Abstract
Background
The G551D mutation in the cystic fibrosis transmembrane conductance regulator (CFTR) is a common cause of cystic fibrosis (CF). G551D-CFTR is characterized by an extremely low open probability despite its normal trafficking to the plasma membrane. Numerous small molecules have been shown to increase the activity of G551D-CFTR presumably by binding to the CFTR protein.
Methods
We investigated the effect of curcumin, genistein and their combined application on G551D-CFTR activity using the patch clamp technique.
Results
Curcumin increased G551D-CFTR whole-cell and single-channel currents less than genistein did at their maximally effective concentrations. However, curcumin further increased the channel activity of G551D-CFTR that had been already maximally potentiated by genistein, up to ~50% of the WT-CFTR level. In addition, the combined application of genistein and curcumin over a lower concentration range synergistically rescued the gating defect of G551D-CFTR.
Conclusions
The additive effects between curcumin and genistein not only support the hypothesis that multiple mechanisms are involved in the action of CFTR potentiators, but also pose pharmaceutical implications in the development of drugs for CF pharmacotherapy.
Keywords: Cystic fibrosis, CFTR, G551D mutation, Genistein, Curcumin, Additive effect
1. Introduction
The cystic fibrosis transmembrane conductance regulator (CFTR) chloride channel is a major player in salt and water transport across epithelia and mutations of CFTR that cause the reduction of its activity result in the genetic disease cystic fibrosis (CF) [18,38]. Cystic fibrosis is characterized by the absence of a cAMP-stimulated Cl− current across a variety of epithelia including the nasal epithelium [27], airway epithelia [46], pancreatic ducts [20], sweat glands [37], and the intestine [38]. There are different mechanisms that cause CFTR channel dysfunction [47], and different pharmacological approaches are currently being explored to alleviate the disease [5]. Many compounds, so called CFTR potentiators, have been found to boost the activity of mutant CFTR channels with low open probability, but few of them seem to be able to completely rescue the defective channels (reviewed by Refs. [5,42]). Only Van Goor et al. [41] have recently shown that 1 μM VX-770 restored the open probability of ΔF508-CFTR to that of wild-type CFTR.
The glycine-to-aspartate missense mutation at position 551 (G551D) is the third most common CF-associated mutation, with a worldwide frequency of 3.1% (www.genet.sickkids.on.ca/cftr). The G551D-CFTR protein is a class III mutation [47]. This mutant CFTR protein can traffic normally to the apical membrane and is phosphorylated by cAMP-dependent protein kinase A (PKA) [12,14,30,47] but it exhibits defective gating [9,11,30,47]. Among the many compounds that potentiate G551D-CFTR currents, genistein is perhaps the most extensively studied. Genistein is a bioflavonoid found in legumes [16] and has been known as a CFTR potentiator for more than a decade [6,21,23]. Interestingly, it has also been demonstrated that genistein has inhibitory actions at high concentrations [28,39,43]. Although the exact mechanism of action for genistein remains elusive, studies have identified putative binding sites for genistein on CFTR [33], while other studies have suggested that the drug acts by altering lipid bilayer mechanics [22].
Curcumin is a component of the spice turmeric and is known for its antioxidant, anti-inflammatory, wound-healing, anti-carcinogenic, antiviral, anti-infectious, anti-amyloidogenic and anti-cancer effects [2,19,26,32,35]. Egan et al. [17] reported that oral administration of curcumin to ΔF508-CFTR mice could correct the trafficking defects of mutant CFTR, although these results remain controversial [e.g., 40]. Interestingly, Berger et al. [7] showed that curcumin potentiated WT- and ΔF508-CFTR and Wang et al. [44] reported that curcumin strongly activated mutant CFTR channels with very low activities, including G551D-CFTR.
In this study, we investigated the effects of genistein, curcumin and their combined application on the G551D-CFTR gating defects.
2. Materials and methods
2.1. Cells, cell culture, DNA constructs and transfection
CHO cells were grown at 37 °C and 5% CO2 in Dulbecco’s modified Eagle’s medium supplemented with 10% FBS. To transiently express CFTR, CHO cells were grown for 1 day in 35-mm tissue culture dishes at an initial seeding density of 0.5–1×105 cells/ml. The DNA constructs, encoding WT or G551D-mutant CFTR, were separately cotransfected with ~0.2 μg of pEGFP-C3 (CLONTECH Laboratories Inc., Mountain View, CA, USA) encoding GFP, using SuperFect transfection reagent (QIAGEN, Hilden, Germany) according to the manufacturer’s protocols. After transfection for 2–6 days, the transfected cells were detached using 0.05% trypsin in phosphate-buffered saline (PBS) (0.25% trypsin/1 mM EDTA diluted with PBS, Sigma-Aldrich, St. Louis, MO, USA), and kept as a cell suspension before use.
2.2. Electrophysiological recording and analysis
All CFTR currents were recorded at room temperature with an EPC9 amplifier (HEKA, Lamberecht/Pfalz. Germany) or an Axopatch 200A patch clamp amplifier (Axon Instruments, Inc, Foster City, CA, USA).
2.2.1. Whole-cell recordings
The pipette resistance was ~5 MΩ in the bath solution. To monitor the effect of drugs on CFTR currents, the membrane potential was held at 0 mV and a 1 s voltage ramp (−100 mV to 100 mV) was applied every 6 s. Currents were filtered at 1 kHz with a built-in 4-pole Bessel filter, and digitized by the computer at a sampling rate of 2 kHz. CFTR was activated with 10 μM forskolin and 100 μM CPT-cAMP before the application of curcumin or genistein. The net current was calculated as the current between −100 mV and +100 mV minus the basal current (leak current) at the same voltages, using Igor software (Wavemetrics, Lake Oswego, OR) or Clampfit software (Axon). Current–Voltage (I–V) relationships were obtained when whole-cell currents reached a steady state under each condition (Figs. 1AB and 3B). The bath solution contained 145 mM NaCl, 5 mM KCl, 2 mM MgCl2, 1 mM CaCl2, 5 mM glucose, and 5 mM HEPES (pH 7.4 with NaOH). 20 mM sucrose was added to the bath solution to prevent activation of swelling-induced currents. The pipette solution contained 10 mM EGTA, 10 mM HEPES, 20 mM TEACl, 10 mM MgATP, 2 mM MgCl2, 101 mM CsCl, and 5.8 mM glucose.
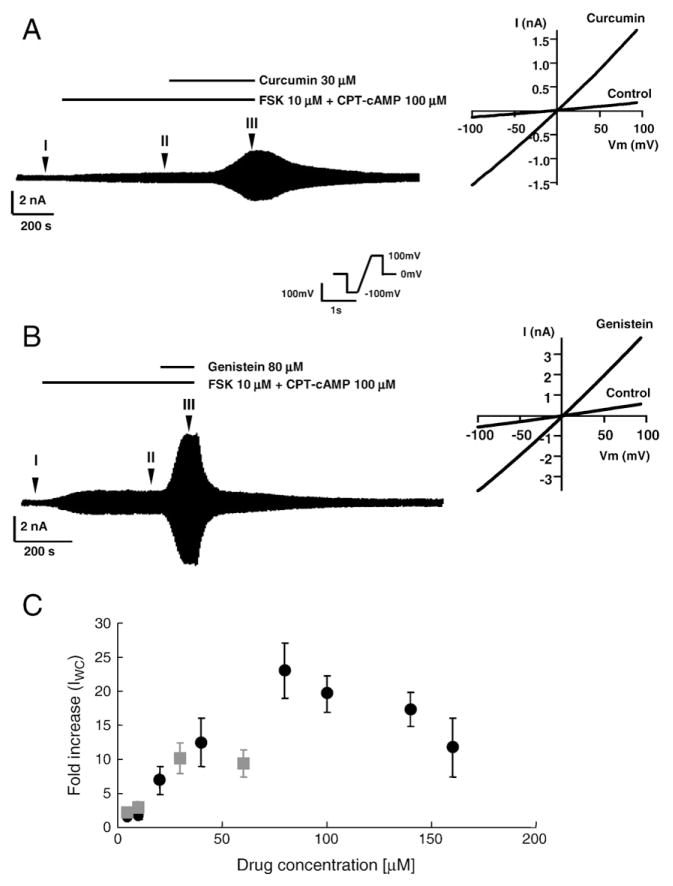
Fig. 1.
Potentiation effects of genistein and curcumin on whole-cell currents of G551D-CFTR channels expressed in CHO cells. (A) Effect of curcumin on G551D-CFTR whole-cell currents stimulated by 10 μM FSK+100 μM CPT-cAMP. Representative traces for G551D-CFTR whole-cell currents affected by 30 μM curcumin. The right panel shows the I–V relationships in control (at II) and curcumin (at III) after the subtraction of the leak-currents in the pulse at the position I. Representative of twelve data. (B) Effect of genistein on G551D-CFTR whole-cell currents stimulated by 10 μM FSK+100 μM CPT-cAMP. Representative traces for G551D-CFTR whole-cell currents affected by 80 μM genistein. The right panel shows the net I–V relationships in control (II–I) and genistein (III–I). Representative of eleven data. (C) The dose response relationship for genistein (●) (n=11) and curcumin (■) (n=12). Error bars represent SEM. The fold-increase in the whole cell current (IWC) was calculated by dividing the mean current at 100 mV in the presence of genistein or curcumin by the mean current in control after the leak current subtraction.
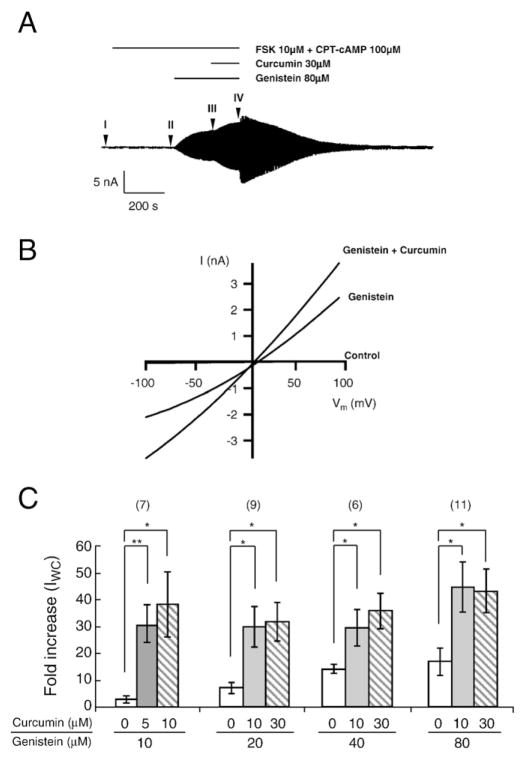
Fig. 3.
Additive effect of curcumin and genistein on G551D-CFTR whole-cell currents. (A) Representative whole-cell current trace from G551D-CFTR expressed in CHO cells, showing the additive effect of curcumin and genistein. Representative of eleven data. (B) The I–V relationships of the net G551D-CFTR whole-cell currents. The net whole-cell currents were obtained by subtracting the (leak) whole-cell currents at I from the whole-cell currents at II (control), III (genistein) and IV (genistein+curcumin) in Fig. 3A. (C) Comparison of fold-increases in the G551D-CFTR whole-cell currents (IWC) in control, induced by 10–80 μM genistein alone and additional applications of 5–30 μM curcumin to different genistein concentrations. Error bars represent SEM. ANOVA, *: P<0.05; **: P<0.01.
2.2.2. Cell-attached recordings
The pipette resistance was 3 to 5 MΩ in the bath solution. The pipette potential (Vp) was held at +50 mV for all experiments. Data were filtered at 100 Hz with an eight-pole Bessel filter (Warner Instrument, Hamden, CT, USA) and digitized by the computer at a sampling rate of 500 Hz. The NPo value was estimated from the equation; NPo=I/i, where n is the number of channels, I is the mean steady-state current and i is the unitary current, using Igor software. The duration of the current recordings used for the NPo analysis was longer than 60 s. The pipette solution contained 140 mM N-methyl-D-glucamine chloride (NMDG-Cl), 2 mM MgCl2, 5 mM CaCl2, and 5 mM HEPES (pH 7.4 with NMDG). The bath solution was the same as used for whole-cell experiments.
2.3. Western blot analysis
Western blot analysis was performed forty-eight hours after transfection, when cells were ~70% confluent on 35-mm dishes. To prepare cell lysates, dishes were washed once with PBS, and then lysis buffer was added (M-PER Protein Extraction reagent, Thermo Scientific, Rockford, IL, USA). DNA was sheared by brief sonication (~3 s). The whole cell lysates were separated on 7.5% Tris–HCl SDS-PAGE gels (Bio-Rad Laboratories Inc., Hercules, CA, USA) and transferred onto a nitrocellulose membrane. The membrane was blocked with 5% milk in a Tris-buffered saline with Tween (TBST) buffer (20 mM Tris, 137 mM NaCl, 0.05% Tween) at room temperature for 1 h and then probed with a primary antibody against CFTR (clone CFTR#569 mAb, a kind gift from Dr. J. R. Riordan) in PBS at 4 °C overnight. The nitrocellulose membrane was washed with TBST three times and then incubated with the horseradish peroxidase (HRP)-conjugated secondary antibody (donkey anti-mouse Ig G; Jackson ImmunoResearch Laboratories, West Grove, PA, USA) for 1 h at room temperature. The membrane was developed with chemiluminescence HRP substrate reagent (Millipore Corp., Billerica, MA, USA) for 5 min and exposed to ImageQuant LAS 4000 mini machine (GE Healthcare Bio-Science Corp., Uppsala, Sweden). The membrane was cut in the region around 37 kDa position, and reprobed with an anti-actin antibody (sc-1616, Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) and then incubated with the HRP-conjugated secondary antibody (goat anti-rabbit IgG; Sigma-Aldrich) for 1 h at room temperature for an internal control of protein loading. For a quantitative comparison of the expression level between WT- and G551D-CFTR, a relative intensity (CFTR/actin) was calculated by dividing the signal intensity of CFTR protein by the signal intensity of actin in each lane.
2.4. Reagents
Forskolin (FSK) was purchased from Alexis (San Diego, CA, USA) and stored as a 20 mM stock in dimethyl sulfoxide (DMSO) at −20 °C. 8-(4-chlorophenylthio) (CPT)-cAMP was purchased from BioLog (Bremen, Germany) and stored as a 50 mM stock in water at −20 °C. Genistein was purchased from Alexis and stored as a 100 mM stock in DMSO at −20 °C. Curcumin was purchased from Sigma-Aldrich (St Louis, MO, USA), stored as a 30 mM stock in DMSO at −20 °C and dissolved in the bath solution just before use.
2.5. Statistics
All of the results are expressed as means±SEM. To compare data, ANOVA and paired or non-paired t-test were performed using SPSS (IBM Corp., Armonk, NY, USA) and Igor or Excel (Microsoft, Redmond, WA, USA). P<0.05 was considered statistically significant.
3. Results
3.1. Effects of curcumin and genistein on G551D-CFTR
We first examined the effects of curcumin on G551D-CFTR whole-cell current at different concentrations and compared them with those of genistein. G551D-CFTR currents were activated with 10 μM forskolin (FSK) and 100 μM CPT-cAMP before the application of curcumin or genistein. Fig. 1A and B are representative whole cell current traces which illustrate the potentiating effects of 30 μM curcumin and 80 μM genistein on G551D-CFTR whole-cell currents, respectively. Note that upon removal of 80 μM genistein, we often observed a transient current increase which might be caused by the release of the inhibitory effect of genistein prior to the recovery of its potentiation effect (data not shown). The I–V relationships were slightly outwardly rectifying before and after the application of the potentiators, with no change in the reversal potential. Fig. 1C shows the dose response relationships for the fold-increase caused by curcumin and genistein. Although it appears that curcumin has a higher apparent affinity than genistein, the maximum fold-increase for curcumin is significantly smaller (10.2±2.2, n=12) than that for genistein (23.0±4.1, n=11). This result indicates that genistein is a more effective potentiator of G551D-CFTR than curcumin. Although the dose–response relationship for curcumin follows a simple saturation function up to 60 μM, the highest soluble concentration (Fig. 1C), a bell-shape dose–response relationship is seen for genistein (Fig. 1C), indicating an inhibitory effect of genistein at high concentrations [31,33], similar to the effect observed for WT channels [28,33,39,43].
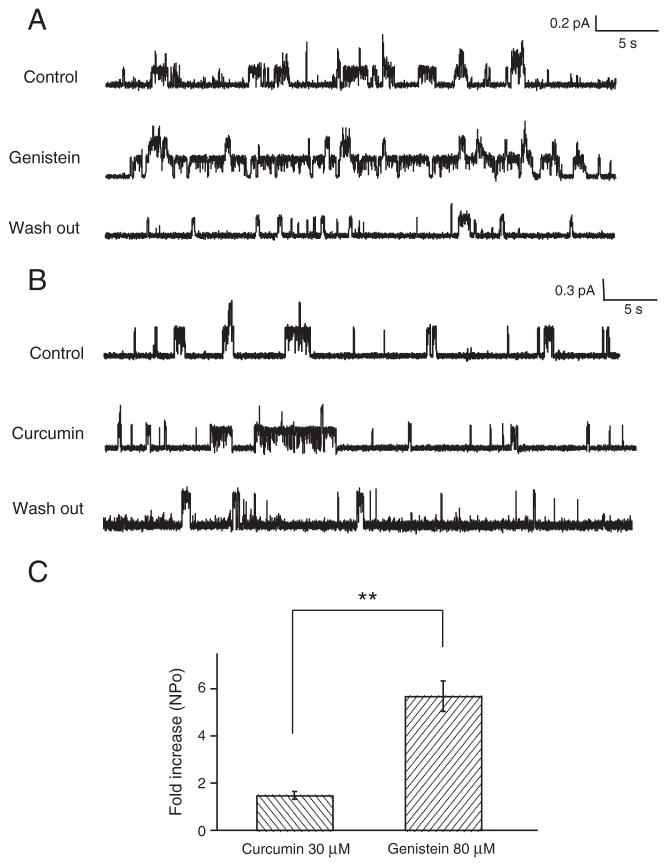
To examine the potentiation of CFTR by both compounds further, we tested their effects on G551D-CFTR in the cell-attached mode that had been activated first by forskolin and cAMP. Fig. 2A and B shows representative single channel current traces before and after exposure to genistein and curcumin, respectively. 80 μM genistein significantly increased the G551D-CFTR channel activity in the cell-attached patch in a reversible manner (Fig. 2A). This effect was apparently due to an increase of the channel burst time and a decrease of the closed time as reported previously [25]. In contrast, 30 μM curcumin only slightly increased the G551D-CFTR channel activity in the cell-attached patch (Fig. 2B). The application of genistein or curcumin also changed the single channel current amplitudes (see Fig. 2B legend). This effect on channel amplitude might be caused by changes in the membrane potential and/or the intracellular Cl− concentration, induced by opening of G551D-CFTR channels in the entire cell membrane, in addition to a pore blocking effect which has been previously reported for genistein [28].
Fig. 2.
Potentiation effects of genistein and curcumin on G551D-CFTR observed in cell-attached mode. Representative cell-attached single-channel recordings of cAMP-stimulated G551D-CFTR channel currents potentiated by genistein (A) and curcumin (B). The G551D-CFTR channels are continuously stimulated by 10 μM FSK and 100 μM CPT-cAMP. Vp=+50 mV. Single channel amplitude: (A) control: 0.26 pA, genistein: 0.26 pA, wash out: 0.22 pA, (B) control: 0.41 pA, curcumin: 0.31 pA, wash out: 0.53 pA. (C) Summary of fold-increase of NPo induced by 80 μM genistein (n=5) and 30 μM curcumin (n=5). G551D-CFTR was expressed in CHO cells. Error bars represent SEM. Non-paired t-test, **: P<0.01.
Fig. 2C summarizes the fold-increase of NPo induced by curcumin and by genistein. Although, compared to data generated from whole-cell experiments, the potentiation effects of genistein or curcumin are dramatically reduced, at the tested concentrations, genistein remains a more effective potentiator than curcumin.
3.2. Additive effects of genistein and curcumin
There are at least two possibilities to explain the results shown above. First, these two potentiators may act through a similar mechanism, but, compared to genistein, curcumin serves as a partial agonist. This scenario is very similar to the one proposed for capsaicin [3]. Second, curcumin and genistein may act through two completely different mechanisms. These two ideas can be differentiated by adding curcumin and genistein together. The first proposition entails a partial inhibition by curcumin applied after CFTR currents are maximally activated by genistein. On the other hand, an additive effect between curcumin and genistein would suggest two different mechanisms of action.
In the whole-cell recording mode, after activating the G551D-CFTR channels by 10 μM FSK and 100 μM CPT-cAMP, we first applied 80 μM genistein which is the maximally effective concentration. Once the whole-cell current reached steady state, 30 μM curcumin was added in the continued presence of genistein (Fig. 3A). The additional application of 30 μM curcumin produced a further increase in the G551D-CFTR whole-cell current already potentiated by 80 μM genistein (Fig. 3AB). A similar additive effect was observed when G551D-CFTR currents were initially activated by 30 μM curcumin and subsequently exposed to 80 μM genistein (data not shown). Fig. 3C summarizes the fold-increase of G551D-CFTR whole cell currents induced by various combinations of different concentrations of genistein and curcumin. This additive effect suggests that curcumin and genistein can potentiate CFTR channel activity through different mechanisms. It is also worth noting that a combination of relatively low concentrations of genistein and curcumin showed a significant fold-increase in G551D-CFTR currents (Fig. 3C). For example the combined application of 10 μM genistein and 5 μM curcumin produced a very large synergistic effect (~30 fold) that increased the G551D whole-cell currents to levels obtained with much higher doses of the two compounds (Fig. 3C). This also contrasts markedly with the much smaller ~2–3 fold potentiation observed when 10 μM genistein or 5 μM curcumin was applied alone (Fig. 1C).
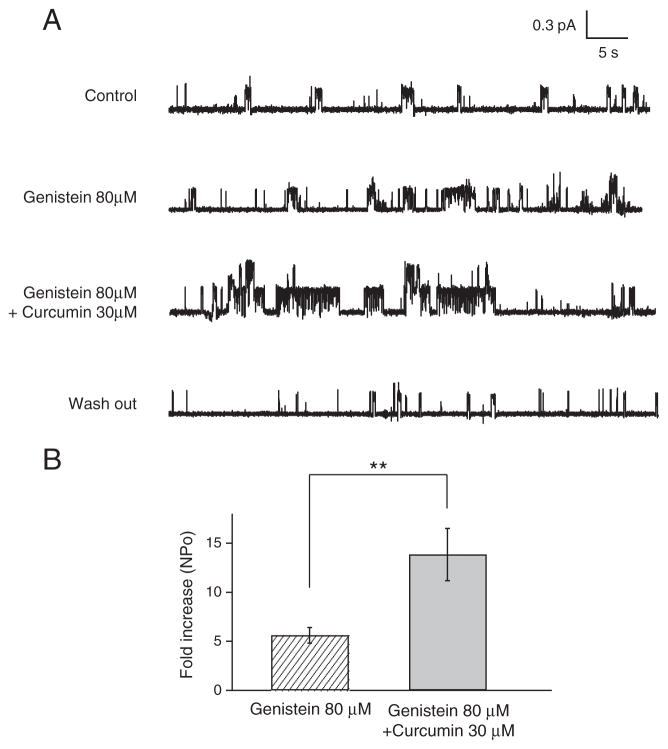
To further confirm these whole-cell experimental results, we carried out similar experiments in the cell-attached mode. Fig. 4A shows representative single channel current data obtained from a cell-attached patch containing G551D-CFTR. 80 μM genistein increased the channel activity of cAMP-stimulated G551D-CFTR in the cell-attached patch. The additional application of 30 μM curcumin further increased the genistein-potentiated G551D-CFTR channel activity (Fig. 4A). Fig. 4B shows the summary of the NPo fold-increases induced by 80 μM genistein (5.6±0.8, n=10) and by 80 μM genistein plus 30 μM curcumin (13.8±2.7, n=10). These results again suggest that curcumin and genistein can potentiate CFTR channel activity through different mechanisms.
Fig. 4.
Additive effect of curcumin and genistein on G551D-CFTR in cell-attached mode. (A) Representative traces for the additive effect of curcumin and genistein on G551D-CFTR single channel currents in cell-attached mode. The G551D-CFTR channels, expressed in CHO cells, are continuously stimulated by 10 μM FSK and 100 μM CPT-cAMP. Vp=+50 mV. Single channel amplitude: control: 0.27 pA, genistein: 0.35 pA, genistein+curcumin: 0.30 pA, wash out: 0.31 pA. (B) Comparisons of relative fold-increase in NPo of G551D-CFTR single channel induced by 80 μM genistein alone and a combined application of 80 μM genistein and 30 μM curcumin (n=10). Error bars represent SEM. Paired t-test, **: P<0.01.
3.3. Estimation of the restoration of G551D gating defect by genistein and curcumin
Finally we attempted to quantify how much the combined application of genistein/curcumin restored the G551D gating defect, in comparison to WT-CFTR.
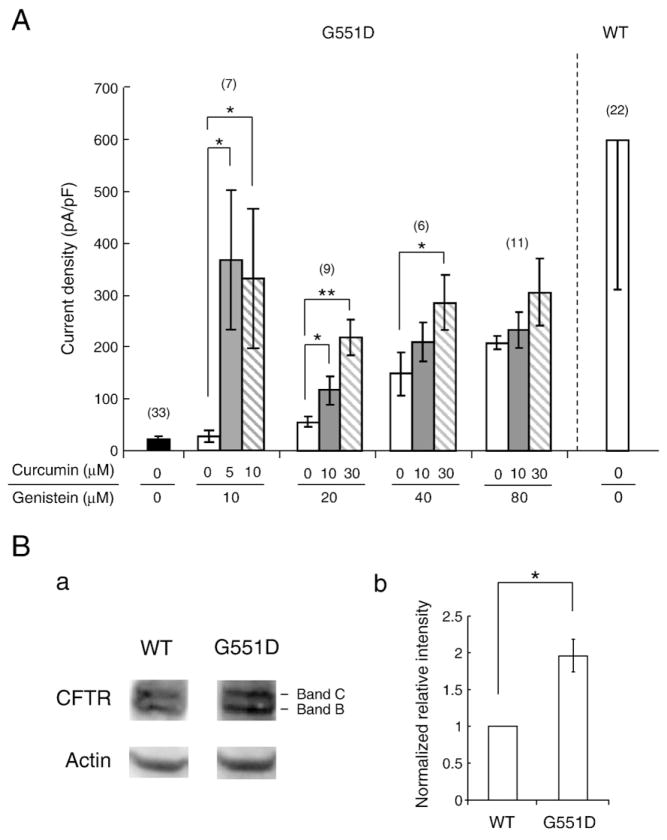
Fig. 5A summarizes the current densities of the G551D-CFTR whole-cell currents obtained after exposure to various combinations of different concentrations of curcumin and genistein. It is clear from the data that for each combination of genistein and curcumin there was a clear and statistically significant potentiation in G551D-CFTR current density, which reached levels that were approximately half of the WT-CFTR level, however, there was no statistical difference between the data sets (Fig. 5A) because of the large variation in the absolute whole-cell CFTR current density probably caused by heterogeneous CFTR expressions in the transient expression system.
Fig. 5.
Summary of the effects of curcumin and genistein on G551D-CFTR whole-cell current density. (A) Current densities of G551D-CFTR expressed in CHO cells obtained from whole cell currents potentiated by various combinations of curcumin and genistein are indicated. Note that the current density (whole-cell current normalized by cell capacitance) data show some differences from the fold-increase data in Fig. 3C because of wide variation in current values. Error bars represent SEM. ANOVA, *: P<0.05; **: P<0.01. (B) Western blot analysis for WT- and G551D-CFTR proteins expressed in CHO cells. (a) Representative of three western-blot data. Band B: ~150 kD; Band C: ~170 kD. (b) Comparison of the relative intensity (CFTR/actin) between WT- and G551D-CFTR proteins (band C) (n=3). Normalized to the relative intensity of WT-CFTR. Non-paired t-test *: P<0.05.
Fig. 5B shows a comparison of Western blots obtained from CHO cells expressing WT- and G551D-CFTR proteins. The CFTR proteins were separated into two bands: one for fully glycosylated mature proteins (band C) and another for non-glycosylated immature proteins (band B) (Fig. 5B-a). The normalized band C signal intensities suggested that the expression level of G551D-CFTR is approximately 2-fold higher than that of WT-CFTR in our transient expression system (Fig. 5B-b), although it should be noted that the Western blot is poorly quantitative.
Based on these data, we speculate that the combined application of curcumin and genistein can compensate for up to 50% of the gating defect of G551D-CFTR compared to the WT-CFTR level.
4. Discussion
In this study, we investigated the potentiation effects of curcumin, genistein and their combined applications on G551D-CFTR and found that the combined application of these two reagents can cause additive effects, which suggests that the two compounds might work through different mechanisms.
4.1. Genistein and curcumin
There are different mechanisms that cause CFTR channel dysfunction [47], but when the defective proteins are present in the apical membrane, pharmacological agents that increase the activity of the mutant channels are of potential clinical value in the treatment of CF. Compounds that increase CFTR activity include isoflavones (e.g. genistein [24,48]), flavones (e.g. apigenin [24]), capsaicin [3], phenylglycines (e.g. PG-01 [36]), sulfonamides (e.g. SF-01 [36]), 1,4-dihydropyridines [36], benzoflavones (e.g. UCCF-029 [10]), benzimidazolones (e.g. NS004 [4] and UCCF-853 [10]), pyrrolo[2,3-b]pyrazines derivatives (e.g., RP107 [34]), benzo[c]quinolizinium derivatives (e.g., MPB-91 [15]) and curcumin [7,8]. Although the detailed mechanism through which these compounds increase CFTR activity is still unclear, some potentiators seem to share a common mechanism of action or binding site since no additive effect is observed when applied together, as shown for genistein and NS004 [4], genistein and UCCF-029 [10] or genistein and capsaicin [3]. On the other hand, UCCF-029 and UCCF-853 seem to work through different binding sites, since they exhibited an additive effect when applied together [10].
Of all the potentiators genistein is perhaps the most studied. Since genistein increases CFTR currents in excised patches, it has been proposed that genistein’s target is the CFTR molecule itself [43,45]. This reagent clearly affects the ATP-dependent gating of CFTR channels, increasing the opening rate and decreasing the closing rate, consistent with what one would expect if the drug acts at the NBD dimer [3,17,28,29,32]. Wang et al. [43] reported that the dose response of genistein for WT channels exhibited a bell shape with the maximum potentiation effect at about 40 μM. In this study, we found that the genistein dose response for G551D-CFTR channels also showed a bell-shape, although the maximally effective concentration was shifted towards higher [genistein] at around 80 μM. This shift is in agreement with previous results [33]. Although it was reported that the amino acid at position 551 is part of the potentiator binding site [33], this may not be the only interpretation for the shift in the genistein dose response. The dose response measurement of the potentiator-induced CFTR current enhancement is just a functional assay in which the ligand binding process itself is not directly measured. A decrease of the Po by the G551D mutation can produce a shift of the dose response curve simply because there is more room for an activity increase in G551D-CFTR versus the wild-type channels. Alternatively, if binding of the potentiator alters gating, then gating will have to alter binding based on thermodynamic principles [13]. Also the pore blocking effect of genistein [28] might affect the dose–response of G551D-CFTR whole cell currents (e.g., Fig. 1C), but its contribution is likely to be small. Thus, one has to exert great caution in interpreting concentration/ effect relationships.
Wang et al. [44] reported that curcumin opened CFTR channels, including G551D-CFTR, by a novel mechanism that required neither ATP nor NBD2 whereas it was strongly dependent on prior phosphorylation by PKA. Moreover a biochemical study [8] showed that curcumin cross-linked a wide variety of CFTR constructs including G551D-CFTR at similar concentrations and durations of exposure employed in this study. However, it is unlikely that such cross-linking itself had a significant influence on the CFTR channel function [8]. Actually it was reported that one cyclic derivative of curcumin, that had no cross-linking activity, could potentiate the G551D-CFTR channels as well as WT-CFTR [8]. Thus the mechanism of curcumin-induced potentiation is still unclear.
4.2. The additive effect of genistein and curcumin
In this present study, we confirm that curcumin potentiates G551D-CFTR reversibly within our experimental time frame, whereas the effect of curcumin is quantitatively smaller than that of genistein up to the highest soluble concentration, 60 μM. A biphasic dose–response relationship for genistein was observed, but there was no evidence for an inhibitory effect of curcumin over the range of concentrations tested. Importantly, genistein and curcumin additively potentiate G551D-CFTR channels, that is, G551D-CFTR channel activity maximally potentiated by genistein was further increased by curcumin (Figs. 3, 4 and 5). These observations are consistent with the conclusion that genistein and curcumin affect G551D-CFTR through different mechanisms. However, Berger et al [7] demonstrated that addition of increasing concentrations of curcumin in the presence of genistein inhibited wild-type CFTR Cl- currents. While further studies are required to understand the synergistic effects of genistein and curcumin on G551D-CFTR, our results highlight the potential benefit of combinations of different CFTR potentiators, as they might result in stronger effects being achieved at lower drug concentrations.
Although we made qualitatively similar observations in both whole-cell and cell-attached recordings, there are significant quantitative differences between the results obtained in the two recording configurations. (compare Figs. 1C and 2C). The reason for this discrepancy is unclear, and beyond the scope of this paper, but one should note that many factors may affect the response of mutant CFTR to potentiators, such as phosphorylation status of the channel [28,43] and differences in the ATP levels present in the cytosol of the two systems. Also the dialysis of the cytoplasmic milieu for whole-cell recordings may also contribute to these differences.
4.3. Clinical implications
Our results also have important clinical implications for the pharmaceutical therapy of CF because few of the known CFTR potentiators, except for VX-770 [41], seem effective enough to restore the activity (Po) of mutants associated with severe CF [5]. This is of concern especially for mutants such as G551D-CFTR which, despite trafficking normally to the membrane, exhibit very low Po [9]. Recently Accurso et al. [1] reported that the CFTR potentiator VX-770 rescued ~30–40% of the G551D gating defect [41], and was associated with within-subject improvements in CFTR and lung function. Zegarra-Moran et al. [48] reported that 200 μM genistein rescued the gating defect of G551D-CFTR of up to 20% of the WT-CFTR level. Importantly, our studies have shown that the combined application of genistein and curcumin in a lower concentration range showed a synergistic effect of restoring the gating defect of G551D-CFTR of up to ~50% of the WT-CFTR level (Figs. 3C and 5A). Thus, our data suggest that even if each compound by itself cannot potentiate the channel activity of the CFTR mutants to a level that is beneficial to patients with CF, a combination of the two compounds might achieve a therapeutic level of correction, while minimizing potential harmful side-effects by lowering each dose.
Acknowledgments
We are grateful to Shenghui Hu (Univ. of Missouri—Columbia) for his technical assistance and Dr. Mutsuo Nuriya (Keio University) for the useful discussions. We appreciate Prof. J. R. Riordan (Univ. of North Carolina—Chapel Hill, NC, USA) for his kind gift of the anti-CFTR antibody 596. This work was supported by NIHR01DK55835, NIHR01HL53455 (TCH) and NIHK01DK075408 (SGB), a research grant from the Cystic Fibrosis Foundation (BOMPAD06G0, SGB) and JSPS KAKENHI 19590215 and 22590212 (YS).
Footnotes
Statement of conflict of interests
The authors declare no competing personal and financial interests.
References
- 1.Accurso FJ, Rowe SM, Clancy JP, Boyle MP, Dunitz JM, Durie PR, et al. Effect of VX-770 in persons with cystic fibrosis and the G551D-CFTR mutation. N Engl J Med. 2010;363:1991–2003. doi: 10.1056/NEJMoa0909825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aggarwal BB, Kumar A, Bharti AC. Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res. 2003;23:363–98. [PubMed] [Google Scholar]
- 3.Ai T, Bompadre SG, Wang X, Hu S, Li M, Hwang T-C. Capsaicin potentiates wild-type and mutant cystic fibrosis transmembrane conductance regulator chloride-channel currents. Mol Pharmacol. 2004;65:1415–26. doi: 10.1124/mol.65.6.1415. [DOI] [PubMed] [Google Scholar]
- 4.Al-Nakkash L, Hu SML, Hwang T-C. A common mechanism for cystic fibrosis transmembrane conductance regulator protein activation by genistein and benzimidazolone analogs. J Pharmacol Exp Ther. 2001;296:464–72. [PubMed] [Google Scholar]
- 5.Amaral MD, Kunzelmann K. Molecular targeting of CFTR as a therapeutic approach to cystic fibrosis. Trends Pharmacol Sci. 2007;28:334–41. doi: 10.1016/j.tips.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 6.Andersson C, Servetnyk Z, Roomans G. Activation of CFTR by genistein in human airway epithelial cell lines. Biochem Biophys Res Commun. 2003;308:518–22. doi: 10.1016/s0006-291x(03)01436-0. [DOI] [PubMed] [Google Scholar]
- 7.Berger AL, Randak C, Ostedgaard LS, Karp P, Vermeer D, Welsh MJ. Curcumin stimulates cystic fibrosis transmembrane conductance regulator Cl– channel activity. J Biol Chem. 2005;280:5221–6. doi: 10.1074/jbc.M412972200. [DOI] [PubMed] [Google Scholar]
- 8.Bernard K, Wang W, Narlawar R, Schmidt B, Kirk KL. Curcumin cross-links cystic fibrosis transmembrane conductance regulator (CFTR) polypeptides and potentiates CFTR channel activity by distinct mechanisms. J Biol Chem. 2009;284:30754–65. doi: 10.1074/jbc.M109.056010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bompadre SG, Sohma Y, Li M, Hwang T-C. G551D and G1349D, two CF-associated mutations in the signature sequence of CFTR, exhibit distinct gating defects. J Gen Physiol. 2007;129:285–98. doi: 10.1085/jgp.200609667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caci E, Folli C, Zegarra-Moran O, Ma T, Springsteel MF, Sammelson RE, et al. CFTR activation in human bronchial epithelial cells by novel benzoflavone and benzimidazolone compounds. Am J Physiol Lung Cell Mol Physiol. 2003;285:L180–8. doi: 10.1152/ajplung.00351.2002. [DOI] [PubMed] [Google Scholar]
- 11.Cai Z, Taddei A, Sheppard DN. Differential sensitivity of the cystic fibrosis (CF)-associated mutants G551D and G1349D to potentiators of the cystic fibrosis transmembrane conductance regulator (CFTR) Cl– channel. J Biol Chem. 2006;281:1970–7. doi: 10.1074/jbc.M510576200. [DOI] [PubMed] [Google Scholar]
- 12.Chang X-B, Tabcharani JA, Hou Y-X, Jensen TJ, Kartner N, Alon N, et al. Protein kinase A (PKA) still activates CFTR chloride channel after mutagenesis of all 10 PKA concensus phosphorylation sites. J Biol Chem. 1993;268:11304–11. [PubMed] [Google Scholar]
- 13.Colquhoun D. Binding, gating, affinity and efficacy: the interpretation of structure–activity relationships for agonists and of the effects of mutating receptors. Br J Pharmacol. 1998;125:924–47. doi: 10.1038/sj.bjp.0702164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cutting GR, Kasch LM, Rosenstein BJ, Zielenski J, Tsui LC, Antonarakis SE, et al. A cluster of cystic fibrosis mutations in the first nucleotide-binding fold of the cystic fibrosis conductance regulator protein. Nature. 1990;346:366–9. doi: 10.1038/346366a0. [DOI] [PubMed] [Google Scholar]
- 15.Dérand R, Bulteau-Pignoux L, Mettey Y, Zegarra-Moran O, Howell LD, Randak C, et al. Activation of G551D CFTR channel with MPB-91: regulation by ATPase activity and phosphorylation. Am J Physiol Cell Physiol. 2001;281:C1657–66. doi: 10.1152/ajpcell.2001.281.5.C1657. [DOI] [PubMed] [Google Scholar]
- 16.Dixon R, Ferreira D. Genistein. Phytochemistry. 2002;60:205–11. doi: 10.1016/s0031-9422(02)00116-4. [DOI] [PubMed] [Google Scholar]
- 17.Egan ME, Pearson M, Weiner SA, Rajendran V, Rubin D, Glockner-Pagel J, et al. Curcumin, a major constituent of turmeric, corrects cystic fibrosis defects. Science. 2004;304:600–2. doi: 10.1126/science.1093941. [DOI] [PubMed] [Google Scholar]
- 18.Gadsby DC, Vergani P, Csanády L. The ABC protein turned chloride channel whose failure causes cystic fibrosis. Nature. 2006;440:477–83. doi: 10.1038/nature04712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gopinath D, Ahmed MR, Gomathi K, Chitra K, Sehgal PK, Jayakumar R. Dermal wound healing processes with curcumin incorporated collagen films. Biomaterials. 2004;25:1911–7. doi: 10.1016/s0142-9612(03)00625-2. [DOI] [PubMed] [Google Scholar]
- 20.Gray MA, Greenwell JR, Argent BE. Secretin-regulated chloride channel on the apical plasma membrane of pancreatic duct cells. J Membr Biol. 1988;105:131–42. doi: 10.1007/BF02009166. [DOI] [PubMed] [Google Scholar]
- 21.Hwang T-C, Wang F, Yang IC, Reenstra WW. Genistein potentiates wild-type and delta F508-CFTR channel activity. Am J Physiol. 1997;273:C988–98. doi: 10.1152/ajpcell.1997.273.3.C988. [DOI] [PubMed] [Google Scholar]
- 22.Hwang TC, Koeppe RE, II, Andersen OS. Genistein can modulate channel function by a phosphorylation-independent mechanism: importance of hydrophobic mismatch and bilayer mechanics. Biochemistry. 2003;42:13646–58. doi: 10.1021/bi034887y. [DOI] [PubMed] [Google Scholar]
- 23.Illek B, Fischer H, Santos GF, Widdicombe JH, Machen TE, Reenstra WW. cAMP-independent activation of CFTR Cl channels by the tyrosine kinase inhibitor genistein. Am J Physiol. 1995;268:C886–93. doi: 10.1152/ajpcell.1995.268.4.C886. [DOI] [PubMed] [Google Scholar]
- 24.Illek B, Fischer N. Flavonoids stimulate Cl conductance on human airway epithelium in vitro and in vivo. Am J Physiol Lung Cell Mol Physiol. 1998;275:L902–10. doi: 10.1152/ajplung.1998.275.5.L902. [DOI] [PubMed] [Google Scholar]
- 25.Illek B, Zhang L, Lewis NC, Moss RB, Dong JY, Fischer H. Defective function of the cystic fibrosis-causing missense mutation G551D is recovered by genistein. Am J Physiol. 1999;277:C833–9. doi: 10.1152/ajpcell.1999.277.4.C833. [DOI] [PubMed] [Google Scholar]
- 26.Joe B, Vijaykumar M, Lokesh BR. Biological properties of curcumin-cellular and molecular mechanisms of action. Crit Rev Food Sci Nutr. 2004;44:97–111. doi: 10.1080/10408690490424702. [DOI] [PubMed] [Google Scholar]
- 27.Knowles MR, Stutts MJ, Spock A, Fischer N, Gatzy JT, Boucher RC. Abnormal ion permeation through cystic fibrosis respiratory epithelium. Science. 1983;221:1067–70. doi: 10.1126/science.6308769. [DOI] [PubMed] [Google Scholar]
- 28.Lansdell K, Cai Z, Kidd JF, Sheppard DN. Two mechanisms of genistein inhibition of cystic fibrosis transmembrane conductance regulator Cl–channels expressed in murine cell line. J Physiol. 2000;524:317–30. doi: 10.1111/j.1469-7793.2000.t01-1-00317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lewis HA, Buchanan SG, Burley SK, Conners K, Dickey M, Dorwart M, et al. Structure of nucleotide-binding domain 1 of the cystic fibrosis transmembrane conductance regulator. EMBO J. 2004;23:282–93. doi: 10.1038/sj.emboj.7600040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li C, Ramjeesingh M, Wang W, Garami E, Hewryk M, Lee D, et al. ATPase activity of the cystic fibrosis transmembrane conductance regulator. J Biol Chem. 1996;271:28463–8. doi: 10.1074/jbc.271.45.28463. [DOI] [PubMed] [Google Scholar]
- 31.Melin P, Thoreau V, Norez C, Bilan F, Kitzis A, Becq F. The cystic fibrosis mutation G1349D within the signature motif LSHGH of NBD2 abolishes the activation of CFTR chloride channels by genistein. Biochem Pharmacol. 2004;67:2187–96. doi: 10.1016/j.bcp.2004.02.022. [DOI] [PubMed] [Google Scholar]
- 32.Miquel J, Bernd A, Sempere JM, Diaz-Alperi J, Ramirez A. The curcuma antioxidants: pharmacological effects and prospects for future clinical use. A review. Arch Geronto Geriatr. 2002;34:37–46. doi: 10.1016/s0167-4943(01)00194-7. [DOI] [PubMed] [Google Scholar]
- 33.Moran O, Galietta LJ, Zegarra-Moran O. Binding site of activators of the cystic fibrosis transmembrane conductance regulator in the nucleotide binding domains. Cell Mol Life Sci. 2005;62:446–60. doi: 10.1007/s00018-004-4422-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Noel S, Faveau C, Norez C, Rogier C, Mettey Y, Becq F. Discovery of pyrrolo[2,3-b]pyrazines derivatives as submicromolar affinity activators of wild type, G551D, and F508del cystic fibrosis transmembrane conductance regulator chloride channels. J Pharmacol Exp Ther. 2006;319:349–59. doi: 10.1124/jpet.106.104521. [DOI] [PubMed] [Google Scholar]
- 35.Ono K, Hasegawa K, Naiki H, Yamada M. Curcumin has potent anti-amyloidogenic effects for Alzheimer’s beta-amyloid fibrils in vitro. J Neurosci Res. 2004;75:742–50. doi: 10.1002/jnr.20025. [DOI] [PubMed] [Google Scholar]
- 36.Pedemonte N, Sonawane ND, Taddei A, Hu J, Zegarra-Moran O, Suen YF, et al. Phenylglycine and sulfonamide correctors of defective ΔF508 and G551D cystic fibrosis transmembrane conductance regulator chloride-channel gating. Mol Pharmacol. 2005;67:1797–807. doi: 10.1124/mol.105.010959. [DOI] [PubMed] [Google Scholar]
- 37.Quinton PM. Missing Cl conductance in cystic fibrosis. Am J Physiol. 1986;251:C649–52. doi: 10.1152/ajpcell.1986.251.4.C649. [DOI] [PubMed] [Google Scholar]
- 38.Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245:1066–73. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- 39.Schmidt A, Hughes LK, Cai Z, Mendes F, Li H, Sheppard DN, et al. Prolonged treatment of cells with genistein modulates the expression and function of the cystic fibrosis transmembrane conductance regulator. Br J Pharmacol. 2008;153:1311–23. doi: 10.1038/sj.bjp.0707663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Song Y, Sonawane ND, Salinas D, Qian L, Pedemonte N, Galietta LJ, et al. Evidence against the rescue of defective DeltaF508-CFTR cellular processing by curcumin in cell culture and mouse models. J Biol Chem. 2004;279:40629–33. doi: 10.1074/jbc.M407308200. [DOI] [PubMed] [Google Scholar]
- 41.Van Goor F, Hadida S, Grootenhuis PD, Burton B, Cao D, Neuberger T, et al. Rescue of CF airway epithelial cell function in vitro by a CFTR potentiator, VX-770. Proc Natl Acad Sci USA. 2009;106:18825–30. doi: 10.1073/pnas.0904709106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Verkman AS, Galietta LJ. Chloride channels as drug targets. Nat Rev Drug Discov. 2009;8:153–71. doi: 10.1038/nrd2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang F, Zeltwanger S, Yang IC-H, Nairn AC, Hwang T-C. Actions of genistein on cystic fibrosis transmembrane conductance regulator channel gating. J Gen Physiol. 1998;111:477–90. doi: 10.1085/jgp.111.3.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang W, Bernard K, Li G, Kirk KL. Curcumin opens cystic fibrosis transmembrane conductance regulator channels by a novel mechanism that requires neither ATP binding nor dimerization of the nucleotide-binding domains. J Biol Chem. 2007;282:4533–44. doi: 10.1074/jbc.M609942200. [DOI] [PubMed] [Google Scholar]
- 45.Weinreich F, Wood PG, Riordan JR, Nagel G. Direct action of genistein on CFTR. Pflugers Arch. 1997;434:484–91. doi: 10.1007/s004240050424. [DOI] [PubMed] [Google Scholar]
- 46.Welsh MJ, Liedtke CM. Chloride and potassium channels in cystic fibrosis airway epithelia. Nature. 1986;332:467–70. doi: 10.1038/322467a0. [DOI] [PubMed] [Google Scholar]
- 47.Welsh MJ, Smith AE. Molecular mechanisms of CFTR chloride channel dysfunction in cystic fibrosis. Cell. 1993;73(7):1251–4. doi: 10.1016/0092-8674(93)90353-r. [DOI] [PubMed] [Google Scholar]
- 48.Zegarra-Moran O, Romio L, Folli C, Caci E, Becq F, Vierfond JM, et al. Correction of G551D-CFTR transport defect in epithelial monolayers by genistein but not by CPX or MPB-07. Br J Pharmacol. 2002;137:504–12. doi: 10.1038/sj.bjp.0704882. [DOI] [PMC free article] [PubMed] [Google Scholar]