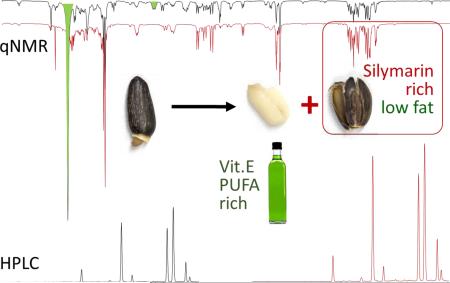
Abstract
An improved method for the purification of silymarin, the flavonolignan complex from the fruits of milk thistle, Silybum marianum, is reported. The method enables a more efficient extraction of silymarin from the pericarp after it has been separated mechanically from the rest of the fruits. Accelerated solvent extraction (ASE) was employed for each extraction procedure. Quantitation of the eight major silymarin components in the pericarp extract was compared to that of the whole fruit extract using two orthogonal analytical methods. The pericarp extract showed higher silymarin content (2.24-fold by HPLC and 2.12- fold by qHNMR) than whole fruit extract using acetone as an extraction solvent following defatting with hexane. Furthermore, the mg/g recovery of silymarin major components was not diminished by eliminating the hexane defatting step from the pericarp extraction procedure. The efficiencies of acetone, ethanol, and methanol as extraction solvents were compared. Methanol pericarp extract showed the highest content of the silymarin major components, 2.72-fold higher than an extract prepared from the whole fruits using acetone. Finally, all of the major silymarin components showed a higher w/w content in the pericarp extract than in a commercial extract.
Keywords: Silybum marianum, silymarin, pericarp, accelerated solvent extraction
Graphical Abstract
1. Introduction
Silybum marianum (L.) Gaertn. (Asteraceae) is an annual or biennial plant native to the Mediterranean region, Sinai, eastwards to Afghanistan, and has been naturalized in other parts of the world [1]. The fruits of this plant are of the achene type and have a shiny dark brown to black color. Extracts prepared from these fruits have been used for the treatment of liver disorders since ancient times [2], and have also been used as a chemopreventive agent for human prostate carcinoma [3]. In 2014, the plant held the sixth position among the top-twenty most selling herbal dietary supplements in the natural and health food market, and at the twelfth position among the forty top-selling herbal supplements in the mainstream multi-outlet channel market in the U.S., at about $9.2 million and $16.4 million in sales, respectively [4]. One S. marianum fruit head can produce 100 to nearly 200 fruits, and about 40-45 fruits yield a gram of crude herbal material [5].
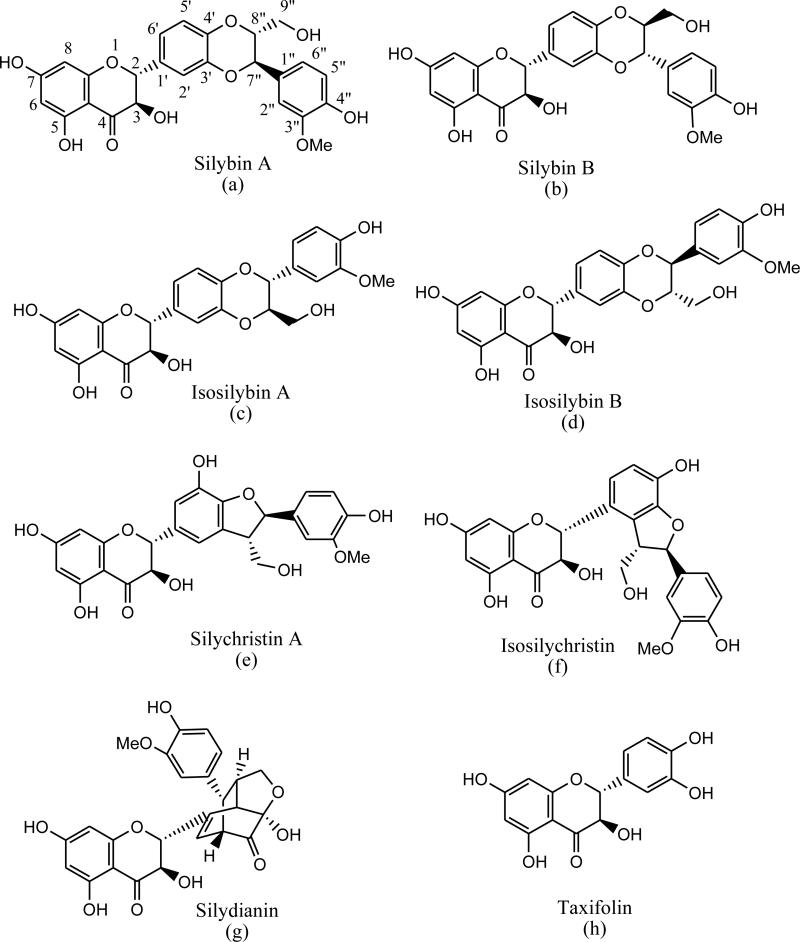
Silymarin is the designated main active principle of standardized S. marianum fruit extracts and represents a complex mixture, of which seven major flavonolignans and the flavanonol, taxifolin, make up 65-80% w/w with minor and unknown related congeners comprising the balance. According to the European and American Pharmacopeias, mature fruits yield no less than 1.5-2% of silymarin-standardized extract. Commercial S. marianum fruit extracts typically contain 70-80% silymarin. The seven major flavonolignans are the silybins A (a) and B (b), the isosilybins A (c) and B (d), silychristin A (e), isosilychristin (f), and silydianin (g) (Fig. 1). The seven major flavonolignans and taxifolin (h) may be considered as silymarin marker compounds for quantitation studies. Recently, silychristin B, isosilybin C, and isosilybin D have been reported as belonging to the group of previously unknown, minor constituents of silymarin [6-7].
Fig. 1.
Chemical structures of the silymarin marker compounds.
S. marianum fruit contain relatively large amounts (ca. 20-25% w/w) of fatty oil that consists of essential phospholipids with a high content of unsaturated fatty acids such as oleic and linoleic acids, and vitamin E [8-9]. Accordingly, the S. marianum fruit lipids possess a high nutritional value. The fatty acid composition of the seeds growing wild in Tunisia has been reported [10]. Linoleic acid had the highest relative content (60%), followed by oleic acid (21%) and palmitic acid (13%). However, the oil is considered an unwanted byproduct of the silymarin production and has to be removed from the fruits prior to silymarin extraction. Therefore, it is common practice that the production of silymarin from the fruits of S. marianum requires an initial defatting step, which uses a highly lipophilic solvent such as hexane or petroleum ether. On a large or even industrial scale, this is associated with long processing times, consumes relatively large amounts of organic solvents, and leads to a significant content of oily, lipophilic contaminants in the silymarin products. Moreover, it is inevitable that lipophilic contaminants and solvent residues will be found in silymarin. These residual components have to be separated from the silymarin complex during sample preparation in botanical quality control, which complicates analysis and interferes with accuracy.
Previous studies have shown that S. marianum fruits consist of four parts: pericarp, seed integument, albumen, and embryo [11]. The pericarp consists of three layers; the epidermis, the subepidermic layer, and the membraniform layer [12]. Some of the cells of the subepidermic layer are filled with dark brown content responsible for the spotted appearance of the fruits. The seed integument consists of the epidermis of the integument and a few cell layers that contain calcium oxalate crystals. The albumen consists of one layer of cells with protein as a storage material. The embryo includes two large cotyledons with fat as storage material. Flavonolignans, the major silymarin components, have been localized in the outer portion of the fruit, which includes all the cell layers from the pericarp epidermis to the albumen [13]. Using a histochemical method which stains silybin, silychristin A, and silydianin as well as the flavanonol, taxifolin, these silymarin constituents were found to be localized in the following cellular layers: the subepidermic cell layer and the membraniform layer of the pericarp, and all the layers of the seed integument [11].
It has been hypothesized in much of the S. marianum literature that flavonolignans are biosynthesized from taxifolin and coniferyl alcohol. The latter is a lignin precursor likely associated with cell-wall materials. These histochemical studies were further supported by a phytochemical study reporting that flavonolignans are mostly situated in the outer covering of the fruits [14]. In the present study, a simplified and more economic method for the production of an enhanced, silymarin-rich, but fatty oil depleted (ideally: free) extract from the fruits of S. marianum, was developed by removing the pericarp from the kernel prior to solvent extraction.
Quantitation of silymarin in a given extract is complicated by the fact that part of what is considered to be silymarin is an unidentified mixture of flavonolignans. In many previous reports of extraction methods, the product quality was quantitatively determined by the 2,4-dinitrophenylhydrazine derivatization followed by spectrophotometry at 490 nm [15]. This method assesses the content of total ketones and is limited in selectivity. It determines all flavonoles in S. marianum fruits, not only the flavonolignans that constitute the silymarin complex. Chromatographic analysis of the product provides more detailed information about the silymarin composition. HPLC quantitation of the eight major silymarin components has the advantage of separation along with quantitation to determine the relative amounts of the major components. NMR may also provide useful qualitative and quantitative information in analysis of complex mixtures such as plant extracts [16-17]. qNMR can offer an overview of the sample composition through quantification of multiple metabolites without the need for chromatographic separation. This method, orthogonal to the aforementioned LC-based analysis, allows quantification of targeted compounds without the need of reference materials. For example, 1H NMR was proposed as a method for authentication of vegetable oils such as olive oil [18]. This is mainly useful in detecting cheaper oils such as sunflower oil, used as an adulterant [19].
2. Materials and methods
2.1. Plant material
S. marianum plants were collected beside the Cairo-Alexandria dessert road, Egypt (31°0′8.45″N, 29°48′20.91″E) in March, 2014. The plant was botanically authenticated by Dr. Abdel Halim Mohamed, Flora and Phytotaxonomy Department, Agricultural Research Center, Cairo Egypt. Ripe fruits were manually separated from the heads, freed from the pappus, and kept at −20°C until use.
2.2. Chemicals
Silymarin (S0292-10G, Sigma, China), silybin (A & B) primary reference standard 92.53% (HWI Analytik GmbH Pharma Solutions, Rülzheim, Germany), and taxifolin analytical standard 85% (Sigma-Aldrich, St. Louis, MO, USA) were used as reference compounds. These purities were assayed by qNMR according to the manufacturer. Isosilychristin, silychristin A, and silydianin were isolated by preparative column chromatography and their purity determined by an absolute qHNMR method [20]. Briefly, samples were weighed (0.01 mg accuracy), dissolved in 600 μl of DMSO-d6 (Lot no. 10E-645, Cambridge Isotope Laboratories, Inc., Andover, MA,USA) using a Pressure-Lok gas syringe (VICI Precision Sampling Inc., Baton Rouge, LA, USA), and transferred into 5-mm standard NMR tubes (NORELL Inc., Landisville, NJ, USA). The qHNMR spectrum was measured for the calibration sample using quantitative acquisition and processing parameters [20]. The percentage of each silymarin component was calculated as silibinin using the external standard method. The qHNMR purities were as follows: isosilychristin 73.96%, silychristin A 80.80%, and silydianin 84.83%. The solvents used in this study, n-hexane and acetone (Pharmaco-AAper, Brookfield, CT, USA) were of reagent grade and re-distilled before use. For analytical HPLC, methanol (Fisher Scientific, Fair Lawn, NJ, USA), water, and formic acid (Sigma-Aldrich, St. Louis, MO, USA) were of HPLC grade.
2.3. Pericarp separation and silymarin extraction
The ground plant material (whole fruits, 1.00 g; pericarp or kernel equivalent to 1.00 g of whole fruits) was used for the extraction of silymarin. The pericarp was separated from the kernel using the following procedure: initial soaking of the fruits in distilled water (10 ml) at room temperature; after 24 h, the water was removed by decantation, and the fruits were subjected to rolling using a wooden roller. During this procedure, the pericarp was separated from the kernel. The pericarp represents 41%, and the kernels 52% of the weight of the water-soaked fruits. The pericarp and kernels were then air-dried, ground, and subjected to organic solvent extraction procedures. The soaking water was extracted with ethyl acetate twice. The combined extracts were evaporated, and subjected to chromatographic analysis.
Accelerated solvent extraction (ASE) was carried out on a Dionex ASE350 (Dionex Corporation, Sunnyvale, CA, USA). The plant material was placed into a 34 ml stainless steel extraction cell containing filter paper at the bottom. The cell was tightly closed and placed in the extractor. In all but one extraction, two extraction cycles were used. First, the plant material was defatted with n-hexane at 50°C, 1600 psi, for 30 min. Second, the content of the cell was extracted with acetone, ethanol or methanol at a temperature of 70°C and a pressure of 1700 psi for 15 min. The extraction volumes were 61-64 ml. The solvent extracts were evaporated to dryness under vacuum, dried in a vacuum desiccator, weighed, and dissolved in methanol for chromatographic analysis.
2.4. Chromatographic analysis of the extracts
A Waters Alliance 2695 High Performance Liquid Chromatography (HPLC) system equipped with a PDA and an Agilent ZORBAX SB-C18 column were used to analyze the content of individual silymarin components according to a reported method [20]. A gradient with MeOH as modifier and H2O/0.1% formic acid as base solvent was used as mobile phase, starting at 30:70 and increasing linearly to 60:40 over 32 min. The flow rate was 1.0 ml/min, and ambient temperature was used. Ten μl of the extract constituted in 5 ml methanol were injected into the HPLC and quantified at 288 nm employing the appropriate response factors. The method offered the following retention times (minutes) for the major silymarin compounds: taxifolin (Rt = 13.2), isosilychristin (Rt = 17.8), silychristin A (Rt= 19.7), silydianin (Rt = 21.5), silybin A (Rt = 27.1), silybin B (Rt = 28.1), isosilybin A (Rt = 30.2), and isosilybin B (Rt = 30.8).
2.5. qHNMR sample preparation
A Bruker AVANCE-400 spectrometer (9.4T/400 MHz, Oxford magnet) equipped with a 5 mm Z-gradient probe BBO (broadband observe) networked with MS Windows-based computer was used for acquiring the 1H NMR spectra. Samples were weighed (whole fruit extract 10.81 mg, defatted and acetone extracted pericarp 10.31 mg), dissolved in 600 μl of DMSO-d6 (Dimethyl sulfoxide-d6, Lot no. 10E-645, Cambridge Isotope Laboratories, Inc., Andover, MA, USA) using a Pressure-Lok gas syringe (VICI Precision Sampling Inc., Baton Rouge, LA, USA), and transferred into 5-mm standard NMR tubes (NORELL Inc., Landisville, NJ, USA). A qHNMR spectrum was acquired using quantitative acquisition and processing parameters as shown in Table 1. 1HNMR data were analyzed using MestReNova 10.0.2-15465. Phase was corrected manually and baseline correction was carried out using a Bernstein polynomial fit. The chemical shift values in the spectra were referenced to the residual solvent signal at 2.500 ppm.
Table 1.
Quantitative acquisition and processing parameters for qHNMR measurements
| Pulse Width | Single 90° pulse with GARP carbon decoupling |
| Relaxation delay | 60 s |
| Acquisition time | 4 s |
| Number of Scans | 64 |
| Spinning status | Non-spinning |
| Sample temperature | 25°C |
| Data points acquired | 64 K |
| Dummy scans | 4 |
| Preacquisition delay | 59.57 μsec |
| Probe adjustments | Tuning and matching were performed on each sample |
2.6. Statistical analysis
One-way ANOVA using Microsoft Excel/XLSTAT was used to analyze variation between different treatments. Tukey's HSD test was used for analysis of differences between the extraction protocols, with a confidence interval of 95%. Dunnett's test was used to analyze the differences between the whole fruits extract (control) and the pericarp extracts using a different protocol as shown in Table 2, also with a confidence interval of 95%.
Table 2.
Contents of individual silymarin constituents in Silybum marianum extracts obtained from whole fruits, pericarp, and kernels (mg/g plant material DW).
| * | Taxifolin | Isosilychristin | Silychristin A | Silydianin | Silybin A | Silybin B | Isosilybin A | Isosilybin B | Silymarin |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.57±0.07 | 0.04±0.01 | 2.77±0.24 | 0.24±0.03 | 2.47±0.23 | 3.91±0.35 | 0.86±0.08 | 0.14±0.02 | 11.02±1.02 |
| 2 | 1.43±0.10 | 0.19±0.01 | 6.31±0.51 | 0.68±0.10 | 5.49±0.37 | 8.09±0.45 | 2.10±0.18 | 0.43±0.03 | 24.72±1.72 |
| 3 | n.d. | n.d. | 0.01±0.01 | n.d. | 0.01±0.00 | 0.05±0.02 | n.d. | n.d. | 0.07±0.03 |
| 4 | 1.27±0.02 | 0.15±0.02 | 6.00±0.07 | 0.66±0.01 | 5.45±0.08 | 8.00±0.09 | 1.98±0.04 | 0.41±0.01 | 23.91±0.30 |
| 5 | 1.11±0.09 | 0.13±0.01 | 4.71±0.11 | 0.48±0.01 | 4.10±0.07 | 6.28±0.12 | 1.44±0.02 | 0.27±0.00 | 18.53±0.42 |
| 6 | 1.74±0.13 | 0.21±0.02 | 6.91±0.29 | 0.79±0.05 | 6.00±0.26 | 8.56±0.26 | 2.24±0.12 | 0.46±0.03 | 29.94±1.15* |
Extraction procedures: 1 = whole fruits extracted with acetone after defatting with n-hexane, 2 = pericarp extracted with acetone after defatting with n-hexane, 3 = kernel extracted with acetone after defatting with n-hexane, 4 = pericarp extracted with acetone without defatting step, 5 = pericarp extracted with ethanol after defatting with n-hexane, 6 = pericarp extracted with methanol after defatting with n-hexane.
3. Results and discussion
3.1. Separation of the pericarp from S. marianum fruits
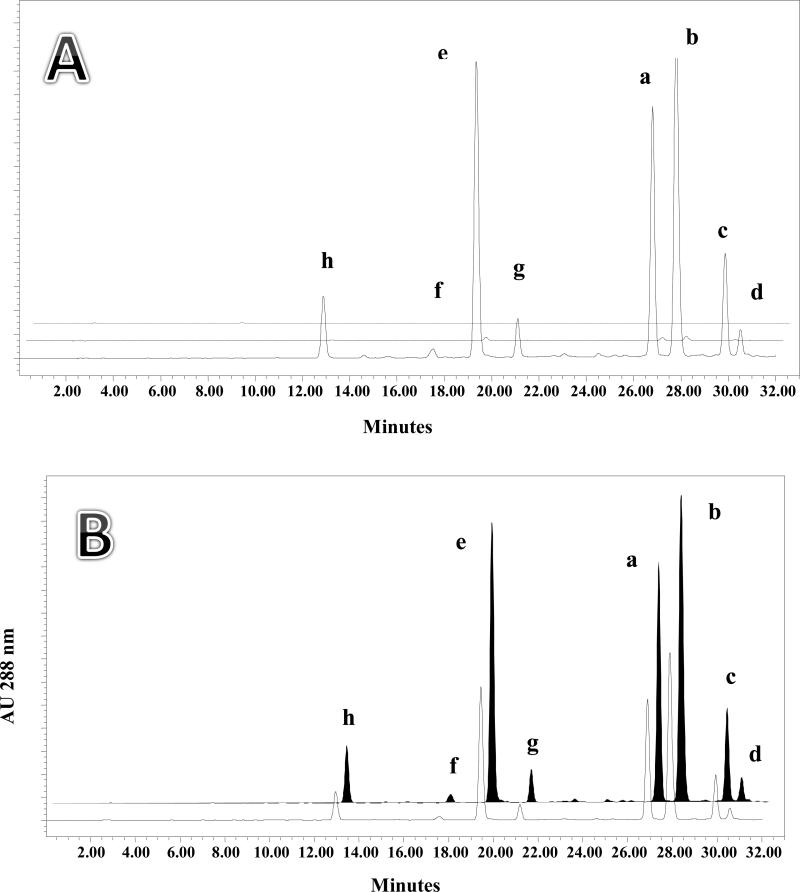
The key to developing a more efficient method for silymarin production was the physical separation of the pericarp from the other tissues (kernel) of the milk thistle fruits. For this purpose, S. marianum fruits were soaked in distilled water for 24 h. After filtration, the fruits were subjected to cold pressure using a wooden roller. The entire pericarp and kernel could thereby be separated, as shown in Fig. 2. The separated pericarp and kernels were then air-dried and, initially, extracted with acetone, after defatting with n-hexane. The extracts were then subjected to HPLC analysis for determination of the individual silymarin compounds. In order to assess potential losses, the kernels were extracted with the same procedure and the soaking water was back-extracted with ethyl acetate and subjected to HPLC. The chromatogram in Fig. 3A shows the eight silymarin marker compounds in the pericarp, kernel, and soaking water. As expected, almost no silymarin constituents were lost into the soaking water, which is consistent with the lipophilic nature of the flavonolignans. Table 2 shows the quantitative data from defatted acetone extracts of the pericarp (2) and kernels (3). The data demonstrate that the silymarin flavonolignans are mainly concentrated in the pericarp of S. marianum fruits. As a result, the use of the outer covering of the fruits can be recommended for the production of silymarin.
Fig. 2.
Silybum marianum fruits pericarp detached from the kernels. Left column: whole fruits, middle column: kernels, and right column: pericarps.
Fig. 3.
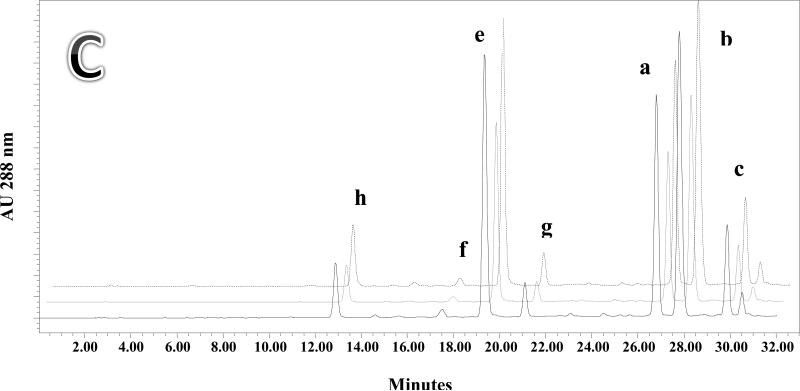
A: HPLC chromatograms showing the analysis of silymarin components contained in Silybum marianum pericarp (front, solid line), kernel (middle, dashed line), and soaking water (back, dotted line). Quantitation data for pericarp and kernel are found in Table 2 extracts 2 and 3 respectively. B: HPLC chromatograms showing the analysis of silymarin components obtained by extraction of Silybum marianum whole fruits (front, solid line) and pericarp (back, black filled peaks). Quantitation data for these extracts are found in Table 2, extracts 1 and 2 respectively. C: HPLC chromatograms showing the content of silymarin components obtained by extracting pericarp with acetone (front, solid line), ethanol (middle, dotted line), and methanol (back, dashed line). Quantitation data for these extracts are found in Table 2, extracts 2, 4 and 5 respectively.
3.2. Comparison of S. marianum whole fruit and pericarp extracts
The quantitative recovery of silymarin marker compounds was measured from parallel extractions of whole fruits and pericarps. Both chromatographic (HPLC) and spectroscopic (qNMR) methods were employed to assess the content of silymarin marker compounds in whole fruits extract and simple pericarp extracts. The whole fruits and pericarp were subjected to identical extraction procedures, including defatting steps for comparison. The HPLC chromatograms of the two extracts are shown in Fig. 3B. Table 2 shows that the total silymarin content in the pericarp extract was 2.24-fold higher than that of the whole fruit extract.
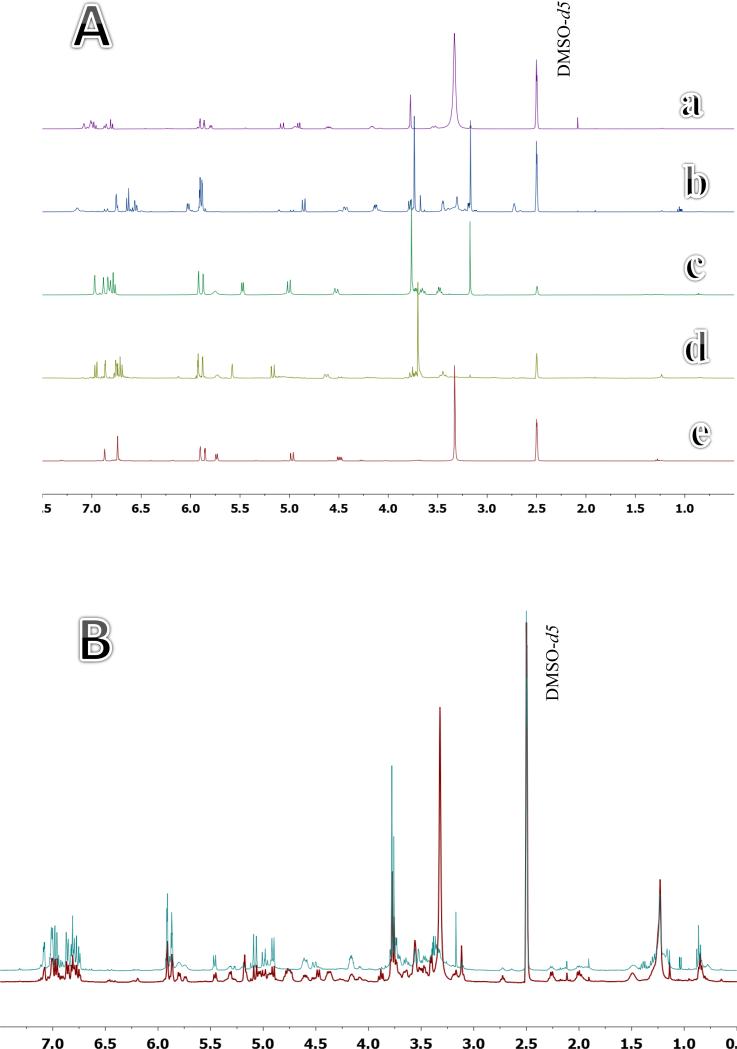
The same whole fruit and pericarp extracts were compared using quantitative nuclear magnetic resonance spectroscopy (qNMR). A quantitative comparison between the two crude extracts may be made by careful analysis of the individual spectral characteristics of the silymarin marker compounds. From previous qNMR studies [21] and the five spectra shown in Fig. 4A, three regions that are distinctive for silymarin marker compounds may be identified. The interval from 4.85 to 5.35 ppm is a group of distinctive aryl protons and phenolic hydroxyl groups. For example, in silychristin A these would be H-7”, OH-9”, and H-2. The interval from 5.72 to 5.95 ppm is a second group of protons that corresponds to OH-3, H-8, and H-6 of silychristin A. The third interval of 6.73-7.15 corresponds to H-5”, H-6”, H-5’, H-6’, H-2”, and H-2’ of silychristin A. The other silymarin marker compounds also have distinctive resonances in these regions.
Fig. 4.
A. 1H NMR spectra of silybin (A & B) (a), silydianin (b), silychristin A (c), isosilychristin (d), and taxifolin (e). B. 1H NMR spectra for whole fruit (red, front) and pericarp (blue, back) extracts (expanded region, 0.5 – 7.5 ppm). Quantitation data for these extracts are presented in Table 3.
The comparison of the integration of each region in the whole fruit extract with the similar regions in the pericarp extract is a reasonable method to determine the relative recovery of silymarin marker compounds. Assumptions include: 1) the composition of the two extracts are similar, 2) there are no impurities with resonances in the designated regions that are present in only one extract, and 3) the relative ratios of silymarin marker compounds in the two extracts are similar.
The 1H NMR spectra of the whole fruit and pericarp extracts are shown in Fig. 4B. Visual inspection of the two spectra shows variation between the two extracts in the contents of silymarin and oily compounds. S. marianum seeds contain 20-25% w/w oily material, localized mainly in the kernel and partly isolated with silymarin during preparation from the whole fruits. The integral values of the three regions corresponding to flavonolignan signals were compared in qHNMR spectra of whole fruit and pericarp extracts. Data are shown in Table 3. The residual protonated solvent signal (DMSO-d5) was used as a calibrant and set to 100. A correction of the chemical shift regions integral values was then applied according to the weights of the two extracts. The ratios of integral values of signal regions corresponding to silymarin complex were then calculated. The average ratio for integral values shows that the silymarin marker content in the pericarp extract was 2.12-fold higher than that of the whole fruit extract which is comparable to the 2.24-fold value obtained with HPLC.
Table 3.
qHNMR comparison between extracts prepared from the whole fruits and pericarp of Silybum marianum (extracts 1 and 2 in Table 2 respectively).
| Chemical shift (ppm) | Whole fruit | Pericarp | Ratio | Ratio (extract wt basis) |
|---|---|---|---|---|
| 2.45 - 2.52 (DMSO-d5) | 1.00 | 1.00 | 1.00 | 1.00 |
| 4.85 - 5.35 | 0.49 | 0.98 | 2.00 | 2.10 |
| 5.72 - 5.95 | 0.26 | 0.55 | 2.12 | 2.22 |
| 6.73 – 7.15 | 0.62 | 1.21 | 1.95 | 2.04 |
3.3. Comparison of S. marianum pericarp extracts with and without defatting
In case of extraction from the pericarp, the defatting process may be omitted without affecting the yield of total silymarin significantly. This is clearly shown by comparing the quantitation of silymarin marker compounds obtained from pericarp acetone extracts without a defatting step 23.91 mg/g (experiment 4 on Table 2) and extracts with n-hexane defatting 24.72 mg/g (experiment 2). The omission of the defatting step allows the extraction of silymarin in an environmentally friendly manner by reducing the consumption of petroleum-based solvents.
3.4. Silymarin extraction with three different solvents
The effect of different organic solvents on the extraction of silymarin was also studied. It has been reported previously that acetone had higher extraction efficiency for the silymarin complex than methanol and ethyl acetate [22]. Fig. 3C shows the HPLC chromatograms of pericarp extracts made using acetone, ethanol, and methanol with accelerated solvent extraction including a defatting step. Table 2, experiments 2 (acetone), 5 (ethanol) and 6 (methanol) show the quantitation of the individual silymarin marker compounds in the extracts as being 24.72, 18.53, and 29.94 mg/g respectively.
3.5. Silymarin marker compounds in a commercial extract
Table 4 compares the content of the individual silymarin marker compounds in commercially available silymarin (Sigma) vs. silymarin prepared from the pericarp using a defatting step and acetone extraction (experiment 2 in Table 2). All of the eight markers showed a higher w/w content in the silymarin complex when prepared with the pericarp only. The silymarin marker content represented 60.0% (w/w) of the extract prepared from the pericarp, in which the two silybins (silybin A & B) represented 29.7% of the total silymarin content. The calculation was based on the HPLC analysis of the seven major silymarin compounds and taxifolin. However, this method does not include minor and unknown components that contribute about 20 ~ 35% of the total silymarin content in a typical silymarin extract [7].
Table 4.
Contents of individual Silymarin constituents in extract prepared from Silybum marianum pericarp (P – extract 2 in Table 2) compared to commercially available Silymarin (S) (% w/w).
| Taxifolin | Isosilychristin | Silychristin A | Silydianin | Silybin A | Silybin B | Isosilybin A | Isosilybin B | Silymarin | |
|---|---|---|---|---|---|---|---|---|---|
| P | 1.22±0.03 | 0.45±0.03 | 9.24±0.04 | 1.47±0.01 | 9.82±0.05 | 15.84±0.02 | 3.40±0.05 | 0.66±0.01 | 42.09±0.13 |
| S | 4.88±0.09 | 1.66±0.03 | 13.22±0.23 | 3.07±0.06 | 11.40±0.17 | 18.28±0.26 | 5.60±0.08 | 1.80±0.02 | 59.91±0.7 |
3.6. One way ANOVA
One-way ANOVA was used for the analysis of the variation between the different extraction procedures. The adjusted coefficient of determination (R2) indicated that 86.6% of the variability in the silymarin content can be explained by the treatments. The other 13.4% are due to random error. Fisher's F test showed that the probability corresponding to the F value is 0.0001, which implies a 0.01% risk for the conclusion that the null hypothesis (i.e., protocols have no effect) is wrong. Therefore, it can be concluded that the protocols do affect the silymarin content in the prepared extracts. Tukey's HSD test was used for the analysis of differences between the protocols with a confidence interval of 95%. This showed that all the protocols using the pericarp for extracting the silymarin compounds were significantly different from the protocols using the whole fruits. Moreover, protocols using ethanol as an extraction solvent showed significant differences from other protocols using acetone or methanol. The REGWQ - Ryan / Einot and Gabriel / Welsch test procedure gives similar results to those obtained by the Tukey's HSD test. Dunnett's test was used to analyze the differences between the protocols using the whole fruits (control) and the other protocols using the pericarp, with a confidence interval of 95%. The Dunnett's test result agrees with the REGWQ outcome, namely: that the extracts of the whole fruits are significantly different from extracts of other materials.
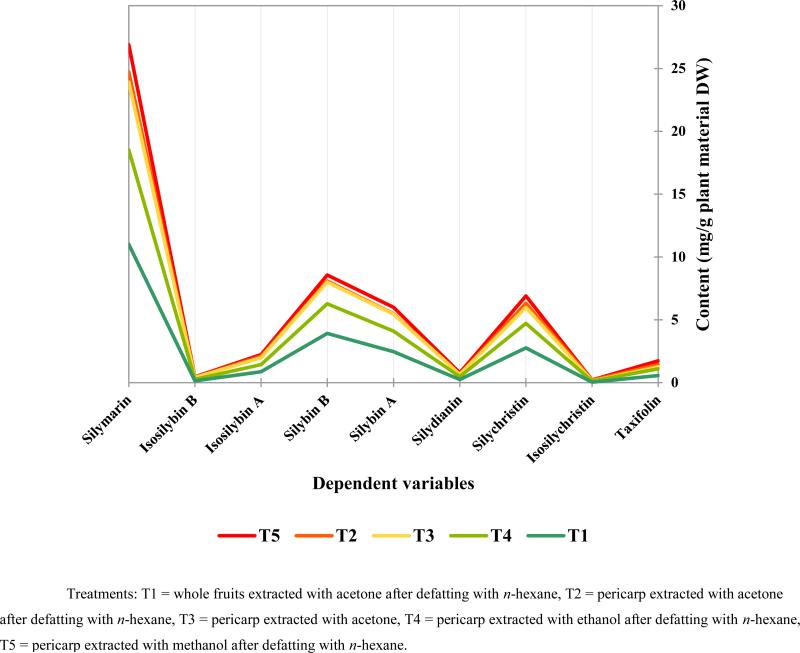
The overall conclusion is that the four protocols (experiments 2, 4, 5, and 6 in Table 2) showed significant difference in the achieved recovery of silymarin marker compounds than the control protocol (experiment 1 in Table 2). The variation in composition of extracts from experiments 1, 2, 4, 5, and 6 in Table 2 are shown in Fig. 5. The protocol using the whole fruit is the conventional method for obtaining silymarin, whereas the protocols that used acetone or methanol for extracting the pericarp showed significant increases in recovery.
Fig. 5.
Graphical representation of the quantitation data presented in Table 2 for extracts 1 (T1), 2 (T2), 4 (T3), 5 (T4), and 6 (T5).
4. Conclusions
Silymarin extraction from S. marianum fruits has been reported using different methods. Gupta et al. [23] loaded the alcoholic extract of the fruits onto a silica gel column and developed the column with benzene-ethyl acetate mixtures of increasing polarity. Silymarin was isolated from the eluate by precipitation from methanol. This method gave 86% pure silybin (m.p. 167-180°C; reported values). However, this method involves the costly step of silica gel column chromatography which is uneconomical when used on an industrial scale.
A second method pre-cooled the fruits to −20°C for 24 h, making the fruits brittle and friable [24]. This was followed by powdering the frozen fruits in a hammer mill. Defatting the fruits by extraction with hexane in a Soxhlet extractor removed the major portion of the fatty oil. The next step was extraction of the defatted fruits with acetonitrile at 20 - 30°C. The obtained silymarin fraction was stirred with cold dichloromethane at 5°C, filtered, and the filtrate was dried with a slow purge of nitrogen gas. The silymarin was further purified by suspension in acetonitrile and precipitation using water at 20°C. The precipitated silymarin was then washed with distilled water and dried in a vacuum oven.
A third method used a hydrocarbon solvent (n-hexane or petroleum ether) for defatting, followed by extraction with acetone, which was considered the most economic and least toxic solvent [25]. The extract was subjected to azeotropic drying with toluene. This was followed by a defatting step with di-isopropyl ether.
An alternative to the use of petroleum ether was suggested by Subramaniam et al. [26]. Pretreatment of the fruit meal with 1.5% H2SO4 (w/w) at 50°C for 18 h was followed by 4 h extraction with ethanol at 60°C. All of the aforementioned methods depend on a two-step phytochemical process for the preparation of silymarin from the crude fruit material.
Finally, hot water has been proposed as a green solvent for the extraction of silymarin from milk thistle [27]. As an extraction solvent, water has the advantage of low purchase and disposal costs. The dielectric constant, surface tension, and viscosity of water can be controlled by adjustment of temperature and pressure. Water extraction also does not require the defatting step. In addition, the time required for extraction of silymarin compounds decreases with increasing extraction temperature. However, the use of high temperatures causes degradation of the silymarin compounds and represents a major drawback of the approach [28].
The new method represented by extraction 4 in Table 2 offers several advantages compared to the previously described methods. First, it provides a higher yield of silymarin marker compounds. Second, the method minimizes thermal degradation of silymarin compounds observed with commonly used elevated extraction temperatures and/or extended extraction times. Third, it also provides a product with better solubility in hydrophilic solvents when compared to commercially available silymarin. This could be due to the reduced content in fatty material. Fourth, the absence in the procedure of low polarity, or highly toxic solvents such as n-hexane, petroleum ether, light petroleum and chloroform, which are otherwise necessary for the defatting step, enhances the environmental friendliness of the extraction method. However, in certain situations, the use of a defatting step may actually have utility as it might reduce the content of unwanted lipophilic compounds such as herbicides, which tend to accumulate in the outer layers of the fruits. Fifth, the proposed method reduces the cost and time of the silymarin preparation process. Because only the pericarp is used, the total amount of solvent per unit of silymarin is further reduced when compared with methods that employ the whole fruits. Sixth, the kernels, which are byproducts from the physical separation process, can be potentially used for the production of, e.g., a nutritionally valuable vegetable oil that is rich in vitamin E and PUFAs.
This study introduced a qNMR method for quantitating silymarin marker compounds in crude extracts. The advantages of the qNMR method over the HPLC method are: (1) no standards are needed to calculate response factors; (2) structural information of both silymarin and non-silymarin constituents is readily available; (3) the same spectra may be used to determine relative quantities of silymarin marker compounds as demonstrated by Napolitano et al. [21] and Cheilari et al. [29]; (4) less solvent is employed for analysis; and (5) the ppm intervals that were chosen are likely to also include resonances from minor flavonolignans in addition to the known silymarin marker compounds.
Silymarin is an important raw material in the pharmaceutical and dietary supplement industry. An upstream strategy for the yield and quality improvement in the production process of silymarin from S. marianum fruits requires the use of a high producing strain and optimum agronomic conditions. On the downstream side, as shown here, an optimized silymarin production procedure utilizes the pericarp after physical separation from the kernels of the fruits, in conjunction with standardized pressurized methanol extraction. Future work will focus on enhancing the efficiency of the physical separation of the pericarp from the fruits and on further optimizing the extraction conditions (solvent, temperature, time) for an even further enhanced silymarin production process.
Acknowledgments
The authors appreciate the support by the Science and Technology Development Fund (STDF-STF), Egypt, project ID 6081 (PI: SAZ), as well as through grant P50 AT000155 from NCCIH and ODS/NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors have indicated that they have no other conflicts of interest regarding the content of this article.
References
- 1.Boulos L. Flora of Egypt, Volume three (Verbenaceae - Compositae) Al Hadara Publishing; Cairo, Egypt: 2002. pp. 157–159. [Google Scholar]
- 2.Flora K, Hahn M, Rosen H, Benner K. Milk thistle (Silybum marianum) for the therapy of liver disease. Am. J. Gastroenterol. 1998;93:139–143. doi: 10.1111/j.1572-0241.1998.00139.x. [DOI] [PubMed] [Google Scholar]
- 3.Singh RP, Tyagi AK, Zhao J, Agarwal R. Silymarin inhibits growth and causes regression of established skin tumors in SENCAR mice via modulation of mitogen-activated protein kinases and induction of apoptosis. Carcinogenesis. 2002;23:499–510. doi: 10.1093/carcin/23.3.499. [DOI] [PubMed] [Google Scholar]
- 4.Smith T, Lynch ME, Johnson J, Kawa K, Bauman H, Blumenthal M. Herbal dietary supplement sales in US increase 6.8% in 2014. HerbalGram. 2015;107:52–59. [Google Scholar]
- 5.AbouZid S, Ahmed OM. Silymarin flavonolignans: structure-activity relationship and biosynthesis. Stud. Nat. Prod. Chem. 2013;40:469–484. [Google Scholar]
- 6.Smith WA, Lauren DR, Burgess EJ, Perry NB, Martin RJ. A silychristin isomer and variation of flavonolignan level in milk thistle (Silybum marianum) fruits. Planta Med. 2005;71:877–880. doi: 10.1055/s-2005-864187. [DOI] [PubMed] [Google Scholar]
- 7.Sy-Cordero A, Graf TN, Nakanishi Y, Wani MC, Agarwal R, Kroll DJ, Oberlies NH. Large-scale isolation of flavonolignans from Silybum marianum (milk thistle) extract affords new minor constituents and preliminary structure-activity relationships. Planta Med. 2010;76:644–647. doi: 10.1055/s-0029-1240624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yin QF, Wang SH, Nan JX, Li SY. The fatty acid compositions of Silybum marianum by GC–MS. J. Yanbian Univ. (Nat. Sci.) 1998;24:26–28. [Google Scholar]
- 9.Hadolin M, Škerget M, Knez Ž, Bauman D. High pressure extraction of vitamin E-rich oil from Silybum marianum. Food Chem. 2001;74:355–364. [Google Scholar]
- 10.Harrabi S, Romdhane H, Daassa M, Fellah H. Fatty acid and triacylglycerol compositions of milk thistle seeds growing wild in Tunisia (Silybum marianum L.). Acta Aliment. Hung. 2015;44:304–310. [Google Scholar]
- 11.Cappelletti EM, Caniato R. Silymarin localization in the fruit and seed of Silybum marianum L. Gaertn. Herba Hungarica. 1984;23:53–66. [Google Scholar]
- 12.Upton R, Graff A, Jolliffe G, Länger R, Williamson E. Milk Thistle Fruits. CRC Press; 2010. American Herbal Pharmacopoeia: Botanical Pharmacognosy - Microscopic Characterization of Botanical Medicines. Silybum marianum (L.) Gaertn. pp. 616–618. [Google Scholar]
- 13.Cappelletti EM, Caniato R, Trevisan R. Atti 1 Conegno Nazionale Societa Italiana Fitochimica. Milano: 1983. p. 219. [Google Scholar]
- 14.Stoiljković Z, Petrović S, Ilić B. Examination of localization of silymarin and fatty oil in Silybum marianum (L.) Gaertn. fruit. Chem. Ind. Chem. Eng. Quart. 2007;13:55–59. [Google Scholar]
- 15.Wagner VH, Diesel P, Seitz M. The chemistry and analysis of silymarin from Silybum marianum Gaertn. Arzneimittel-Forsch. 1974;24:466–471. [PubMed] [Google Scholar]
- 16.Pauli GF, Gödecke T, Jaki BU, Lankin DC. Quantitative 1H NMR. Development and potential of an analytical method: an update. J. Nat. Prod. 2012;75:834–851. doi: 10.1021/np200993k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simmler C, Napolitano JG, McAlpine JB, Chen SN, Pauli GF. Universal quantitative NMR analysis of complex natural samples. Curr. Opin. Biotechnol. 2014;25:51–59. doi: 10.1016/j.copbio.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sacchi R, Patumi M, Fontanazza G, Barone P, Fiordiponti P, Mannina L, Rossi E, Segre AL. A high-field 1H nuclear magnetic resonance study of the minor components in virgin olive oils. J. Am. Oil Chem. Soc. 1996;73:747–758. [Google Scholar]
- 19.Fauhl C, Reniero F, Guillou C. 1H NMR as a tool for the analysis of mixtures of virgin olive oil with oils of different botanical origin. Magn. Reson. Chem. 2000;38:436–443. [Google Scholar]
- 20.AbouZid S, Chen SN, Pauli GF. Silymarin content in Silybum marianum populations growing in Egypt. Ind. Crop. Prod. 2016;83:729–737. doi: 10.1016/j.indcrop.2015.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Napolitano JG, Lankin D, Graf T, Friesen JB, Chen SN, McAlpine JB, Oberlies NH, Pauli GF. HiFSA fingerprinting applied to isomers with near identical NMR spectra: the Silybin/Isosilybin case. J. Org. Chem. 2013;78:2827–2839. doi: 10.1021/jo302720h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wianowska D, Wiśniewski M. Simplified procedure of silymarin extraction from Silybum marianum L. Gaertner. J. Chromatogr. Sci. 2015;53:366–372. doi: 10.1093/chromsci/bmu049. [DOI] [PubMed] [Google Scholar]
- 23.Gupta GK, Raj S, Rao PR. Isolation of antihepatotoxic agents from seeds of Silybum marianum. Res. Ind. 1982;27:37–42. [Google Scholar]
- 24.Kahol AP, Singh KL, Tandon S, Kumar S. Process for isolation of hepatoprotective agent silymarin from the seeds of the plant Silybum marianum. U.S. Patent and Trademark Office; Washington, DC: 2001. U.S. Patent No. 6,309,678. [Google Scholar]
- 25.Leko V. Method for isolation of silymarin from Silybum marianum seeds. U.S. Patent and Trademark Office; Washington, DC: 2008. U.S. Patent No. 7,318,940. [Google Scholar]
- 26.Subramaniam S, Vaughn K, Carrier DJ, Clausen EC. Pretreatment of milk thistle seed to increase the silymarin yield: an alternative to petroleum ether defatting. Bioresource Technol. 2008;99:2501–2506. doi: 10.1016/j.biortech.2007.04.071. [DOI] [PubMed] [Google Scholar]
- 27.Barreto JFA, Wallace SN, Carrier DJ, Clausen EC. Extraction of nutraceuticals from milk thistle. Appl. Biochem. Biotechnol. 2003;108:881–889. doi: 10.1385/abab:108:1-3:881. [DOI] [PubMed] [Google Scholar]
- 28.Duan L, Carrier DJ, Clausen EC. Silymarin extraction from milk thistle using hot water.. In proceedings of the twenty-fifth symposium on biotechnology for fuels and chemicals held May 4-7, 2003, in Breckenridge; Humana Press; 2004. pp. 559–568. [DOI] [PubMed] [Google Scholar]
- 29.Cheilari A, Sturm S, Intelmann D, Seger C, Stuppner H. Head to head comparison of uHPLC-DAD versus qNMR for the quantitative analysis of the silymarin complex in Silybum marianum fruit extracts. J. Agric. Food Chem. 2016;64:1618–1626. doi: 10.1021/acs.jafc.5b05494. [DOI] [PubMed] [Google Scholar]