Abstract
Introduction
Management of locally advanced non-small cell lung cancer is among the most highly contested areas in thoracic oncology. In this population, a history of prior cancer frequently results in exclusion from clinical trials and may influence therapeutic decisions. We therefore determined prevalence and prognostic impact of prior cancer among these patients.
Materials and Methods
We identified patients >65 years of age diagnosed 1992–2009 with locally advanced lung cancer in the Surveillance, Epidemiology, and End Results-Medicare linked dataset. We characterized prior cancer by prevalence, type, stage, and timing. We compared all-cause and lung cancer-specific survival between patients with and without prior cancer using propensity score-adjusted Cox regression.
Results
51,542 locally advanced lung cancer patients were included; 15.8% had a history of prior cancer. Prostate (25%), gastrointestinal (17%), breast (16%), and other genitourinary (15%) were the most common types of prior cancer, and 76% percent of prior cancers were localized or in situ stage. Approximately half (54%) of prior cancers were diagnosed within 5 years of the index lung cancer date. Patients with prior cancer had similar (propensity-score adjusted hazard ratio [HR] 0.96; 95% CI, 0.94–0.99; P=0.005) and improved lung cancer-specific (HR 0.84; 95% CI, 0.81–0.86; P<0.001) survival compared to patients with no prior cancer.
Conclusions
For patients with locally advanced lung cancer, prior cancer does not adversely impact clinical outcomes. Patients with locally advanced lung cancer and a history of prior cancer should not be excluded from clinical trials, and should be offered aggressive, potentially curative therapies if otherwise appropriate.
Keywords: Lung cancer, prior malignancy, survival, clinical trial design
1. INTRODUCTION
Locally advanced lung cancer represents a critical unmet need in thoracic oncology research. Characterized by an unresectable primary tumor or regional lymph node involvement, it accounts for more than 30 percent of all U.S. lung cancer cases [1, 2]. Despite numerous large clinical trials examining the role of multi-modality therapy, high-dose radiation, and consolidation and maintenance medical therapy [3–8], outcomes remain poor, with five-year survival rates under 20 percent.
Clinical trials are essential to improving outcomes for these patients. Yet well under five percent of U.S. adult cancer patients are enrolled in cancer clinical trials [9, 10]. Increasingly numerous and stringent eligibility criteria represent a major barrier to clinical trial accrual [11, 12]. For instance, over 80 percent of lung cancer clinical trials sponsored or endorsed by the National Cancer Institute (NCI)-affiliated Eastern Cooperative Oncology Group (ECOG) exclude patients with prior cancer, resulting in exclusion of up to 18% of potential participants for this reason alone [13]. Until recently, limited research has examined whether this common exclusion criterion was evidence-based; that is, whether lung cancer patients with a history of prior cancer did worse than those without a prior cancer. Likewise, little was known about the characteristics of lung cancer patients with a history of prior cancer such as the type, stage, and timing of the prior cancers. Of the few existing studies, most were single-center, relatively small, and limited to early-stage surgically resected disease [14–18]. We recently employed nationally representative data to examine prevalence and characteristics of prior cancer history among patients with metastatic lung cancer. We found that the majority of prior cancers were early-stage and were diagnosed within five years of the index lung cancer diagnosis [19]. We also found that mortality among metastatic patients with a prior cancer history was equivalent—if not slightly better than—patients without a prior cancer history.
However, findings in metastatic lung cancer cannot be extrapolated to locally advanced disease, a setting in which complex multimodality therapy is employed and 15–20% of patients achieve long-term survival. Therefore, we examined the prevalence, type, stage, timing, and prognostic impact of prior cancer diagnoses among patients with locally advanced lung cancer using a representative national dataset.
2. MATERIALS AND METHODS
2.1 Data Acquisition
This study was approved by the Institutional Review Board of the University of Texas Southwestern Medical Center (IRB# STU 082012-040). Data were obtained from linked 1992–2009 National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) program data and 1991–2011 Medicare claims data. SEER is a nationally representative collection of population-based cancer registries. Linked SEER-Medicare data provides additional clinical information on SEER patients with Medicare. Ninety-four percent of cancer patients reported to SEER aged 65 years or older have been successfully linked with Medicare data [20]. Data for this study were available from 17 registries broadly representing approximately 26% of the U.S. population [21].
2.2 Study Population
This study included patients >65 years of age diagnosed with locally advanced primary lung cancer between 1992–2009. We defined locally advanced disease as American Joint Committee on Cancer (AJCC) Stage III, ascertained from SEER data per the AJCC Cancer Staging Manual, Third and Sixth Editions [22]. We used 1992 as the initial year for our study because Medicare claims were first available in 1991, providing a one-year lead-in time to capture any pre-existing comorbidies. The last year of the study period, 2009, was the most recent year of data available at the time of the data request. We included individuals age >65 years to allow for one-year of complete Medicare claims data pre-diagnosis to capture pre-existing comorbidies. All patients had full coverage of Medicare Parts A and B from one year before and one year after their lung cancer diagnosis. To ensure complete claims data, we excluded HMO members and patients with only autopsy or death certificate records. We included only patients with non-small cell lung cancer (NSCLC) or small cell lung cancer (SCLC) histology. We excluded patients with incomplete diagnosis or death dates or discrepancies in SEER and Medicare birth dates of a year or more.
2.3 Measures
Primary outcomes were all-cause and lung cancer-specific mortality. Survival was measured as the interval in months between diagnosis date (defined as the 15th of the month because SEER provides only month and year of diagnosis) and death date per SEER. Patients were followed until date of death or censored at the end of 2009 (last date of death in 2011 SEER submission).
A history of prior cancer was determined as described in our previous studies.[13, 19] In brief, prior cancers were identified using the SEER variable tumor site recode; patients whose first primary cancer was a lung cancer (siterkm1=39) were defined as not having a prior cancer; whereas those whose second or subsequent primary cancer was a lung cancer (siterkm2–10=39) were defined as having a prior cancer. We measured features of prior cancers including cancer type, stage, and timing in relation to the index lung cancer. For patients with more than one primary lung cancer, the first primary lung cancer was considered the index lung cancer. We did not consider these patients to have a prior cancer history because (1) it is challenging to accurately identify same-site second primary cancers using registry data [23]; (2) in clinical practice it is difficult to distinguish between a second primary lung cancer versus recurrent disease; and (3) a history of resected early-stage lung cancer is typically allowed in clinical trials of locally advanced disease [23, 24].
We examined multiple covariates previously shown to be associated with lung cancer prognosis [19, 25]. Surgery within 120 days of diagnosis, chemotherapy within 120 days of diagnosis, and radiotherapy within one year of diagnosis were identified using Medicare inpatient, outpatient, and physician claims (coded yes/no) [26]. ICD-9 and CPT codes used to identify these measures were previously described [19, 26]. We measured comorbidity by searching inpatient, outpatient, or carrier claims for multiple chronic conditions (e.g., myocardial infarction, diabetes, dementia, or AIDS) within 12 months pre-diagnosis using the Charlson comorbidity index-Klabunde adaptation [27, 28]. Patients with Medicaid were identified using the state buy-in variable [29].
2.4 Statistical Analysis
Using descriptive statistics, we reported the prevalence and correlates of prior cancer history. We quantified the type and stage of prior cancer in addition to time elapsed between the prior cancer diagnosis and index lung cancer diagnosis. We used unadjusted Kaplan-Meier (K-M) analysis to compare survival functions for patients with and without a prior cancer history for both outcomes. K-M curves were also constructed according to characteristics of the most recent prior cancer, including: 1) timing of diagnosis (within ≤1 year; ≤3 years; ≤5 years of the index lung cancer); 2) cancer stage; and 3) cancer type.
We used three Cox proportional hazard models for each outcome: propensity score-adjusted models, univariable, and multivariable models. Propensity scores were constructed to adjust for observable differences (i.e. potential confounders) between patients with and without a prior cancer diagnosis. Our propensity score logistic regression model included all measured covariates (see Table 1). We examined propensity score overlap and balance across covariates using multiple regression, chi-square analysis, and histograms. We fitted Cox models adjusting for propensity scores as a continuous variable. We compared these models to multivariable covariate-adjusted models including all measured covariates.
Table 1.
Baseline patient characteristics of the locally advanced lung cancer SEER-Medicare cohort (N=51,542)
| Patient Characteristics | Total Patients N | Prior Cancer N (%) | P-Value | Adjusted P-Value* |
|---|---|---|---|---|
| Age | <.001 | 0.919 | ||
| Age<75 | 23,416 | 3,127 (13.4) | ||
| 75<=Age<85 | 22,434 | 3,923 (17.5) | ||
| Age>=85 | 5,692 | 1,068 (18.8) | ||
| Sex | <.001 | 0.956 | ||
| Female | 23,358 | 3,251 (13.5) | ||
| Male | 28,184 | 4,967 (17.7) | ||
| Race/Ethnicity | <.001 | 0.958 | ||
| White | 43,933 | 7,129 (16.2) | ||
| Black | 4,642 | 664 (14.3) | ||
| Other | 556 | 52 (9.4) | ||
| Hispanic | 2,411 | 282 (11.7) | ||
| Marriage Status | <.001 | 0.984 | ||
| Married | 26,104 | 4,479 (17.2) | ||
| Sep/Div/Wid † | 20,170 | 2,909 (14.4) | ||
| Single | 3,596 | 496 (13.8) | ||
| Unknown | 1,672 | 243 (14.5) | ||
| Histology | <.001 | 0.855 | ||
| Adenocarcinoma | 16,264 | 2,842 (17.5) | ||
| Squamous | 12,853 | 1,978 (15.4) | ||
| Small cell | 7,277 | 975 (13.4) | ||
| NSCLC‡ | 15,148 | 2,332 (15.4) | ||
| Charlson Comorbidity | <.001 | 0.945 | ||
| 0 | 20,444 | 3,332 (16.3) | ||
| 1 | 14,954 | 2,373 (15.9) | ||
| 2+ | 13,160 | 2,154 (16.4) | ||
| Not available | 2,984 | 268 (9.0) | ||
| Medicaid | <.001 | 0. 498 | ||
| Yes | 9,265 | 1,052 (11.4) | ||
| No | 42,277 | 7,075 (16.7) | ||
| Treatment | <.001 | 0. 719 | ||
| Surgery only | 2,794 | 503 (18.0) | ||
| Chemotherapy only | 5,145 | 905 (17.6) | ||
| Radiation only | 8,894 | 1,438 (16.2) | ||
| ≥ 2 treatments | 17,738 | 2,750 (15.5) | ||
| No Surg/Chemo/Rad§ | 16,971 | 2,531 (14.9) | ||
| Cause of death|| | <.001 | |||
| Alive | 5,857 | 1,027 (17.5) | ||
| Lung cancer specific | 37,150 | 5,122 (13.8) | ||
| All other causes | 8,535 | 1,978 (23.2) | ||
| Total | ||||
| 51,542 | 8,127 | |||
Propensity Score Adjusted P-value: non-significance denotes groups are well balanced for covariates of interest
Separated/Divorced/Widowed
Non-small cell lung carcinoma
No surgery/chemotherapy/radiation
Dependent variable, no adjusted P-value necessary
Finally, we conducted a subset analysis to better represent a clinical trial eligible population. In this analysis, we included only patients who were age <75 years, had no comorbidities, and had received surgery and/or radiotherapy as treatment of the index locally advanced lung cancer. Analyses were performed using SAS 9.3 (SAS Institute Inc. Cary, NC, 2013) and Stata 13.1 (StataCorp. LP., College Station, TX, 2013).
3. RESULTS
In total, 51,542 patients with locally advanced lung cancer diagnosed 1992–2009 were identified, of whom 15.8% (n=8,127) had a history of prior cancer. Baseline characteristics are listed in Table 1. A history of prior cancer was associated with increasing age, male sex, white race, adenocarinoma histology, and absence of Medicaid enrollment. Three-quarters of these patients received at least one lung cancer therapy. Characteristics did not differ between groups after adjustment for propensity scores.
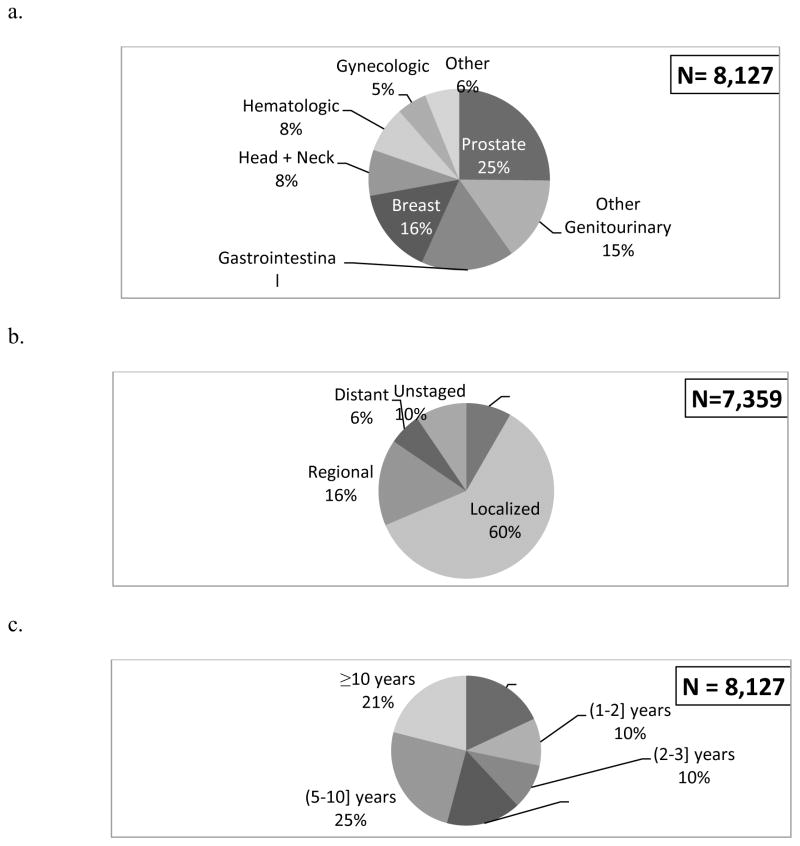
Figure 1 depicts the type (a), stage (b), and timing (c) of the most recent prior cancer. Prostate, gastrointestinal, breast, and other genitourinary cancers were the most common prior cancer types. Over two-thirds of prior cancers were localized or in situ stage and only 6% were distant stage. Approximately half of prior cancers were diagnosed within five years of the index lung cancer. The median time between the prior cancer and the index lung cancer was 4.5 years (SD=6.2). Two prior cancers occurred in 2% of patients; three prior cancers occurred in 0.3%. These second most recent prior cancer occurred a median of 8.6 years (SD=7.1) prior to index lung cancer diagnosis. The type and stage of the second most recent prior cancer are shown in e-Figure 1.
Figure 1.
Type (a), stage (b) and timing (c) of the most recent prior cancers. (Note: Cell sizes less than 11 are suppressed per the SEER-Medicare data use agreement; Denominators are not equal due to missing data.)
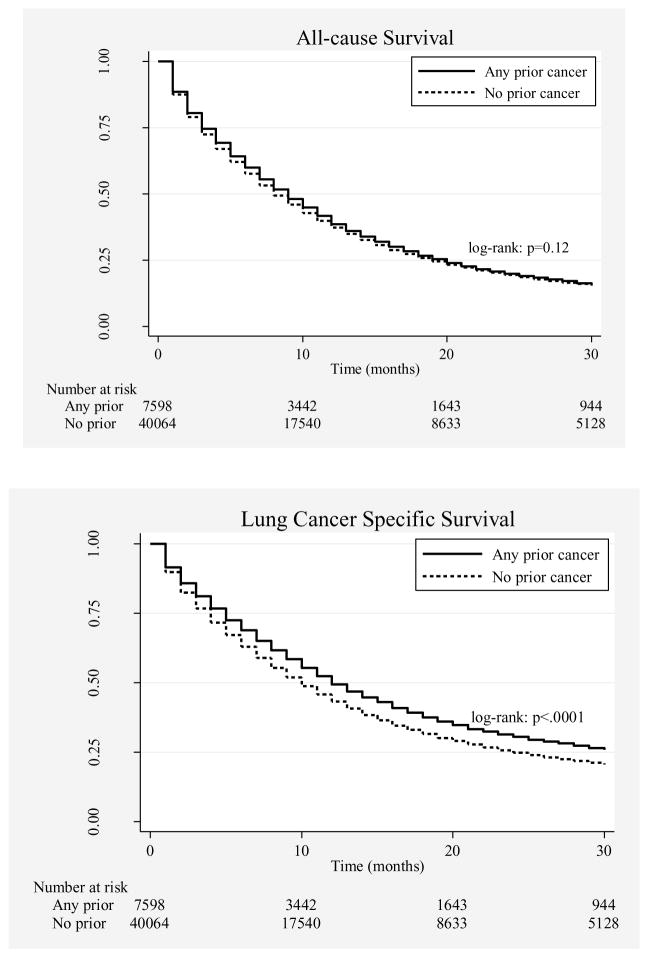
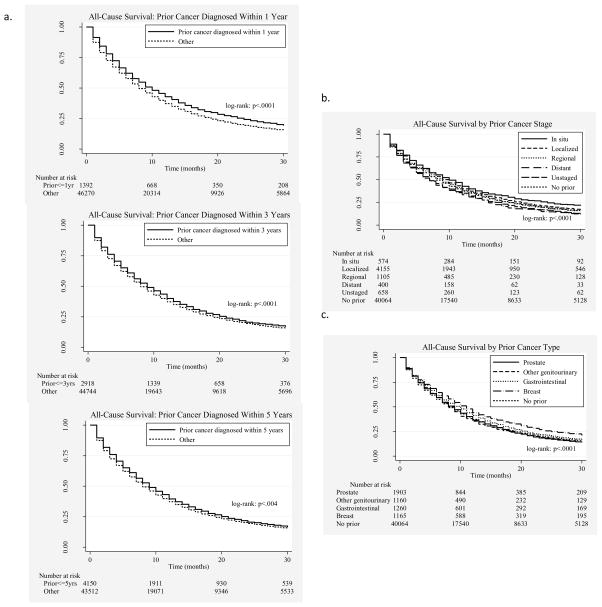
In unadjusted K-M curves, patients with prior cancer demonstrated similar all-cause and improved lung cancer-specific survival compared to patients with no prior cancer (Figure 2). Figure 3 shows all-cause survival curves stratified by the timing, stage, and type of the most recent prior cancer. In propensity-score-adjusted Cox proportional hazard models, patients with a prior cancer history had slightly better all-cause (hazard ratio [HR]=0.96; 95% CI 0.94–0.99, p=0.005) and lung cancer-specific (HR=0.84; 95% CI 0.81–0.86, p<0.001) mortality when compared to patients without a prior cancer history. Results from the unadjusted models (data not shown) and multivariable covariate-adjusted models (Table 2) were similar in effect size, direction, and significance.
Figure 2.
All-cause and lung cancer-specific survival for patients with and without any prior malignancy
Figure 3.
All-cause survival according to type (a), stage (b), and timing (c) of prior cancer diagnosis (“Other” denotes patients with no prior malignancy or a history of prior malignancy diagnosed outside the referenced time frame) Table 1. Baseline patient characteristics of the locally advanced lung cancer SEER-Medicare cohort (N=51,542)
Table 2.
Multivariable covariate-adjusted hazard ratios for all cause and lung cancer specific mortality
| Patient Characteristics | All Cause HR* (95% CI†) | All Cause P-value | Lung Cancer-specific HR* (95% CI†) | Lung Cancer-specific P-value |
|---|---|---|---|---|
| Prior cancer diagnosis (vs. None) | ||||
| Yes (any) | 0.95 (0.92–0.97) | <0.001 | 0.82 (0.80–0.85) | <0.001 |
| Age (years; vs. 66–74) | ||||
| 75 – 85 | 1.16 (1.14–1.19) | <0.001 | 1.15 (1.13–1.18) | <0.001 |
| > 85 | 1.33 (1.29–1.37) | <0.001 | 1.28 (1.23–1.32) | <0.001 |
| Sex (vs. female) | ||||
| Male | 1.14 (1.12–1.17) | <0.001 | 1.14 (1.12–1.17) | <0.001 |
| Race/ethnicity (vs. white) | ||||
| Black | 0.96 (0.93–0.99) | 0.009 | 0.94 (0.91–0.98) | 0.001 |
| Hispanic | 0.91 (0.83–1.00) | 0.039 | 0.91 (0.83–1.01) | 0.078 |
| Other | 0.85 (0.81–0.89) | <0.001 | 0.86 (0.81–0.90) | <0.001 |
| Marital Status (vs. married) | ||||
| Sep/Div/Wid‡ | 1.05 (1.03–1.07) | <0.001 | 1.03 (1.01–1.06) | 0.005 |
| Single | 1.09 (1.05–1.13) | <0.001 | 1.08 (1.03–1.13) | <0.001 |
| Unknown | 1.07 (1.01–1.12) | 0.016 | 1.05 (0.99–1.12) | 0.091 |
| Histology (vs. other non-small cell lung cancer [NSCLC]) | ||||
| Small Cell | 1.20 (1.17–1.24) | <0.001 | 1.29 (1.25–1.33) | <0.001 |
| Adenocarcinoma | 0. 88 (0.86–0.90) | <0.001 | 0.92 (0.90–0.94) | <0.001 |
| Squamous | 0.94 (0.92–0.97) | <0.001 | 0.97 (0.95–1.00) | 0.071 |
| Comorbidity (vs. none) | ||||
| One | 1.11 (1.08–1.13) | <0.001 | 1.06 (1.04–1.09) | <0.001 |
| Two or more | 1.24 (1.21–1.27) | <0.001 | 1.12 (1.09–1.15) | <0.001 |
| Rule out | 0.93 (0.90–0.97) | 0.001 | 0.93 (0.88–0.97) | <0.001 |
| Payer Status (vs. no) | ||||
| Medicaid | 1.04 (1.01–1.06) | 0.006 | 1.02 (0.99–1.05) | 0.247 |
| Treatment Status (vs. no Treatment) | ||||
| Surgery only | 0.3 (0.29–0.31) | <0.001 | 0.27 (0.26–0.29) | <0.001 |
| Chemotherapy only | 0.52 (0.50–0.53) | <0.001 | 0.55 (0.53–0.57) | <0.001 |
| Radiation only | 0.62 (0.60–0.64) | <0.001 | 0.66 (0.64–0.68) | <0.001 |
| Two or more | 0.36 (0.35–0.37) | <0.001 | 0.38 (0.37–0.39) | <0.001 |
HR denotes hazard ratio of all cause and lung cancer specific death for the above covariates
CI denotes confidence interval
Separated/Divorced/Widowed
We also determined the prevalence and prognostic impact in a subset of patients likely to be eligible for clinical trials (defined as age <75 years, no recorded comorbidities, and receiving surgery and/or radiation for the locally advanced lung cancer diagnosis) (N=2,211). In this population, 14.8% had a history of prior cancer. A prior cancer diagnosis was associated with similar all-cause (HR 1.01; 95% CI, 0.89–1.15; P=0.886) and superior lung cancer-specific (HR 0.85; 95% CI, 0.73–0.99; P=0.035) survival in the propensity score-adjusted model.
4. DISCUSSION
Understanding the clinical significance of prior cancer diagnoses is central to clinical practice and to clinical trial design. Assumptions about the prognostic effect of prior cancer diagnoses can impact clinical decisions to administer or withhold potentially curative therapy. Furthermore, there is a longstanding and widespread practice of excluding patients with a history of prior cancer from lung cancer clinical trials [13]. Prior research has suggested that only approximately one-quarter of stage 3 lung cancer patients are eligible for clinical trials, with medical comorbidities—including prior cancer diagnoses—serving as the primary reason for exclusion [30]. Given persistently low rates of clinical trial participation among cancer patients and persistently poor outcomes among those with locally advanced lung cancer, the possibility of expanding the pool of eligible patients for clinical trials could benefit researchers, physicians, and patients alike by reducing study duration, increasing trial completion rates, enhancing generalizability, and ultimately delivering new treatments to more patients sooner.
Our findings suggest that prior cancer-related eligibility criteria may substantially limit accrual to clinical trials for locally advanced lung cancer. Almost 16% of the patients in our sample had a history of prior cancer. This proportion exceeds the 13% described among all cancer types [31]. perhaps reflecting the influence of smoking or the advanced average age at diagnosis. Importantly, the majority of prior cancer diagnoses occurred within five years before the index cancer and would therefore result exclusion from clinical trials that employ the common 5-year cancer-free window for eligibility. When restricting by age, comorbidity burden, and receipt of lung cancer treatment to examine prior cancer in a clinical trial-type population, the prevalence of prior cancer diagnosis was similar. An earlier study demonstrated that, among patients with stage 1–3 lung cancer, prior cancer is considerably more common than other medical comorbidities that may exclude patients from clinical trials, such as renal disease (three times more common), liver disease (seven times more common), and HIV (14 times more common) [25]. Given the near four-fold increase in U.S. cancer survivors over the past 30 years [32], this proportion will only increase in the future.
In this study, the most common type of prior cancer was prostate, which usually has such an indolent clinical course that the United States Preventive Services Task Force no longer recommends routine screening for it [33]. More than two-thirds of prior cancers in our study cohort were localized or in situ stage. Thus it seems unlikely that these earlier cancer diagnoses would have a detrimental impact on the outcomes of patients facing locally advanced lung cancer.
Our observation that patients with a history of prior cancer may have better lung cancer-specific survival than those without a prior cancer diagnosis is perhaps counterintuitive but mirrors our findings among patients with metastatic lung cancer [19]. There are few other data to which our observations can be compared. A subset analysis of 30 patients with locally advanced lung cancer and a history of prior cancer found that they had equivocal survival to locally advanced cases without prior cancer diagnoses [18]. Potential explanations include an advantageous cancer survivor phenotype, reflecting favorable cancer biology and improved response to therapy [13, 34]. Alternatively, prior cancer may result in lead- and length-time biases, with clinical and radiographic surveillance of the earlier cancer resulting in detection and treatment of a clinically silent locally advanced lung cancer earlier than might otherwise occur.
In addition to potential concerns about survival impact, there are other reasons why a prior cancer diagnosis might warrant exclusion from a trial. For instance, patients with a prior cancer may have been previously exposed to radiation therapy or chemotherapy that could hypothetically result in either (1) lower tolerance for or (2) decreased efficacy of treatment for the current locally advanced lung cancer. However, excluding prior cancer diagnoses generally for these reasons is an inefficient and overly broad approach to these issues. In the current analysis, over two-thirds of prior cancers were in situ or localized stage, for which radiation therapy and chemotherapy may not have been administered. An alternative strategy would be to exclude prior cancer treatment (which has been done in approximately 40% of Eastern Cooperative Oncology Group [ECOG] lung cancer trials[13]) and/or restrict enrollment according to organ function.
Certain limitations apply to our work. First, our results are not generalizable to other cancer types. Patients with locally advanced lung cancer may be older, have greater smoking histories (and therefore at greater risk for multiple cancer types [30, 35]), and have worse outcomes than patients with other cancers. SEER-Medicare data, although arguably representative of the general U.S. lung cancer population, does not resemble a clinical trial population. However, our clinical trial-like subset, similar to the overall population, demonstrated no survival detriment associated with prior cancer. Due to delays in case ascertainment by contributing local registries and the fact that SEER-Medicare is updated every two years, the time period of our analysis is not current (most recent year 2009). However, in contrast to advanced (stage 4 lung cancer), the diagnosis, staging, and treatment of locally advanced (stage 3) lung cancer have not meaningfully changed over the past seven years. Thus, it is unlikely that the inclusion of more recent data would alter our conclusions. Any recent therapeutic advances for other cancers during this time period would presumably improve the outcomes of the prior cancer cohort in this analysis, thus supporting our current conclusions. Importantly, we were unable to address other factors relevant to clinical trial conduct and outcomes such as tolerability of therapy. However, we did observe that the percent of patients receiving cancer-directed therapy was similar among patients with and without a prior cancer history (68.8% vs 67.1%) suggesting that intensive therapy is feasible in this population.
In conclusion, locally advanced lung cancer represents a major area of unmet need in thoracic oncology. Despite the emergence of new radiation techniques, molecularly targeted therapies, cytotoxic agents, and vaccine approaches, overall outcomes have not changed in recent years. Key to future improvements will be informative design and efficient conduct of clinical trials. The results of our present study demonstrate that clinical investigators, sponsors, and physicians should no longer conclude that a prior cancer diagnosis in patients with locally advanced lung cancer is a poor prognostic factor or a limitation to treatment. These patients should no longer be excluded from clinical trials, and unless other factors preclude it, they should be offered potentially curative therapy.
Supplementary Material
Highlights.
Stringent eligibility criteria represent a major barrier to clinical trial accrual.
The majority of lung cancer clinical trials exclude patients with prior cancer.
15.8% of locally advanced lung cancer patients have a history of prior cancer.
Prior cancer does not adversely impact survival in locally advanced lung cancer.
These patients should be considered for clinical trials and for aggressive therapy.
Acknowledgments
Funding/Acknowledgment:
This work was supported by the National Cancer Institute (NCI) (1R03CA191875-01A1; to DEG, SLP; EAH, LX); NCI Clinical Investigator Team Leadership Award (1P30 CA142543-01 supplement; to DEG); an NCI Midcareer Investigator Award in Patient-Oriented Research (K24CA201543-01; to DEG) Cancer Prevention Research Institute of Texas (CPRIT; R1208; to SLP); the Agency for Healthcare Research and Quality (1R24HS022418-01; to EAH, SLP, LX) and National Center for Advancing Translational Sciences UT Southwestern Center for Translational Medicine (U54 RFA-TR-12-006; to EAH, SLP).
The authors thank Helen Mayo, MLS, from the UT Southwestern Medical Library for assistance performing literature searches. The authors also acknowledge the efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database. Contents of this paper are solely the responsibility of the authors and do not necessarily represent the official view of the NIH.
Footnotes
Author Contributions
A.L. was responsible for literature search, data interpretation and manuscript writing. S.P. contributed to study design, statistical analysis and manuscript writing. L.X. assisted with study design, performed statistical methods, and was involved in manuscript writing. E.H. was involved with study conceptualization, data interpretation, and manuscript writing. D.G. was responsible study conceptualization/design, data interpretation and manuscipt writing.
Conflict of Interest
All authors indicated no potential conflicts of interest.
Author Declaration/Conflict of Interest Statement
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us.
We confirm that we have given due consideration to the protection of intellectual property associated with this work and that there are no impediments to publication, including the timing of publication, with respect to intellectual property. In so doing we confirm that we have followed the regulations of our institutions concerning intellectual property.
We understand that the Corresponding Author is the sole contact for the Editorial process (including Editorial Manager and direct communications with the office). He is responsible for communicating with the other authors about progress, submissions of revisions and final approval of proofs. We confirm that we have provided a current, correct email address which is accessible by the Corresponding Author and which has been configured to accept email from (david.gerber@utsouthwestern.edu)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Iyengar DEGP. Chapter 8: Locally Advanced Non-Small Cell Lung Cancer. In: Gerber AKGDE, editor. Oxford American Oncology Library: Lung Cancer. Oxford University Press; New York, NY: 2013. [Google Scholar]
- 2.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA: a cancer journal for clinicians. 2014;64(1):9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 3.Albain KS, Swann RS, Rusch VW, Turrisi AT, 3rd, Shepherd FA, Smith C, Chen Y, Livingston RB, Feins RH, Gandara DR, Fry WA, Darling G, Johnson DH, Green MR, Miller RC, Ley J, Sause WT, Cox JD. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet. 2009;374(9687):379–86. doi: 10.1016/S0140-6736(09)60737-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belani CP, Choy H, Bonomi P, Scott C, Travis P, Haluschak J, Curran WJ., Jr Combined chemoradiotherapy regimens of paclitaxel and carboplatin for locally advanced non-small-cell lung cancer: a randomized phase II locally advanced multimodality protocol. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2005;23(25):5883–91. doi: 10.1200/JCO.2005.55.405. [DOI] [PubMed] [Google Scholar]
- 5.Bradley JD, Paulus R, Komaki R, Masters GA, Forster K, Schild SE, Bogart J, Garces YI, Narayan S, Kavadi V, Nedzi LA, Michalski JM, Johnson D, MacRae RM, Curran WJ, Choy H. A randomized phase III comparison of standard-dose (60 Gy) versus high-dose (74 Gy) conformal chemoradiotherapy with or without cetuximab for stage III non-small cell lung cancer: Results on radiation dose in RTOG 0617. ASCO Annual Meeting; Chicago, IL. 2013.. [Google Scholar]
- 6.Hanna N, Neubauer M, Yiannoutsos C, McGarry R, Arseneau J, Ansari R, Reynolds C, Govindan R, Melnyk A, Fisher W, Richards D, Bruetman D, Anderson T, Chowhan N, Nattam S, Mantravadi P, Johnson C, Breen T, White A, Einhorn L. Phase III study of cisplatin, etoposide, and concurrent chest radiation with or without consolidation docetaxel in patients with inoperable stage III non-small-cell lung cancer: the Hoosier Oncology Group and U.S. Oncology. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2008;26(35):5755–60. doi: 10.1200/JCO.2008.17.7840. [DOI] [PubMed] [Google Scholar]
- 7.Kelly K, Chansky K, Gaspar LE, Albain KS, Jett J, Ung YC, Lau DH, Crowley JJ, Gandara DR. Phase III trial of maintenance gefitinib or placebo after concurrent chemoradiotherapy and docetaxel consolidation in inoperable stage III non-small-cell lung cancer: SWOG S0023. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2008;26(15):2450–6. doi: 10.1200/JCO.2007.14.4824. [DOI] [PubMed] [Google Scholar]
- 8.Ciardiello F, Tortora G. EGFR antagonists in cancer treatment. The New England journal of medicine. 2008;358(11):1160–74. doi: 10.1056/NEJMra0707704. [DOI] [PubMed] [Google Scholar]
- 9.Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA: the journal of the American Medical Association. 2004;291(22):2720–6. doi: 10.1001/jama.291.22.2720. [DOI] [PubMed] [Google Scholar]
- 10.Lara PN, Jr, Higdon R, Lim N, Kwan K, Tanaka M, Lau DH, Wun T, Welborn J, Meyers FJ, Christensen S, O’Donnell R, Richman C, Scudder SA, Tuscano J, Gandara DR, Lam KS. Prospective evaluation of cancer clinical trial accrual patterns: identifying potential barriers to enrollment. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2001;19(6):1728–33. doi: 10.1200/JCO.2001.19.6.1728. [DOI] [PubMed] [Google Scholar]
- 11.Denicoff AM, McCaskill-Stevens W, Grubbs SS, Bruinooge SS, Comis RL, Devine P, Dilts DM, Duff ME, Ford JG, Joffe S, Schapira L, Weinfurt KP, Michaels M, Raghavan D, Richmond ES, Zon R, Albrecht TL, Bookman MA, Dowlati A, Enos RA, Fouad MN, Good M, Hicks WJ, Loehrer PJ, Sr, Lyss AP, Wolff SN, Wujcik DM, Meropol NJ. The national cancer institute-american society of clinical oncology cancer trial accrual symposium: summary and recommendations. Journal of oncology practice/American Society of Clinical Oncology. 2013;9(6):267–76. doi: 10.1200/JOP.2013.001119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fuks A, Weijer C, Freedman B, Shapiro S, Skrutkowska M, Riaz A. A study in contrasts: eligibility criteria in a twenty-year sample of NSABP and POG clinical trials. National Surgical Adjuvant Breast and Bowel Program. Pediatric Oncology Group. Journal of clinical epidemiology. 1998;51(2):69–79. doi: 10.1016/s0895-4356(97)00240-0. [DOI] [PubMed] [Google Scholar]
- 13.Gerber DE, Laccetti AL, Xuan L, Halm EA, Pruitt SL. Impact of prior cancer on eligibility for lung cancer clinical trials. Journal of the National Cancer Institute. 2014;106(11) doi: 10.1093/jnci/dju302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aguilo R, Macia F, Porta M, Casamitjana M, Minguella J, Novoa AM. Multiple independent primary cancers do not adversely affect survival of the lung cancer patient. Eur J Cardiothorac Surg. 2008;34(5):1075–80. doi: 10.1016/j.ejcts.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 15.Duchateau CS, Stokkel MP. Second primary tumors involving non-small cell lung cancer: prevalence and its influence on survival. Chest. 2005;127(4):1152–8. doi: 10.1378/chest.127.4.1152. [DOI] [PubMed] [Google Scholar]
- 16.Lopez-Encuentra A, Gomez de la Camara A, Rami-Porta R, Duque-Medina JL, de Nicolas JL, Sayas JP. Bronchogenic Carcinoma Cooperative Group of the Spanish Society of, S. Thoracic, Previous tumour as a prognostic factor in stage I non-small cell lung cancer. Thorax. 2007;62(5):386–90. doi: 10.1136/thx.2005.051615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu YY, Chen YM, Yen SH, Tsai CM, Perng RP. Multiple primary malignancies involving lung cancer-clinical characteristics and prognosis. Lung Cancer. 2002;35(2):189–94. doi: 10.1016/s0169-5002(01)00408-1. [DOI] [PubMed] [Google Scholar]
- 18.Massard G, Ducrocq X, Beaufigeau M, Elia S, Kessler R, Herve J, Wihlm J. Lung cancer following previous extrapulmonary malignancy. Eur J Cardiothorac Surg. 2000;18(5):524–8. doi: 10.1016/s1010-7940(00)00571-6. [DOI] [PubMed] [Google Scholar]
- 19.Laccetti AL, Pruitt SL, Xuan L, Halm EA, Gerber DE. Effect of prior cancer on outcomes in advanced lung cancer: implications for clinical trial eligibility and accrual. Journal of the National Cancer Institute. 2015;107(4) doi: 10.1093/jnci/djv002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER- Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40(8 Suppl):IV-3-18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 21.Altekruse SF, Kosary CL, Krapcho M, Neyman N, Aminou R, Waldron W, Ruhl J, Howlader N, Tatalovich Z, Cho H, Mariotto A, Eisner MP, Lewis DR, Cronin K, Chen HS, Feuer EJ, Stinchcomb DG, Edwards BKe. SEER Cancer Statistics Review, 1975–2007. National Cancer Institute; Bethesda, MD: 2010. http://seer.cancer.gov/csr/1975_2007/, based on November 2009 SEER data submission, posted to the SEER web site. [Google Scholar]
- 22.Adamo MDL, Ruhl J. [accessed July 10.2015];National Cancer Institute SEER Coding and Staging Manual. 2015 < http://seer.cancer.gov/manuals/2015/SPCSM_2015_maindoc.pdf>.
- 23.Coyte A, Morrison DS, McLoone P. Second primary cancer risk - the impact of different definitions of multiple primaries: results from a retrospective population-based cancer registry study. BMC cancer. 2014;14:272. doi: 10.1186/1471-2407-14-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, Lilenbaum R, Johnson DH. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. The New England journal of medicine. 2006;355(24):2542–50. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 25.Ahn DH, Mehta N, Yorio JT, Xie Y, Yan J, Gerber DE. Influence of Medical Comorbidities on the Presentation and Outcomes of Stage I–III Non-Small-Cell Lung Cancer. Clinical lung cancer. 2013 doi: 10.1016/j.cllc.2013.06.009. [DOI] [PMC free article] [PubMed]
- 26.Warren JL, Yabroff KR, Meekins A, Topor M, Lamont EB, Brown ML. Evaluation of trends in the cost of initial cancer treatment. Journal of the National Cancer Institute. 2008;100(12):888–97. doi: 10.1093/jnci/djn175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 28.National Cancer Institute. [accessed Accessed on: 7-18-2011];SEER-Medicare: Calculation of comorbidity weights. < http://healthservices.cancer.gov/seermedicare/program/comorbidity.html>.
- 29.Koroukian SM, Dahman B, Copeland G, Bradley CJ. The utility of the state buy-in variable in the Medicare denominator file to identify dually eligible Medicare-Medicaid beneficiaries: a validation study. Health services research. 2010;45(1):265–82. doi: 10.1111/j.1475-6773.2009.01051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horn L, Keedy VL, Campbell N, Garcia G, Hayes A, Spencer B, Carbone DP, Sandler A, Johnson DH. Identifying barriers associated with enrollment of patients with lung cancer into clinical trials. Clinical lung cancer. 2013;14(1):14–8. doi: 10.1016/j.cllc.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 31.Levi F, Randimbison L, Rafael BM, Manuela MC, Vecchia CL. Second primary cancers in the Vaud and Neuchatel Cancer Registries. European journal of cancer prevention: the official journal of the European Cancer Prevention Organisation (ECP) 2014 doi: 10.1097/CEJ.0000000000000085. [DOI] [PubMed] [Google Scholar]
- 32.de Moor JS, Mariotto AB, Parry C, Alfano CM, Padgett L, Kent EE, Forsythe L, Scoppa S, Hachey M, Rowland JH. Cancer survivors in the United States: prevalence across the survivorship trajectory and implications for care, Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research cosponsored by the American Society of Preventive. Oncology. 2013;22(4):561–70. doi: 10.1158/1055-9965.EPI-12-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moyer VA. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Annals of internal medicine. 2012;157(2):120–34. doi: 10.7326/0003-4819-157-2-201207170-00459. [DOI] [PubMed] [Google Scholar]
- 34.Sankila R, Hakulinen T. Survival of patients with colorectal carcinoma: effect of prior breast cancer. Journal of the National Cancer Institute. 1998;90(1):63–5. doi: 10.1093/jnci/90.1.63. [DOI] [PubMed] [Google Scholar]
- 35.Bunn PA, Jr, Lilenbaum R. Chemotherapy for elderly patients with advanced non-small-cell lung cancer. Journal of the National Cancer Institute. 2003;95(5):341–3. doi: 10.1093/jnci/95.5.341. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.