Abstract
Melanoma frequently metastasizes to the brain, with CNS involvement being clinically evident in ~30% of patients (as high as 75% at autopsy). In ~5% cases melanoma cells also metastasize to the leptomeninges, the sub-arachnoid space and cerebrospinal fluid (CSF). Patients with leptomeningeal melanoma metastases (LMM) have the worst prognosis and are characterized by rapid disease progression (mean survival 8-10 weeks) and a death from neurological causes. The recent years have seen tremendous progress in the development of targeted and immune therapies for melanoma that has translated into an increased survival benefit. Despite these gains, the majority of patients fail therapy and there is a suspicion that the brain and the leptomeninges are a “sanctuary” sites for melanoma cells that escape both targeted therapy and immunologic therapies. Emerging evidence suggests that 1) Cancer cells migrating to the CNS may have unique molecular properties and 2) the CNS/leptomeningeal microenvironment represents a pro-survival niche that influences therapeutic response. In this Mini-Review we will outline the clinical course of LMM development and will describe how the intracranial immune and cellular microenvironments offer both opportunities and challenges for the successful management of this disease. We will further discuss the latest data demonstrating the potential use of BRAF inhibitors and immune therapy in the management of LMM, and will review future potential therapeutic strategies for the management of this most devastating complication of advanced melanoma.
Introduction
Melanoma progression in the leptomeninges
Cutaneous melanomas are tumors that derive from melanocytes, the pigment producing cells of the skin. They represent the most deadly of all skin cancers, and account for the majority of skin cancer fatalities. Melanomas are notorious for their ability to metastasize early, with even lesions <1 mm thick (in 5-15% of cases) frequently disseminating to other organs. Melanomas often metastasize to the brain, with CNS involvement being clinically evident in ~30% of patients (as high as 75% at autopsy)1. Melanoma brain metastases (MBM) are associated with a poor prognosis and a median survival of 17-22 weeks2, 3. Although stereotactic radiosurgery (SRS) and radiation can provide some local disease control in the brain, most systemic therapies - including chemotherapy and immune therapy - are associated low rates of response (~10%)4-6.
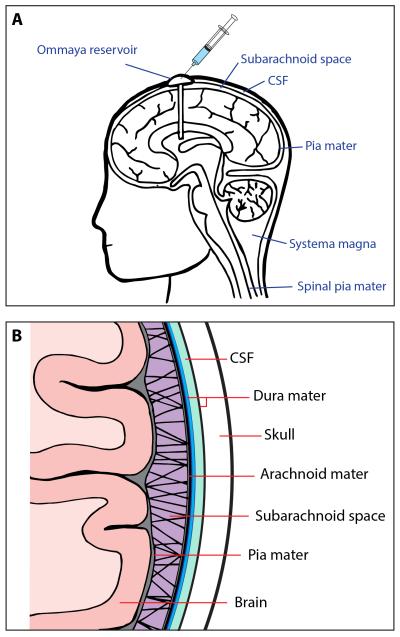
One of the most serious complications of advanced melanoma is the metastasis of cancer cells to intracranial structures and their infiltration into the cerebrospinal fluid (CSF)7-9. The involved tissues include the membranes that surround the brain; the arachnoid mater and the pia mater, which are collectively known as the leptomeninges (Figures 1A,B)10. Of these, the arachnoid mater consists of an avascular membrane of fibroblasts lined with epithelial-like mesothelial cells that prevent the escape of CSF into the sub-dural space11. The sub-arachnoid space is spanned by numerous arachnoid trabeculae that form a “spiders web” pattern between the arachnoid membrane and the pia mater11. The sub-arachnoid space contains numerous blood vessels and is filled with CSF that may also contain macrophages and lymphocytes. The pia mater adheres directly to the surface of the brain and consists of several layers of fibroblasts, capillaries and collagen fibrils (Figure 1B). The inner membrane of the pia mater sits on top of a basement membrane directly over joined astrocyte end feet, the latter of which form the membrane gliae limitans supeficialis (which is part of the BBB)11. Normal human melanocytes also reside in both the pia mater and arachnoid mater12. In rare cases, (1 in 10 million) these can develop into primary leptomeningeal melanoma12.
Figure 1. The anatomy of the CNS and leptomeninges.
A). Representative figure of the head and neck showing the brain, meninges and the placement of the Ommaya reservoir into the ventricles.
B). High powered representation of the leptomeninges showing the relationship of the arachnoid mater, pia mater and brain parenchyma. Note the CSF is found between the two layers of the dura and within the sub-arachnoid space.
Leptomeningeal metastases primarily occur following the spread of cancer cells through the vascular system to the vessels of the arachnoid or choroid plexus13. After invading the leptomeninges, the tumor cells gain access to the subarachnoid space and circulate freely through the CSF13. Other potential mechanisms of leptomeningeal infiltration include direct migration from the brain parenchyma (possibly from existing brain metastases) and perineural spread where the tumor cells migrate along cranial or spinal nerves before entering the subarachnoid space14. The perineural route of entry into the CSF of systemic melanomas has been rarely reported, though some melanomas (particularly desmoplastic melanomas) exhibit perineural migration and can spread along the cranial nerves15.
Leptomeningeal metastasis typically affects ~5% of all patients with cancer. Tumor histologies with high rates of leptomeningeal metastasis development include breast cancer (3-5% with metastatic disease), small cell lung cancer (SCLC) (11%) and melanoma (5-7%) 10, 14. Patients with LMM have the worst prognosis of all, with a mean survival 8-10 weeks and a death from neurological causes9, 16, 17. In melanoma, a link has been suggested between the presence of parenchymal brain metastases and the development of LMM, with up to 19% of patients having concurrent tumor in the leptomeninges and the brain7. The reported incidence of leptomeningeal metastasis is rising across all tumor types probably due to improved detection (higher resolution contrast imaging), longer survival of patients with better controlled systemic cancer and the “sanctuary” nature of the CSF space.
Diagnosis and treatment
The diagnosis of LMM is a challenge. Initial disease presentation includes acute neurological deterioration associated with headaches, impaired sensory/motor function, weakness, pain and possible cognitive impairment. Diagnoses are typically made on the basis of MRI with T1-weighted gadolinium enhancement of the entire neuroaxis (although this is often false negative) and the cytological evaluation of the CSF for tumor cells, which remains the “Gold Standard”9, 14 (Figure 2). Cytology, which is only a morphologic description of rare cells in the CSF, is the most definitive diagnostic with a very high specificity (>95%) but very low sensitivity (<50%) 18. Typically, only 55% patients with LMM show a positive cytology at initial examination. The poor sensitivity of CSF cytology, and its subjective nature, makes quantification of therapeutic responses difficult. The development of new techniques, such as those designed to identify circulating tumor cells (CTCs) in the blood, are promising. A recent study described the use of the Veridex CellSearch® technology to enumerate CTCs from the CSF of two patients with LMM19. Melanoma cells were identified on the basis of positive staining for CD146 (Mel-CAM) and HMW-MAA and negativity for CD45 and CD34. CTCs in the range of 5-1090 cells per 5 ml of CSF were enumerated and broadly agreed with the cytology counts (low vs high CTC numbers)19. Assessing cell free DNA, single cell analysis or CSF proteomics may prove useful as diagnostics in the future (e.g. assessing for circulating mutant BRAF).
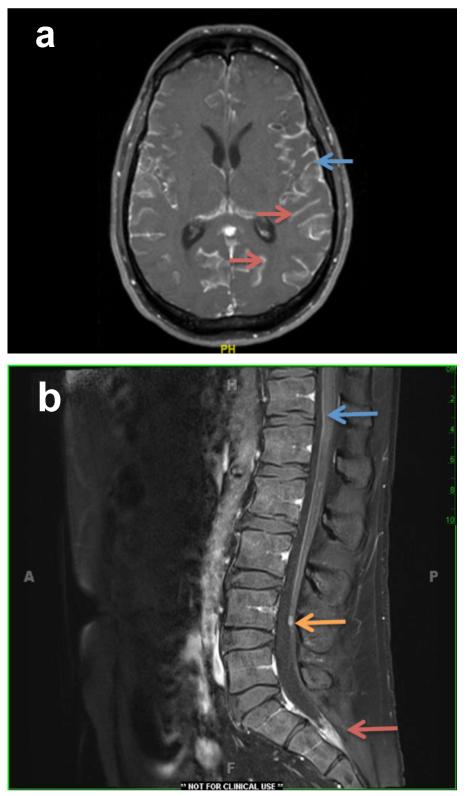
Figure 2. MRI imaging of leptomeningeal disease.
A). MRI image of a 46-year-old man with metastatic melanoma (BRAF V600E mutant) on his left upper back that was resected in 2011. In 12/2014 after developing fatigue, fevers, abdominal pain and syncope, he was found to have multiple masses in his liver and subcutaneous masses. He was started on dabrafenib/trametinib and shortly thereafter, he presented with headaches. An MRI brain demonstrated extensive supratentorial and infratentorial leptomeningeal carcinomatosis with involvement of the cranial nerves and mild hydrocephalus. Enhancement is seen on the surface of the brain on the surface of the gyri (blue arrow) and deep within the sulci (red arrows). He had an Ommaya reservoir placed and was treated with IT Thiotepa followed by whole brain radiation therapy. Cytology was diagnostic for malignancy.
B). MRI image of the spine of a 44 year old man who was successfully treated for metastatic melanoma in his lungs with Ipilimumab. Four years later he had a small brain metastasis and had SRS. Six months later he had back pain and a MRI showed characteristic findings of LMM. There is a small nodule in the cauda equina (orange arrow), an accumulation of enhancing tumor in the thecal sac (red arrow), and fine linear enhancement on the ventral and dorsal surfaces of the spinal cord (blue arrow). His cytology was positive for malignant cells. He was treated with intrathecal thiotepa and local RT to his LS spine.
At this time there are no effective treatments for LMM. Until recently, most patients received off-label therapies such as intrathecal (IT) chemotherapy and whole brain radiotherapy (WBRT)20-23. Commonly used drugs include thiotepa, methotrexate and liposomal cytarabine, which are typically administered through a surgically implanted Ommaya reservoir20-22 (Figure 1A). Although no large-scale clinical trials have directly evaluated these therapies, most anecdotal reports suggest they have little impact upon patient survival9. Isolated cases have been reported in which intrathecal chemotherapy, combined with systemic chemotherapy, leads to clinical benefit9. One of the most comprehensive clinical trials to date was a phase II multi-center trial of intrathecal topotecan in individuals with newly diagnosed or recurrent leptomeningeal metastases resulting from either primary CNS tumors or other hematological/solid tumors24. It was found that 30% of those treated achieved the primary endpoint of a progression-free survival (PFS) of 13 weeks, with a median overall survival (mOS) of 15 weeks. Poor prognostic factors included an MRI showing enhancement of the leptomeninges and positive cytology at diagnosis24. A second study, evaluating the efficacy of systemic temozolomide in patients with leptomeningeal metastases arising from breast cancer and lung cancer, was associated with a response rate of 15% and a median time to progression of 43 days25. Since melanomas are relatively radioresistant, WBRT is typically used as a palliative measure to control headaches and other neurologic symptoms.
The role of targeted therapy in the management of leptomeningeal melanoma metastases
Disseminated melanoma is highly refractory to most standard anti-cancer therapies such as chemotherapy and radiotherapy. The discovery in 2002 that approximately half of all cutaneous melanomas harbored activating mutations in the serine/threonine kinase BRAF revolutionized care and led to the clinical development of small molecule BRAF kinase inhibitors26, 27. BRAF inhibitors such as vemurafenib (and now dabrafenib and encorafenib) have proven highly effective at shrinking the tumors of patients whose melanomas have activating position 600 mutations in BRAF (V600E, V600D, V600K)28-31. Despite rapid and dramatic responses, most patients develop resistance with reactivation of the MAPK pathway (resulting from acquired NRAS and MEK mutations, and BRAF-splice mutants) 32-34. Vertical targeting of the MAPK pathway in BRAF-mutant melanoma using the combination of a BRAF and MEK inhibitor (such as dabrafenib-trametinib and now vemurafenib-cobimetinib) limits resistance and increases overall survival (mOS 25.1 months)35, 36.
The CNS is a “privileged” site and drug penetration across the blood-brain-barrier (BBB) is typically low. The BBB serves to limit the influx of potentially toxic chemotherapy drugs and activated immunocytes (induced by immunotherapies) into the brain. Balancing the highly protected nature of the brain/CSF microenvironment while exposing it to potentially neurotoxic therapies is a major challenge. Preclinical studies found that dabrafenib and vemurafenib were substrates for the drug efflux pumps, ABCB1 (P-glycoprotein) and ABCG2 (breast cancer resistance protein, BCRP) which reduce CSF drug levels through active transport mechanisms37, 38. In mouse models, the distribution of vemurafenib in the CSF was 3-logs lower than that of the plasma. Interestingly, the brain penetration of dabrafenib in mice was higher than vemurafenib, perhaps making this a better choice for patients with BRAF-mutant LMM and MBM 37, 38. Recent clinical data on vemurafenib levels in the CSF of melanoma patients with MBM confirmed the animal findings and showed CSF levels to be significantly lower (0.47 +/− 0.37 mg/ml) than those in plasma (53.4 +/− 26.2 mg/ml)39. No relationship was noted between the levels of the drug in the CSF and the plasma39. The high variability of vemurafenib levels in the CSF between individual patients may reflect differences in BBB permeability. Integrity of the BBB is compromised in MBMs as well as following interventions such as surgery and radiotherapy. Indeed, two of the patients with the highest CSF penetration of vemurafenib had received prior SRS39. Despite these caveats, BRAF inhibitors do have activity against intracranial disease with >30% of patients with BRAF-mutant MBM showing a response to dabrafenib 40.
It is likely that the CNS environment itself may confer resistance to BRAF-targeted drugs, and that this may arise in part through crosstalk between melanoma cells and cells of the host41, 42. There is already evidence that normal host cells, including fibroblasts and macrophages, protect melanoma cells from BRAF inhibitor treatment43-45. Importantly, melanoma cells residing in the brain environment have different signaling than those at extracranial sites. Melanomas that metastasize to the CNS have higher AKT signaling, which is frequently secondary to a loss of function of the tumor suppressor PTEN41, 46. The increased AKT signaling in brain-resident melanoma cells is probably due to crosstalk with the neighboring astrocytes or other brain specific factors. Studies have shown that astrocyte-conditioned media decreases the sensitivity of melanoma cells to BRAF inhibition by increasing intratumoral PI3K/AKT signaling, which in turn suppressed the apoptotic response41. It is known that host astrocytes can modulate PTEN expression directly in nearby tumor cells through the release of exosomes that contain PTEN-targeting microRNAs47. Secreted factors within the brain microenvironment can also modulate drug response, with CSF (from normal rat brains) being found to decrease the sensitivity of melanoma cell lines to BRAF inhibition48. Mechanistically, the addition of CSF decreased the kinetics of phospho-ERK inhibition in the melanoma cells leading to early signal recovery and reduced cell death. The addition of a PI3K inhibitor reversed the protective effects of the rat CSF48.
One question that remains is whether leptomeningeal melanoma metastases are genetically distinct from extracranial disease and if so, whether these have unique signatures that are therapeutically tractable. Importantly, up to 53% of brain metastases (non melanoma) have therapeutically actionable mutations in genes that confer sensitivity to PI3K/AKT/mTOR, CDK and HER2 inhibitors. These are lacking in matched non-cranial metastases49. One of the only genetic profiling studies on leptomeningeal metastases to date was a comparative genomic (array CGH) analysis of primary and leptomeningeal disease in metastatic breast cancer 50. Like the brain metastasis study, it was found that although genomic alterations were conserved between the primary tumor and the CTCs in the CSF, the CTCs tended to have more extensive genomic changes (including the c-Myc locus)50.
Clinical experience with BRAF inhibitors in LMM
The relatively small numbers of patients with LMM has made the assessment of BRAF inhibitor therapy challenging. Despite this, there are now at least 5 published case reports suggesting that vemurafenib and dabrafenib may have some efficacy. One of the first reported cases described a 47 year old female patient with multiple brain metastases who underwent surgical resection and WBRT51. The patient then received systemic chemotherapy and intrathecal cytarabine before testing positive for Melan-A expressing tumor cells in her CSF. Her tumor was BRAF mutant and she was started on systemic vemurafenib treatment. After 12 weeks of therapy, a marked clinical improvement was seen associated with a regression of the leptomeningeal disease and the disappearance of melanoma cells from the CSF51.
A second study reported the case of a 53-year old woman with extensive BRAF-mutant metastatic disease who progressed on two cycles of biochemotherapy before receiving vemurafenib as part of a clinical trial52. The patient remained on BRAF inhibitor therapy for over 14 months and experienced a significant reduction in extracranial disease burden before a brain MRI showed an enhancement that was suggestive of LMM. Following the detection of melanoma cells in her CSF, the individual received WBRT and was restarted on vemurafenib. No further progression of her extracranial disease or the LMM was noted over the proceeding 18 months52. Similarly impressive responses were also reported in a 60-year old patient with LMM in which systemic vemurafenib treatment led to a near-complete resolution of leptomeningeal enhancement by MRI and an improvement in neurological symptoms53.
The fourth case concerned a 61 year-old man with prior melanoma lung metastases who received ipilimumab and chemotherapy before presenting with disease in the brain parenchyma and LMM54. Vemurafenib treatment was initiated and responses were observed for 3 months before the onset of disease progression in the brain, leptomeninges and extracranial sites. After receiving WBRT the patient was then switched to dabrafenib-trametinib and again responded in the brain and leptomeninges54. Responses at these sites continued for a further 5 months before disease progression in the peritoneum. The patient died 19 months after the diagnosis of MBM and LMM. The response of this individual to dabrafenib-trametinib at intracranial sites following failure on single agent BRAF inhibitor therapy was unexpected, as there is overlap between the mechanisms of resistance to BRAF monotherapy and the BRAF-MEK inhibitor combination55. Indeed, Phase II studies in melanomas at non-CNS sites have demonstrated that BRAF inhibitor monotherapy failure precludes any further response to the BRAF-MEK inhibitor combination56. The reasons underlying the secondary response to the combination are unclear but could be a consequence of the prior radiation therapy (BRAF inhibitors can be radiosensitizing) or the sanctuary status of BRAF mutant melanoma cells in the CSF/brain that shielded them from the initial round of therapy.
Single agent dabrafenib has also been reported to have activity in LMM57. A recent report detailed the case of a 57-year old woman who initially presented in 2006 with a primary melanoma on her foot. In 2012 she exhibited neurological symptoms and a diagnosis of LMM was confirmed by MRI enhancement of the leptomeninges and positive CSF cytology. Upon confirmation of a positive BRAF mutation test the patient was started on systemic dabrafenib therapy57. An immediate clinical improvement of neurological function was seen, accompanied by resolution of the leptomeningeal lesions by MRI 4 weeks after treatment initiation. At the last recorded follow up (15 weeks after treatment initiation), the patient remained in complete remission. Although together these reports suggest that BRAF inhibitor therapy may be effective in LMM, the small sample size and the bias towards reporting positive cases make the true level of efficacy hard to judge. It is worth noting that at least one case report to date has demonstrated systemic dabrafenib to be ineffective in an individual with BRAF-mutant LMM 58.
Clinical experience with immune therapies in LMM
Like targeted therapy, there has been little published experience with immunotherapies in LMM. The rationale behind this approach is that the CSF in LMM is remarkably non-inflammatory and stimulating an immune response might provoke an anti-tumor response. The paucity of data reflects both the rarity of this disease and concerns about the possibility of inducing “too much” inflammation in the CNS. And, though rare, when they occur, the neurologic side effects associated with the immune checkpoint inhibitors can be devastating 59, 60.
One of the earliest reports of immunotherapy approaches centered upon use of intrathecal interferon (IFN)-α in patients with LM disease associated with multiple tumor types (including melanoma)61. Another early immune therapy that was often used in advanced melanoma was the cytokine interleukin (IL)-2. A number of cases have reported complete responses to IL-2 in patients with MBM, raising interest in its potential use in LMM62, 63. A recent report details a study in which IL-2 was administered intrathecally at 1-5 doses/week for four weeks, then weekly for four weeks, then every other week followed by monthly64. Although the data have not reached maturity, responses were seen in patients with LMM. A median survival of 9.1 months was reported with 16% of patients showing a median survival of >24 months64. Severe side effects of raised intracranial pressure and ICU admissions were reported.
Adoptive cell therapy is an immunological approach in which T-cells [e.g. extracted from the tumor itself (Tumor infiltrating lymphocytes; (TILS) or engineered to express a specific T-cell receptor)] are expanded ex vivo before being infused back in to the patient. The potential utility of TIL therapy in LMM was suggested by a retrospective analysis of patients undergoing adoptive cell therapy who were discovered to also have coincidental MBMs. Within this group, seven of seventeen (41%) patients treated with TILs had a CR in the brain and six patients achieved a partial response (PR). Similarly, two out of nine patients that received TCR-transduced lymphocytes had CRs in the brain. These data suggest that T-cells may have trafficked into the CNS, perhaps with the potential to infiltrate the leptomeninges and CSF65. One example pointing the potential of adoptive cell therapy in LMM comes from a case report of an LMM patient who failed intrathecal IL-266. Cytotoxic T-lymphocytes (CTLs) were then generated using autologous dendritic cells pulsed with peptides from melanoma-associated antigens tyrosinase, Melan-A/MART, and gp100/Pmel 17. Delivery of the cells via an Ommaya resulted in retention in the brain for 48 hours and led to elevated levels of TNF-α, INF-γ and IL-6 in the CSF66. The patient showed improvement in neurologic symptoms and remained alive >18 months after the diagnosis of LMM.
Recently, a single patient was reported in which the LMM was successfully treated with the combination of intrathecal autologous TILs and IL-267. The individual had previously received TILs, derived from a systemic metastasis, and had developed LMM. He was treated with intrathecal IL-2 and progressed in his CSF and systemically. A compassionate use IND was obtained and intrathecal TILs were administered once a week in conjunction with intrathecal IL-2 twice a week. Five months after the first TIL infusion, the patient died from systemic disease but his LMM remained stable67.
Another strategy that has changed the face of melanoma treatment is the use of immune checkpoint inhibitors to upregulate T-cell function. This approach is predicated on the use of antibodies that block the inhibitory signals through receptors such as cytotoxic T-lymphocyte antigen (CTLA)-4, programmed cell death (PD)-1 and programmed cell death like (PD-L)-1. Antibodies targeted against CTLA-4, PD-1 or PD-L1 are associated with increased survival in patients with advanced melanoma (with BRAF mutant and BRAF wild-type disease) both alone and in combination. There is also evidence from a retrospective analysis of the phase II trial of ipilimumab that included patients with small symptomatic MBM that the drug may have activity68, 69. These reports led to a prospective Phase II study of ipilimumab designed specifically to treat MBMs where response rates were similar to systemic melanoma and equally as durable6. From a MBM perspective the drug seems safe although it is not yet known whether the treatment will be more effective in LMM where a differentially permeable BBB may allow a greater ingress of activated cytotoxic T-cells. As noted above, rare neurological syndromes have been reported in Ipilimumab in patients with systemic melanoma.
At this time there has only been one report of Ipilimumab being successfully used against LMM70. The patient had metastatic melanoma and developed headaches with MRI scans characteristic of LMM. The individual then received WBRT without clinical improvement and Ipilimumab was started. Ipilimumab therapy led to an improvement of symptoms after the first dose and was associated with a complete radiographic response70. The patient was still alive 18 months after her diagnosis of LMM.
Conclusions and future perspectives
The recent years have seen incredible progress in the treatment of advanced melanoma. One consequence of these breakthroughs has been the increase in patients developing MBM and LMM. Whether this is a direct cause of the treatment (the brain/leptomeninges being a sanctuary site) or is simply a result of the disease timeline being extended remains to be determined. What is clear is that the development of CNS disease, and in particular LMM, will limit the long-term therapeutic responses for some patients. The rapid progression of melanoma in the leptomeninges and the lack of research in this area offers both challenges and opportunities. More research is urgently needed into the biology that underlies the dissemination of melanoma cells to the leptomeninges and the CSF compartment. One important question is whether CTCs from the blood eventually migrate into the leptomeningeal environment as a result of transendothelial migration. There is already in vivo evidence that melanoma cells migrate into the brain following the co-option of cerebral blood vessels 71. The genetic profiles of CTCs in the CSF need to be analyzed to establish the absence or presence of therapeutically actionable mutations (such as those seen in brain metastases). These genetic analyses may also allow novel biomarkers of LMM to be defined. New insights are also needed into the immune microenvironment of the brain and leptomeninges/CSF, as this may give important insights into the potential utility of immune therapies.
At this time our understanding of MBM is slightly more advanced, with new spontaneous mouse melanoma models showing roles for both AKT1 and PTEN loss in brain metastasis development72. A number of other GEM models of melanoma brain metastasis are being developed. Many clinical trials are also ongoing to evaluate the efficacy of targeted therapies (including the BRAF inhibitors, the BRAF-MEK inhibitor combination, BRAF inhibition + radiation, CDK4/6 inhibitors and PI3K inhibitors), immune therapies (anti-PD-1, anti-CTAL-4, anti-CTAL4 + anti anti-PD-1 and anti-CTLA-4 + radiation) and immune-targeted therapy combinations (anti-PD-1 + anti-VEGFR antibody) in patients with MBMs. The outcome of these trials is eagerly awaited.
To date, there are only a handful of reports suggesting that BRAF-MEK inhibitor therapy or immune therapy may have utility against LMM. Clearly these approaches need to be evaluated in prospective clinical trials with detailed tissue and CSF interrogation to learn about mechanisms, efficacy and side effects. Clinical trial designs also need to be amended, so that patients with LMM can be evaluated. This is challenging in a rare disease in which patients deteriorate rapidly, so careful patient selection will be paramount. Progress will require the active collaboration between clinician-investigators at several major medical centers, and between basic scientists and clinicians. Only a better understanding of this disease will lead to better treatments. This most devastating disease is a significant challenge but, as the melanoma field recently proven, nothing should be taken for granted and intense collaboration can achieve significant progress.
Acknowledgments
Grant Support: Inna Fedorenko is supported by a career award from the Melanoma Research Foundation. Keiran S.M. Smalley is supported by R01 CA161107, R21 CA198550 and SPORE grant CA168536 from the National Institutes of Health. Peter Forsyth is supported by the Moffitt Foundation, the ABC2 Foundation and SPORE grant CA168536 from the National Institutes of Health
References
- 1.Kenchappa RS, Tran N, Rao NG, Smalley KS, Gibney GT, Sondak VK, Forsyth PA. Novel Treatments for Melanoma Brain Metastases. Cancer Control. 2013;20:298–306. doi: 10.1177/107327481302000407. [DOI] [PubMed] [Google Scholar]
- 2.Davies MA, Liu P, McIntyre S, Kim KB, Papadopoulos N, Hwu WJ, Hwu P, Bedikian A. Prognostic Factors for Survival in Melanoma Patients With Brain Metastases. Cancer. 2011;117:1687–96. doi: 10.1002/cncr.25634. [DOI] [PubMed] [Google Scholar]
- 3.Fife KM, Colman MH, Stevens GN, Firth IC, Moon D, Shannon KF, Harman R, Petersen-Schaefer K, Zacest AC, Besser M, Milton GW, McCarthy WH, et al. Determinants of outcome in melanoma patients with cerebral metastases. J Clin Oncol. 2004;22:1293–300. doi: 10.1200/JCO.2004.08.140. [DOI] [PubMed] [Google Scholar]
- 4.Carlino MS, Fogarty GB, Long GV. Treatment of melanoma brain metastases: a new paradigm. Cancer J. 2012;18:208–12. doi: 10.1097/PPO.0b013e31824b2890. [DOI] [PubMed] [Google Scholar]
- 5.Agarwala SS, Kirkwood JM, Gore M, Dreno B, Thatcher N, Czarnetski B, Atkins M, Buzaid A, Skarlos D, Rankin EM. Temozolomide for the treatment of brain metastases associated with metastatic melanoma: a phase II study. J Clin Oncol. 2004;22:2101–7. doi: 10.1200/JCO.2004.11.044. [DOI] [PubMed] [Google Scholar]
- 6.Margolin K, Ernstoff MS, Hamid O, Lawrence D, McDermott D, Puzanov I, Wolchok JD, Clark JI, Sznol M, Logan TF, Richards J, Michener T, et al. Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncol. 2012;13:459–65. doi: 10.1016/S1470-2045(12)70090-6. [DOI] [PubMed] [Google Scholar]
- 7.Raizer JJ, Hwu WJ, Panageas KS, Wilton A, Baldwin DE, Bailey E, von Althann C, Lamb LA, Alvarado G, Bilsky MH, Gutin PH. Brain and leptomeningeal metastases from cutaneous melanoma: survival outcomes based on clinical features. Neuro Oncol. 2008;10:199–207. doi: 10.1215/15228517-2007-058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harstad L, Hess KR, Groves MD. Prognostic factors and outcomes in patients with leptomeningeal melanomatosis. Neuro Oncol. 2008;10:1010–8. doi: 10.1215/15228517-2008-062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pape E, Desmedt E, Zairi F, Baranzelli MC, Dziwniel V, Dubois F, Bonneterre J, Mortier L, Le Rhun E. Leptomeningeal metastasis in melanoma: a prospective clinical study of nine patients. In Vivo. 2012;26:1079–86. [PubMed] [Google Scholar]
- 10.Leal T, Chang JE, Mehta M, Robins HI. Leptomeningeal Metastasis: Challenges in Diagnosis and Treatment. Curr Cancer Ther Rev. 2011;7:319–27. doi: 10.2174/157339411797642597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vandenabeele F, Creemers J, Lambrichts I. Ultrastructure of the human spinal arachnoid mater and dura mater. J Anat. 1996;189(Pt 2):417–30. [PMC free article] [PubMed] [Google Scholar]
- 12.Hsieh YY, Yang ST, Li WH, Hu CJ, Wang LS. Primary leptomeningeal melanoma mimicking meningitis: a case report and literature review. J Clin Oncol. 2015;33:e57–61. doi: 10.1200/JCO.2013.50.0264. [DOI] [PubMed] [Google Scholar]
- 13.Kokkoris CP. Leptomeningeal carcinomatosis. How does cancer reach the pia-arachnoid? Cancer. 1983;51:154–60. doi: 10.1002/1097-0142(19830101)51:1<154::aid-cncr2820510130>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 14.Le Rhun E, Taillibert S, Chamberlain MC. Carcinomatous meningitis: Leptomeningeal metastases in solid tumors. Surg Neurol Int. 2013;4:S265–88. doi: 10.4103/2152-7806.111304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang PC, Fischbein NJ, McCalmont TH, Kashani-Sabet M, Zettersten EM, Liu AY, Weissman JL. Perineural spread of malignant melanoma of the head and neck: clinical and imaging features. AJNR Am J Neuroradiol. 2004;25:5–11. [PMC free article] [PubMed] [Google Scholar]
- 16.Raizer JJ, Hwu WJ, Panageas KS, Wilton A, Baldwin DE, Bailey E, von Althann C, Lamb LA, Alvarado G, Bilsky MH, Gutin PH. Brain and leptomeningeal metastases from cutaneous melanoma: Survival outcomes based on clinical features. Neuro-Oncology. 2008;10:199–207. doi: 10.1215/15228517-2007-058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harstad L, Hess KR, Groves MD. Prognostic factors and outcomes in patients with leptomeningeal melanomatosis. Neuro-Oncology. 2008;10:1010–8. doi: 10.1215/15228517-2008-062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glantz MJ, Cole BF, Glantz LK, Cobb J, Mills P, Lekos A, Walters BC, Recht LD. Cerebrospinal fluid cytology in patients with cancer: minimizing false-negative results. Cancer. 1998;82:733–9. doi: 10.1002/(sici)1097-0142(19980215)82:4<733::aid-cncr17>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 19.Le Rhun E, Tu Q, De Carvalho Bittencourt M, Farre I, Mortier L, Cai H, Kohler C, Faure GC. Detection and quantification of CSF malignant cells by the CellSearch technology in patients with melanoma leptomeningeal metastasis. Med Oncol. 2013;30:538. doi: 10.1007/s12032-013-0538-3. [DOI] [PubMed] [Google Scholar]
- 20.Comte A, Jdid W, Guilhaume MN, Kriegel I, Piperno-Neumann S, Dieras V, Dorval T, Pierga JY, Cottu PH, Mignot L, Bidard FC. Survival of breast cancer patients with meningeal carcinomatosis treated by intrathecal thiotepa. J Neurooncol. 2013;115:445–52. doi: 10.1007/s11060-013-1244-x. [DOI] [PubMed] [Google Scholar]
- 21.Schaefer N, Rasch K, Moehlenbruch M, Urbach H, Stuplich M, Blasius E, Scheffler B, Herrlinger U, Glas M. Leptomeningeal melanomatosis: stabilization of disease due to radiation, temozolomide and intrathecal liposomal cytarabine. Acta Oncol. 2011;50:1260–2. doi: 10.3109/0284186X.2011.586001. [DOI] [PubMed] [Google Scholar]
- 22.Salgia S, Fleming GF, Lukas RV. Leptomeningeal carcinomatosis from breast cancer treated with intrathecal topotecan with concomitant intravenous eribulin. J Clin Neurosci. 2014;21:1250–1. doi: 10.1016/j.jocn.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 23.Morris PG, Reiner AS, Szenberg OR, Clarke JL, Panageas KS, Perez HR, Kris MG, Chan TA, DeAngelis LM, Omuro AM. Leptomeningeal metastasis from non-small cell lung cancer: survival and the impact of whole brain radiotherapy. J Thorac Oncol. 2012;7:382–5. doi: 10.1097/JTO.0b013e3182398e4f. [DOI] [PubMed] [Google Scholar]
- 24.Groves MD, Glantz MJ, Chamberlain MC, Baumgartner KE, Conrad CA, Hsu S, Wefel JS, Gilbert MR, Ictech S, Hunter KU, Forman AD, Puduvalli VK, et al. A multicenter phase II trial of intrathecal topotecan in patients with meningeal malignancies. Neuro Oncol. 2008;10:208–15. doi: 10.1215/15228517-2007-059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Segura PP, Gil M, Balana C, Chacon I, Langa JM, Martin M, Bruna J. Phase II trial of temozolomide for leptomeningeal metastases in patients with solid tumors. J Neurooncol. 2012;109:137–42. doi: 10.1007/s11060-012-0879-3. [DOI] [PubMed] [Google Scholar]
- 26.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, Davis N, Dicks E, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–54. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 27.Smalley KSM. A pivotal role for ERK in the oncogenic behaviour of malignant melanoma? International Journal of Cancer. 2003;104:527–32. doi: 10.1002/ijc.10978. [DOI] [PubMed] [Google Scholar]
- 28.Tsai J, Lee JT, Wang W, Zhang J, Cho H, Mamo S, Bremer R, Gillette S, Kong J, Haass NK, Sproesser K, Li L, et al. Discovery of a selective inhibitor of oncogenic B-Raf kinase with potent antimelanoma activity. Proc Natl Acad Sci U S A. 2008;105:3041–6. doi: 10.1073/pnas.0711741105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bollag G, Hirth P, Tsai J, Zhang J, Ibrahim PN, Cho H, Spevak W, Zhang C, Zhang Y, Habets G, Burton EA, Wong B, et al. Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature. 2010;467:596–9. doi: 10.1038/nature09454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Flaherty KT, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA, O'Dwyer PJ, Lee RJ, Grippo JF, Nolop K, Chapman PB. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809–19. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.King AJ, Arnone MR, Bleam MR, Moss KG, Yang J, Fedorowicz KE, Smitheman KN, Erhardt JA, Hughes-Earle A, Kane-Carson LS, Sinnamon RH, Qi H, et al. Dabrafenib; preclinical characterization, increased efficacy when combined with trametinib, while BRAF/MEK tool combination reduced skin lesions. PLoS ONE. 2013;8:e67583. doi: 10.1371/journal.pone.0067583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fedorenko IV, Gibney GT, Sondak VK, Smalley KSM. Beyond BRAF: where next for melanoma therapy? British Journal of Cancer. 2015;112:217–26. doi: 10.1038/bjc.2014.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van Allen EM, Wagle N, Sucker A, Treacy DJ, Johannessen CM, Goetz EM, Place CS, Taylor-Weiner A, Whittaker S, Kryukov GV, Hodis E, Rosenberg M, et al. The Genetic Landscape of Clinical Resistance to RAF Inhibition in Metastatic Melanoma. Cancer Discov. 2013 doi: 10.1158/2159-8290.CD-13-0617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rizos H, Menzies AM, Pupo GM, Carlino MS, Fung C, Hyman J, Haydu LE, Mijatov B, Becker TM, Boyd SC, Howle J, Saw R, et al. BRAF Inhibitor Resistance Mechanisms in Metastatic Melanoma: Spectrum and Clinical Impact. Clinical Cancer Research. 2014;20:1965–77. doi: 10.1158/1078-0432.CCR-13-3122. [DOI] [PubMed] [Google Scholar]
- 35.Long GV, Stroyakovskiy D, Gogas H, Levchenko E, de Braud F, Larkin J, Garbe C, Jouary T, Hauschild A, Grob JJ, Chiarion-Sileni V, Lebbe C, et al. Overall survival for dabrafenib and trametinib versus dabrafenib and placebo in V600 BRAF-mutant melanoma: a multi-center, double-blind, phase 3 randomised controlled trial. Lancet. 2015 doi: 10.1016/S0140-6736(15)60898-4. [DOI] [PubMed] [Google Scholar]
- 36.Larkin J, Ascierto PA, Dreno B, Atkinson V, Liszkay G, Maio M, Mandala M, Demidov L, Stroyakovskiy D, Thomas L, de la Cruz-Merino L, Dutriaux C, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med. 2014;371:1867–76. doi: 10.1056/NEJMoa1408868. [DOI] [PubMed] [Google Scholar]
- 37.Mittapalli RK, Vaidhyanathan S, Dudek AZ, Elmquist WF. Mechanisms limiting distribution of the threonine-protein kinase B-RaF(V600E) inhibitor dabrafenib to the brain: implications for the treatment of melanoma brain metastases. J Pharmacol Exp Ther. 2013;344:655–64. doi: 10.1124/jpet.112.201475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mittapalli RK, Vaidhyanathan S, Sane R, Elmquist WF. Impact of P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) on the brain distribution of a novel BRAF inhibitor: vemurafenib (PLX4032) J Pharmacol Exp Ther. 2012;342:33–40. doi: 10.1124/jpet.112.192195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sakji-Dupre L, Le Rhun E, Templier C, Desmedt E, Blanchet B, Mortier L. Cerebrospinal fluid concentrations of vemurafenib in patients treated for brain metastatic BRAF-V600 mutated melanoma. Melanoma Res. 2015;25:302–5. doi: 10.1097/CMR.0000000000000162. [DOI] [PubMed] [Google Scholar]
- 40.Long GV, Trefzer U, Davies MA, Kefford RF, Ascierto PA, Chapman PB, Puzanov I, Hauschild A, Robert C, Algazi A, Mortier L, Tawbi H, et al. Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): a multicentre, open-label, phase 2 trial. The lancet oncology. 2012;13:1087–95. doi: 10.1016/S1470-2045(12)70431-X. [DOI] [PubMed] [Google Scholar]
- 41.Niessner H, Forschner A, Klumpp B, Honegger JB, Witte M, Bornemann A, Dummer R, Adam A, Bauer J, Tabatabai G, Flaherty K, Sinnberg T, et al. Targeting hyperactivation of the AKT survival pathway to overcome therapy resistance of melanoma brain metastases. Cancer Med. 2013;2:76–85. doi: 10.1002/cam4.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seifert H, Hirata E, Gore M, Khabra K, Messiou C, Larkin J, Sahai E. Extrinsic factors can mediate resistance to BRAF inhibition in CNS melanoma metastases. Pigment Cell Melanoma Res. 2015 doi: 10.1111/pcmr.12424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fedorenko IV, Wargo JA, Flaherty KT, Messina JL, Smalley KS. BRAF Inhibition Generates a Host-Tumor Niche that Mediates Therapeutic Escape. J Invest Dermatol. 2015;135:3115–24. doi: 10.1038/jid.2015.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hirata E, Girotti MR, Viros A, Hooper S, Spencer-Dene B, Matsuda M, Larkin J, Marais R, Sahai E. Intravital imaging reveals how BRAF inhibition generates drug-tolerant microenvironments with high integrin beta1/FAK signaling. Cancer Cell. 2015;27:574–88. doi: 10.1016/j.ccell.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang T, Xiao M, Ge Y, Krepler C, Belser E, Lopez-Coral A, Xu X, Zhang G, Azuma R, Liu Q, Liu R, Li L, et al. BRAF Inhibition Stimulates Melanoma-Associated Macrophages to Drive Tumor Growth. Clin Cancer Res. 2015;21:1652–64. doi: 10.1158/1078-0432.CCR-14-1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Davies MA, Stemke-Hale K, Lin E, Tellez C, Deng W, Gopal YN, Woodman SE, Calderone TC, Ju Z, Lazar AJ, Prieto VG, Aldape K, et al. Integrated Molecular and Clinical Analysis of AKT Activation in Metastatic Melanoma. Clin Cancer Res. 2009;15:7538–46. doi: 10.1158/1078-0432.CCR-09-1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang L, Zhang S, Yao J, Lowery FJ, Zhang Q, Huang WC, Li P, Li M, Wang X, Zhang C, Wang H, Ellis K, et al. Microenvironment-induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth. Nature. 2015;527:100–4. doi: 10.1038/nature15376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seifert H, Hirata E, Gore M, Khabra K, Messiou C, Larkin J, Sahai E. Extrinsic factors can mediate resistance to BRAF inhibition in central nervous system melanoma metastases. Pigment Cell Melanoma Res. 2016;29:92–100. doi: 10.1111/pcmr.12424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brastianos PK, Carter SL, Santagata S, Cahill DP, Taylor-Weiner A, Jones RT, Van Allen EM, Lawrence MS, Horowitz PM, Cibulskis K, Ligon KL, Tabernero J, et al. Genomic Characterization of Brain Metastases Reveals Branched Evolution and Potential Therapeutic Targets. Cancer Discov. 2015 doi: 10.1158/2159-8290.CD-15-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Magbanua MJ, Melisko M, Roy R, Sosa EV, Hauranieh L, Kablanian A, Eisenbud LE, Ryazantsev A, Au A, Scott JH, Park JW. Molecular profiling of tumor cells in cerebrospinal fluid and matched primary tumors from metastatic breast cancer patients with leptomeningeal carcinomatosis. Cancer Res. 2013;73:7134–43. doi: 10.1158/0008-5472.CAN-13-2051. [DOI] [PubMed] [Google Scholar]
- 51.Schafer N, Scheffler B, Stuplich M, Schaub C, Kebir S, Rehkamper C, Mack F, Niehusmann P, Simon M, Greschus S, Kuchelmeister K, Herrlinger U, et al. Vemurafenib for leptomeningeal melanomatosis. J Clin Oncol. 2013;31:e173–4. doi: 10.1200/JCO.2012.46.5773. [DOI] [PubMed] [Google Scholar]
- 52.Lee JM, Mehta UN, Dsouza LH, Guadagnolo BA, Sanders DL, Kim KB. Long-term stabilization of leptomeningeal disease with whole-brain radiation therapy in a patient with metastatic melanoma treated with vemurafenib: a case report. Melanoma Res. 2013;23:175–8. doi: 10.1097/CMR.0b013e32835e589c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Floudas CS, Chandra AB, Xu Y. Vemurafenib in leptomeningeal carcinomatosis from melanoma: a case report of near-complete response and prolonged survival. Melanoma Res. 2016 doi: 10.1097/CMR.0000000000000257. [DOI] [PubMed] [Google Scholar]
- 54.Kim DW, Barcena E, Mehta UN, Rohlfs ML, Kumar AJ, Penas-Prado M, Kim KB. Prolonged survival of a patient with metastatic leptomeningeal melanoma treated with BRAF inhibition-based therapy: a case report. BMC Cancer. 2015;15:400. doi: 10.1186/s12885-015-1391-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wagle N, Van Allen EM, Treacy DJ, Frederick DT, Cooper ZA, Taylor-Weiner A, Rosenberg M, Goetz EM, Sullivan RJ, Farlow DN, Friedrich DC, Anderka K, et al. MAP kinase pathway alterations in BRAF-mutant melanoma patients with acquired resistance to combined RAF/MEK inhibition. Cancer Discov. 2014;4:61–8. doi: 10.1158/2159-8290.CD-13-0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Johnson DB, Flaherty KT, Weber JS, Infante JR, Kim KB, Kefford RF, Hamid O, Schuchter L, Cebon J, Sharfman WH, McWilliams RR, Sznol M, et al. Combined BRAF (Dabrafenib) and MEK inhibition (Trametinib) in patients with BRAFV600-mutant melanoma experiencing progression with single-agent BRAF inhibitor. J Clin Oncol. 2014;32:3697–704. doi: 10.1200/JCO.2014.57.3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wilgenhof S, Neyns B. Complete Cytologic Remission of V600E BRAF-Mutant Melanoma-Associated Leptomeningeal Carcinomatosis Upon Treatment With Dabrafenib. J Clin Oncol. 2015;33:e109–11. doi: 10.1200/JCO.2013.48.7298. [DOI] [PubMed] [Google Scholar]
- 58.Simeone E, De Maio E, Sandomenico F, Fulciniti F, Lastoria S, Aprea P, Staibano S, Montesarchio V, Palmieri G, Mozzillo N, Ascierto PA. Neoplastic leptomeningitis presenting in a melanoma patient treated with dabrafenib (a V600EBRAF inhibitor): a case report. J Med Case Rep. 2012;6:131. doi: 10.1186/1752-1947-6-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Manousakis G, Koch J, Sommerville RB, El-Dokla A, Harms MB, Al-Lozi MT, Schmidt RE, Pestronk A. Multifocal radiculoneuropathy during ipilimumab treatment of melanoma. Muscle Nerve. 2013;48:440–4. doi: 10.1002/mus.23830. [DOI] [PubMed] [Google Scholar]
- 60.Abdallah AO, Herlopian A, Ravilla R, Bansal M, Chandra-Reddy S, Mahmoud F, Ong S, Gokden M, Hutchins L. Ipilimumab-induced necrotic myelopathy in a patient with metastatic melanoma: A case report and review of literature. J Oncol Pharm Pract. 2015 doi: 10.1177/1078155215572932. [DOI] [PubMed] [Google Scholar]
- 61.Chamberlain MC. A phase II trial of intra-cerebrospinal fluid alpha interferon in the treatment of neoplastic meningitis. Cancer. 2002;94:2675–80. doi: 10.1002/cncr.10547. [DOI] [PubMed] [Google Scholar]
- 62.Guirguis LM, Yang JC, White DE, Steinberg SM, Liewehr DJ, Rosenberg SA, Schwartzentruber DJ. Safety and efficacy of high-dose interleukin-2 therapy in patients with brain metastases. J Immunother. 2002;25:82–7. doi: 10.1097/00002371-200201000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Powell S, Dudek AZ. Single-institution outcome of high-dose interleukin-2 (HD IL-2) therapy for metastatic melanoma and analysis of favorable response in brain metastases. Anticancer Res. 2009;29:4189–93. [PubMed] [Google Scholar]
- 64.Glitza IC, Rohlfs M, Bassett RL, Ida J, Richard J, Iqbal M, Bernzen T, Gerber D, Lacey C, Diab A, Amaria R, Woodman SE, et al. Therapeutic outcomes of intrathecal IL-2 in metastatic melanoma patients with leptomeningeal disease. Society for NeuroOncology. 2015 ICMT-07. [Google Scholar]
- 65.Hong JJ, Rosenberg SA, Dudley ME, Yang JC, White DE, Butman JA, Sherry RM. Successful treatment of melanoma brain metastases with adoptive cell therapy. Clin Cancer Res. 2010;16:4892–8. doi: 10.1158/1078-0432.CCR-10-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Clemons-Miller AR, Chatta GS, Hutchins L, Angtuaco EJ, Ravaggi A, Santin AD, Cannon MJ. Intrathecal cytotoxic T-cell immunotherapy for metastatic leptomeningeal melanoma. Clin Cancer Res. 2001;7:917s–24s. [PubMed] [Google Scholar]
- 67.Glitza IC, Haymaker C, Bernatchez C, Vence L, Rohlfs M, Richard J, Lacey C, Mansaray R, Fulbright OJ, Ramachandran R, Toth C, Wardell S, et al. Intrathecal Administration of Tumor-Infiltrating Lymphocytes Is Well Tolerated in a Patient with Leptomeningeal Disease from Metastatic Melanoma: A Case Report. Cancer Immunol Res. 2015;3:1201–6. doi: 10.1158/2326-6066.CIR-15-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schartz NE, Farges C, Madelaine I, Bruzzoni H, Calvo F, Hoos A, Lebbe C. Complete regression of a previously untreated melanoma brain metastasis with ipilimumab. Melanoma Res. 2010;20:247–50. doi: 10.1097/CMR.0b013e3283364a37. [DOI] [PubMed] [Google Scholar]
- 69.Weber JS, Amin A, Minor D, Siegel J, Berman D, O'Day SJ. Safety and clinical activity of ipilimumab in melanoma patients with brain metastases: retrospective analysis of data from a phase 2 trial. Melanoma Res. 2011;21:530–4. doi: 10.1097/CMR.0b013e32834d3d88. [DOI] [PubMed] [Google Scholar]
- 70.Bot I, Blank CU, Brandsma D. Clinical and radiological response of leptomeningeal melanoma after whole brain radiotherapy and ipilimumab. J Neurol. 2012;259:1976–8. doi: 10.1007/s00415-012-6488-4. [DOI] [PubMed] [Google Scholar]
- 71.Kienast Y, von Baumgarten L, Fuhrmann M, Klinkert WE, Goldbrunner R, Herms J, Winkler F. Real-time imaging reveals the single steps of brain metastasis formation. Nat Med. 2010;16:116–22. doi: 10.1038/nm.2072. [DOI] [PubMed] [Google Scholar]
- 72.Cho JH, Robinson JP, Arave RA, Burnett WJ, Kircher DA, Chen G, Davies MA, Grossmann AH, VanBrocklin MW, McMahon M, Holmen SL. AKT1 Activation Promotes Development of Melanoma Metastases. Cell Rep. 2015;13:898–905. doi: 10.1016/j.celrep.2015.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]