Abstract
Neurons in the suprachiasmatic nucleus (SCN), the master circadian pacemaker in mammals, display daily rhythms in electrical activity with more depolarized resting potentials and higher firing rates during the day than at night. Although these daily variations in the electrical properties of SCN neurons are required for circadian rhythms in physiology and behavior, the mechanisms linking changes in neuronal excitability to the molecular clock are not known. Recently, we reported that mice deficient for either Kcna4 (Kv1.4−/−) or Kcnd2 (Kv4.2−/−; but not Kcnd3, Kv4.3−/−), voltage-gated K+ (Kv) channel poreforming subunits that encode subthreshold, rapidly activating, and inactivating K+ currents (IA), have shortened (0.5 h) circadian periods in SCN firing and in locomotor activity compared with wild-type (WT) mice. In the experiments here, we used a mouse (Per2Luc) line engineered with a bioluminescent reporter construct, PERIOD2::LUCIFERASE (PER2::LUC), replacing the endogenous Per2 locus, to test the hypothesis that the loss of Kv1.4- or Kv4.2-encoded IA channels also modifies circadian rhythms in the expression of the clock protein PERIOD2 (PER2). We found that SCN explants from Kv1.4−/−Per2Luc and Kv4.2−/−Per2Luc, but not Kv4.3−/−Per2Luc, mice have significantly shorter (by approximately 0.5 h) circadian periods in PER2 rhythms, compared with explants from Per2Luc mice, revealing that the membrane properties of SCN neurons feedback to regulate clock (PER2) expression. The combined loss of both Kv1.4- and Kv4.2-encoded IA channels in Kv1.4−/−/Kv4.2−/−Per2Luc SCN explants did not result in any further alterations in PER2 rhythms. Interestingly, however, mice lacking both Kv1.4 and Kv4.2 show a striking (approximately 1.8 h) advance in their daily activity onset in a light cycle compared with WT mice, suggesting additional roles for Kv1.4- and Kv4.2-encoded IA channels in controlling the light-dependent responses of neurons within and/or outside of the SCN to regulate circadian phase of daily activity.
Keywords: clock gene expression, A-type K+ channels, entrainment, periodicity
In mammals, a master circadian pacemaker in the suprachiasmatic nucleus (SCN) drives daily rhythms in physiology and behavior (Dibner et al., 2010; Moore, 2013; Welsh et al., 2010). SCN neurons regulate behavior in both nocturnal and diurnal species by exhibiting daily rhythms in electrical activity. During the day, for example, SCN neurons have higher repetitive firing rates and membrane potentials are depolarized. Conversely, during the night, the repetitive firing rates of SCN neurons are reduced and membrane potentials are more hyperpolarized (Colwell, 2011; Wang et al., 2012; Welsh et al., 1995). These spontaneous rhythmic alterations in the excitability of SCN neurons are accompanied by changes in input resistance, suggesting a role for diurnal variations in K+ conductance(s) (De Jeu et al., 2002, 1998; Kuhlman and McMahon, 2004, 2006).
Rhythmicity in the SCN derives from a set of clock genes that generate a transcription-translation feedback loop (King and Takahashi, 2000; Partch et al., 2014; Wilsbacher and Takahashi, 1998), and the daily rhythms in the membrane potentials and the repetitive firing rates of SCN neurons depend on the expression of these clock genes (Albus et al., 2002; Brown and Piggins, 2007; Herzog et al., 1998; Kuhlman and McMahon, 2006; Liu et al., 1997). In addition, several lines of evidence suggest that the daily alterations in the excitability of SCN neurons participate in the feedback regulation of clock genes, thereby regulating behavior (Jones et al., 2015; Khalsa et al., 1990; Lundkvist and Block, 2005). Hyperpolarization of SCN neurons in vitro by exposure to low extracellular K+, for example, has been shown to disrupt the rhythmicity of clock gene expression (Lundkvist et al., 2005). It is not known, however, whether the daily rhythms in the electrical activity of SCN function to regulate the circadian transcription-translation feedback loop and daily rhythms in physiology and behavior (King and Takahashi, 2000; Partch et al., 2014; Wilsbacher and Takahashi, 1998).
In recent studies focused on exploring the roles of subthreshold, voltage-gated A-type K+ channels in mediating the daily rhythms in the resting membrane potentials and the repetitive firing rates of SCN neurons, we found that the firing rates are increased in SCN neurons in mice deficient for either Kcna4 (Kv1.4−/−) or Kcnd2 (Kv4.2−/−), but not Kcnd3 (Kv4.3−/−), which encode the pore-forming (α) subunits of subthreshold, rapidly activating and inactivating A-type neuronal K+ currents (IA) (Granados-Fuentes et al., 2012). In addition, Kv1.4−/− and Kv4.2−/− mice, but not Kv4.3−/− mice, have shortened (by approximately 0.5 h) circadian periods of locomotor activity and start their daily activity 0.5 h earlier than wild-type (WT) mice (Granados-Fuentes et al., 2012). The experiments here were designed to test directly the hypothesis that the loss of Kv1.4- or Kv4.2-encoded IA channels also modifies circadian rhythms in the protein expression levels of the clock gene, PERIOD2 (PER2), taking advantage of the availability of (Per2Luc) mice generated with a PER2::LUCIFERASE (PER2::LUC) reporter construct replacing the endogenous mouse Per2 locus (Yoo et al., 2004). Here, we demonstrate that Kv1.4−/−Per2Luc or Kv4.2−/−Per2Luc, but not Kv4.3−/−Per2Luc, SCN explants have significantly shorter (by ~0.5 h) circadian periods in PER2::LUC expression compared with Per2Luc SCN explants. The combined loss of both Kv1.4- and Kv4.2-encoded IA channels in Kv1.4−/−/Kv4.2−/−Per2Luc SCN explants, however, does not result in further alterations in PER2::LUC rhythms.
MATERIALS AND METHODS
Animals
Mice were maintained on a C57BL/6JN background (WT) in the Danforth animal facility at Washington University. The Per2Luc mouse line, generated by replacing the endogenous mouse Per2 locus with a PERIOD2::LUCIFERASE (PER2::LUC) reporter construct (Yoo et al., 2004), was obtained from Dr. J. Takahashi (University of Texas Southwestern, Dallas, TX). These mice were crossed with mice harboring targeted disruptions of the Kcna4 (Kv1.4−/−) (London et al., 1998), Kcnd2 (Kv4.2−/−) (Guo et al., 2005), or Kcnd3 (Kv4.3−/−) (Niwa et al., 2008) locus to generate the Kv1.4−/−Per2Luc, Kv4.2−/−Per2Luc, and Kv4.3−/−Per2Luc, respectively, mouse lines. Several additional crosses provided Kv1.4−/−/Kv4.2−/−Per2Luc mice expressing the PER2::LUC reporter and lacking both the Kv1.4 and Kv4.2 α subunits. All procedures conformed to US National Institutes of Health guidelines and were approved by the Animal Care and Use Committee of Washington University.
Real-Time Gene Expression Recordings
We recorded bioluminescence from 300-μm coronal SCN slices prepared from adult (2- to 3-mo-old) Per2Luc (n = 15), Kv1.4−/−Per2Luc (n = 18), Kv4.2−/− Per2Luc (n = 11), Kv4.3−/−Per2Luc (n = 12), and Kv1.4−/−/Kv4.2−/−Per2Luc (n = 10) mice. Briefly, male mice were sacrificed with CO2 and decapitated. Brains were quickly collected in chilled Hank’s balanced salt solution (HBSS), pH 7.2 (Sigma), supplemented with 0.01 M HEPES (Sigma), 100 U/mL penicillin, 0.1 mg/mL streptomycin, and 4 mM NaHCO3 (Invitrogen). Brain sections were obtained with a vibratome slicer (OTS-5000; Electron Microscopy Sciences). The SCN was dissected from animals of each genotype and cultured individually on a Millicell-CM membrane (Millipore) in a Petri dish with 1 mL of DMEM (Sigma) supplemented with 10 mM HEPES (Sigma), 2.2 mg/mL NaHCO3 (Invitrogen), and 0.1 mM beetle D-luciferin (Biosynth). Petri dishes were sealed with vacuum grease and placed under photomultiplier tubes (HC135-11MOD; Hamamatsu) at 36 °C in the dark. Bioluminescence was recorded in 10-min bins for at least 6 d.
Acquired in vitro bioluminescence traces were fitted with a damped sine function (Chronostar 1.0; Maier et al., 2009), and data with a coefficient of correlation >0.80 were defined as circadian. The period of PER2::LUC expression was also calculated using Chronostar and compared using a 1-way analysis of variance (ANOVA) followed by a Tukey post hoc test (Origin 9).
Electrophysiological Recordings
Acute SCN slices were prepared from adult (1 to 9 mo) WT Kv1.4−/−, Kv4.2−/−, or Kv1.4−/−/Kv4.2−/− mice maintained in a standard 12:12 h light:dark (LD) cycle with lights on at 0700 h (Zeitgeber time [ZT] 0) and lights off at 0700 h (ZT 12). For experiments, mice were deeply anesthetized with 1.25% Avertin (2,2,2-tribromoethanol and tert-amyl alcohol in 0.9% NaCl; 0.025 mL/g body weight) between ZT5 and ZT6 (1200–1300 h); brains were then rapidly removed and placed in ice-cold cutting solution containing the following (in mM): 240 sucrose, 2.5 KCl, 1.25 NaH2PO4, 25 NaHCO3, 0.5 CaCl2, and 7 MgCl2, saturated with 95% O2/5% CO2. Coronal slices (300 μm) were cut on a Leica VT1000 S vibrating blade microtome (Leica Microsystems Inc.) and incubated in a holding chamber with oxygenated artificial cerebrospinal fluid (ACSF) containing the following (in mM): NaCl, 125; KCl, 2.5; NaH2PO4, 1.25; NaHCO3, 25; CaCl2, 2; MgCl2, 1; and dextrose, 25 (~310 mOsmol/l), saturated with 95% O2/5% CO2 at room temperature for at least 1 h before transfer to the recording chamber.
Whole-cell voltage-clamp recordings were obtained at room temperature from WT, Kv1.4−/−, Kv4.2−/−, and Kv1.4−/−/Kv4.2−/− SCN neurons visually identified in slices using differential interference contrast optics with infrared illumination. Slices were perfused continuously with ACSF saturated with 95% O2/5% CO2. Recordings were obtained between ZT6 and ZT12 in ACSF containing tetraethylammonium (3 mM), CdCl2 (0.1 mM), and tetrodotoxin (150 nM) using glass pipettes (3–5 MΩ) filled with intracellular solution containing (in mM) 130 KCl, 10 HEPES, 10 glucose, 0.83 CaCl2, 2.6 BAPTA, 3 MgATP, and 0.5 NaGTP (pH 7.4; 300 mOsm). About 85% of the whole-cell voltage-clamp recordings were obtained from the ventral SCN. Voltage-clamp paradigms were generated and data were collected using a Multiclamp 700B patch clamp amplifier (Molecular Devices, Sunnyvale, CA) interfaced to a Dell personal computer with a Digidata 1332 and the pCLAMP 10 software package (Molecular Devices). Tip potentials were zeroed before membrane-pipette seals were formed. Following formation of a gigaOhm seal and establishing the whole-cell configuration, membrane capacitances and series resistances were compensated electronically. Series resistances prior to compensation were in the range of 15 to 25 M, and were routinely corrected by 70% to 80%. If the series resistance changed ≥20% during the course of the experiment, acquired data were not included in the analyses. Voltage signals were acquired at 20 kHz, filtered at 10 kHz, and stored for offline analysis.
Whole-cell membrane capacitances were determined from analyses of capacitative currents elicited by brief (25 ms) voltage steps (±20 mV) from the holding potential (–70 mV). The mean whole-cell capacitances of Kv1.4−/−/Kv4.2−/− SCN cells (n = 39) and WT SCN cells (n = 38) were 9.5 ± 0.6 pF and 11.1 ± 0.8 pF, respectively.
Rapidly activating and rapidly inactivating Kv currents, IA, were isolated using a 2-step voltage-clamp protocol, as previously described (Norris and Nerbonne, 2010; Granados-Fuentes et al., 2012). Briefly, whole-cell Kv currents in each cell, evoked in response to 4-s depolarizing voltage steps to potentials between −40 and +30 mV (in 10-mV increments) from a holding potential of −70 mV, were first recorded. Currents were measured again with a prepulse paradigm that included a brief (60-ms) step to −10 mV before the 4-s depolarizing voltage steps to potentials between −40 and +30 mV (in 10-mV increments). Off-line subtraction of the Kv currents recorded with the prepulse paradigm from the currents evoked without the prepulse were performed to isolate IA (Norris and Nerbonne, 2010; Granados-Fuentes et al., 2012).
Locomotor Activity Recordings
Adult (8 to 10 wk) Kv1.4−/−/Kv4.2−/− (n = 17) and WT (n = 12) male mice were housed individually in cages equipped with a running wheel in light-tight chambers illuminated with fluorescent bulbs (2.4 ± 0.5 × 1018 photons/s*m2; General Electric). Running-wheel activity was recorded in 6-min bins (ClockLab software; Actimetrics) for 5 to 15 d in a 12-h light:12-h dark (LD) cycle (lights-on at 0700 h), 14 to 19 d in constant dark (DD), 15 to 22 d in the LD cycle (lights-on at 0700 h), 15 to 18 d in a 6-h delayed LD cycle (lights-on at 1300 h), and finally for 15 to 28 d in a 6-h advanced LD cycle (lights-on at 0700 h).
The period of behavioral rhythmicity of each mouse was determined using χ2 periodogram analysis (Sokolove and Bushell, 1978) from continuous recordings of 10 d in DD (ClockLab software). Rhythmicity was considered statistically significant if the χ2 periodogram value exceeded the 99.9% confidence interval (Qp value). In addition, the phase angle of entrainment in LD, number of days to reentrain after 6-h shifts in the LD cycle, and total daily activity counts in LD and DD were calculated for each mouse (ClockLab), as described previously (Granados-Fuentes et al., 2004) and compared with a Student t test (Origin 9.0).
RESULTS
Loss of Kv1.4 or Kv4.2 Shortens Circadian Period in PER2::LUC Expression
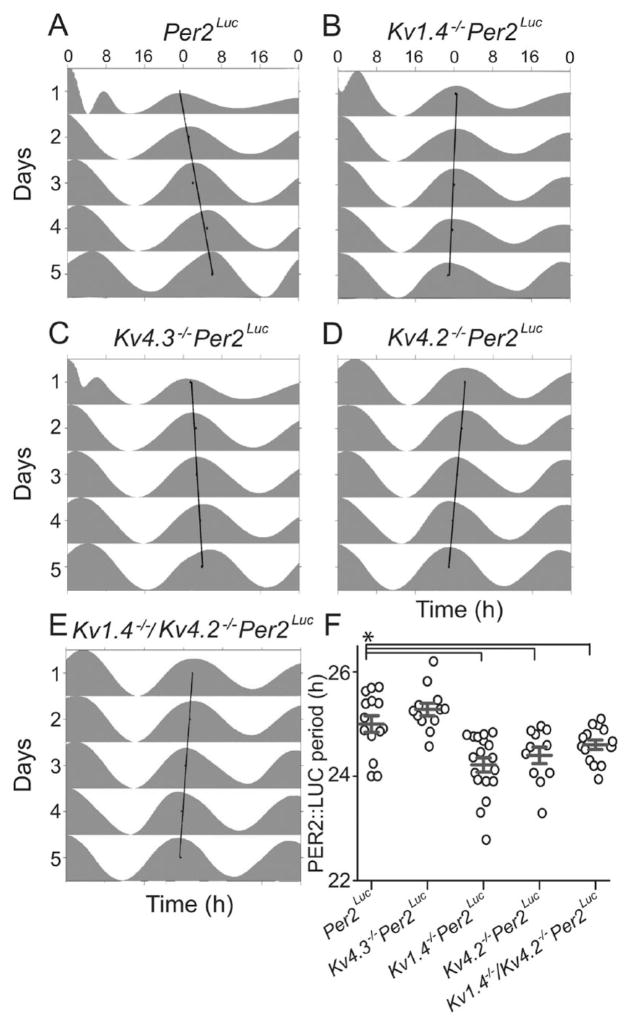
To determine if A-type voltage-gated K+ (Kv) channels regulate circadian gene expression in the SCN, we measured PERIOD2::LUCIFERASE (PER2::LUC) bioluminescence in explanted SCN from Per2Luc mice and from mice lacking the individual Kv α subunits Kv4.3 (Kv4.3−/−Per2Luc), Kv1.4 (Kv1.4−/−Per2Luc), or Kv4.2 (Kv4.2−/−Per2Luc). SCN explants from all 4 genotypes exhibited reliable, high-amplitude circadian rhythms in PER2::LUC bioluminescence. As illustrated in Figure 1, the mean ± SEM periods of PER2::LUC expression in Kv1.4−/−Per2Luc (24.2 ± 0.1 h) and Kv4.2−/−Per2Luc (24.4 ± 0.2 h) SCN explants were significantly (p < 0.001 1-way ANOVA) shorter than in Per2Luc (25.0 ± 0.1) and Kv4.3−/−Per2Luc SCN explants (25.3 ± 0.1). The magnitude (~0.5 h) of the differences in the periods of PER2::LUC expression in the Kv1.4−/−Per2Luc and the Kv4.2−/−Per2Luc, compared with the Per2Luc and the Kv4.3−/−Per2Luc, SCN explants is similar to the (~0.5 h) shift in the period of locomotor behavior reported for mice lacking either Kv1.4 or Kv4.2 (Granados-Fuentes et al., 2012). SCN explants prepared from mice (Kv1.4−/−/Kv4.2−/−Per2Luc) lacking both Kv1.4 and Kv4.2 had PER2::LUC rhythms with a mean ± SEM circadian period (24.6 ± 0.1 h) that is also significantly (p < 0.001 1-way ANOVA) shorter than in the explants from Per2Luc (25.0 ± 0.1 h) mice (Fig. 1) but not different from the values measured in Kv1.4−/−Per2Luc or Kv4.2−/−Per2Luc SCN explants (see the Discussion section).
Figure 1.
L oss of Kv1.4 or Kv4.2 shortens the circadian period of clock gene expression in isolated suprachiasmatic nucleus (SCN). PERIOD2::LUCIFERASE (PER2::LUC) bioluminescence was measured in vitro in SCN slices prepared from mice expressing the PER2::LUC reporter construct in place of the endogenous mouse Per2 (Yoo et al., 2004) and lacking Kv1.4, Kv4.2, Kv4.3, or both the Kv1.4 and Kv4.2 α subunits (see the Materials and Methods section). (A–E) Representative examples of PER2::LUC bioluminescence are shown as double-plotted actograms and normalized to their daily peak in Per2Luc (A), Kv1.4−/−Per2Luc (B), Kv4.3−/−Per2Luc (C), Kv4.2−/−Per2Luc (D), and Kv1.4−/−/Kv4.2−/−Per2Luc (E) slices. In each plot, the linear regression through the daily peaks illustrates the intrinsic period of the slice. In (F), the average periods (gray lines) of PER2::LUC expression in SCN slices from Kv1.4−/−Per2Luc (n = 18), Kv4.2−/−Per2Luc (n = 11), and Kv1.4−/−/Kv4.2−/−Per2Luc (n = 12) were significantly (*p < 0.02 1-way analysis of variance) shorter than in SCN slices from Per2Luc (n = 15) or Kv4.3−/−Per2Luc (n = 14) animals. Open circles represent individual SCN slice values; error bars show SEM.
Although marked differences in the circadian periods of PER2::LUC expression were observed (Fig. 1), the absolute amplitudes of the oscillations measured in explants of all 5 genotypes did not differ significantly (p = 0.2, 1-way ANOVA). The mean ± SEM counts/10 min were 715 087 ± 137 233 (Per2Luc), 566 498 ± 83 883 (Kv1.4−/−Per2Luc), 670 356 ± 148 615 (Kv4.2−/−Per2Luc), 586 824 ± 96 869 (Kv4.3−/−Per2Luc), and 334 381 ± 103 147 (Kv1.4−/−/Kv4.2−/−Per2Luc). Taken together, these results suggest that the loss of Kv1.4- and Kv 4.2-, but not Kv4.3-, encoded IA channels affects the period of PER2::LUC expression in the SCN. Notably, the combined loss of both Kv1.4- and Kv4.2-encoded IA channels did not lead to an additional shortening in the period of PER2::LUC expression compared with the periods measured in explants from the Kv1.4−/−Per2Luc and Kv4.2−/−Per2Luc single knockouts.
IA Densities Are Reduced in Kv1.4−/−/Kv4.2−/− SCN Neurons
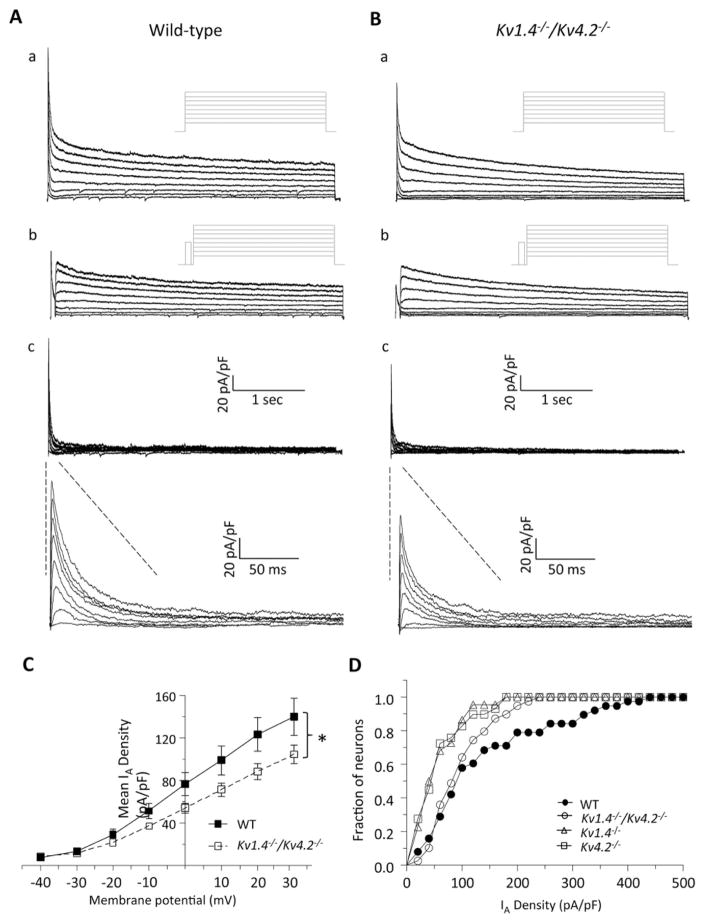
Whole-cell voltage-clamp recordings were obtained from SCN neurons in acute slices prepared from WT and Kv1.4−/−/Kv4.2−/− animals, and IA amplitudes/densities were quantified. As in previous studies (Granados-Fuentes et al., 2012; Norris and Nerbonne, 2010), IA was isolated using a 2-step voltage-clamp paradigm (see the Materials and Methods section). As illustrated in Figure 2, rapidly activating and inactivating, A-type, Kv currents were clearly evident in Kv1.4−/−/Kv4.2−/− (n = 39) SCN neurons, and the wave-forms of the currents (Fig. 2B) were indistinguishable from those measured in WT (n = 38) SCN neurons (Fig. 2A). Quantitative analyses revealed that IA densities were lower (by ~30%) in Kv1.4−/−/Kv4.2−/− than in WT, SCN neurons (Fig. 2C). At +30 mV, for example, mean ± SEM IA densities were 145 ± 18 pA/pF (n = 38) and 104 ± 9 pA/pF (n = 39) in WT and Kv1.4−/−/Kv4.2−/− SCN neurons, respectively (Fig. 2C). Comparison of the cumulative distribution plots of IA densities measured in individual WT and Kv1.4−/−/Kv4.2−/− SCN neurons revealed a significant (p < 0.05; 2-sample Kolmogorov-Smirnov test) shift to the left (to lower densities) for the Kv1.4−/−/Kv4.2−/− SCN neurons (Fig. 2D). Interestingly, the magnitude of the shift in the cumulative IA density distribution plot for the Kv1.4−/−/Kv4.2−/− SCN neurons was smaller (Fig. 2D) than that seen for SCN neurons lacking only Kv1.4 or only Kv4.2 (see the Discussion section).
Figure 2.
IA is reduced in Kv1.4−/−/Kv4.2−/− suprachiasmatic nucleus (SCN) neurons. (A, B) Representative whole-cell Kv currents recorded from SCN neurons in acute slices prepared from wild-type (WT; A) and Kv1.4−/−/Kv4.2−/− (B) animals are shown. In WT (Aa) and Kv1.4−/−/Kv4.2−/− (Ba) SCN neurons, whole-cell Kv currents evoked in response to (4-s) voltage steps to potentials ranging from −40 to +30 mV (in 10-mV increments) from a holding potential of −70 mV were recorded. A second voltage-clamp paradigm, which included a brief (60-ms) prepulse to −10 mV to inactivate IA, was then presented, and Kv currents in WT (Ab) and Kv1.4−/−/Kv4.2−/− (Bb) SCN neurons were again recorded. The voltage-clamp paradigms are illustrated adjacent to the records. Digital offline subtraction of the recordings with the prepulse (Ab, Bb) from those without the prepulse (Aa, Ba) provided IA in WT (Ac) and Kv1.4−/−/Kv4.2−/− (Bc) SCN neurons; the IA records are also shown on an expanded time scale. Mean (±SEM) IA densities are plotted as a function of test potential in (C); mean ± SEM was lower in Kv1.4−/−/Kv4.2−/− (n = 39) SCN neurons compared with WT (n = 38) neurons at different test potentials (*p < 0.05, Student t test). (D) The cumulative distribution of IA densities in Kv1.4−/−/Kv4.2−/− SCN neurons (n = 39) was shifted significantly (p < 0.05; 2-sample Kolmogorov-Smirnov test) to the left (toward lower densities) compared with WT SCN neurons (n = 38), similar to the reduction seen in slices from animals lacking only Kv1.4 (n = 23) or Kv4.2 (n = 28). Data were collected from 11 Kv1.4−/−/Kv4.2−/−, 7 Kv1.4−/−, 8 Kv4.2−/−, and 8 WT SCN slices; 1 SCN slice was generated from each mouse.
Kv1.4−/−/Kv4.2−/− Mice Run with a Shorter Circadian Period and Show an Early Chronotype
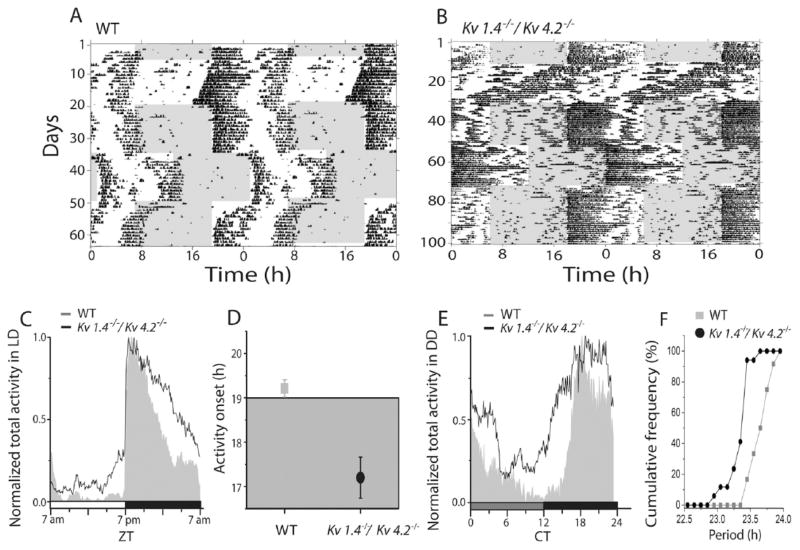
Mice deficient in either Kv1.4 or Kv4.2 express shorter circadian periods and earlier activity onsets than WT mice (Granados-Fuentes et al., 2012). To determine if the combined loss of Kv1.4 and Kv4.2 would affect the period or the activity onset in locomotor behavior to a greater extent than the loss of either Kv1.4 or Kv4.2 alone, we recorded wheel-running activity in Kv1.4−/−/Kv4.2−/− mice (Fig. 3A and B). Strikingly, in a LD cycle, the Kv1.4−/−/Kv4.2−/− mice initiated their daily wheel-running activity significantly (p < 0.01) earlier, by approximately 1.8 h, than WT mice (Fig. 3C and D). The mean ± SEM activity onset times determined for WT and Kv1.4−/−/Kv4.2−/− mice maintained in an LD cycle were at 1906 h (±0.2 h) and 1718 h (±0.5 h), respectively.
Figure 3.
Mice lacking Kv1.4 and Kv4.2 show an early chronotype and shortened circadian period of locomotor activity. Representative recordings of wheel-running activity in a wild-type (WT; A) and a Kv1.4−/−/Kv4.2−/− (B) mouse over 100 consecutive days in different light (gray) and dark (white) cycles. Each line shows wheel revolutions per minute over a 48-h period; data from the subsequent days are replotted on the line below in these double-plotted actograms. Normalized daily activity plots show that, on average, mice lacking both Kv1.4 and Kv4.2 (n = 17) ran more during the day (C) than WT (n =12) and started running significantly (*p < 0.02, Student t test) earlier during the LD cycle (D). Similarly, in constant darkness, Kv1.4−/−/Kv4.2−/− mice (n = 17) also ran more than WT mice (n = 12) during the subjective day (E). The cumulative distribution of intrinsic periods reveals that nearly 90% of the Kv1.4−/−/Kv4.2−/− mice showed shorter circadian periods in constant darkness compared with WT (F).
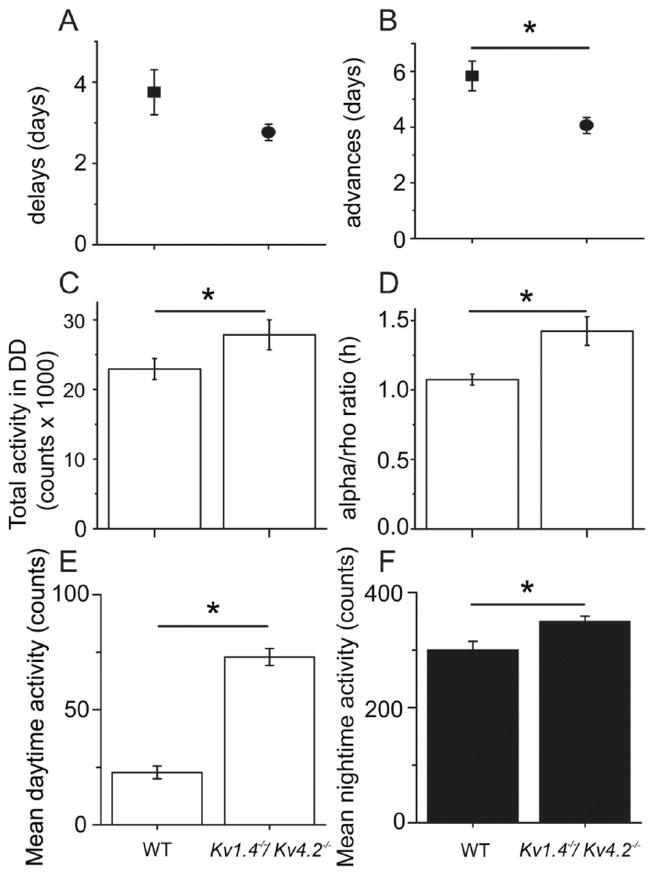
In constant darkness, mice lacking both Kv1.4 and Kv4.2 ran with a significantly (p < 0.01) shorter mean ± SEM circadian period compared with WT (23.7 ± 0.05 h for WT and 23.4 ± 0.04 h for Kv1.4−/−/Kv4.2−/−). More than 90% of the Kv1.4−/−/Kv4.2−/− mice had periods shorter than WT (Fig. 3F; p < 0.04, Kolmogorov-Smirnov test). Entrainment of locomotor activity to a 6-h advance in the light cycle occurred significantly (p < 0.001) faster in Kv1.4−/−/Kv4.2−/− than in WT mice (Fig. 4B), with mean ± SEM entrainment times of 5.8 ± 0.5 d for WT and 4.0 ± 0.3 d for Kv1.4−/−/Kv4.2−/− mice. In contrast, Kv1.4−/−/Kv4.2−/− (2.8 ± 0.2 d) and WT (3.8 ± 0.6 days) mice entrained similarly to a 6-h delay (Fig. 4A). In addition, the Kv1.4−/−/Kv4.2−/− mice ran approximately 3 times (p < 0.001) more than WT mice during the day (Figs. 3C and 4E), with mean ± SEM values of 23 ± 3 revolutions/min for WT and 73 ± 4 rpm for Kv1.4−/−/Kv4.2−/− animals. The Kv1.4−/−/Kv4.2−/− animals also ran significantly (p < 0.001) more at night (393 ± 11 rpm) than WT (300 ± 15 rpm) mice (Figs. 3C and 4F). In constant darkness, Kv1.4−/−/Kv4.2−/− mice also ran more than WT mice (Figs. 3E and 4C; p < 0.05; 22 935 ± 1515 revolutions/d for WT and 27 851 ± 2157 revolutions/d for Kv1.4−/−/Kv4.2−/−). Consequently, the mean ± SEM ratio of time spent running to resting each day (termed alpha/rho) was significantly (p = 0.01) higher in Kv1.4−/−/Kv4.2−/− (1.7 ± 0.1 h) than in WT (1.3 ± 0.05 h) mice (Fig. 4D).
Figure 4.
Mice lacking Kv1.4 and Kv4.2 adjust to an advanced light cycle faster and are more active during the day and at night than wild-type (WT) mice. The Kv1.4−/−/Kv4.2−/− (n = 17) mice shifted their onsets of activity to match the light cycle faster than WT (n = 12) mice when the light cycle was advanced by 6 h (B) but not following a 6-h delay in the light cycle (A). The Kv1.4−/−/Kv4.2−/− mice also ran more (C) and ran for longer (D) in constant darkness. In a light cycle, the Kv1.4−/−/Kv4.2−/− mice were more active at all times, especially during the light phase (E and F). Data shown are means ± SEM. *p < 0.01, Student t test.
DISCUSSION
Native K+ Channels Regulate Circadian Rhythms in PER2 Expression in the SCN
The results presented here demonstrate that the loss of Kv1.4- or Kv4.2-encoded IA channels alters the circadian regulation of PER2 expression. These observations clearly link changes in membrane properties with clock gene expression and are consistent with previous findings in flies demonstrating that chronic overexpression of depolarizing or hyperpolarizing channels in circadian neurons abolishes circadian rhythms in behavior and, importantly, in gene expression (Cao and Nitabach, 2008; Nitabach et al., 2002, 2005, 2004; Wu et al., 2008). Hyperpolarization of SCN neurons in vitro by exposure to low extracellular K+ has also been shown to disrupt the rhythmicity of clock gene expression (Lundkvist et al., 2005). In addition, in recent studies using optogenetic strategies to activate or inhibit SCN neurons expressing channel-rhodopsin or halorhodopsin, Jones and colleagues (2015) demonstrated shifts in the phase and period of the molecular clock, elegantly illustrating that the pattern of neuronal firing in the SCN is key to circadian rhythm generation, entrainment, and output. To our knowledge, however, the results of the studies completed here provide the first demonstration that alterations in the expression/functioning of an endogenous (native) ionic current modulate the molecular circadian timing loop.
The observation that the loss of Kv1.4 or Kv4.2 shortens the period of PER2 expression suggests that Kv1.4- and Kv4.2-encoded IA channels normally function to regulate periodicity. The functioning of these channels and the resulting effects on the membrane potentials and/or the repetitive firing properties of SCN neurons may directly or indirectly modulate the expression or the activity of 1 or more of the various genes/proteins shown to be involved in circadian rhythm generation (Colwell, 2011; Dibner et al., 2010; King and Takahashi, 2000). Loss of Kv1.4- or Kv4.2-encoded IA channels could, for example, modulate the stability or the phosphorylation state of the PER2 protein. A mutation in a phosphorylation site within the casein kinase I-binding domain of PER2 (Lowrey et al., 2000; Xu et al., 2005) has been linked to a phenotype similar to that shown here in the Kv1.4−/−/Kv4.2−/− double knockout: shortened circadian period and advanced chronotype in human familial advanced phase sleep syndrome (Jones et al., 1999). Alternatively, because silencing of circadian neurons only in adulthood led to reversible behavioral arrhythmicity in flies but did not perturb circadian protein expression (Depetris-Chauvin et al., 2011), we cannot rule out the possibility that chronic loss of Kv1.4 or Kv4.2 leads to a compensatory gain of function (of an unidentified molecule) that modulates the circadian clock in a novel way. Notably, chronic loss of Kv4.3 did not produce a circadian phenotype. Future studies, using acute knockdown of Kv1.4 (Kcna4) and/or Kv4.2 (Kcnd2) expression (Norris et al., 2010a) should further define the specific roles of these channels in circadian period determination in mature SCN neurons.
Kv1.4- and Kv4.2-Encoded IA Channels Regulate the Intrinsic Excitability of SCN Neurons
Previously, we demonstrated that the loss of Kv1.4 or Kv4.2, but not Kv4.3, reduced IA densities in SCN neurons by ~50% (Granados-Fuentes et al., 2012). Crossing the Kv1.4−/− and Kv4.2−/− mice to create the Kv1.4−/−/Kv4.2−/− double knockouts allowed us to test whether the combined loss of both Kv1.4 and Kv4.2 would further decrease, or possibly eliminate, IA in SCN neurons. Unexpectedly, we found that the mean ± SEM IA density in Kv1.4−/−/Kv4.2−/− SCN neurons was similar to the mean ± SEM IA densities measured in SCN neurons lacking Kv1.4 or Kv4.2. The overall reduction in mean IA density in Kv1.4−/−/Kv4.2−/− SCN neurons, however, was ~30%, somewhat smaller than the ~50% reductions in IA densities measured in both Kv1.4−/− and Kv4.2−/− SCN neurons. In addition, the cumulative distribution plot of IA densities in Kv1.4−/−/Kv4.2−/− SCN neurons was shifted to the right (more cells with higher IA densities) compared with both Kv1.4−/− and Kv4.2−/− SCN neurons (Fig. 2D). These combined observations suggest that the combined loss of Kv1.4 and Kv4.2 results in the compensatory up-regulation of IA channels encoded by other Kv α subunits and, in addition, that the magnitude of the up-regulation is variable among neurons in the SCN.
The persistence of rapidly activating and inactivating A-type currents in the absence of both Kv1.4 and Kv4.2 suggests a role for other Kv α subunits, such as Kv4.1, which has been shown to produce rapidly activating and inactivating A-type currents in heterologous cells (Pak et al., 1991; Serodio and Rudy, 1998). Interestingly, Kv4.1 is expressed at the transcript and protein levels in the mouse SCN (Itri et al., 2010, and www.brain-map.org). Experiments focused on defining the contribution of Kv4.1-encoded K+ channels to IA in SCN neurons and to the regulation of circadian rhythms will be of interest.
It has previously been reported that IA densities are higher during the day than at night in a subpopulation of SCN neurons, although Western blot analyses revealed no diurnal differences in the expression levels of the Kv4.1 or Kv4.2 α subunit proteins (Itri et al., 2010). The observed changes in IA densities (Itri et al., 2010) could, however, reflect circadian regulation of the transcripts encoding 1 or more of the critical accessory or regulatory subunits linked to the regulation of native neuronal IA channels (Covarrubias et al., 2008; Jerng and Pfaffinger, 2014; Norris et al., 2010a, 2010b). Time-dependent changes in the post-translational processing of IA pore-forming and/or accessory proteins could certainly also regulate day-night differences in IA densities (Itri et al., 2010). Additional experiments are needed to explore these hypotheses directly and to delineate the underlying molecular mechanisms for diurnal variation in IA densities.
Deletion of Both Kv1.4 and Kv4.2 Leads to an Advanced Chronotype
The results presented indicate that the loss of both the Kv1.4 and Kv4.2 α subunits affects running-wheel activity and the time of daily activity onset in a light cycle. These phenotypes are similar to those observed in the single knockouts, Kv1.4−/− and Kv4.2−/− mice (Granados-Fuentes et al., 2012), except that Kv1.4−/−/Kv4.2−/− mice become active about 1.8 h earlier in a light cycle. This is one of the earliest chronotypes in an animal model reported, surpassed only by the ~5-h advanced phase of mice lacking both Kcnc1 and Kcnc2 genes (Kudo et al., 2011). Indeed, the 1.8-h advanced phase of Kv1.4−/−/Kv4.2−/− mice is greater than the 0.5-h positive phase angle of entrainment seen in Per2−/− mice and the 1.5 h seen in (Per2−/−Dec1−/−Dec2−/−) mice lacking Per2 and the Dec transcription factors, Dec1 and Dec2, linked to the circadian regulation of the transcription-translation feedback loop in the SCN (Bode et al., 2011).
The early chronotype was associated with a shortening in the period of locomotor behavior by 0.5 h. Although period shortening is not necessary to produce an advanced phase angle of entrainment (cf. the normal circadian period in mice lacking Kcnc1 and Kcnc2; Kudo et al., 2011), it likely contributes to the early chronotype of mice with reduced IA densities or with impaired PER2 phosphorylation (Lowrey et al., 2000; Xu et al., 2005). This shortening is opposite to the 0.5-h lengthening of circadian behavior in mice lacking 1 copy of Scn1a, which encodes the voltage-gated sodium channel α subunit, Nav1.1 (Han et al., 2012). Other channel deficiencies, including deletion of BK (Kcnma1; Meredith et al., 2006; Montgomery et al., 2013), Cav2.2 (Cacna1b; Beuckmann et al., 2003), Kv3.1, or Kv3.2 (Kcnc1 or Kcnc2; Kudo et al., 2011), in contrast, have not been found to change circadian period. The reductions in the circadian period of locomotor activity observed in mice lacking Kv1.4 or Kv4.2 (Granados-Fuentes et al., 2012), or both Kv1.4 and Kv4.2 (the present study), correlate with the periods observed for PER2::LUC expression in the SCN. Similarly, the lack of a phenotype in the SCN lacking Kv4.3 correlates with their normal circadian behavior (Granados-Fuentes et al., 2012). The findings further implicate Kv1.4 and Kv4.2, but not Kv4.3, α subunits in the regulation of excitability in the SCN, as well as in the regulation of the circadian periodicity in PER2 gene expression in the SCN, to modulate the circadian period of locomotor behavior. Interestingly, however, the findings that mice lacking both Kv1.4 and Kv4.2 show a striking (approximately 1.8 h) advance in their daily activity onset and an increase of locomotor activity in a LD cycle suggest additional roles for Kv1.4- and Kv4.2-encoded K+ channels in controlling the light-dependent response properties of SCN neurons and/or roles for these channels outside of the SCN in regulating the circadian phase of locomotor activity.
Acknowledgments
The authors thank Rebecca Mellor and Tatiana Simon for expert technical assistance. In addition, we acknowledge financial support of the National Institute of General Medical Sciences (GM10499102 to E.D.H. and J.M.N.).
Footnotes
Conflict Of Interest Statement
The authors have no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Albus H, Bonnefont X, Chaves I, Yasui A, Doczy J, van der Horst GT, Meijer JH. Cryptochrome-deficient mice lack circadian electrical activity in the suprachiasmatic nuclei. Curr Biol. 2002;12:1130–1133. doi: 10.1016/s0960-9822(02)00923-5. [DOI] [PubMed] [Google Scholar]
- Beuckmann CT, Sinton CM, Miyamoto N, Ino M, Yanagisawa M. N-type calcium channel {alpha}1B subunit (CaV2.2) knock-out mice display hyperactivity and vigilance state differences. J Neurosci. 2003;23:6793–6797. doi: 10.1523/JNEUROSCI.23-17-06793.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode B, Taneja R, Rossner MJ, Oster H. Advanced light-entrained activity onsets and restored free-running suprachiasmatic nucleus circadian rhythms in per2/dec mutant mice. Chronobiol Int. 2011;28:737–750. doi: 10.3109/07420528.2011.607374. [DOI] [PubMed] [Google Scholar]
- Brown TM, Piggins HD. Electrophysiology of the suprachiasmatic circadian clock. Prog Neurobiol. 2007;82:229–255. doi: 10.1016/j.pneurobio.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Cao G, Nitabach MN. Circadian control of membrane excitability in Drosophila melanogaster lateral ventral clock neurons. J Neurosci. 2008;28:6493–6501. doi: 10.1523/JNEUROSCI.1503-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colwell CS. Linking neural activity and molecular oscillations in the SCN. Nat Rev Neurosci. 2011;12:553–569. doi: 10.1038/nrn3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covarrubias M, Bhattacharji A, De Santiago-Castillo JA, Dougherty K, Kaulin YA, Na-Phuket TR, Wang G. The neuronal Kv4 channel complex. Neurochem Res. 2008;33:1558–1567. doi: 10.1007/s11064-008-9650-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jeu M, Geurtsen A, Pennartz C. A Ba(2+)- sensitive K(+) current contributes to the resting membrane potential of neurons in rat suprachiasmatic nucleus. J Neurophysiol. 2002;88:869–878. doi: 10.1152/jn.2002.88.2.869. [DOI] [PubMed] [Google Scholar]
- De Jeu M, Hermes M, Pennartz C. Circadian modulation of membrane properties in slices of rat suprachiasmatic nucleus. Neuroreport. 1998;9:3725–3729. doi: 10.1097/00001756-199811160-00028. [DOI] [PubMed] [Google Scholar]
- Depetris-Chauvin A, Berni J, Aranovich EJ, Muraro NI, Beckwith EJ, Ceriani MF. Adult-specific electrical silencing of pacemaker neurons uncouples molecular clock from circadian outputs. Curr Biol. 2011;21:1783–1793. doi: 10.1016/j.cub.2011.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dibner C, Schibler U, Albrecht U. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Ann Rev Physiol. 2010;72:517–549. doi: 10.1146/annurev-physiol-021909-135821. [DOI] [PubMed] [Google Scholar]
- Granados-Fuentes D, Norris AJ, Carrasquillo Y, Nerbonne JM, Herzog ED. I(A) channels encoded by Kv1.4 and Kv4.2 regulate neuronal firing in the suprachiasmatic nucleus and circadian rhythms in locomotor activity. J Neurosci. 2012;32:10045–10052. doi: 10.1523/JNEUROSCI.0174-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granados-Fuentes D, Saxena MT, Prolo LM, Aton SJ, Herzog ED. Olfactory bulb neurons express functional, entrainable circadian rhythms. Eur J Neurosci. 2004;19:898–906. doi: 10.1111/j.0953-816x.2004.03117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Jung WE, Marionneau C, Aimond F, Xu H, Yamada KA, Schwarz TL, Demolombe S, Nerbonne JM. Targeted deletion of Kv4.2 eliminates I(to,f) and results in electrical and molecular remodeling, with no evidence of ventricular hypertrophy or myocardial dysfunction. Circ Res. 2005;97:1342–1350. doi: 10.1161/01.RES.0000196559.63223.aa. [DOI] [PubMed] [Google Scholar]
- Han S, Yu FH, Schwartz MD, Linton JD, Bosma MM, Hurley JB, Catterall WA, de la Iglesia HO. PNAS Plus: NaV1.1 channels are critical for intercellular communication in the suprachiasmatic nucleus and for normal circadian rhythms. Proc Natl Acad Sci. 2012;109:E368–E377. doi: 10.1073/pnas.1115729109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog ED, Takahashi JS, Block GD. Clock controls circadian period in isolated suprachiasmatic nucleus neurons. Nat Neurosci. 1998;1:708–713. doi: 10.1038/3708. [DOI] [PubMed] [Google Scholar]
- Itri JN, Vosko AM, Schroeder A, Dragich JM, Michel S, Colwell CS. Circadian regulation of a-type potassium currents in the suprachiasmatic nucleus. J Neurophysiol. 2010;103:632–640. doi: 10.1152/jn.00670.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerng HH, Pfaffinger PJ. Modulatory mechanisms and multiple functions of somatodendritic A-type K (+) channel auxiliary subunits. Front Cell Neurosci. 2014;8:82. doi: 10.3389/fncel.2014.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CR, Campbell SS, Zone SE, Cooper F, DeSano A, Murphy PJ, Jones B, Czajkowski L, Ptacek LJ. Familial advanced sleep-phase syndrome: a shortperiod circadian rhythm variant in humans. Nat Med. 1999;5:1062–1065. doi: 10.1038/12502. [DOI] [PubMed] [Google Scholar]
- Jones JR, Tackenberg MC, McMahon DG. Manipulating circadian clock neuron firing rate resets molecular circadian rhythms and behavior. Nat Neurosci. 2015;18:373–375. doi: 10.1038/nn.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalsa SBS, Ralph MR, Block GD. Chloride conductance contributes to period determination of a neuronal circadian pacemaker. Brain Res. 1990;520:166–169. doi: 10.1016/0006-8993(90)91702-i. [DOI] [PubMed] [Google Scholar]
- King DP, Takahashi JS. Molecular genetics of circadian rhythms in mammals. Ann Rev Neurosci. 2000;23:713–742. doi: 10.1146/annurev.neuro.23.1.713. [DOI] [PubMed] [Google Scholar]
- Kudo T, Loh DH, Kuljis D, Constance C, Colwell CS. Fast delayed rectifier potassium current: critical for input and output of the circadian system. J Neurosci. 2011;31:2746–2755. doi: 10.1523/JNEUROSCI.5792-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlman SJ, McMahon DG. Rhythmic regulation of membrane potential and potassium current persists in SCN neurons in the absence of environmental input. Eur J Neurosci. 2004;20:1113–1117. doi: 10.1111/j.1460-9568.2004.03555.x. [DOI] [PubMed] [Google Scholar]
- Kuhlman SJ, McMahon DG. Encoding the ins and outs of circadian pacemaking. J Biol Rhythms. 2006;21:470–481. doi: 10.1177/0748730406294316. [DOI] [PubMed] [Google Scholar]
- Liu C, Weaver DR, Strogatz SH, Reppert SM. Cellular construction of a circadian clock: period determination in the suprachiasmatic nuclei. Cell. 1997;91:855–860. doi: 10.1016/s0092-8674(00)80473-0. [DOI] [PubMed] [Google Scholar]
- London B, Wang DW, Hill JA, Bennett PB. The transient outward current in mice lacking the potassium channel gene Kv1.4. J Physiol. 1998;509(pt 1):171–182. doi: 10.1111/j.1469-7793.1998.171bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowrey PL, Shimomura K, Antoch MP, Yamazaki S, Zemenides PD, Ralph MR, Menaker M, Takahashi JS. Positional syntenic cloning and functional characterization of the mammalian circadian mutation tau. Science. 2000;288:483–492. doi: 10.1126/science.288.5465.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundkvist GB, Block GD. Role of neuronal membrane events in circadian rhythm generation. Methods Enzymol. 2005;393:623–642. doi: 10.1016/S0076-6879(05)93033-4. [DOI] [PubMed] [Google Scholar]
- Lundkvist GB, Kwak Y, Davis EK, Tei H, Block GD. A calcium flux is required for circadian rhythm generation in mammalian pacemaker neurons. J Neurosci. 2005;25:7682–7686. doi: 10.1523/JNEUROSCI.2211-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier B, Wendt S, Vanselow JT, Wallach T, Reischl S, Oehmke S, Schlosser A, Kramer A. A large-scale functional RNAi screen reveals a role for CK2 in the mammalian circadian clock. Genes Dev. 2009;23:708–718. doi: 10.1101/gad.512209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith AL, Wiler SW, Miller BH, Takahashi JS, Fodor AA, Ruby NF, Aldrich RW. BK calcium-activated potassium channels regulate circadian behavioral rhythms and pacemaker output. Nat Neurosci. 2006;9:1041–1049. doi: 10.1038/nn1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery JR, Whitt JP, Wright BN, Lai MH, Meredith AL. Mis-expression of the BK K(+) channel disrupts suprachiasmatic nucleus circuit rhythmicity and alters clock-controlled behavior. Am J Physiol Cell Physiol. 2013;304:C299–C311. doi: 10.1152/ajpcell.00302.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RY. The suprachiasmatic nucleus and the circadian timing system. Prog Mol Biology Transl Sci. 2013;119:1–28. doi: 10.1016/B978-0-12-396971-2.00001-4. [DOI] [PubMed] [Google Scholar]
- Nitabach MN, Blau J, Holmes TC. Electrical silencing of Drosophila pacemaker neurons stops the free-running circadian clock. Cell. 2002;109:485–495. doi: 10.1016/s0092-8674(02)00737-7. [DOI] [PubMed] [Google Scholar]
- Nitabach MN, Holmes TC, Blau J. Membranes, ions, and clocks: testing the Njus-Sulzman-Hastings model of the circadian oscillator. Methods Enzymol. 2005;393:682–693. doi: 10.1016/S0076-6879(05)93036-X. [DOI] [PubMed] [Google Scholar]
- Nitabach MN, Sheeba V, Vera DA, Blau J, Holmes TC. Membrane electrical excitability is necessary for the free-running larval Drosophila circadian clock. J Neurobiol. 2004;62:1–13. doi: 10.1002/neu.20053. [DOI] [PubMed] [Google Scholar]
- Niwa N, Wang W, Sha Q, Marionneau C, Nerbonne JM. Kv4.3 is not required for the generation of functional Ito,f channels in adult mouse ventricles. J Mol Cell Cardiol. 2008;44:95–104. doi: 10.1016/j.yjmcc.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris AJ, Foeger NC, Nerbonne JM. Interdependent roles for accessory KChIP2, KChIP3, and KChIP4 subunits in the generation of Kv4-encoded IA channels in cortical pyramidal neurons. J Neurosci. 2010a;30:13644–13655. doi: 10.1523/JNEUROSCI.2487-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris AJ, Foeger NC, Nerbonne JM. Neuronal voltage-gated K(+) (Kv) channels function in macromolecular complexes. Neurosci Lett. 2010b;486:73–77. doi: 10.1016/j.neulet.2010.08.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris AJ, Nerbonne JM. Molecular dissection of I(A) in cortical pyramidal neurons reveals three distinct components encoded by Kv4.2, Kv4.3, and Kv1.4 alpha-subunits. J Neurosci. 2010;30:5092–5101. doi: 10.1523/JNEUROSCI.5890-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pak MD, Baker K, Covarrubias M, Butler A, Ratcliffe A, Salkoff L. mShal, a subfamily of A-type K+ channel cloned from mammalian brain. Proc Natl Acad Sci USA. 1991;88:4386–4390. doi: 10.1073/pnas.88.10.4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partch CL, Green CB, Takahashi JS. Molecular architecture of the mammalian circadian clock. Trends Cell Biol. 2014;24:90–99. doi: 10.1016/j.tcb.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serodio P, Rudy B. Differential expression of Kv4 K+ channel subunits mediating subthreshold transient K+ (A-type) currents in rat brain. J Neurophysiol. 1998;79:1081–1091. doi: 10.1152/jn.1998.79.2.1081. [DOI] [PubMed] [Google Scholar]
- Sokolove PG, Bushell WN. The chi square periodogram: its utility for analysis of circadian rhythms. J Theor Biol. 1978;72:131–160. doi: 10.1016/0022-5193(78)90022-x. [DOI] [PubMed] [Google Scholar]
- Wang TA, Yu YV, Govindaiah G, Ye X, Artinian L, Coleman TP, Sweedler JV, Cox CL, Gillette MU. Circadian rhythm of redox state regulates excitability in suprachiasmatic nucleus neurons. Science. 2012;337:839–842. doi: 10.1126/science.1222826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh DK, Logothetis DE, Meister M, Reppert SM. Individual neurons dissociated from rat suprachiasmatic nucleus express independently phased circadian firing rhythms. Neuron. 1995;14:697–706. doi: 10.1016/0896-6273(95)90214-7. [DOI] [PubMed] [Google Scholar]
- Welsh DK, Takahashi JS, Kay SA. Suprachiasmatic nucleus: cell autonomy and network properties. Ann Rev Physiol. 2010;72:551–577. doi: 10.1146/annurev-physiol-021909-135919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilsbacher LD, Takahashi JS. Circadian rhythms—molecular basis of the clock. Curr Opin Genet Dev. 1998;8:595–602. doi: 10.1016/s0959-437x(98)80017-8. [DOI] [PubMed] [Google Scholar]
- Wu Y, Cao G, Nitabach MN. Electrical silencing of PDF neurons advances the phase of non-PDF clock neurons in Drosophila. J Biol Rhythms. 2008;23:117–128. doi: 10.1177/0748730407312984. [DOI] [PubMed] [Google Scholar]
- Xu Y, Padiath QS, Shapiro RE, Jones CR, Wu SC, Saigoh N, Saigoh K, Ptacek LJ, Fu YH. Functional consequences of a CKIdelta mutation causing familial advanced sleep phase syndrome. Nature. 2005;434:640–644. doi: 10.1038/nature03453. [DOI] [PubMed] [Google Scholar]
- Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, Siepka SM, Hong HK, Oh WJ, Yoo OJ, et al. Period2::luciferase real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci USA. 2004;12:1–8. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]