Abstract
The lack of effective therapies for neurodegenerative disorders is one of the most relevant challenges of this century, considering that, as the global population ages, the incidence of these type of diseases is quickly on the rise. Among these disorders, synucleinopathies, which are characterized by the abnormal accumulation and spreading of the synaptic protein alpha-synuclein in the brain, already constitute the second leading cause of parkinsonism and dementia in the elderly population. Disorders with alpha-synuclein accumulation include Parkinson’s disease, dementia with Lewy bodies and multiple system atrophy. Numerous therapeutic alternatives for synucleinopathies are being tested in pre-clinical models and in the clinic, however only palliative treatments addressing the dopaminergic deficits are approved to date, and no disease-modifying options are available yet. In this manuscript we provide a brief overview of therapeutic approaches currently being explored for synucleinopathies, and suggest possible explanations to the clinical trials outcomes. Finally, we propose that a deeper understanding of the pathophysiology of synucleinopathies, together with a combination of therapies tailored to each disease stage, may lead to better therapeutic outcomes in synucleinopathy patients.
Introduction
Neurodegenerative diseases are the leading cause of death in the elderly, and the World Health Organization predicts that by 2040, as the world population gets older, neurodegenerative diseases will become the second overall leading cause of death after cardiovascular disease (Dua et al. 2004). Therefore, developing effective treatments for these disorders is a major priority in the research and pharmaceutical fields. Neurodegenerative diseases can be clinically classified according to their behavioral correlates (e.g., dementias, motor disorders). However, from a neuropathological perspective, neurodegenerative disorders are usually characterized by the abnormal aggregation of misfolded proteins in the brain (Soto & Estrada 2008, Ross & Poirier 2004). Among these, synucleinopathies are the group of disorders that accumulate alpha-synuclein (Goedert et al. 2001, Spillantini 1999) (α-syn), and they include Parkinson’s disease (PD), PD dementia (PDD), dementia with Lewy Bodies (DLB), and multiple System Atrophy (MSA). Synucleinopathies constitute the second leading cause of parkinsonism and dementia in the elderly population, and they are often associated with degeneration of the dopaminergic system and non-dopaminergic cells in the limbic system and the periphery (Jellinger 2003). A-syn is a synaptic protein involved in synaptic transmission and vesicle release (Fortin et al. 2005, George et al. 1995, Uéda et al. 1993, Iwai et al. 1994) that pathologically aggregates within neurons and glial cells in the form of Lewy bodies, neuronal cytoplasmic inclusions (NCIs) and glial cytoplasmic inclusions (GCIs) (Goedert et al. 2001, Spillantini 1999, Takeda et al. 1998, Wakabayashi et al. 1998a, Wakabayashi et al. 1998b, Wakabayashi et al. 1997, Papp et al. 1989). It is believed that oligomers and/or protofibrils are the toxic conformations of α-syn (Lashuel et al. 2013, Winner et al. 2011), and that they can propagate from cell to cell in a prion-like fashion (Frost & Diamond 2010, Lee et al. 2010, Desplats et al. 2009, Prusiner et al. 2015), thus explaining the progression of the disease and its spreading from basal brain regions to neocortical areas (Braak et al. 2003).
Although the accumulation of α-syn is the most prominent neuropathological feature in synucleinopathies, other molecular factors are also involved in the progression of the pathology, and co-aggregation of α-syn with proteins such as amyloid beta and tau has also been detected (Masliah et al. 2001, Ishizawa et al. 2003, Clinton et al. 2010). Moreover, genome-wide association studies (GWAS) have identified several susceptibility genes for synucleinopathies, and the proteins encoded by these genes may also be involved in the molecular mechanisms of the pathology. These include mitochondrial and lysosomal components such as LRRK2 (Zimprich et al. 2004), Parkin/PARK2 (Matsumine et al. 1998), PINK1 (Valente et al. 2004) and DJ-1/PARK7 (Bonifati et al. 2003) in PD (Singleton et al. 2013), and COQ2 in MSA (The Multiple-System Atrophy Research Collaboration 2013), highlighting a role of cell metabolism and protein clearance mechanisms in the disease pathophysiology. In this sense, gene therapy has been recently suggested for PARK2 (Kubo et al. 2013, Winklhofer 2007), and use of the neuroprotective DJ-1 products glycolate and D-lactate has also been explored (Toyoda et al. 2014). However, more research is still needed to elucidate how these proteins may be mechanistically involved in the origin and progression of synucleinopathies.
Therapeutic approaches for synucleinopathies
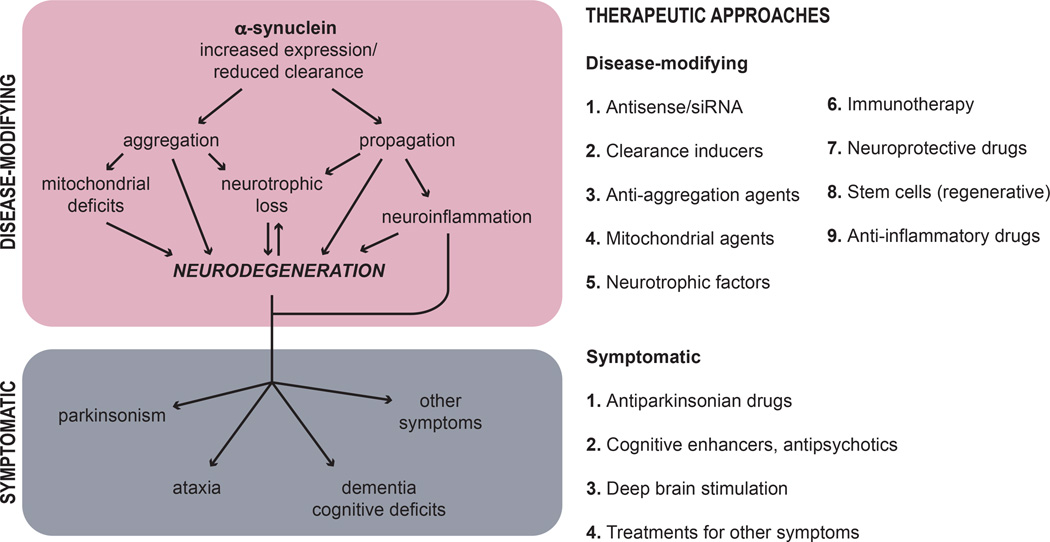
Broadly, all therapeutic approaches can be considered as either disease-modifying or symptomatic (Figure 1). Disease-modifying therapies are those able to delay, stop or revert the progression of the neurodegenerative pathology, while symptomatic approaches are aimed to manage the disease symptoms. Although both type of approaches are necessary and should complement each other, unfortunately there are no approved disease-modifying therapies for synucleinopathies and the available treatments are only symptomatic. However, despite the great deal of effort currently being put into finding effective disease-modifying alternatives, developing new and improved symptomatic approaches with less side effects is also extremely relevant, as they would provide a much needed quality of life improvement for the patients. It is safe to assume that combining symptomatic and disease-modifying approaches would greatly benefit the outcome of the therapeutic regime, therefore researching into safer symptomatic treatments should go hand in hand with disease-modifying efforts. Examples of symptomatic therapies include those aimed at reducing parkinsonism (e.g., L-DOPA and dopaminergic agonists, monoamine oxidase inhibitors) (Cotzias et al. 1969, Rascol et al. 2000, Holloway et al. 2004), cognitive deficits (cholinesterase inhibitors) (Ikeda et al. 2015, Dubois et al. 2012, Reingold et al. 2007, Edwards et al. 2007), orthostatic hypotension (e.g., droxidopa for MSA) (Kaufmann et al. 2014), REM disorders, gastrointestinal and urinary dysfunctions, and other non-motor manifestations (Poewe 2010, Schrag et al. 2015). It is worth mentioning that non-pharmacological treatments are being increasingly explored due to the lack of effective pharmacological approaches with few side effects. These include deep brain stimulation (DBS) of the nucleus basalis of Meynert (Freund et al. 2009) or other basal ganglia circuits (DeLong & Wichmann 2015, Wichmann & Delong 2011), an approach that has been successful at improving motor symptoms in patients suffering from PD and PDD. Exercise and an active lifestyle have also been proven beneficial for reducing symptoms in PD (Ahlskog 2011), results that are likely linked to the neuroprotective effects of physical exercise (e.g., treadmill walking). Finally, calorie restriction (Maswood et al. 2004) and other diet modifications, including ketogenic (Paoli et al. 2014) and phytochemical-rich diets (Seidl et al. 2014, Gao et al. 2007), have also been suggested to improve disease symptoms.
Figure 1. Disease-modifying and symptomatic approaches for the treatment of synucleinopathies.
Disease-modifying therapies delay, stop or revert the progression of the neurodegenerative pathology by targeting α-syn accumulation (including α-syn synthesis, aggregation and clearance), mitochondrial dysfunction, loss of neurotrophic factors, neuroinflammation and/or neurodegeneration. Symptomatic approaches are aimed at managing the disease symptoms, which may include parkinsonism, ataxia, dementia and cognitive deficits, among others. On the right, we provide a brief summary of the recent disease-modifying and symptomatic approaches targeting different molecular mechanisms in synucleinopathies.
Recent pharmacological approaches for synucleinopathies
The pharmacological approaches for the treatment of synucleinopathies can be classified according to their molecular targets. Among these, α-syn is the more prominent element as its abnormal accumulation in the brain is associated to the origin and progression of the pathology in synucleinopathies (Lashuel et al. 2002, Tsigelny et al. 2007, Braak et al. 2003). Therefore the molecular processes leading to α-syn accumulation, including its synthesis, aggregation and clearance, can be targeted for disease modification. Thus, recent approaches focused on α-syn include active and passive immunotherapy (Valera & Masliah 2013), α-syn siRNA delivery (Cooper et al. 2014), anti-aggregation compounds (Caruana et al. 2011, Ono et al. 2004, Wobst et al. 2015), autophagy enhancers (Renna et al. 2010, Lynch-Day et al. 2012), degrading enzymes (Sevlever et al. 2008, Spencer et al. 2013, Devi & Ohno 2015), and molecular chaperones (Danzer et al. 2011, Evans et al. 2006, Voss et al. 2012, Hashimoto et al. 2004), among others. However, other neuropathological processes in synucleinopathies are also susceptible to modulation. For example, the degeneration of dopaminergic and cholinergic neurons can be prevented or restored using neuroprotective compounds (Ilijic et al. 2011, Quik et al. 2015, Stefanova et al. 2008), regenerative therapy with stem cells (Schwerk et al. 2015), or stimulating neurogenesis (Foltynie 2015). The loss of trophic support to neurons due to neuronal and/or glial degeneration can be treated using neurotrophic factors (Hoban et al. 2015, Allen et al. 2013); and the pathological (maladaptive) neuroinflammation that accompanies late disease stages might be susceptible to anti-inflammatory treatment (Valera et al. 2014, Valera et al. 2015). Therapies targeting these processes are potentially disease modifying, as they can delay, revert or compensate for the neurodegeneration that lead to motor and non-motor impairments (Figure 1).
Current clinical trials for synucleinopathies include more than 500 open studies for PD, 28 for DLB and 28 for MSA (see clinicaltrials.gov). Unfortunately, several trials have recently failed to meet expected criteria or were terminated due to significant adverse effects. These include treatment with neurturin (neurotrophin) (Warren Olanow et al. 2015), CoQ10 (antioxidant) (Yoritaka et al. 2015), creatine (ATP production) (Writing Group for the NINDS Exploratory Trials in Parkinson Disease (NET-PD) Investigators et al. 2015), pramipexole (dopamine agonist) (Schapira et al. 2013) and pioglitazone (PPAR-γ agonist) (NINDS Exploratory Trials in Parkinson Disease (NET-PD) FS-ZONE Investigators 2015) for PD; lithium (autophagy inducer) (Sacca et al. 2013), rifampicin (α-syn anti-aggregation agent) (Low et al. 2014) and rasagiline (monoamine oxidase B inhibitor) (Poewe et al. 2015) for MSA. These negative results draw attention to the very common discrepancy found between the effects of pharmacological agents in animal models versus their effects on humans. This dichotomy demonstrates that a deeper understanding of the differences between the model pathology and the human disease is required, together with improved translational research protocols. Moreover, the multifactorial nature of neurodegenerative disorders and the stage-dependent windows for therapeutic intervention should be also taken into account in our efforts to develop more effective therapeutic strategies (Valera & Masliah 2015).
Ongoing clinical trials targeting α-syn (Table 1) include active immunotherapy with PD01A and PD03A, peptides that mimic abnormal α-syn conformations, for the treatment of both PD and MSA (NCT02270489, NCT02267434). Passive immunization trials with antibodies targeting the C-terminus of α-syn (PRX002, NCT02157714) or other regions of the protein (BIIB054, NCT02459886) for the treatment of PD are also ongoing. Compounds designed to stabilize or reduce the formation of toxic α-syn aggregates are also being developed, such as the conformational stabilizer NPT200-11 (Neuropore Therapies). Finally, examples of ongoing clinical trials in synucleinopathies for targets other than α-syn (Table 1) include isradipine (calcium channel blocker, neuroprotective) (NCT02168842), caffeine (adenosine A2A receptor antagonist, neuroprotective) (NCT01738178), nicotine (acetylcholine receptor agonist, neuroprotective) (NCT01560754), glutathione (antioxidant) (NCT02424708), N-acetylcysteine (glutathione precursor) (NCT02212678, NCT01470027), GDNF (neurotrophic factor) (NCT01621581), sargramostim (cytokine) (NCT01882010) and adipose-derived stromal stem cells (regenerative, anti-inflammatory, neurotrophic effects) (NCT01453803) for PD; and autologous mesenchymal stem cells (regenerative) (NCT02315027), epigallocatechin gallate (polyphenol, antioxidant) (NCT02008721), and AZD3241 (myeloperoxidase inhibitor, microglia inhibition) (NCT02388295) for MSA.
Table 1. Selection of relevant ongoing clinical trials for synucleinopathies.
Information regarding clinical trials was found at clinicaltrials.gov as of January 2016.
| Drug name | Phase | Identifier(s) | Target/mechanism | Condition | Sponsor | References |
|---|---|---|---|---|---|---|
| TARGETING ALPHA-SYNUCLEIN | ||||||
| Active immunotherapy: vaccines | ||||||
| AFFITOPE PD01A | I | NCT02270489 | NP | Early MSA | Affiris | Schneeberger et al. 2012, Mandler et al. 2014 |
| AFFITOPE PD03A | I | NCT02270489, NCT02267434 | NP | Early MSA; Early PD | Affiris | Schneeberger et al. 2012, Mandler et al. 2014 |
| Passive immunotherapy: antibodies | ||||||
| BIIB054 | I | NCT02459886 | NP | Healthy participants | Biogen | |
| PRX002 | I | NCT02157714 | C-terminus α-syn | PD | Prothena Biosciences | Games et al. 2014 |
| OTHER TARGETS | ||||||
| GDNF (gene therapy) | I | NCT01621581 | Neurotrophic factor | PD | National Institute of Neurological Disorders and Stroke | Richardson et al. 2011 |
| Mesenchymal stem cells | I | NCT02315027 | Regenerative | MSA | Mayo Clinic | Lee et al. 2012 |
| Sargramostim (leukine) | I | NCT01882010 | Cytokine | PD | Howard Gendelman, MD | |
| Adipose-derived stromal stem cells | II | NCT01453803 | Regenerative, anti-inflammatory, neurotrophic effects | PD | Ageless Regenerative Institute | |
| AZD3241 | II | NCT02388295 | Myeloperoxidase inhibitor, microglia modulation | MSA | AstraZeneca | Stefanova et al. 2012, Kaindlstorfer et al. 2015 |
| Glutathione (intranasal) | II | NCT02424708 | Antioxidant | PD | Bastyr University | |
| N-acetylcysteine | II | NCT02212678, NCT01470027 | Glutathione precursor, antioxidant | PD | University of Minnesota; Cornell University | |
| Nicotine (transdermal) | II | NCT01560754 | Acetylcholine receptor agonist, neuroprotective | Early PD | James Boyd, MD | |
| Caffeine | III | NCT01738178 | Adenosine A2A receptor antagonist, neuroprotective | PD | McGill University Health Center | |
| Epigallocatechin gallate | III | NCT02008721 | Polyphenol, antioxidant | MSA | Johannes Levin, MD | |
| Isradipine | III | NCT02168842 | Calcium channel blocker, neuroprotective | Early PD | University of Rochester |
NP, not provided; MSA, multiple system atrophy; PD, Parkinson’s disease.
New pre-clinical studies are constantly published reporting possible treatments for synucleinopathies. In our laboratory we have recently explored the use of alternative anti-inflammatory treatments with immunomodulatory drugs (e.g., lenalidomide) (Valera et al. 2015), gene therapy with extracellular α-syn degrading enzymes (e.g., neurosin) (Spencer et al. 2015) and single chain antibodies against α-syn (Spencer et al. 2014). Recent reviews have summarized the ever-growing number approaches for synucleinopathies that could eventually progress to the clinic (Dehay et al. 2015, Siebert et al. 2014, Schneeberger et al. 2015).
Final remarks
In conclusion, pre-clinical and clinical evidence suggest that, in order to obtain significant positive results in clinical trials for synucleinopathies, a better knowledge of the human pathology is necessary. In this sense, research priorities in this area include analyzing the intrinsic differences existing between animal models and the human disease. For example, it would be important to determine why the extent of the pathology (e.g., neurodegeneration, neuroinflammation) greatly differs between some transgenic animal models and human synucleinopathy brains. In this sense, the use of double or conditional transgenic models, or combining a transgenic background plus an exogenous insult (e.g. paraquat, maneb (Desplats et al. 2012, Nuber et al. 2014)) (double-hit models), may answer some of these questions and shed light on the molecular mechanisms of the pathology. Another outstanding question is the mechanistic involvement of susceptibility genes in the origin of the disease, due to its relevance to precision medicine approaches (e.g. LRRK2 manipulation in patients with LRRK2 mutations). Finally, identifying biomarkers for early detection in animal models with predicted values for the human disease would undoubtedly improve the outcome of early stage therapeutics, such as immunotherapy. Moreover, although efforts toward the development of effective disease-modifying alternatives for PD and related disorders have increased in the past few years, better symptomatic relief treatments with fewer side effects are still necessary. Finally, it has also been suggested that a logical combination of therapies might be a more potent and broad approach for the treatment of synucleinopathies (Valera & Masliah 2015).
Acknowledgments
Supported by National Institutes of Health (NIH) grants AG18440, AG022074, NS044233.
Footnotes
Conflict of interest: The authors declare no conflict of interest.
REFERENCES
- Ahlskog JE. Does vigorous exercise have a neuroprotective effect in Parkinson disease? Neurology. 2011;77:288–294. doi: 10.1212/WNL.0b013e318225ab66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen SJ, Watson JJ, Shoemark DK, Barua NU, Patel NK. GDNF, NGF and BDNF as therapeutic options for neurodegeneration. Pharmacol Ther. 2013;138:155–175. doi: 10.1016/j.pharmthera.2013.01.004. [DOI] [PubMed] [Google Scholar]
- Bonifati V, Rizzu P, van Baren MJ, et al. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science. 2003;299:256–259. doi: 10.1126/science.1077209. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Caruana M, Högen T, Levin J, Hillmer A, Giese A, Vassallo N. Inhibition and disaggregation of α-synuclein oligomers by natural polyphenolic compounds. FEBS Lett. 2011;585:1113–1120. doi: 10.1016/j.febslet.2011.03.046. [DOI] [PubMed] [Google Scholar]
- Clinton LK, Blurton-Jones M, Myczek K, Trojanowski JQ, LaFerla FM. Synergistic Interactions between Abeta, tau, and alpha-synuclein: acceleration of neuropathology and cognitive decline. J Neurosci. 2010;30:7281–7289. doi: 10.1523/JNEUROSCI.0490-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collaboration, The Multiple-System Atrophy Research. Mutations in COQ2 in familial and sporadic multiple-system atrophy. The New England journal of medicine. 2013;369:233–244. doi: 10.1056/NEJMoa1212115. [DOI] [PubMed] [Google Scholar]
- Cooper JM, Wiklander PB, Nordin JZ, et al. Systemic exosomal siRNA delivery reduced alpha-synuclein aggregates in brains of transgenic mice. Mov Disord. 2014;29:1476–1485. doi: 10.1002/mds.25978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotzias GC, Papavasiliou PS, Gellene R. L-dopa in parkinson's syndrome. The New England journal of medicine. 1969;281:272. doi: 10.1056/NEJM196907312810518. [DOI] [PubMed] [Google Scholar]
- Danzer KM, Ruf WP, Putcha P, Joyner D, Hashimoto T, Glabe C, Hyman BT, McLean PJ. Heat-shock protein 70 modulates toxic extracellular alpha-synuclein oligomers and rescues trans-synaptic toxicity. Faseb J. 2011;25:326–336. doi: 10.1096/fj.10-164624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehay B, Bourdenx M, Gorry P, et al. Targeting alpha-synuclein for treatment of Parkinson's disease: mechanistic and therapeutic considerations. Lancet Neurol. 2015;14:855–866. doi: 10.1016/S1474-4422(15)00006-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLong MR, Wichmann T. Basal Ganglia Circuits as Targets for Neuromodulation in Parkinson Disease. JAMA neurology. 2015:1–7. doi: 10.1001/jamaneurol.2015.2397. [DOI] [PubMed] [Google Scholar]
- Desplats P, Lee HJ, Bae EJ, Patrick C, Rockenstein E, Crews L, Spencer B, Masliah E, Lee SJ. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc Natl Acad Sci USA. 2009;106:13010–13015. doi: 10.1073/pnas.0903691106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desplats P, Patel P, Kosberg K, Mante M, Patrick C, Rockenstein E, Fujita M, Hashimoto M, Masliah E. Combined exposure to Maneb and Paraquat alters transcriptional regulation of neurogenesis-related genes in mice models of Parkinson's disease. Mol Neurodegener. 2012;7:49. doi: 10.1186/1750-1326-7-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devi L, Ohno M. A combination Alzheimer's therapy targeting BACE1 and neprilysin in 5XFAD transgenic mice. Mol Brain. 2015;8:19. doi: 10.1186/s13041-015-0110-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dua T World Federation of Neurology., World Health Organization. Programme for Neurological Diseases and Neuroscience. and World Health Organization. Department of Mental Health and Substance Abuse. Programme for Neurological Diseases and Neuroscience. Geneva: Department of Mental Health and Substance Abuse, World Health Organization; 2004. Atlas : country resources for neurological disorders 2004 : results of a collaborative study of the World Health Organization and the World Federation of Neurology. [Google Scholar]
- Dubois B, Tolosa E, Katzenschlager R, et al. Donepezil in Parkinson's disease dementia: a randomized, double-blind efficacy and safety study. Mov Disord. 2012;27:1230–1238. doi: 10.1002/mds.25098. [DOI] [PubMed] [Google Scholar]
- Edwards K, Royall D, Hershey L, Lichter D, Hake A, Farlow M, Pasquier F, Johnson S. Efficacy and safety of galantamine in patients with dementia with Lewy bodies: a 24-week open-label study. Dement Geriatr Cogn Disord. 2007;23:401–405. doi: 10.1159/000101512. [DOI] [PubMed] [Google Scholar]
- Evans CG, Wisen S, Gestwicki JE. Heat shock proteins 70 and 90 inhibit early stages of amyloid beta-(1–42) aggregation in vitro. J Biol Chem. 2006;281:33182–33191. doi: 10.1074/jbc.M606192200. [DOI] [PubMed] [Google Scholar]
- Foltynie T. Can Parkinson's disease be cured by stimulating neurogenesis? The Journal of clinical investigation. 2015;125:978–980. doi: 10.1172/JCI80822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin DL, Nemani VM, Voglmaier SM, Anthony MD, Ryan TA, Edwards RH. Neural activity controls the synaptic accumulation of alpha-synuclein. J Neurosci. 2005;25:10913–10921. doi: 10.1523/JNEUROSCI.2922-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund HJ, Kuhn J, Lenartz D, Mai JK, Schnell T, Klosterkoetter J, Sturm V. Cognitive functions in a patient with Parkinson-dementia syndrome undergoing deep brain stimulation. Archives of neurology. 2009;66:781–785. doi: 10.1001/archneurol.2009.102. [DOI] [PubMed] [Google Scholar]
- Frost B, Diamond MI. Prion-like mechanisms in neurodegenerative diseases. Nat Rev Neurosci. 2010;11:155–159. doi: 10.1038/nrn2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Games D, Valera E, Spencer B, et al. Reducing C-terminal-truncated alpha-synuclein by immunotherapy attenuates neurodegeneration and propagation in Parkinson's disease-like models. J Neurosci. 2014;34:9441–9454. doi: 10.1523/JNEUROSCI.5314-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Chen H, Fung TT, Logroscino G, Schwarzschild MA, Hu FB, Ascherio A. Prospective study of dietary pattern and risk of Parkinson disease. The American journal of clinical nutrition. 2007;86:1486–1494. doi: 10.1093/ajcn/86.5.1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George JM, Jin H, Woods WS, Clayton DF. Characterization of a novel protein regulated during the critical period for song learning in the zebra finch. Neuron. 1995;15:361–372. doi: 10.1016/0896-6273(95)90040-3. [DOI] [PubMed] [Google Scholar]
- Goedert M, Jakes R, Anthony Crowther R, Grazia Spillantini M. Parkinson's Disease, Dementia with Lewy Bodies, and Multiple System Atrophy as alpha-Synucleinopathies. Methods Mol Med. 2001;62:33–59. doi: 10.1385/1-59259-142-6:33. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Rockenstein E, Mante M, Crews L, Bar-On P, Gage FH, Marr R, Masliah E. An antiaggregation gene therapy strategy for Lewy body disease utilizing beta-synuclein lentivirus in a transgenic model. Gene therapy. 2004;11:1713–1723. doi: 10.1038/sj.gt.3302349. [DOI] [PubMed] [Google Scholar]
- Hoban DB, Howard L, Dowd E. GDNF-secreting mesenchymal stem cells provide localized neuroprotection in an inflammation-driven rat model of Parkinson's disease. Neuroscience. 2015;303:402–411. doi: 10.1016/j.neuroscience.2015.07.014. [DOI] [PubMed] [Google Scholar]
- Holloway RG, Shoulson I, Fahn S, et al. Pramipexole vs levodopa as initial treatment for Parkinson disease: a 4-year randomized controlled trial. Archives of neurology. 2004;61:1044–1053. doi: 10.1001/archneur.61.7.1044. [DOI] [PubMed] [Google Scholar]
- Ikeda M, Mori E, Matsuo K, Nakagawa M, Kosaka K. Donepezil for dementia with Lewy bodies: a randomized, placebo-controlled, confirmatory phase III trial. Alzheimer's research & therapy. 2015;7:4. doi: 10.1186/s13195-014-0083-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilijic E, Guzman JN, Surmeier DJ. The L-type channel antagonist isradipine is neuroprotective in a mouse model of Parkinson's disease. Neurobiology of disease. 2011;43:364–371. doi: 10.1016/j.nbd.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Investigators, NINDS Exploratory Trials in Parkinson Disease (NET-PD) FS-ZONE. Pioglitazone in early Parkinson's disease: a phase 2, multicentre, double-blind, randomised trial. Lancet Neurol. 2015;14:795–803. doi: 10.1016/S1474-4422(15)00144-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizawa T, Mattila P, Davies P, Wang D, Dickson DW. Colocalization of tau and alpha-synuclein epitopes in Lewy bodies. J Neuropathol Exp Neurol. 2003;62:389–397. doi: 10.1093/jnen/62.4.389. [DOI] [PubMed] [Google Scholar]
- Iwai A, Masliah E, Yoshimoto M, De Silva R, Ge N, Kittel A, Saitoh T. The precursor protein of non-Ab component of Alzheimer's disease amyloid (NACP) is a presynaptic protein of the central nervous system. Neuron. 1994;14:467–475. doi: 10.1016/0896-6273(95)90302-x. [DOI] [PubMed] [Google Scholar]
- Jellinger KA. Neuropathological spectrum of synucleinopathies. Mov Disord. 2003;18(Suppl 6):S2–S12. doi: 10.1002/mds.10557. [DOI] [PubMed] [Google Scholar]
- Kaindlstorfer C, Sommer P, Georgievska B, Mather RJ, Kugler AR, Poewe W, Wenning GK, Stefanova N. Failure of Neuroprotection Despite Microglial Suppression by Delayed-Start Myeloperoxidase Inhibition in a Model of Advanced Multiple System Atrophy: Clinical Implications. Neurotox Res. 2015;28:185–194. doi: 10.1007/s12640-015-9547-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann H, Freeman R, Biaggioni I, et al. Droxidopa for neurogenic orthostatic hypotension: a randomized, placebo-controlled, phase 3 trial. Neurology. 2014;83:328–335. doi: 10.1212/WNL.0000000000000615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo S, Hatano T, Takanashi M, Hattori N. Can parkin be a target for future treatment of Parkinson's disease? Expert Opin Ther Targets. 2013;17:1133–1144. doi: 10.1517/14728222.2013.827173. [DOI] [PubMed] [Google Scholar]
- Lashuel HA, Overk CR, Oueslati A, Masliah E. The many faces of α-synuclein: from structure and toxicity to therapeutic target. Nat Rev Neurosci. 2013;14:38–48. doi: 10.1038/nrn3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lashuel HA, Petre BM, Wall J, Simon M, Nowak RJ, Walz T, Lansbury PT. Alpha-synuclein, especially the Parkinson's disease-associated mutants, forms pore-like annular and tubular protofibrils. J Mol Biol. 2002;322:1089–1102. doi: 10.1016/s0022-2836(02)00735-0. [DOI] [PubMed] [Google Scholar]
- Lee PH, Lee JE, Kim HS, et al. A randomized trial of mesenchymal stem cells in multiple system atrophy. Ann Neurol. 2012;72:32–40. doi: 10.1002/ana.23612. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Desplats P, Sigurdson C, Tsigelny I, Masliah E. Cell-to-cell transmission of non-prion protein aggregates. Nat Rev Neurol. 2010;6:702–706. doi: 10.1038/nrneurol.2010.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low PA, Robertson D, Gilman S, et al. Efficacy and safety of rifampicin for multiple system atrophy: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2014;13:268–275. doi: 10.1016/S1474-4422(13)70301-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch-Day MA, Mao K, Wang K, Zhao M, Klionsky DJ. The role of autophagy in Parkinson's disease. Cold Spring Harb Perspect Med. 2012;2:a009357. doi: 10.1101/cshperspect.a009357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandler M, Valera E, Rockenstein E, et al. Next-generation active immunization approach for synucleinopathies: implications for Parkinson's disease clinical trials. Acta neuropathologica. 2014;127:861–879. doi: 10.1007/s00401-014-1256-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliah E, Rockenstein E, Veinbergs I, Sagara Y, Mallory M, Hashimoto M, Mucke L. beta-amyloid peptides enhance alpha-synuclein accumulation and neuronal deficits in a transgenic mouse model linking Alzheimer's disease and Parkinson's disease. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:12245–12250. doi: 10.1073/pnas.211412398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maswood N, Young J, Tilmont E, et al. Caloric restriction increases neurotrophic factor levels and attenuates neurochemical and behavioral deficits in a primate model of Parkinson's disease. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:18171–18176. doi: 10.1073/pnas.0405831102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumine H, Yamamura Y, Hattori N, Kobayashi T, Kitada T, Yoritaka A, Mizuno Y. A microdeletion of D6S305 in a family of autosomal recessive juvenile parkinsonism (PARK2) Genomics. 1998;49:143–146. doi: 10.1006/geno.1997.5196. [DOI] [PubMed] [Google Scholar]
- Nuber S, Tadros D, Fields J, et al. Environmental neurotoxic challenge of conditional alpha-synuclein transgenic mice predicts a dopaminergic olfactory-striatal interplay in early PD. Acta neuropathologica. 2014;127:477–494. doi: 10.1007/s00401-014-1255-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono K, Hasegawa K, Naiki H, Yamada M. Curcumin has potent anti-amyloidogenic effects for Alzheimer's beta-amyloid fibrils in vitro. J Neurosci Res. 2004;75:742–750. doi: 10.1002/jnr.20025. [DOI] [PubMed] [Google Scholar]
- Paoli A, Bianco A, Damiani E, Bosco G. Ketogenic diet in neuromuscular and neurodegenerative diseases. BioMed research international. 2014;2014:474296. doi: 10.1155/2014/474296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp MI, Kahn JE, Lantos PL. Glial cytoplasmic inclusions in the CNS of patients with multiple system atrophy (striatonigral degeneration, olivopontocerebellar atrophy and Shy-Drager syndrome) J Neurol Sci. 1989;94:79–100. doi: 10.1016/0022-510x(89)90219-0. [DOI] [PubMed] [Google Scholar]
- Poewe W. Parkinson disease: treatment of the nonmotor symptoms of Parkinson disease. Nat Rev Neurol. 2010;6:417–418. doi: 10.1038/nrneurol.2010.87. [DOI] [PubMed] [Google Scholar]
- Poewe W, Seppi K, Fitzer-Attas CJ, et al. Efficacy of rasagiline in patients with the parkinsonian variant of multiple system atrophy: a randomised, placebo-controlled trial. Lancet Neurol. 2015;14:145–152. doi: 10.1016/S1474-4422(14)70288-1. [DOI] [PubMed] [Google Scholar]
- Prusiner SB, Woerman AL, Mordes DA, et al. Evidence for alpha-synuclein prions causing multiple system atrophy in humans with parkinsonism. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:E5308–E5317. doi: 10.1073/pnas.1514475112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quik M, Zhang D, McGregor M, Bordia T. Alpha7 nicotinic receptors as therapeutic targets for Parkinson's disease. Biochem Pharmacol. 2015 doi: 10.1016/j.bcp.2015.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rascol O, Brooks DJ, Korczyn AD, De Deyn PP, Clarke CE, Lang AE. A five-year study of the incidence of dyskinesia in patients with early Parkinson's disease who were treated with ropinirole or levodopa. The New England journal of medicine. 2000;342:1484–1491. doi: 10.1056/NEJM200005183422004. [DOI] [PubMed] [Google Scholar]
- Reingold JL, Morgan JC, Sethi KD. Rivastigmine for the treatment of dementia associated with Parkinson's disease. Neuropsychiatric disease and treatment. 2007;3:775–783. doi: 10.2147/ndt.s1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renna M, Jimenez-Sanchez M, Sarkar S, Rubinsztein DC. Chemical inducers of autophagy that enhance the clearance of mutant proteins in neurodegenerative diseases. J Biol Chem. 2010;285:11061–11067. doi: 10.1074/jbc.R109.072181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson RM, Kells AP, Rosenbluth KH, et al. Interventional MRI-guided putaminal delivery of AAV2-GDNF for a planned clinical trial in Parkinson's disease. Mol Ther. 2011;19:1048–1057. doi: 10.1038/mt.2011.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross CA, Poirier MA. Protein aggregation and neurodegenerative disease. Nat Med. 2004;10(Suppl):S10–S17. doi: 10.1038/nm1066. [DOI] [PubMed] [Google Scholar]
- Sacca F, Marsili A, Quarantelli M, et al. A randomized clinical trial of lithium in multiple system atrophy. J Neurol. 2013;260:458–461. doi: 10.1007/s00415-012-6655-7. [DOI] [PubMed] [Google Scholar]
- Schapira AH, McDermott MP, Barone P, et al. Pramipexole in patients with early Parkinson's disease (PROUD): a randomised delayed-start trial. Lancet Neurol. 2013;12:747–755. doi: 10.1016/S1474-4422(13)70117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneeberger A, Mandler M, Mattner F, Schmidt W. Vaccination for Parkinson's disease. Parkinsonism Relat Disord. 2012;18(Suppl 1):S11–S13. doi: 10.1016/S1353-8020(11)70006-2. [DOI] [PubMed] [Google Scholar]
- Schneeberger A, Tierney L, Mandler M. Active immunization therapies for Parkinson's disease and multiple system atrophy. Mov Disord. 2015 doi: 10.1002/mds.26377. [DOI] [PubMed] [Google Scholar]
- Schrag A, Sauerbier A, Chaudhuri KR. New clinical trials for nonmotor manifestations of Parkinson's disease. Mov Disord. 2015;30:1490–1504. doi: 10.1002/mds.26415. [DOI] [PubMed] [Google Scholar]
- Schwerk A, Altschuler J, Roch M, Gossen M, Winter C, Berg J, Kurtz A, Akyuz L, Steiner B. Adipose-derived human mesenchymal stem cells induce long-term neurogenic and anti-inflammatory effects and improve cognitive but not motor performance in a rat model of Parkinson's disease. Regenerative medicine. 2015;10:431–446. doi: 10.2217/rme.15.17. [DOI] [PubMed] [Google Scholar]
- Seidl SE, Santiago JA, Bilyk H, Potashkin JA. The emerging role of nutrition in Parkinson's disease. Frontiers in aging neuroscience. 2014;6:36. doi: 10.3389/fnagi.2014.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevlever D, Jiang P, Yen SH. Cathepsin D is the main lysosomal enzyme involved in the degradation of alpha-synuclein and generation of its carboxy-terminally truncated species. Biochemistry. 2008;47:9678–9687. doi: 10.1021/bi800699v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebert M, Sidransky E, Westbroek W. Glucocerebrosidase is shaking up the synucleinopathies. Brain : a journal of neurology. 2014;137:1304–1322. doi: 10.1093/brain/awu002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton AB, Farrer MJ , Bonifati V. The genetics of Parkinson's disease: progress and therapeutic implications. Mov Disord. 2013;28:14–23. doi: 10.1002/mds.25249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto C, Estrada LD. Protein misfolding and neurodegeneration. Archives of neurology. 2008;65:184–189. doi: 10.1001/archneurol.2007.56. [DOI] [PubMed] [Google Scholar]
- Spencer B, Emadi S, Desplats P, et al. ESCRT-mediated uptake and degradation of brain-targeted alpha-synuclein single chain antibody attenuates neuronal degeneration in vivo. Mol Ther. 2014;22:1753–1767. doi: 10.1038/mt.2014.129. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Spencer B, Michael S, Shen J, Kosberg K, Rockenstein E, Patrick C, Adame A, Masliah E. Lentivirus mediated delivery of neurosin promotes clearance of wild-type alpha-synuclein and reduces the pathology in an alpha-synuclein model of LBD. Mol Ther. 2013;21:31–41. doi: 10.1038/mt.2012.66. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Spencer B, Valera E, Rockenstein E, Trejo-Morales M, Adame A, Masliah E. A brain-targeted, modified neurosin (kallikrein-6) reduces alpha-synuclein accumulation in a mouse model of multiple system atrophy. Mol Neurodegener. 2015;10:48. doi: 10.1186/s13024-015-0043-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillantini MG. Parkinson's disease, dementia with Lewy bodies and multiple system atrophy are alpha-synucleinopathies. Parkinsonism Relat Disord. 1999;5:157–162. doi: 10.1016/s1353-8020(99)00031-0. [DOI] [PubMed] [Google Scholar]
- Stefanova N, Georgievska B, Eriksson H, Poewe W, Wenning GK. Myeloperoxidase inhibition ameliorates multiple system atrophy-like degeneration in a transgenic mouse model. Neurotox Res. 2012;21:393–404. doi: 10.1007/s12640-011-9294-3. [DOI] [PubMed] [Google Scholar]
- Stefanova N, Poewe W, Wenning GK. Rasagiline is neuroprotective in a transgenic model of multiple system atrophy. Exp Neurol. 2008;210:421–427. doi: 10.1016/j.expneurol.2007.11.022. [DOI] [PubMed] [Google Scholar]
- Takeda A, Mallory M, Sundsmo M, Honer W, Hansen L, Masliah E. Abnormal accumulation of NACP/alpha-synuclein in neurodegenerative disorders. The American journal of pathology. 1998;152:367–372. [PMC free article] [PubMed] [Google Scholar]
- Toyoda Y, Erkut C, Pan-Montojo F, Boland S, Stewart MP, Muller DJ, Wurst W, Hyman AA, Kurzchalia TV. Products of the Parkinson's disease-related glyoxalase DJ-1, D-lactate and glycolate, support mitochondrial membrane potential and neuronal survival. Biology open. 2014;3:777–784. doi: 10.1242/bio.20149399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsigelny IF, Bar-On P, Sharikov Y, et al. Dynamics of alpha-synuclein aggregation and inhibition of pore-like oligomer development by beta-synuclein. The FEBS journal. 2007;274:1862–1877. doi: 10.1111/j.1742-4658.2007.05733.x. [DOI] [PubMed] [Google Scholar]
- Uéda K, Fukushima H, Masliah E, et al. Molecular cloning of cDNA encoding an unrecognized component of amyloid in Alzheimer disease. Proc Natl Acad Sci USA. 1993;90:11282–11286. doi: 10.1073/pnas.90.23.11282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valente EM, Abou-Sleiman PM, Caputo V, et al. Hereditary early-onset Parkinson's disease caused by mutations in PINK1. Science. 2004;304:1158–1160. doi: 10.1126/science.1096284. [DOI] [PubMed] [Google Scholar]
- Valera E, Mante M, Anderson S, Rockenstein E, Masliah E. Lenalidomide reduces microglial activation and behavioral deficits in a transgenic model of Parkinson's disease. J Neuroinflammation. 2015;12:93. doi: 10.1186/s12974-015-0320-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valera E, Masliah E. Immunotherapy for neurodegenerative diseases: Focus on α-synucleinopathies. Pharmacol Ther. 2013 doi: 10.1016/j.pharmthera.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valera E, Masliah E. Combination therapies: The next logical Step for the treatment of synucleinopathies? Mov Disord. 2015 doi: 10.1002/mds.26428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valera E, Ubhi K, Mante M, Rockenstein E, Masliah E. Antidepressants reduce neuroinflammatory responses and astroglial alpha-synuclein accumulation in a transgenic mouse model of multiple system atrophy. Glia. 2014;62:317–337. doi: 10.1002/glia.22610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss K, Combs B, Patterson KR, Binder LI, Gamblin TC. Hsp70 alters tau function and aggregation in an isoform specific manner. Biochemistry. 2012;51:888–898. doi: 10.1021/bi2018078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi K, Hayashi S, Kakita A, Yamada M, Toyoshima Y, Yoshimoto M, Takahashi H. Accumulation of alpha-synuclein/NACP is a cytopathological feature common to Lewy body disease and multiple system atrophy. Acta neuropathologica. 1998a;96:445–452. doi: 10.1007/s004010050918. [DOI] [PubMed] [Google Scholar]
- Wakabayashi K, Matsumoto K, Takayama K, Yoshimoto M, Takahashi H. NACP, a presynaptic protein, immunoreactivity in Lewy bodies in Parkinson's disease. Neurosci Lett. 1997;239:45–48. doi: 10.1016/s0304-3940(97)00891-4. [DOI] [PubMed] [Google Scholar]
- Wakabayashi K, Yoshimoto M, Tsuji S, Takahashi H. Alpha-synuclein immunoreactivity in glial cytoplasmic inclusions in multiple system atrophy. Neurosci Lett. 1998b;249:180–182. doi: 10.1016/s0304-3940(98)00407-8. [DOI] [PubMed] [Google Scholar]
- Warren Olanow C, Bartus RT, Baumann TL, et al. Gene delivery of neurturin to putamen and substantia nigra in Parkinson disease: A double-blind, randomized, controlled trial. Ann Neurol. 2015;78:248–257. doi: 10.1002/ana.24436. [DOI] [PubMed] [Google Scholar]
- Wichmann T, Delong MR. Deep-Brain Stimulation for Basal Ganglia Disorders. Basal ganglia. 2011;1:65–77. doi: 10.1016/j.baga.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winklhofer KF. The parkin protein as a therapeutic target in Parkinson's disease. Expert Opin Ther Targets. 2007;11:1543–1552. doi: 10.1517/14728222.11.12.1543. [DOI] [PubMed] [Google Scholar]
- Winner B, Jappelli R, Maji SK, et al. In vivo demonstration that alpha-synuclein oligomers are toxic. Proc Natl Acad Sci USA. 2011;108:4194–4199. doi: 10.1073/pnas.1100976108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wobst HJ, Sharma A, Diamond MI, Wanker EE, Bieschke J. The green tea polyphenol (−)-epigallocatechin gallate prevents the aggregation of tau protein into toxic oligomers at substoichiometric ratios. FEBS Lett. 2015;589:77–83. doi: 10.1016/j.febslet.2014.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Writing Group for the NINDS Exploratory Trials in Parkinson Disease (NET-PD) Investigators. Kieburtz K, Tilley BC, et al. Effect of creatine monohydrate on clinical progression in patients with Parkinson disease: a randomized clinical trial. JAMA. 2015;313:584–593. doi: 10.1001/jama.2015.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoritaka A, Kawajiri S, Yamamoto Y, Nakahara T, Ando M, Hashimoto K, Nagase M, Saito Y, Hattori N. Randomized, double-blind, placebo-controlled pilot trial of reduced coenzyme Q10 for Parkinson's disease. Parkinsonism Relat Disord. 2015;21:911–916. doi: 10.1016/j.parkreldis.2015.05.022. [DOI] [PubMed] [Google Scholar]
- Zimprich A, Biskup S, Leitner P, et al. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron. 2004;44:601–607. doi: 10.1016/j.neuron.2004.11.005. [DOI] [PubMed] [Google Scholar]