Abstract
Background
Several new types of polyomavirus have been discovered in recent years mainly because of the recent state-of-the-art detection technologies. Among the polyomaviruses, Merkel cell polyomavirus (MCPyV) has attracted the most attention because of its possible role in the etiology of Merkel cell carcinoma, a rare but lethal form of skin cancer.
Objectives
This study aimed to determine age-specific seroprevalence of MCPyV in Tehran.
Patients and Methods
In this cross-sectional study, we collected 440 serum samples from healthy individuals 2 to 78 years of age who visited the Pasteur Institute’s clinic in Tehran, Iran, using a convenience sampling strategy. We developed a virus-like particle-based enzyme-linked immunosorbent assay that uses VP1, the major capsid protein of MCPyV, to detect and quantitate serum antibodies to MCPyV. We compared the prevalence of MCPyV between males and females and across eight age groups.
Results
A total of 255 (57.9%) of the serum samples were MCPyV positive. The seroprevalence in children under 10 years of age was 25%. The seroprevalence increased to 56% over the next decade of life (10 - 19 years of age). The seroprevalence rate in males and females was 56.1% and 59.7% respectively, and a binary logistic regression showed no significant difference between males and females (P = 0.77). However, the prevalence of MCPyV increased with age (P = 0.012).
Conclusions
Our results suggest that human exposure to MCPyV occurs throughout life. The MCPyV antibody levels remained high among older adults in our population, consistent with reports from other populations.
Keywords: Merkel Cell Polyomavirus, Virus-Like Particle, Enzyme-Linked Immunosorbet Assay
1. Background
Polyomaviruses are small, naked, double-stranded DNA viruses packaged in a capsid that is about 45 nm in diameter (1). Capsids of polyomaviruses consist of three proteins, namely, VP1, VP2, and VP3. Nine polyomaviruses are known to infect humans, including BK polyomavirus (BKV) (2) and JC polyomavirus (JCV) (3), which persist latently in various organs such as the urogenital system and brain, WU polyomavirus (4), KI polyomavirus (5), Merkel cell polyomavirus (MCPyV) (6), and three other newly discovered polyomaviruses called the human polyomavirus 6 - 9 (7-9). In general, primary infection with polyomaviruses is asymptomatic in humans, and only under severe immunosuppression can clinical symptoms be observed (1). Since 1953, the transforming potential of polyomaviruses has attracted much attention, and ultimately in 1962, Rabson et al.reported the oncogenic potential of a newly discovered polyomavirus called the simian vacuolating virus 40 (SV40) in vitro (10, 11).
MCPyV was discovered in a patient with Merkel cell carcinoma (MCC), a relatively rare skin cancer found among elderly and immunosuppressed persons (6). Despite the rarity of MCC, its mortality rate (30%) is very high compared with that of other skin cancers (12). Although tangible evidence suggests that the combination of immunodeficiency and exposure to ultra-violet radiation plays a significant role in MCC pathogenesis, a research group led by Patrick Moore at the University of Pittsburgh started investigating MCC for a possible infectious origin. Subsequent studies indicated that MCPyV DNA is clonally integrated into the cellular DNA of 80% - 90% of MCC tumors in patients from different geographic locations (13-15). Substantial evidence now supports the hypothesis that the nonproductive integration of MCPyV DNA into the human genome has an etiopathological role in the development of MCC (16).
MCPyV, similar to the majority of oncogenic viruses, is rendered incapable of replication in MCC tumors by a combination of events, but it retains the potential to induce cellular proliferation (17). A substantial number of reports have shown that mutations in MCPyV VP1 genes isolated from MCC cell lines prevent efficient viral replication and assembly (6, 14). In addition, transcripts of MCPyV T antigens are frequently detected in MCC tumors, and T antigen expression is essential for growth in MCC tumor cell lines (8, 18). According to previous studies, patients with MCC who are also infected with MCPyV have uniformly high titers of MCPyV antibody compared with patients with MCC who are uninfected (19). Whether MCPyV infection plays a role in other forms of cancer in addition to MCC remains unclear. MCPyV DNA has been isolated from non-melanoma, non-MCC malignancies, but the etiological link between this virus and these forms of skin cancer has not yet been documented.
As little information is known about the natural history of MCPyV infection in the general population, serological studies can clarify the extent of past exposure to the virus and give new insights into the epidemiology of MCPyV. The majority of past serological studies used MCPyV virus-like particles (VLPs) that contain conformational epitopes recognized by MCPyV-infected sera. According to recent studies, specific anti-MCPyV IgGs are detected in 40% - 90% of adults (19-22) and in 20% - 45% of children 1 - 5 years of age (19, 20). In addition, two recent studies revealed that the levels of anti-MCPyV antibodies in patients with MCC were significantly higher than those in healthy control individuals (19, 23).
2. Objectives
We employed a VLP-based enzyme-linked immunosorbent assay (ELISA) to detect anti-MCPyV antibodies to investigate the age-specific seroprevalence of MCPyV in Tehran. We produced empty viral capsids using VP1, the major capsid protein of MCPyV, expressed in a baculovirus expression system.
3. Patients and Methods
3.1. Human Subjects
The study was performed according to the declaration of Helsinki and relevant local regulations. The sampling protocols were approved by the ethical committee of the Tehran University of Medical Sciences (Code and date of ethical approval: 91-10-29-21091, 19 Jan 2013). In this cross-sectional study, we collected 440 serum samples from September 2013 to May 2014 using convenience sampling strategy, which is a non-probability sampling technique, and all the admitted samples were evaluated. We considered the eligibility of the participants of this study based on the following eligibility criteria: a) desire to participate in this study, b) age ≥2 years of age, and c) Iranian nationality. As mentioned previously, the samples were collected from individuals who agreed to participate in the study and provided written consent (for participants under 18, informed consent was provided by their parents or legal guardians). All of the participants had been referred to the Pasteur Institute’s clinic in Tehran (referral and governmental center) for health certificate tests. The participants ranged from 2 to 78 years of age and comprised 219 males and 221 females. All serum samples were stored at -20°C.
3.2. Generation of recombinant baculovirus
The VP1 coding sequence from MCPyV strain 339 was commercially synthesized (GenScript, USA) and codon optimized for expression in a Spodoptera frugiperda insect cell line (sf9 cells); the synthesized VP1 gene was kindly provided by Prof. Klaus Hedman. Using the Bac-to-BacR Baculovirus expression system (Invitrogen, Carlsbad, USA), we subcloned the VP1 coding sequence into a pFastBacTM HT A plasmid. Purified plasmids containing VP1 were subsequently transformed into DH10BacTM Escherichia coli cells for transposition into the bacmid vector according to the manufacturer’s instructions. The recombinant bacmid was purified from DH10BacTM E. Coli using the PureLinkTM HiPure Plasmid DNA Miniprep Kit (Life Technologies, USA). Sf9 cells were transfected with the recombinant bacmid vector using CellfectinR II Reagent (Invitrogen) according to the manufacturer’s instructions. Six days after transfection, the recombinant baculovirus was harvested by collecting the supernatant and centrifugation for 5 min at 3000 rpm to remove cell debris. The viral stock was stored at 4°C.
3.3. Generation of MCPyV-Like Particles
Sf9 cells were grown in supplemented Grace’s Insect Medium (Life Technologies) containing 10% fetal bovine serum (Life Technologies) and were subsequently infected with the recombinant baculovirus. VLPs were purified using the protocol described by Chen et al. (24). Briefly, sf9 cells were harvested approximately 4 - 5 days after infection by centrifugation at 1500 rpm for 10 minutes. The pellets were resuspended in an extraction buffer (50 mM Tris-HCL, pH 7.0, 150 mM NaCl, 0.01% Triton), and the VLPs were released by four freeze–thaw cycles. The cell lysates were clarified by centrifugation at 10,000 rpm for 10 minutes. One milliliter clarified cell lysate was then centrifuged in 28% CsCl in a swing SW41Ti rotor (Beckman) at 24,000 rpm at 4°C for 24 hours. The visible band was collected by puncturing the tubes with an 18-gauge needle and was subsequently dialyzed against phosphate-buffered saline (PBS) two times to remove the CsCl.
3.4. Transmission Electron Microscopy
The expression and the self-assembly of the VP1 protein was confirmed by transmission electron microscopy (TEM). Negative staining of the purified VLPs was performed as described previously (25). Briefly, a formware/carbon-coated 400-mesh grid was floated on 50 μL drops of purified VLP suspension. Following incubation for 30 min at room temperature, the grid was washed briefly with double-distilled water, and then 2% aqueous phosphotungstic acid was added for 2 minutes and allowed to dry for 45 minutes. After the grid was air dried, the negatively stained samples were viewed by TEM (Zeiss 10A). Micrographs of the sections were taken randomly at × 50,000 nominal magnification.
3.5. VLP-Based Enzyme-Linked Immunosorbent Assay
The VLP concentration was determined using a NanoDrop spectrophotometer (Thermo Scientific). Ninety-six-well polystyrene microplates (Nunc) were coated with 200 ng VLP diluted in PBS (pH 7.4) at 4°C overnight. The VLP-coated wells were blocked with 300 μL PBS containing 2% nonfat dried milk (Sigma-Aldrich) for 2 hours at room temperature. The sera were diluted 1:200 in the blocking solution, applied in duplicate, and incubated at 37°C for 1 hour on a shaker. After incubation, the plates were washed three times with PBS–Tween washing solution. Peroxidase-conjugated goat anti-human immunoglobulin G (H and L, Abcam) was diluted 1:5000 and added to each well. The plates were incubated at 37°C for 1 hour and then washed as described previously. One hundred microliters TMB ELISA substrate (Abcam) was added to each well and incubated at room temperature for 15 minutes in the dark. The reaction was stopped by adding 100 μL 1 M sulfuric acid to each well. Readings were taken at 450 nm in an automated microplate reader (Bio Hit) with a 690 nm filter used as reference.
3.6. Statistics
Previous studies have suggested that human exposure to polyomaviruses occurs early in life, and thus infants and young children are expected to have a low likelihood of exposure. To calculate the cut-off values, we used samples from children less than five years of age; the lower cut-off (mean + three standard deviations) was 0.36 optical density (OD) units, and the higher cut-off (mean + four standard deviations) was 0.38 OD units. By using these cut-offs, we dichotomized the serology results so that those above the higher cut-off were considered MCPyV IgG positive and those below the lower cut-off were considered MCPyV IgG negative. Serum samples with absorbance units between the two cut-offs were considered undetermined.
The differences in the age-specific seroprevalence between males and females were examined by logistic regression. Binary logistic regression was used to analyze the relationships between the presence of MCPyV IgG (the dependent variable) and sex and age (independent variables), as well as the possible interaction between sex and age, which was included as a third independent variable in the model. A set of binary logistic regression models was applied to calculate the adjusted odds ratios (OR) along with a 95% confidence interval, and the significance level was set to 0.05.
4. Results
4.1. Generation and Purification of MCPyV-Like Particles
The VP1 gene of MCPyV strain 339 was cloned into the recombinant baculovirus using the Bac-to-Bac system and expressed in sf9 insect cell lines. Four days after infection with a high titer of the recombinant baculovirus, cell lysate containing VLPs was harvested and subsequently purified by the CsCl gradient. The assembled VLPs were observed by TEM, and they had an approximate size of 40 nm (Figure 1).
Figure 1. Electron Micrograph of MCPyV VP1 Virus-Like Particles at × 50,000 Magnification.

Scale bar = 100 nm.
4.2. Age-Specific Seroprevalence of MCPyV
The age-specific seroprevalence of MCPyV is shown in Table 1. The overall MCPyV IgG seropositivity in our study sample was 57.9%. MCPyV seroprevalence increased with age. The steepest increase (from 25% to 56%) was between children less than 10 years of age and individuals 10 - 19 years of age. The MCPyV seroprevalence fluctuated at around 60% among individuals aged 20 years or above, peaking at 68% in individuals 60 - 69 years of age. Binary logistic regression indicated that neither sex nor the interaction between sex and age had a significant effect on seropositivity, whereas age had a highly significant effect on seropositivity (P = 0.012; Table 2).
Table 1. Age-Specific Seroprevalence of MCPyV.
| Age, y | Total Number of Samples | Seroprevalence in Males | Seroprevalence in Females | Total Seroprevalence |
|---|---|---|---|---|
| ≤ 10 | 53 | |||
| Positive | 6 | 7 | 13 | |
| Negative | 25 | 28 | 53 | |
| Positive percentage | 24 | 25 | 25 | |
| 10 – 19 | 45 | |||
| Positive | 14 | 11 | 25 | |
| Negative | 23 | 22 | 45 | |
| Positive percentage | 61% | 50 | 56 | |
| 20 – 29 | 43 | |||
| Positive | 10 | 16 | 26 | |
| Negative | 20 | 23 | 43 | |
| Positive percentage | 50 | 70 | 60 | |
| 30 – 39 | 68 | |||
| Positive | 22 | 20 | 42 | |
| Negative | 31 | 37 | 68 | |
| Positive percentage | 71 | 54 | 62 | |
| 40 – 49 | 86 | |||
| Positive | 25 | 30 | 55 | |
| Negative | 40 | 46 | 86 | |
| Positive percentage | 63 | 65 | 64 | |
| 50 – 59 | 70 | |||
| Positive | 22 | 23 | 45 | |
| Negative | 36 | 34 | 70 | |
| Positive percentage | 61 | 68 | 64 | |
| 60 – 69 | 40 | |||
| Positive | 13 | 14 | 27 | |
| Negative | 19 | 21 | 40 | |
| Positive percentage | 68 | 67 | 68 | |
| ≥ 70 | 35 | |||
| Positive | 11 | 11 | 22 | |
| Negative | 17 | 18 | 35 | |
| Positive percentage | 65 | 67 | 63 |
Table 2. Seroprevalence of MCPyV and its Association With Selected Variables.
| Variables | Seropositivitya | ORb | 95% CI | P Value | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Gender | 0.47 | 2.75 | .77 | ||
| Male | 123 (56.1) | 1 | |||
| Female | 132 (59.7) | 1.138 | |||
| Age group | 1.03 | 1.33 | .012 | ||
| ≤ 10 | 25 (13.53) | 1 | |||
| 10 - 19 | 56 (25.45) | 1.177 | |||
| 20 - 29 | 60 (26.43) | 1.177 | |||
| 30 - 39 | 62 (42.68) | 1.177 | |||
| 40 - 49 | 64 (55.86) | 1.177 | |||
| 50 - 59 | 64 (45.70) | 1.177 | |||
| 60 - 69 | 68 (27.40) | 1.177 | |||
| ≥ 70 | 63 (22.35) | 1.177 | |||
Abbreviations: CI, confidence interval; OR, odds ratio.
aData are presented as No. (%).
bOdds ratio adjusted for age and gender.
4.3. Age-Specific Antibody Titers
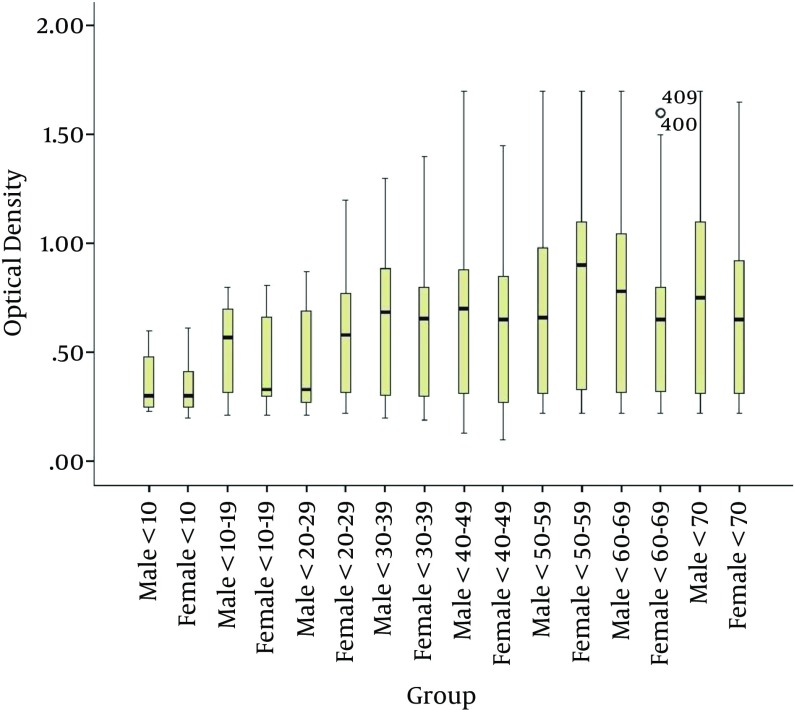
The MCPyV IgG antibody levels tended to increase with age (P = 0.012; Figure 2) and were not significantly different between males and females (Table 2).
Figure 2. Comparison of the Amount of MCPyV IgG in Sera Between Males and Females in Different Age Groups.
5. Discussion
Serological methods to determine exposure to viral pathogens are a useful and conventional way to document viral infection. We used the VLP-based ELISA to investigate the prevalence of MCPyV IgG among 440 persons divided among 10-year age classes. The VLP-based ELISA is a newly developed method first introduced by Tolstov et al. (19), who produced MCPyV-based VLPs by co-expressing two capsid proteins, VP1 and VP2. Subsequently, Touze et al. generated MCPyV-based VLPs using VP1 alone in a baculovirus expression system (22). We generated our MCPyV-based VLPs by expressing the VP1 coding sequence from MCPyV strain 339 in insect cells using the recombinant baculovirus. We produced self-assembling VLPs 20 nm-50 nm in diameter, consistent with other reports about the size variation of VLPs generated in vitro (19, 26).
The total MCPyV seropositivity in our sample taken from the general population of Tehran was 57.9%, and this finding represents the first report on the seroprevalence of MCPyV in the Middle East. Our result is consistent with those for other populations, mostly in Europe, where seroprevalence is estimated to be between 45% and 90% (19, 20, 22, 26). In our study, MCPyV IgG prevalence was around 25% in children less than 10 years of age and increased drastically over the next decade of life. This pattern fits the exposure patterns observed for other human polyomaviruses (BKV and JCV) (20, 27, 28). The highest seroprevalence (68%) was observed among adults 60 - 70 years of age.
Consistent with the results of Viscidi et al. our results indicate a positive correlation between MCPyV IgG prevalence and age (26). Likewise, our finding that no association exists between MCPyV prevalence and sex in the population confirms previous results published by Tolstov et al. (19) and Kean et al. (20). The increase in MCPyV seropositivity with age supports the hypothesis that the transmission of MCPyV occurs throughout life. The route of MCPyV transmission is unknown, but the presence of the virus in a wide variety of organs and anatomical locations supports several probable modes of transmission. For instance, MCPyV has been detected in the saliva, the gastrointestinal tract, and in urban sewage, thus suggesting fecal–oral transmission (29, 30). MCPyV DNA has also been detected in the upper respiratory tract, thus suggesting airborne transmission (31, 32).
Our findings suggest an association between MCPyV IgG levels and age, which was previously hypothesized by Touze et al. (22). However, we found no statistical difference in the MCPyV antibody levels based on sex. As mentioned previously, BKV and JCV establish latent infections that can persist for years (1), and for these human polyomaviruses, the reactivation and shedding of virions is directly correlated with humoral responses to the viral surface proteins (33). Therefore, considering that MCPyV can also establish a persistent infection that is resistant to be neutralized by antibodies is possible. Such latent infections may serve as a permanent source of immunogenic agents that result in the induction of high levels of MCPyV antibody during a lifetime.
This age-dependent seroprevalence of MCPyV, in accordance with previous studies (21, 22), showed a similar pattern with those of BKV and JCV in widespread exposure in the early years of life. However, this study in agreement with the previous reports indicating that MCPyV has more unique features than BKV and JCV. For instance, MCPyV seroprevalence remains high among adults, whereas BKV seroprevalence declines and JCV seroprevalence continues to increase in older adults.
To the best our knowledge, this study is the first to report on the seroprevalence of MCPyV in Iran and even in the Middle East, and it has great significance in revealing the epidemiological aspects of this newly discovered virus in general. However, in this study, we encountered some limitations, including its cross-sectional design and the fact that the samples could not represent the general population of the country.
In seroprevalence studies, the possibility of serological cross-reactivity among viruses within the same family exists. A number of previous studies have confirmed the specificity of MCPyV VLP-based ELISA using competitive inhibition assays and have found no evidence of cross-reactivity with other polyomaviruses, including BKV and JCV (22, 26). Despite the fact that MCPyV infection is common in most populations, investigating the prevalence of MCPyV in developing countries such as Iran may still open up new horizons in the search for the etiological roles of MCPyV and similar viruses in specific diseases.
Acknowledgments
The authors would like to acknowledge the directors and staff of the Hepatitis and AIDS Department of the Pasteur Institute of Iran.
Footnotes
Authors’ Contribution:Rouhollah Vahabpour, Mohammad Reza Aghasadeghi, Mostafa Salehi-Vaziri, and Seyed Hamidreza Monavari developed the study concept, performed the experimental protocols, and prepared the manuscript. Nasir Mohajel, Hossein Keyvani, Maryam Nasimi, and Maryam Esghaei were involved in the administrative, technical, and material support.
Funding/Support:This work was supported by a grant from the Tehran University of Medical Sciences (Project code: 21091).
References
- 1.Jiang M, Abend JR, Johnson SF, Imperiale MJ. The role of polyomaviruses in human disease. Virology. 2009;384(2):266–73. doi: 10.1016/j.virol.2008.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gardner SD, Field AM, Coleman DV, Hulme B. New human papovavirus (B.K.) isolated from urine after renal transplantation. Lancet. 1971;1(7712):1253–7. doi: 10.1016/s0140-6736(71)91776-4. [DOI] [PubMed] [Google Scholar]
- 3.Padgett BL, Walker DL, ZuRhein GM, Eckroade RJ, Dessel BH. Cultivation of papova-like virus from human brain with progressive multifocal leucoencephalopathy. Lancet. 1971;1(7712):1257–60. doi: 10.1016/s0140-6736(71)91777-6. [DOI] [PubMed] [Google Scholar]
- 4.Gaynor AM, Nissen MD, Whiley DM, Mackay IM, Lambert SB, Wu G, et al. Identification of a novel polyomavirus from patients with acute respiratory tract infections. PLoS Pathog. 2007;3(5):e26097. doi: 10.1371/journal.ppat.0030064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allander T, Andreasson K, Gupta S, Bjerkner A, Bogdanovic G, Persson MA, et al. Identification of a third human polyomavirus. J Virol. 2007;81(8):4130–6. doi: 10.1128/JVI.00028-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feng H, Shuda M, Chang Y, Moore PS. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science. 2008;319(5866):1096–100. doi: 10.1126/science.1152586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schowalter RM, Pastrana DV, Pumphrey KA, Moyer AL, Buck CB. Merkel cell polyomavirus and two previously unknown polyomaviruses are chronically shed from human skin. Cell Host Microbe. 2010;7(6):509–15. doi: 10.1016/j.chom.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scuda N, Hofmann J, Calvignac-Spencer S, Ruprecht K, Liman P, Kuhn J, et al. A novel human polyomavirus closely related to the african green monkey-derived lymphotropic polyomavirus. J Virol. 2011;85(9):4586–90. doi: 10.1128/JVI.02602-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van der Meijden E, Janssens RW, Lauber C, Bouwes Bavinck JN, Gorbalenya AE, Feltkamp MC. Discovery of a new human polyomavirus associated with trichodysplasia spinulosa in an immunocompromized patient. PLoS Pathog. 2010;6(7):e26097. doi: 10.1371/journal.ppat.1001024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rabson AS, Kirschstein RL. Induction of Malignancy in vitro in Newborn Hamster Kidney Tissue Infected with Simian Vacuolating Virus (SV40). Exp Biol Med. 1962;111(2):323–8. doi: 10.3181/00379727-111-27780. [DOI] [PubMed] [Google Scholar]
- 11.Rabson AS, Malmgren RA, O'Conor GT, Kirschstein RL. Simian vacuolating virus (SV40) infection in cell cultures derived from adult human thyroid tissue. J Natl Cancer Inst. 1962;29:1123–45. [PubMed] [Google Scholar]
- 12.Hodgson NC. Merkel cell carcinoma: changing incidence trends. J Surg Oncol. 2005;89(1):1–4. doi: 10.1002/jso.20167. [DOI] [PubMed] [Google Scholar]
- 13.Foulongne V, Kluger N, Dereure O, Brieu N, Guillot B, Segondy M. Merkel cell polyomavirus and Merkel cell carcinoma, France. Emerg Infect Dis. 2008;14(9):1491–3. doi: 10.3201/eid1409.080651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kassem A, Schopflin A, Diaz C, Weyers W, Stickeler E, Werner M, et al. Frequent detection of Merkel cell polyomavirus in human Merkel cell carcinomas and identification of a unique deletion in the VP1 gene. Cancer Res. 2008;68(13):5009–13. doi: 10.1158/0008-5472.CAN-08-0949. [DOI] [PubMed] [Google Scholar]
- 15.Ridd K, Yu S, Bastian BC. The presence of polyomavirus in non-melanoma skin cancer in organ transplant recipients is rare. J Invest Dermatol. 2009;129(1):250–2. doi: 10.1038/jid.2008.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buck CB, Lowy DR. Getting stronger: the relationship between a newly identified virus and Merkel cell carcinoma. J Invest Dermatol. 2009;129(1):9–11. doi: 10.1038/jid.2008.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moore PS, Chang Y. Why do viruses cause cancer? Highlights of the first century of human tumour virology. Nat Rev Cancer. 2010;10(12):878–89. doi: 10.1038/nrc2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Houben R, Shuda M, Weinkam R, Schrama D, Feng H, Chang Y, et al. Merkel cell polyomavirus-infected Merkel cell carcinoma cells require expression of viral T antigens. J Virol. 2010;84(14):7064–72. doi: 10.1128/JVI.02400-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tolstov YL, Pastrana DV, Feng H, Becker JC, Jenkins FJ, Moschos S, et al. Human Merkel cell polyomavirus infection II. MCV is a common human infection that can be detected by conformational capsid epitope immunoassays. Int J Cancer. 2009;125(6):1250–6. doi: 10.1002/ijc.24509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kean JM, Rao S, Wang M, Garcea RL. Seroepidemiology of human polyomaviruses. PLoS Pathog. 2009;5(3):e26097. doi: 10.1371/journal.ppat.1000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pastrana DV, Tolstov YL, Becker JC, Moore PS, Chang Y, Buck CB. Quantitation of human seroresponsiveness to Merkel cell polyomavirus. PLoS Pathog. 2009;5(9):e26097. doi: 10.1371/journal.ppat.1000578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Touze A, Gaitan J, Arnold F, Cazal R, Fleury MJ, Combelas N, et al. Generation of Merkel cell polyomavirus (MCV)-like particles and their application to detection of MCV antibodies. J Clin Microbiol. 2010;48(5):1767–70. doi: 10.1128/JCM.01691-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carter JJ, Paulson KG, Wipf GC, Miranda D, Madeleine MM, Johnson LG, et al. Association of Merkel cell polyomavirus-specific antibodies with Merkel cell carcinoma. J Natl Cancer Inst. 2009;101(21):1510–22. doi: 10.1093/jnci/djp332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen T, Hedman L, Mattila PS, Jartti T, Ruuskanen O, Soderlund-Venermo M, et al. Serological evidence of Merkel cell polyomavirus primary infections in childhood. J Clin Virol. 2011;50(2):125–9. doi: 10.1016/j.jcv.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 25.Biel SS, Gelderblom HR. Diagnostic electron microscopy is still a timely and rewarding method. J Clin Virol. 1999;13(1-2):105–19. doi: 10.1016/S1386-6532(99)00027-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Viscidi RP, Rollison DE, Sondak VK, Silver B, Messina JL, Giuliano AR, et al. Age-specific seroprevalence of Merkel cell polyomavirus, BK virus, and JC virus. Clin Vaccine Immunol. 2011;18(10):1737–43. doi: 10.1128/CVI.05175-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brown DW, Gardner SD, Gibson PE, Field AM. BK virus specific IgM responses in cord sera, young children and healthy adults detected by RIA. Arch Virol. 1984;82(3-4):149–60. doi: 10.1007/BF01311159. [DOI] [PubMed] [Google Scholar]
- 28.Flaegstad T, Traavik T, Kristiansen BE. Age-dependent prevalence of BK virus IgG and IgM antibodies measured by enzyme-linked immunosorbent assays (ELISA). J Hyg (Lond). 1986;96(3):523–8. doi: 10.1017/s0022172400066328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bofill-Mas S, Rodriguez-Manzano J, Calgua B, Carratala A, Girones R. Newly described human polyomaviruses Merkel cell, KI and WU are present in urban sewage and may represent potential environmental contaminants. Virol J. 2010;7:141. doi: 10.1186/1743-422X-7-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Campello C, Comar M, D'Agaro P, Minicozzi A, Rodella L, Poli A. A molecular case-control study of the Merkel cell polyomavirus in colon cancer. J Med Virol. 2011;83(4):721–4. doi: 10.1002/jmv.22004. [DOI] [PubMed] [Google Scholar]
- 31.Babakir-Mina M, Ciccozzi M, Lo Presti A, Greco F, Perno CF, Ciotti M. Identification of Merkel cell polyomavirus in the lower respiratory tract of Italian patients. J Med Virol. 2010;82(3):505–9. doi: 10.1002/jmv.21711. [DOI] [PubMed] [Google Scholar]
- 32.Kantola K, Sadeghi M, Lahtinen A, Koskenvuo M, Aaltonen LM, Mottonen M, et al. Merkel cell polyomavirus DNA in tumor-free tonsillar tissues and upper respiratory tract samples: implications for respiratory transmission and latency. J Clin Virol. 2009;45(4):292–5. doi: 10.1016/j.jcv.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen Y, Trofe J, Gordon J, Du Pasquier RA, Roy-Chaudhury P, Kuroda MJ, et al. Interplay of cellular and humoral immune responses against BK virus in kidney transplant recipients with polyomavirus nephropathy. J Virol. 2006;80(7):3495–505. doi: 10.1128/JVI.80.7.3495-3505.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]