Abstract
The apparent Sc3+ adduct of [FeIV(O)-(TMC)]2+ (1, TMC = 1,4,8,11-tetramethyl-1,4,8,11-tetraazacyclotetradecane) has been synthesized in amounts sufficient to allow its characterization by various spectroscopic techniques. Contrary to the earlier assignment of a +4 oxidation state for the iron center of 1, we establish that 1 has a high-spin iron(III) center based on its Mössbauer and EPR spectra and its quantitative reduction by 1 equiv of ferrocene to [FeII(TMC)]2+. Thus, 1 is best described as a ScIII–O–FeIII complex, in agreement with previous DFT calculations (Swart, M. Chem. Commun. 2013, 49, 6650.). These results shed light on the interaction of Lewis acids with high-valent metal-oxo species.
The role of redox-inactive Lewis-acidic metal ions in modulating the chemistry of redox-active metal-oxo centers has recently attracted considerable attention due to the requirement for a Ca2+ or Sr2+ ion to be an integral part of the oxygen evolving Mn4O5 cluster of photosystem II.1–3 Seminal efforts of Agapie have addressed how the binding of Lewis acidic metal ions can affect properties of manganese-oxo clusters,4–6 while complementary investigations of Fukuzumi and Nam have demonstrated the significant acceleration (by up to 8 orders of magnitude in some cases) of various oxidative transformations carried out by FeIV═O complexes upon addition of Lewis acidic metal ions, particularly Sc3+.7,8 Sc3+ binding has also been shown to facilitate trapping high-valent metal-oxo and imido complexes of late first row transition metal ions,9–11 and heterobimetallic complexes with MIII–(μ-OH)–MII cores (MIII = Fe, Mn, Ga; MII = Ca, Sr, and Ba) have also been structurally characterized.12
An exciting development was the report of a crystal structure of the Sc3+-bound [FeIV(O)(TMC)]2+ adduct (TMC = 1,4,8,11-tetramethyl-1,4,8,11-tetraazacyclotetradecane) by Fu-kuzumi and Nam in 2010,13 which provided the first crystallographic evidence for Sc3+ binding to an FeIV(O) moiety and formulated the adduct as the neutral [(TMC)-(FeIV–O–ScIII)(OTf)4(OH)] complex (1). However, scrutiny of the crystallographic data led to some concern about the iron(IV) oxidation state assignment.14 Specifically, the observed average Fe–NTMC bond length of 2.18 Å was 0.08 Å longer than that found in the crystal structure of the bona fide [FeIV(O)(TMC)(NCMe)]2+ complex.15 Additionally, the observed Sc–O bond length of 2.19 Å was more typical of Sc–OH2 than Sc–OH distances.16 These discrepancies prompted Swart to carry out DFT calculations to investigate the oxidation state of the Fe atom.14 An iron(III) oxidation state was required to reproduce the metal–ligand bond lengths found in the crystal structure, leading Swart to conclude that the adduct should instead be formulated as [(TMC)FeIII–O–ScIII(OTf)4(OH2)].
Although there are obviously methods for ascertaining iron oxidation state, 1 has only been characterized by X-ray crystallography. We surmised that the absence of additional data to characterize this intriguing complex was probably due to a lack of sufficient material, so we embarked on an effort to obtain larger amounts of the complex. Via a modification of the preparation method, we were able to obtain the desired adduct in about 50% yield, an amount sufficient to carry out characterization of the complex by Mössbauer and X-ray absorption spectroscopy of the crystals as well as electrospray ionization mass spectrometry and EPR spectroscopy of the solutions obtained from these crystals. Taken together, our studies establish the iron center in the adduct to be high-spin Fe(III). We thus confirm Swart’s formulation of adduct 1 as [(TMC)FeIII–O–ScIII(OTf)4(OH2)].
Our attempts to obtain larger amounts of 1 led us to make a small modification of the procedure reported by Fukuzumi et al.13 and afforded a structural analogue of the target complex, designated 1a, in which the apical water-derived Sc3+ ligand was replaced by MeCN (Figure 1). Instead of PhIO, 2-(tBuSO2)-C6H4IO (ArIO dissolved in trifluoroethanol)17 was used as oxidant in the synthetic procedure, resulting in a dramatic increase in the yield of these crystals to ~50%. Upon reaction of FeII(TMC)(OTf)2 with ArIO, the characteristic blue-green chromophore of the oxoiron(IV) complex was obtained as expected. Subsequent addition of 1 equiv of ScOTf3 elicited no immediate change in the near-IR band of the FeIV═O precursor, but the solution turned yellow over the course of a week, during which time yellow crystals of 1a were obtained by vapor diffusion of Et2O into the reaction solution at −20 °C. The crystals of 1a used for spectroscopic analysis were harvested by decanting off most of the mother liquor followed by carefully removing the remaining mother liquor with tissues; the crystals were then washed quickly with cold diethyl ether and dried under vacuum at −20 °C. In addition to X-ray crystallography, we were able to characterize 1a in the solid state by X-ray absorption and Mössbauer spectroscopy, and in MeCN solution by EPR, ICP-MS, and ESI-MS techniques.
Figure 1.
Overlay of X-ray structures of 1a (solid lines) and 1 (dashed lines). For clarity, the atoms of 1a were drawn as spheres to the 25% probability level and the atoms of 1 were drawn as small dots. Hydrogen atoms were omitted in both structures. The rmsd between the Sc, μ-O, Fe, and all non-hydrogen TMC atoms was found to be 0.0742 Å and is illustrated by the small deviation in bonds drawn above. The crystallographic R-factor (R1) is 0.0791, and complete XRD experimental and refinement details are reported in the SI.
X-ray analysis of the crystals of 1a confirmed the earlier structure reported by Fukuzumi et al.,13 except for the replacement of the water-derived ligand on the Sc3+ in 1 by CH3CN in 1a. Despite this change, the crystallographic parameters (Figure S1 and Tables S1A and S1B) obtained for the (N4)Fe–O–Sc(OTf)4 core are essentially identical, a conclusion illustrated by the overlay of the two structures (Figure 1). The root-mean-square deviation (rmsd, calculated by OFIT SHELX) for the overlay of the equivalent atoms in the two structures, namely Sc, μ-O, Fe, and all non-hydrogen atoms of the TMC macrocycle, is remarkably small at 0.0742 Å. As previously noted, the oxo bridge is coordinated syn to the TMC methyl groups, opposite to the anti configuration found for [FeIV(O)(TMC)(NCMe)]2+.15a Importantly, key bond distances are in close agreement, such as the Fe–O distances of 1.748(5) Å for 1a and 1.753(3) Å for 1 and an average Fe–NTMC distance of 2.167(6) Å for 1a and 2.175(3) Å for 1. The Fe–O bond lengths are typical of 5-coordinate FeIII–O–FeIII complexes,18 while the Fe–NTMC distances are associated with FeIII(TMC) complexes.19–21 Importantly, the apical CH3CN ligand in 1a removes the ambiguity of assigning the iron oxidation state based solely on the presence or absence of protons on the solvent-derived apical ligand, which is not advisable with X-ray diffraction experiments. Indeed, two triflate oxygen atoms in the structure of 1 are found at appropriate distances to act as hydrogen bond acceptors for the water-derived ligand in 1 (Figure S2), supporting its assignment as a neutral aqua species, rather than a hydroxide as proposed in ref 13.
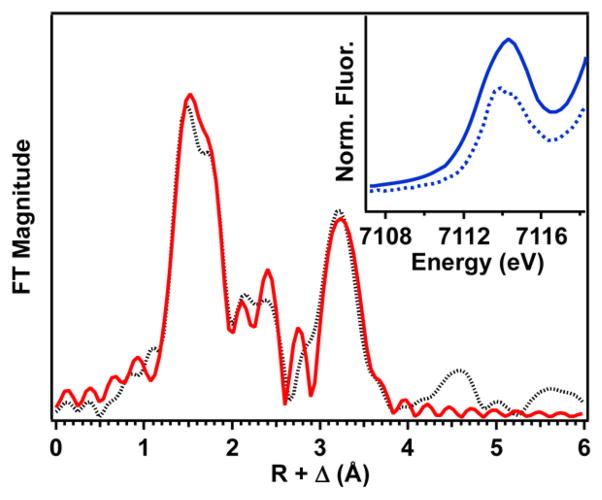
Fe K-edge X-ray absorption spectroscopic studies were also performed on a solid sample of 1a. The Fe K-edge of 1a is observed at 7122.6 eV (Figure S3), which falls within the range of known high-spin Fe(III) species.20,22–24 Moreover, there is a pre-edge peak at 7114.3 eV (Figure 2 inset) with an area of 32 units, which is by far the largest pre-edge area known for any 5-or 6-coordinate high-spin Fe(III) species in the literature.20,22–24 This large area indicates a high degree of distortion from centrosymmetry upon scandium binding, reflecting the square-pyramidal geometry around the iron(III) ion that moves the Fe 0.53 Å away from the mean N4-plane of the TMC framework. Although not as large as 1a, the pre-edge areas of related [FeIII(TMC)(η1-OOH)]2+ (2) and [FeIII(TMC)(η2-O2)]+ (3) complexes are also quite large (22.4 and 17.9 units, respectively),20 reflecting the large differences between the bond lengths Fe–NTMC (~2.2 Å) and Fe–O (1.74 Å for 1a, 1.92 Å for 2, and 1.93 Å for 3) and the number of O ligands. An EXAFS analysis was performed on this solid sample to ensure that the bulk solid contained the same material as the single crystal of 1a. Fits of the EXAFS region (Figures 2 and S4 and Table S2) revealed four N scatterers at 2.17 Å, one O scatterer at 1.74 Å, and one Sc scatterer at 3.69 Å, in excellent agreement with the X-ray structure. Importantly, the Sc scatterer in this sample exhibits a much more prominent feature in the Fourier-transformed data than in the spectrum of the Sc3+ adduct of [FeIII(TMC)(η2-O2)]+.20b,21
Figure 2.
Fourier transform of the Fe K-edge EXAFS data for 1a over a k range of 2–14.5 Å−1 (the black dotted line is experimental data; the solid red line is the best fit with one O scatterer at 1.74 Å, four N scatterers at 2.17 Å, and one Sc scatterer at 3.69 Å). The inset compares the pre-edge features of 1a (solid line) and [FeIII(η1-OOH)(TMC)]2+ (dotted line).
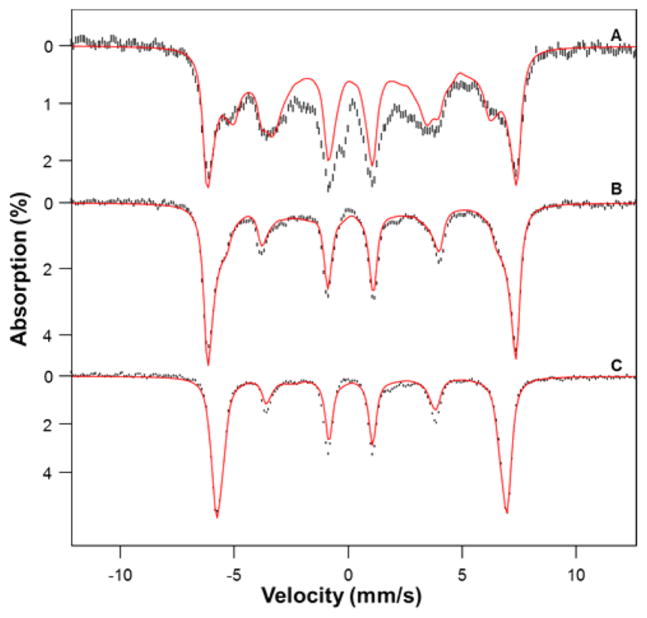
In order to gain insight into the iron oxidation state, we have studied Mössbauer and EPR spectra of 1a. Mössbauer spectra of crystals of 1a were collected at 4.2 K in parallel applied magnetic fields, B, up to 7.5 T. For B < 2 T the spectra were found to be broadened due to spin–spin interactions between neighboring molecules in the crystals. For B > 4 T, however, the applied field sufficiently decouples these interactions so that well-resolved spectra were obtained. The spectra shown in Figure 3 unambiguously show that 1a is a high-spin ferric complex. The red lines in Figure 3 are spectral simulations based on the S = 5/2 spin Hamiltonian,
Figure 3.
4.2 K Mössbauer spectra of crystals of 1a recorded in parallel applied fields of 1.0 (A), 4.0 (B), and 7.5 T (C). From spectral simulations using an S = 5/2 spin Hamiltonian, we obtained D = 3.25 cm−1, E/D = 0.14(2), g0 = 2.00, A0/gnβn = −19.1(2) T, ΔEQ = −1.02(5) mm s−1, η ≈ 0, and δ = 0.36(3) mm s−1. We have subtracted from the data a spectral simulation for two FeIV═O contaminants (representing 8% of Fe), namely [FeIV(O)(TMC)(NCMe)]2+ (5%) and [FeIV(O)(TMC)(OH)]+ (3%), using the parameters reported in ref 15.
where
and all symbols have their conventional meanings; the parameters used are listed in the caption of Figure 3. The salient features of the spectra are as follows. Complex 1a has an isomer shift δ = 0.36(3) mm s−1 and a quadrupole splitting ΔEQ = −1.02(5) mm s−1, and the major component of the electric field gradient tensor is negative (ΔEQ < 0). The δ and ΔEQ values agree within the experimental uncertainty with respective values of 0.39(5) and −0.99(50) mm s−1 from DFT calculations of Swart who postulated 1 to be a high-spin ferric complex.14 The 57Fe A-tensor of 1a is isotropic as expected for high-spin FeIII. The zero-field splitting parameter D ≈ +3–4 cm−1 was determined from the intensities of the nuclear Δm = 0 transitions (lines 2 and 5 counting from the left). The rhombicity parameter E/D was determined as follows. The splitting between the outermost lines of the B = 1.0 T spectrum reflects the internal magnetic field, Bint = 〈Sy〉A0/gnβn, associated with the ground Kramers doublet. The expectation value of 〈Sy〉, like the effective g-value geff,y of the doublet, depends sensitively on E/D (〈Sy〉 = −geff,y/4 for βB/D ≪ 1 for the spin-down level). With A0 known from the 4.0 and 8.0 T spectra (which are quite insensitive to E/D along the critical y direction), the magnetic splitting of the 1.0 T spectrum can be used to determine E/D. We found E/D = 0.14 ± 0.02, in excellent agreement with the EPR value E/D = 0.14 obtained for 1a dissolved in MeCN (see below), showing that both in the solid state and in frozen solution 1a has a high-spin FeIII site, with the same structure. At least 90% of the iron in the sample can be attributed to 1a; the sample contains two FeIV═O contaminants (see Figure 3 caption), estimated to represent ~8% of the Fe in the sample.
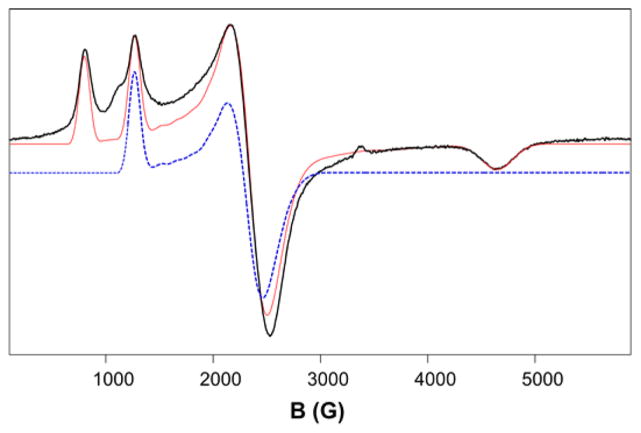
The findings obtained by Mössbauer spectroscopy are fully corroborated by the EPR spectra of 1a obtained in frozen MeCN solution (Figure 4), which display resonances arising from two Kramers doublets of a high-spin FeIII system with D > 0 and E/D = 0.14. A simulation of the whole spectrum (red) is shown in Figure 4, together with a simulation (blue) of the signal associated with the first excited Kramers doublet. The ground Kramers doublet of 1a gives rise to a signal with effective g-values at geff = (2.87, 8.57, 1.48), the intensities of which increase as the temperature is decreased from 30 to 2 K (Figure S5). Concomitantly, the intensity of the geff = (3.00, 2.64, 5.4) signal decreases, which assigns this feature as the gmax signal of the middle Kramers doublet. Some features of the EPR spectrum of 1a are worth noting. First, the sample lacks a signal at g = 4.3 often associated with rhombic FeIII. Second, complex 1a is one of the rare examples of a high-spin FeIII species with intermediate E/D for which all of the expected signals from the ground Kramers doublet are clearly resolved. Finally, the EPR results establish that the iron center has a +3 oxidation state in solution.
Figure 4.
X-band EPR spectrum (black trace) of 1a in MeCN. The red line is a SpinCount simulation using D = 3.25 cm−1, E/D = 0.14, and g0 = 2.00. Two of the Kramers doublets of the high-spin iron(III) center contribute to the spectrum. For E/D = 0.14 the ground doublet has geff = (2.87, 8.57, 1.48), while the spectrum of the middle doublet has geff = (3.00, 2.64, 5.4) (dashed blue line, offset for clarity). The upper doublet would yield a very weak resonance (not seen) at geff = 9.95. Conditions: T = 30 K; microwave power, 20 μW; modulation amplitude, 1 mT.
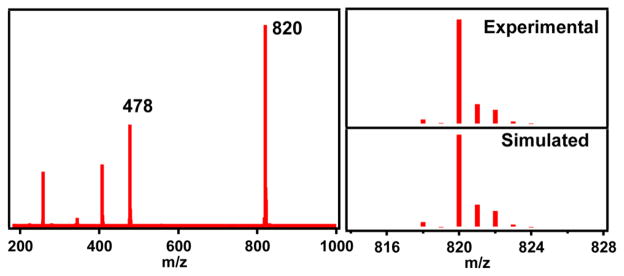
We have characterized other properties of 1a in MeCN solution. Consistent with the assignment of an iron(III) oxidation state for 1a, its electronic absorption spectrum (Figure S6) did not exhibit the NIR feature characteristic of [FeIV(O)(TMC)(NCMe)]2+ (λmax = 824 nm).15 The only feature observed was an intense UV band at λmax = 307 nm (ε = 9500 M−1 cm−1) that is likely associated with the Fe–O–Sc unit of 1a. Low-temperature ESI-MS analysis showed a prominent ion cluster peak at m/z = 820 and an associated isotope pattern corresponding to the formulation [(TMC)-(Fe)(O)(Sc)(OTf)3]+ (Figure 5), confirming the persistence of the solid state structure in solution. There was also a less intense peak at m/z = 478 corresponding to the [(TMC)-(Fe)(OH)(OTf)]+ ion, presumably due to a small amount of hydrolysis of 1a under the experimental conditions. An ICP-MS analysis of 1a in MeCN solution revealed a Sc:Fe ratio of 0.9, consistent with the Mössbauer finding showing that 1a represents 90% of the Fe in the bulk sample.
Figure 5.
ESI-MS spectrum of 1a crystals dissolved in MeCN obtained by injecting the solution at −30 °C into the mass spectrometer preset at a low dry gas temperature of 25 °C.
[FeIV(O)(TMC)(NCMe)]2+ was previously shown to undergo 2-e− reduction by 2 equiv of ferrocene in the presence of Sc3+.13 In contrast, 1a was reduced by only 1 equiv of ferrocene (even when excess ferrocene is used, Figure S7), affording [FeII(TMC)]2+ quantitatively, as indicated by the quantitative formation of [FeIV(O)(TMC)(NCMe)]2+ upon treating the ferrocene-reduced sample with PhIO. There was also a significant difference in the rates of ferrocene oxidation between [FeIV(O)(TMC)(NCMe)]2+ and 1a. At −20 °C, the oxidation of 1 equiv of ferrocene by FeIV(O)(TMC)-(NCMe)]2+ in the presence of 1 equiv of Sc3+ takes ~4 h, but the corresponding reaction with 1a was complete within 10 min, making 1a 24-fold more reactive than [FeIV(O)(TMC)-(NCMe)]2+ in the presence of 1 equiv Sc3+. These results also provide support for the assignment of a +3 oxidation state for the iron center in 1a.
In summary, we have re-investigated the nature of the apparent Sc3+ adduct of [FeIV(O)(TMC)]2+ 13 by obtaining sufficient amounts of the isolated complex to allow thorough spectroscopic investigation in both the solid state and in solution. Our studies conclusively establish that the iron center in 1a is not iron(IV) as was previously assigned on the basis of X-ray crystallography data alone,13 but is in fact in a high-spin iron(III) state as proposed by Swart.14 This oxidation state assignment is also supported by the observation that only 1 equiv of ferrocene is required to reduce 1a to [FeII(TMC)]2+. We are actively pursuing studies to address important mechanistic questions regarding the identity of the 1-e− reductant that converts [FeIV(O)(TMC)]2+ to 1a upon Sc3+ binding and how the oxo atom becomes coordinated syn to the methyl groups on the TMC ligand.
Supplementary Material
Acknowledgments
This work was supported by grants from the NSF (CHE-1361773 to L.Q. and CHE-1305111 to E.M.). We thank the University of Minnesota for a dissertation fellowship to G.T.R. and the NIH for a postdoctoral fellowship (GM-093479) to K.M.V.H. XAS data were collected on beamline X3B at the National Synchrotron Radiation Lightsource, which is supported by the U.S. Department of Energy under Contract No. DE-AC02-98CH10886. Use of beamline X3B was made possible by the Center for Synchrotron Biosciences grant, P30-EB-00998, from the National Institute of Biomedical Imaging and Bioengineering. Lastly, we thank Prof. M. Swart and Dr. Victor G. Young, Jr. for valuable discussions.
Footnotes
Notes
The authors declare no competing financial interest.
Experimental details, additional spectroscopic data, and a crystallographic file in CIF format for 1a. This material is available free of charge via the Internet at http://pubs.acs.org.
Contributor Information
Eckard Münck, Email: emunck@cmu.edu.
Lawrence Que, Jr., Email: larryque@umn.edu.
References
- 1.Yano J, Yachandra V. Chem Rev. 2014;114:4175. doi: 10.1021/cr4004874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Umena Y, Kawakami K, Shen JR, Kamiya N. Nature. 2011;473:55. doi: 10.1038/nature09913. [DOI] [PubMed] [Google Scholar]
- 3.Rivalta I, Brudvig GW, Batista VS. Curr Opin Chem Biol. 2012;16:11. doi: 10.1016/j.cbpa.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kanady JS, Tsui EY, Day MW, Agapie T. Science. 2011;333:733. doi: 10.1126/science.1206036. [DOI] [PubMed] [Google Scholar]
- 5.Tsui EY, Tran R, Yano J, Agapie T. Nat Chem. 2013;5:293. doi: 10.1038/nchem.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsui EY, Agapie T. Proc Natl Acad Sci USA. 2013;110:10084. doi: 10.1073/pnas.1302677110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fukuzumi S. Coord Chem Rev. 2013;257:1564. doi: 10.1016/j.ccr.2012.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park J, Morimoto Y, Lee YM, Nam W, Fukuzumi S. Inorg Chem. 2014;53:3618. doi: 10.1021/ic403124u. [DOI] [PubMed] [Google Scholar]
- 9.Chen J, Lee YM, Davis KM, Wu X, Seo MS, Cho KB, Yoon H, Park YJ, Fukuzumi S, Pushkar YN, Nam W. J Am Chem Soc. 2013;135:6388. doi: 10.1021/ja312113p. [DOI] [PubMed] [Google Scholar]
- 10.(a) Pfaff FF, Kundu S, Risch M, Pandian S, Heims F, Pryjomska-Ray I, Haack P, Metzinger R, Bill E, Dau H, Comba P, Ray K. Angew Chem, Int Ed. 2011;50:1711. doi: 10.1002/anie.201005869. [DOI] [PubMed] [Google Scholar]; (b) Kundu S, Miceli E, Farquhar E, Pfaff FF, Kuhlmann U, Hildebrandt P, Braun B, Greco C, Ray K. J Am Chem Soc. 2012;134:14710. doi: 10.1021/ja306674h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hong S, Pfaff FF, Kwon E, Wang Y, Seo MS, Bill E, Ray K, Nam W. Angew Chem, Int Ed. 2014;53:10403. doi: 10.1002/anie.201405874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.(a) Lacy DC, Park YJ, Ziller JW, Yano J, Borovik AS. J Am Chem Soc. 2012;134:17526. doi: 10.1021/ja304525n. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Park YJ, Cook SA, Sickerman NS, Sano Y, Ziller JW, Borovik AS. Chem Sci. 2013;4:717. doi: 10.1039/C2SC21400H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fukuzumi S, Morimoto Y, Kotani H, Naumov P, Lee YM, Nam W. Nat Chem. 2010;2:756. doi: 10.1038/nchem.731. [DOI] [PubMed] [Google Scholar]
- 14.Swart M. Chem Commun. 2013;49:6650. doi: 10.1039/c3cc42200c. [DOI] [PubMed] [Google Scholar]
- 15.(a) Rohde J-U, In J-H, Lim MH, Brennessel WW, Bukowski MR, Stubna A, Münck E, Nam W, Que L., Jr Science. 2003;299:1037. doi: 10.1126/science.299.5609.1037. [DOI] [PubMed] [Google Scholar]; (b) Jackson TA, Rohde J-U, Seo MS, Sastri CV, DeHont R, Stubna A, Ohta T, Kitagawa T, Münck E, Nam W, Que L., Jr J Am Chem Soc. 2008;130:12394. doi: 10.1021/ja8022576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meehan PR, Aris DR, Willey GR. Coord Chem Rev. 1999;181:121. [Google Scholar]
- 17.Macikenas D, Skrzypczak-Jankun E, Protasiewicz JD. J Am Chem Soc. 1999;121:7164. doi: 10.1002/1521-3773(20000602)39:11<2007::aid-anie2007>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 18.Kurtz DM., Jr Chem Rev. 1990;90:585. [Google Scholar]
- 19.Cho J, Jeon S, Wilson SA, Liu LV, Kang EA, Braymer JJ, Lim MH, Hedman B, Hodgson KO, Valentine JS, Solomon EI, Nam W. Nature. 2011;478:502. doi: 10.1038/nature10535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.(a) Li F, Meier KK, Cranswick MA, Chakrabarti M, Van Heuvelen KM, Münck E, Que L., Jr J Am Chem Soc. 2011;133:7256. doi: 10.1021/ja111742z. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Li F, Van Heuvelen KM, Meier KK, Münck E, Que L., Jr J Am Chem Soc. 2013;135:10198. doi: 10.1021/ja402645y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee YM, Bang S, Kim YM, Cho J, Hong S, Nomura T, Ogura T, Troeppner O, Ivanovic-Burmazovic I, Sarangi R, Fukuzumi S, Nam W. Chem Sci. 2013;4:3917. doi: 10.1039/C3SC51864G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.(a) Shan X, Rohde JU, Koehntop KD, Zhou Y, Bukowski MR, Costas M, Fujisawa K, Que L., Jr Inorg Chem. 2007;46:8410. doi: 10.1021/ic700649w. [DOI] [PubMed] [Google Scholar]; (b) Koehntop KD, Rohde J-U, Costas M, Que L., Jr Dalton Trans. 2004:3191. doi: 10.1039/B409727K. [DOI] [PubMed] [Google Scholar]
- 23.Westre TE, Kennepohl P, DeWitt JG, Hedman B, Hodgson KO, Solomon EI. J Am Chem Soc. 1997;119:6297. [Google Scholar]
- 24.Roe AL, Schneider DJ, Mayer RJ, Pyrz JW, Widom J, Que L., Jr J Am Chem Soc. 1984;106:1676. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.