SN, Siegel DM. Pharmacokinetics, covalent binding and subcellular distribution of [3H]doxorubicin after intravenous administration in the mouse. J Pharmacol Exp Ther. 1981;216:55–61. [PubMed] [Google Scholar]
- 99.Laginha KM, Verwoert S, Charrois GJ, Allen TM. Determination of doxorubicin levels in whole tumor and tumor nuclei in murine breast cancer tumors. Clin Cancer Res. 2005;11:6944–6949. doi: 10.1158/1078-0432.CCR-05-0343. [DOI] [PubMed] [Google Scholar]
- 100.Burden-Gulley SM, Qutaish MQ, Sullivant KE, Lu H, Wang J, Craig SE, Basilion JP, Wilson DL, Brady-Kalnay SM. Novel cryo-imaging of the glioma tumor microenvironment reveals migration and dispersal pathways in vivid three-dimensional detail. Cancer Res. 2011;71:5932–5940. doi: 10.1158/0008-5472.CAN-11-1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Furnari FB, Fenton T, Bachoo RM, Mukasa A, Stommel JM, Stegh A, Hahn WC, Ligon KL, Louis DN, Brennan C, et al. Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev. 2007;21:2683–2710. doi: 10.1101/gad.1596707. [DOI] [PubMed] [Google Scholar]
- 102.Zagzag D, Esencay M, Mendez O, Yee H, Smirnova I, Huang Y, Chiriboga L, Lukyanov E, Liu M, Newcomb EW. Hypoxia- and vascular endothelial growth factor-induced stromal cell-derived factor-1alpha/CXCR4 expression in glioblastomas: one plausible explanation of Scherer’s structures. Am J Pathol. 2008;173:545–560. doi: 10.2353/ajpath.2008.071197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kislin KL, McDonough WS, Eschbacher JM, Armstrong BA, Berens ME. NHERF-1: modulator of glioblastoma cell migration and invasion. Neoplasia. 2009;11:377–387. doi: 10.1593/neo.81572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nakada M, Niska JA, Miyamori H, McDonough WS, Wu J, Sato H, Berens ME. The phosphorylation of EphB2 receptor regulates migration and invasion of human glioma cells. Cancer Res. 2004;64:3179–3185. doi: 10.1158/0008-5472.can-03-3667. [DOI] [PubMed] [Google Scholar]
- 105.Nakada M, Drake KL, Nakada S, Niska JA, Berens ME. Ephrin-B3 ligand promotes glioma invasion through activation of Rac1. Cancer Res. 2006;66:8492–8500. doi: 10.1158/0008-5472.CAN-05-4211. [DOI] [PubMed] [Google Scholar]
- 106.Brazel CS. Magnetothermally-responsive nanomaterials: combining magnetic nanostructures and thermally-sensitive polymers for triggered drug release. Pharm Res. 2009;26:644–656. doi: 10.1007/s11095-008-9773-2. [DOI] [PubMed] [Google Scholar]
- 107.Gay LJ, Felding-Habermann B. Contribution of platelets to tumour metastasis. Nat Rev Cancer. 2011;11:123–134. doi: 10.1038/nrc3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Felding-Habermann B, Habermann R, Saldivar E, Ruggeri ZM. Role of beta3 integrins in melanoma cell adhesion to activated platelets under flow. J Biol Chem. 1996;271:5892–5900. doi: 10.1074/jbc.271.10.5892. [DOI] [PubMed] [Google Scholar]
- 109.McCarty OJ, Mousa SA, Bray PF, Konstantopoulos K. Immobilized platelets support human colon carcinoma cell tethering, rolling, and firm adhesion under dynamic flow conditions. Blood. 2000;96:1789–1797. [PubMed] [Google Scholar]
- 110.Arnaout MA, Mahalingam B, Xiong JP. Integrin structure, allostery, and bidirectional signaling. Annu Rev Cell Dev Biol. 2005;21:381–410. doi: 10.1146/annurev.cellbio.21.090704.151217. [DOI] [PubMed] [Google Scholar]
- 111.Felding-Habermann B, O’Toole TE, Smith JW, Fransvea E, Ruggeri ZM, Ginsberg MH, Hughes PE, Pampori N, Shattil SJ, Saven A, et al. Integrin activation controls metastasis in human breast cancer. Proc Natl Acad Sci U S A. 2001;98:1853–1858. doi: 10.1073/pnas.98.4.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lorger M, Krueger JS, O’Neal M, Staflin K, Felding-Habermann B. Activation of tumor cell integrin alphavbeta3 controls angiogenesis and metastatic growth in the brain. Proc Natl Acad Sci U S A. 2009;106:10666–10671. doi: 10.1073/pnas.0903035106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10:9–22. doi: 10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Schnell O, Krebs B, Carlsen J, Miederer I, Goetz C, Goldbrunner RH, Wester HJ, Haubner R, Popperl G, Holtmannspotter M, et al. Imaging of integrin alpha(v)beta(3) expression in patients with malignant glioma by [18F] Galacto-RGD positron emission tomography. Neuro Oncol. 2009;11:861–870. doi: 10.1215/15228517-2009-024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Reardon DA, Nabors LB, Stupp R, Mikkelsen T. Cilengitide: an integrin-targeting arginine-glycine-aspartic acid peptide with promising activity for glioblastoma multiforme. Expert Opin Investig Drugs. 2008;17:1225–1235. doi: 10.1517/13543784.17.8.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Danhier F, Vroman B, Lecouturier N, Crokart N, Pourcelle V, Freichels H, Jerome C, Marchand-Brynaert J, Feron O, Preat V. Targeting of tumor endothelium by RGD-grafted PLGA-nanoparticles loaded with paclitaxel. J Control Release. 2009;140:166–173. doi: 10.1016/j.jconrel.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 117.Bello L, Francolini M, Marthyn P, Zhang J, Carroll RS, Nikas DC, Strasser JF, Villani R, Cheresh DA, Black PM. Alpha(v)beta3 and alpha(v)beta5 integrin expression in glioma periphery. Neurosurgery. 2001;49:380–389. doi: 10.1097/00006123-200108000-00022. discussion 390. [DOI] [PubMed] [Google Scholar]
- 118.Phuphanich S, Brat DJ, Olson JJ. Delivery systems and molecular targets of mechanism-based therapies for GBM. Expert Rev Neurother. 2004;4:649–663. doi: 10.1586/14737175.4.4.649. [DOI] [PubMed] [Google Scholar]
- 119.Brooks PC, Clark RA, Cheresh DA. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science. 1994;264:569–571. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- 120.Brooks PC, Stromblad S, Klemke R, Visscher D, Sarkar FH, Cheresh DA. Antiintegrin alpha v beta 3 blocks human breast cancer growth and angiogenesis in human skin. J Clin Invest. 1995;96:1815–1822. doi: 10.1172/JCI118227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Saul JM, Annapragada AV, Bellamkonda RV. A dual-ligand approach for enhancing targeting selectivity of therapeutic nanocarriers. J Control Release. 2006;114:277–287. doi: 10.1016/j.jconrel.2006.05.028. [DOI] [PubMed] [Google Scholar]
- 122.Takara K, Hatakeyama H, Kibria G, Ohga N, Hida K, Harashima H. Size-controlled, dual-ligand modified liposomes that target the tumor vasculature show promise for use in drug-resistant cancer therapy. Journal of Controlled Release. 2012;162:225–232. doi: 10.1016/j.jconrel.2012.06.019. [DOI] [PubMed] [Google Scholar]
- 123.Cheng CJ, Saltzman WM. Enhanced siRNA delivery into cells by exploiting the synergy between targeting ligands and cell-penetrating peptides. Biomaterials. 2011;32:6194–6203. doi: 10.1016/j.biomaterials.2011.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gao H, Xiong Y, Zhang S, Yang Z, Cao S, Jiang X. RGD and interleukin-13 peptide functionalized nanoparticles for enhanced glioblastoma cells and neovasculature dual targeting delivery and elevated tumor penetration. Mol Pharm. 2014;11:1042–1052. doi: 10.1021/mp400751g. [DOI] [PubMed] [Google Scholar]
- 125.Kluza E, Jacobs I, Hectors SJ, Mayo KH, Griffioen AW, Strijkers GJ, Nicolay K. Dual-targeting of alphavbeta3 and galectin-1 improves the specificity of paramagnetic/fluorescent liposomes to tumor endothelium in vivo. J Control Release. 2012;158:207–214. doi: 10.1016/j.jconrel.2011.10.032. [DOI] [PubMed] [Google Scholar]
- 126.Doolittle E, Peiris PM, Doron G, Goldberg A, Tucci S, Rao S, Shah S, Sylvestre M, Govender P, Turan O, et al. Spatiotemporal Targeting of a Dual-Ligand Nanoparticle to Cancer Metastasis. ACS Nano. 2015;9:8012–8021. doi: 10.1021/acsnano.5b01552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cheng Z, Al Zaki A, Hui JZ, Muzykantov VR, Tsourkas A. Multifunctional nanoparticles: cost versus benefit of adding targeting and imaging capabilities. Science. 2012;338:903–910. doi: 10.1126/science.1226338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Toy R, Bauer L, Hoimes C, Ghaghada KB, Karathanasis E. Targeted nanotechnology for cancer imaging. Adv Drug Deliv Rev. 2014;76:79–97. doi: 10.1016/j.addr.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Guryanova OA, Wu Q, Cheng L, Lathia JD, Huang Z, Yang J, MacSwords J, Eyler CE, McLendon RE, Heddleston JM, et al. Nonreceptor tyrosine kinase BMX maintains self-renewal and tumorigenic potential of glioblastoma stem cells by activating STAT3. Cancer Cell. 2011;19:498–511. doi: 10.1016/j.ccr.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Eyler CE, Wu Q, Yan K, MacSwords JM, Chandler-Militello D, Misuraca KL, Lathia JD, Forrester MT, Lee J, Stamler JS, et al. Glioma stem cell proliferation and tumor growth are promoted by nitric oxide synthase-2. Cell. 2011;146:53–66. doi: 10.1016/j.cell.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gubernator J. Active methods of drug loading into liposomes: recent strategies for stable drug entrapment and increased in vivo activity. Expert Opin Drug Deliv. 2011;8:565–580. doi: 10.1517/17425247.2011.566552. [DOI] [PubMed] [Google Scholar]
- 132.Norvaisas P, Ziemys A. The role of payload hydrophobicity in nanotherapeutic pharmacokinetics. J Pharm Sci. 2014;103:2147–2156. doi: 10.1002/jps.23996. [DOI] [PubMed] [Google Scholar]
- 133.Crist RM, Grossman JH, Patri AK, Stern ST, Dobrovolskaia MA, Adiseshaiah PP, Clogston JD, McNeil SE. Common pitfalls in nanotechnology: lessons learned from NCI’s Nanotechnology Characterization Laboratory. Integr Biol (Camb) 2013;5:66–73. doi: 10.1039/c2ib20117h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Stern ST, Hall JB, Yu LL, Wood LJ, Paciotti GF, Tamarkin L, Long SE, McNeil SE. Translational considerations for cancer nanomedicine. J Control Release. 2010;146:164–174. doi: 10.1016/j.jconrel.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Fatehi L, Wolf SM, McCullough J, Hall R, Lawrenz F, Kahn JP, Jones C, Campbell SA, Dresser RS, Erdman AG, et al. Recommendations for nanomedicine human subjects research oversight: an evolutionary approach for an emerging field. J Law Med Ethics. 2012;40:716–750. doi: 10.1111/j.1748-720X.2012.00703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.McNeil SE. Characterization of Nanoparticles Intended for Drug Delivery. Vol. 697. TOTOWA, NJ 07512–1165 USA: HUMANA PRESS INC; 2011. [Google Scholar]
- 137.Tinkle S, McNeil SE, Muhlebach S, Bawa R, Borchard G, Barenholz YC, Tamarkin L, Desai N. Nanomedicines: addressing the scientific and regulatory gap. Ann N Y Acad Sci. 2014;1313:35–56. doi: 10.1111/nyas.12403. [DOI] [PubMed] [Google Scholar]
- 138.Zamboni WC, Torchilin V, Patri AK, Hrkach J, Stern S, Lee R, Nel A, Panaro NJ, Grodzinski P. Best practices in cancer nanotechnology: perspective from NCI nanotechnology alliance. Clin Cancer Res. 2012;18:3229–3241. doi: 10.1158/1078-0432.CCR-11-2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Houghton PJ, Kurmasheva RT, Kolb EA, Gorlick R, Maris JM, Wu J, Tong Z, Arnold MA, Chatterjee M, Williams TM, et al. Initial testing (stage 1) of the tubulin binding agent nanoparticle albumin-bound (nab) paclitaxel (Abraxane((R))) by the Pediatric Preclinical Testing Program (PPTP) Pediatr Blood Cancer. 2015;62:1214–1221. doi: 10.1002/pbc.25474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Khanna C, London C, Vail D, Mazcko C, Hirschfeld S. Guiding the optimal translation of new cancer treatments from canine to human cancer patients. Clin Cancer Res. 2009;15:5671–5677. doi: 10.1158/1078-0432.CCR-09-0719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Vail DM, MacEwen EG. Spontaneously occurring tumors of companion animals as models for human cancer. Cancer Invest. 2000;18:781–792. doi: 10.3109/07357900009012210. [DOI] [PubMed] [Google Scholar]
- 142.Yu LX, Amidon G, Khan MA, Hoag SW, Polli J, Raju GK, Woodcock J. Understanding pharmaceutical quality by design. AAPS J. 2014;16:771–783. doi: 10.1208/s12248-014-9598-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Dobrovolskaia MA, Neun BW, Clogston JD, Grossman JH, McNeil SE. Choice of method for endotoxin detection depends on nanoformulation. Nanomedicine (Lond) 2014;9:1847–1856. doi: 10.2217/nnm.13.157. [DOI] [PubMed] [Google Scholar]
- 144.Dobrovolskaia MA. Pre-clinical immunotoxicity studies of nanotechnology-formulated drugs: Challenges, considerations and strategy. J Control Release. 2015 doi: 10.1016/j.jconrel.2015.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Dobrovolskaia MA, McNeil SE. Understanding the correlation between in vitro and in vivo immunotoxicity tests for nanomedicines. J Control Release. 2013;172:456–466. doi: 10.1016/j.jconrel.2013.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]