Abstract
Genistein and curcumin are major components of Asian foods, soybean and curry turmeric respectively. These compounds have been intensively investigated for their chemical and biological features conferring their anti-cancer activity. Genistein and curcumin have also been investigated for their potentiation effects on disease-associated CFTR mutants such as ΔF508 and G551D.
Recently, we investigated the combined effect of genistein and curcumin on G551D-CFTR, which exhibits gating defects without abnormalities in protein synthesis or trafficking using the patch-clamp technique. We found that genistein and curcumin showed additive effects on their potentiation of G551D-CFTR in high concentration range and also, more importantly, showed a significant synergistic effect in their minimum concentration ranges. These results are consistent with the idea that multiple mechanisms are involved in the action of these CFTR potentiators.
In this review, we revisit the pharmacology of genistein and curcumin on CFTR and also propose new pharmaceutical implications of combined use of these compounds in the development of drugs for CF pharmacotherapy.
Keywords: Additive effect, synergistic effect, G551D
INTRODUCTION
The Cystic fibrosis transmembrane conductance regulator (CFTR) chloride channel plays an essential role in salt and water transport across epithelia and mutations of CFTR causing its dysfunction result in the autosomal recessive fatal genetic disease cystic fibrosis (CF) [1, 2]. The symptoms of CF, such as high sweat Cl− concentration, exocrine pancreatic insufficiency, male infertility, and airway disease [3], are secondary to the absence of a cAMP-stimulated anion conductance in transport epithelia including sweat glands, pancreatic ducts, epididymal epithelia and airway epithelia [4–8].
More than 1500 disease-associated CFTR mutations have been identified. Among them, the ΔF508 mutation, the most common one, causes not only impaired CFTR protein maturation but also, like the third most common G551D mutation, exhibits gating defects (www.genet.sickkids.on.ca/cftr, also see below) [3]. Thus, pharmacological agents (so-called CFTR potentiators) that can potentiate the channel activity of the mutant CFTRs are of potential clinical value in the therapy of CF.
Various pharmacological approaches are currently being explored to develop new reagents for the treatment of CF [9]. Recent advances in high throughput screening (HTS) [10, 11] enable us to test the effects of a huge number of compounds on mutated CFTRs. This technology has successfully discovered novel candidate compounds for CFTR modulation including a potentiator Kalydeco (VX-770) [12] and a corrector VX-809 [13]. Clinical trials have confirmed the effectiveness of Kalydeco in patients [14, 15] and now Kalydeco is approved by the Food and Drug Administration (FDA) as the first and only CFTR potentiator available for the treatment of CF patients with G551D mutation (Vertex Pharmaceuticals Incorporated, Cambridge, MA). However this does NOT mean that the research for the mechanism of the previous compounds is useless and unnecessary because they could be important for further improvement of the CF pharmacotherapy and discovery of a new category of the drugs.
In this review, we revisit the well-studied CFTR modulator genistein and a unique allosteric CFTR modulator, curcumin. Both compounds have a wide spectrum of targets and effects. We also consider their combined effects, both additive and synergistic, on the gating of G551D-CFTR.
STRUCTURE AND GATING MECHANISM OF CFTR
CFTR is a member of ABC transporter superfamily [2]. The basic structure of CFTR consists two tandem repeats of a membrane spanning domain (MSD) and a nucleotide binding domain (NBD) with a regulatory domain (RD) unique to CFTR sandwiched between the two MSD-NBD repeats (Fig. 1A). The NBD1 and NBD2 form an ATP-driven gating engine that is common among all ABC transporter super family members. On the other hand, the structure of MSDs is widely varied among different ABC transporters and confers their specific functions. In the case of CFTR, two MSDs (MSD1 and MSD2) form an anion conducting pore [2]. Four intracellular loops (ICL1 – 4) connect MSDs and NBDs presumably in domain swapping formation [16–18] and transmit the mechanical force generated in NBDs to MSDs.
Fig. (1). Basic structures of Cystic Fibrosis Transmembrane conductance Regulator (CFTR).
(A) Membrane topology of CFTR. MSD: membrane spanning domain; NBD: nucleotide binding domain; RD: regulatory domain. (B) Schematic presentation of structure of two NBDs in CFTR. The 551st glycine in the NBD1 signature sequence is marked by square. Note that the ABC signature sequence in NBD2 is different from consensus.
Each NBD contains the prototypical Walker A and Walker B motifs in the ”head’ subdomain and the ABC signature sequence in the “tail” subdomain. In a canonical “head-to-tail” NBD dimer, two ATP binding pockets (ABP1 and ABP2) with the Walker A/B motif in one NBD and the ABC signature sequence in another NBD are formed (Fig. 1B). In CFTR, two NBDs have different amino acid sequences and different functions (Fig. 1B) [2]. Whereas the signature sequence in NBD1 forming ABP2 is well conserved (LSGGQ), the signature sequence in NBD2 forming ABP1 (LSHGH) is different from the consensus sequence (Fig. 1B). Residue E1371 is thought to provide the catalytic base for hydrolyzing ATP bound to ABP2. On the other hand, in ABP1, the amino acid residue at equivalent position 573 is a serine (Fig. 1B) and in fact a number of experimental results [19–21] suggest that ATP bound at ABP1 is not hydrolyzed during the gating cycle. In addition, W401 and Y1219 support stable binding of ATP to ABP1 and ABP2 respectively by a π–π stacking between their aromatic rings and the adenine ring of ATP [22] and mutations in W401 and Y1219 induced a defect in the ATP-dependent gating [23].
RD underlies the most significant physiological regulation of CFTR activity by the cAMP – protein kinase A (PKA) pathway [24]. Biochemical [25] and NMR [26] studies supported an idea that unphosphorylated RD obstructed the NBD1–NBD2 dimerization and PKA phosphorylation of the RD relieved its obstructive effect for the NBD dimerization which initiated the ATP-dependent gating (Fig. 2). However it was recently reported that RD might work through an allosteric regulatory mechanism independent from the ATP-induced NBD dimerization [27]
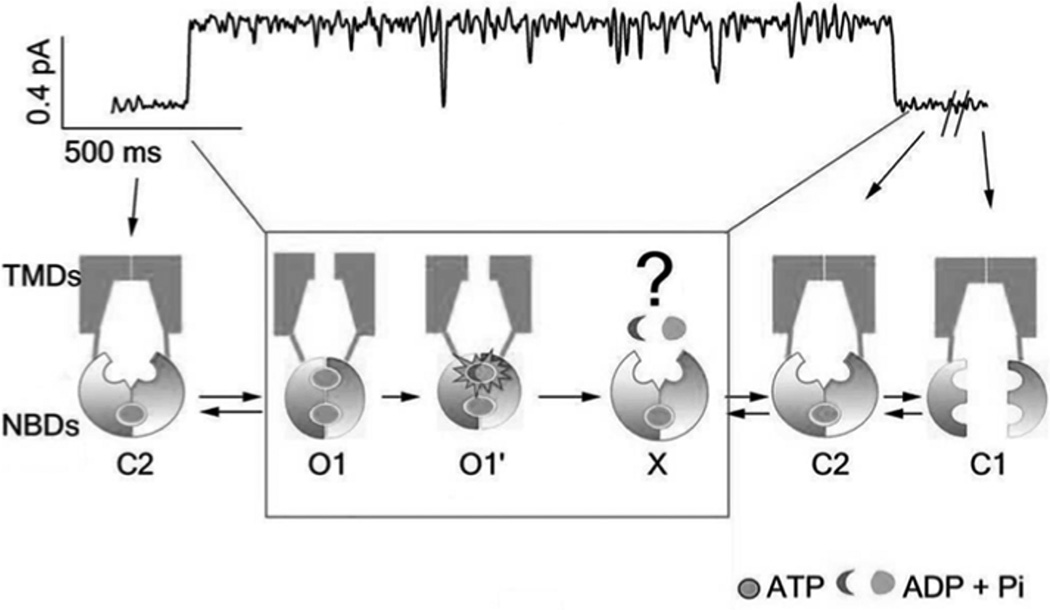
Fig. (2). Mechanism of ATP-dependent gating of CFTR channel.
The latest version of consensus model for the ATP-dependent gating of CFTR channel based on the NBD dimerization hypothesis [29, 30]. The trace on top represents an opening burst of WT-CFTR. Note the MSD conformation (open or closed) in the state X is not identified whereas it is likely to be open [29, 30].
Opening of the CFTR chloride channel is associated with the dimerization of its two NBDs in a head-to-tail configuration following ATP binding to the two ABPs (Fig. 2). ATP hydrolysis initiates the separation of the NBD dimer and thereby closes the CFTR channel pore (Fig. 2) [28], (reviewed by [19]). Recent studies [20, 21] suggest that ATP hydrolysis occurs only for the ATP bound in ABP2 and the hydrolysis induces the separation of the NBD dimer limited to the vicinity of ABP2 (partial dimer). The partial dimer is very stable (τ: ~ 50 s) [20] and two NBDs are thought to transit between the state of a full dimer and that of a partial dimer in the physiological ATP concentration range (Fig. 2). The channel pore closes following the partial separation of the NBD dimer (Fig. 2). A novel state X with the partial NBD dimer has been identified between the ATP hydrolysis (O1’) and the closed state with the partial NBD dimer (C2). The state X seemed to be an open state, which suggested a nonintegral stoichiometry between the gating cycle and ATP consumption in CFTR channel [29, 30].
Because the activation of CFTR channel (pore opening) should follow NBD dimerization and stabilizing the full NBD dimer lengthens the open time duration (Fig. 2), the NBD has been an important target for the CFTR poteniators.
CFTR MUTATIONS
Now CF-associated mutations (www.genet.sickkids.on.ca/cftr) are categorized into five classes I – V according to the mechanisms by which mutations disrupt CFTR function [3, 31].
-
Class I: defective protein production (e.g., W1282X)
Class I mutations fail to produce full-length CFTR protein because of nonsense mutations that introduce stop codons into the CFTR gene.
-
Class II: defective protein processing (e.g., ΔF508)
In normal, CFTR is synthesized in the endoplasmic reticulum (ER), transported to the Golgi for maturation, and delivered to the apical membrane. However Class II mutations cause a misfolding of CFTR protein, and the misfolded proteins are retained in the ER and subsequently degraded by the proteasome.
-
Class III: defective channel regulation (e.g., G551D)
CFTR channel is tightly-regulated by (i) RD phosphorylation and (ii) cycles of ATP-binding and hydrolysis at the NBDs (Fig. 2). Class III mutations cause a loss of CFTR channel function by disrupting the channel regulation.
The mutant proteins of this class are those that appear to be fully processed and correctly located in the apical membrane but are unable to function as chloride channels.
-
Class IV: defective channel conduction (e.g., R347P)
The MSDs forms the channel pore. Some CF mutants in the MSDs impair the rate of Cl− flow through open channels, whereas the regulation by PKA-dependent phosphorylation and intracellular ATP appears to be normal.
-
Class V: reduced synthesis (e.g., A455E, P574H)
Class V mutants reduce the synthesis and trafficking of the CFTR protein, not as severely as the levels of class I and II mutants, by promoter mutations reducing CFTR gene transcription and mutations inducing alternative splicing variants [31].
The glycine-to-aspartate missense mutation at position 551 (G551D) we focus on in this review is the third most common CF-associated mutation categorized into the class III with a worldwide frequency of 3.1 % [3]. The G551 residue is located in the ABC transporter signature sequence in NBD1 which forms ABP2 with the Walker A/B motif in NBD2 (Fig. 1B). G551D-CFTR protein can be normally trafficked to the plasma membrane and is phosphorylated by cAMP-dependent PKA [3, 32–34] but it exhibits defective gating (shows a very low open probability) [7, 34–36].
DRUGS
Numerous compounds of diverse chemical structures have been reported to increase CFTR activity, for example, isoflavones, e.g., genistein [37, 38], flavones, e.g., apigenin [37], capsaicin [39], phenylglycines, e.g., PG-01 [40], sulfonamides, e.g., SF-01 [40], 1,4-dihydropyridines [40], benzoflavones, e.g., UCCF-029 [41], benzimidazolones, e.g., NS004 [42] and UCCF-853 [43], pyrrolo [2,3-b]pyrazines derivatives, e.g., RP107 [44], benzo[c]quinolizinium derivatives, e.g., MPB-91 [45], and curcumin [46, 47]. Recently Ivacaftor (VX-770, Kalydeco) has been developed [12]. Although these CFTR potentiators potentiate mutant CFTR channels with low open probability including G551D-CFTR, few of them can completely rescue the defective channels (reviewed by [9, 10]). Also the detailed mechanism of these CFTR potentiators including VX-770 is still unclear.
GENISTEIN AND ITS EFFECTS ON CFTR
Genistein (4,5,7-trihydroxyisoflavone) (Fig. 3A) has been recognized as one of the predominant isoflavones found in legumes [48]. Genistein has a similar structure to the potent estrogen 17-β-estradiol, especially the phenolic rings and the distance between its 4’- and 7-hydroxyl groups and also to testosterone (Fig. 3B). These structural similarities likely explain genistein’s estrogenic or androgenic activity by binding to estrogen or androgen receptors and sex hormone binding proteins. Alternatively, genistein may have an anti-estrogenic or anti-androgenic activity by competing with estradiol or testosterone for their respective receptor. Thus, genistein could potentially affect homeostasis of estrogens and androgens in target cells such as breast cancer cells and prostate cancer cells.
Fig. (3). Structures of (A) genistein and (B) 17-Δ-estradiol and testosterone, sex hormones that genistein might cross-react.
Genistein and 17-β-estradiol share structural features of the phenolic ring and the 11.5 Å distance between its 4’- and 7-hydroxyl groups [48].
Genistein may inhibit human cancer cell growth through the modulation of genes related to the control of cell cycle and apoptosis (e.g. nuclear factor (NF) - κB and protein kinase B/Akt). Genistein antagonizes estrogen- and androgen-mediated signaling pathways during carcinogenesis. It also has antioxidant capacity and inhibits angiogenesis and metastasis. Taken together, these properties identify genistein as a promising agent for cancer chemoprevention and cancer therapy. Unlike other isoflavonoids, the minimum effective concentration of each of its effect is much lower than the maximum toxic concentration, providing a great advantage for dietary cancer chemoprevention
Genistein has been studied most intensively as a CFTR potentiator for more than a decade [49–53]. Since genistein could increase CFTR currents in excised patches, genistein’s target is thought to be the CFTR molecule itself [54–57]. This compound clearly affects the ATP-dependent gating of CFTR channels by increasing the opening rate and decreasing the closing rate (Fig. 4A). This observation suggested that the drug acts at NBDs (Figs. 1 and 2) [39, 58–60]. On the other hand, genistein is also suggested to act by altering lipid bilayer mechanics [61]. Thus the detail mechanism underlying genistein’s effects is still unclear.
Fig. (4). Effects of genistein on CFTR channel.
(A) Representative single channel currents obtained from (a) WT- and (b) ΔF508-CFTR channels in cell-attached membrane patches. The bath and pipette solutions contained 154 µM Cl−. The membrane potential was clamped at +50 mV and downward deflections of the trace represent channel openings. See [77] for more detail. Reproduced from [77] with permission.
(B) A molecular model showing the possible interactions between genistein and NBD1–NBD2 heterodimer of human CFTR at the five putative binding sites [62]. The genistein molecules are shown in ball-and-stick mode and the residues important for interacting with genistein are indicated. The five model-predicted potential genistein binding sites are superimposed (dashed circles). See [62] for more detail. Modified from [62] with permission.
Genistein potentiates the channel activity of CFTR in a low concentration range but inhibits it in a higher concentration range [56, 59]. The inhibitory effect of genistein is due to two different mechanisms: one is a direct interaction with NBD1 by competing with ATP and the other a direct pore blocking from the cytoplasmic side [59].
Genistein potentiates the gating of G551D- and ΔF508-CFTR as well as WT-CFTR [50, 52]. Genistein-induced activation requires a prior phosphorylation of RD [52]. This suggests that genistein might affect the CFTR channel activity at a point downstream from the RD phosphorylation, perhaps during ATP binding and/or hydrolysis in NBDs. For the molecular mechanism of the potentiation effect, Ai et al [39] proposed that genistein as well as other CFTR potentiators might enhance CFTR channel gating by affecting NBD dimerization. That is, genistein binding at the interface of the NBD dimer accelerates channel opening by lowering the free energy of the transition state and decreases the closing rate by stabilizing the NBD dimer conformation [39].
A recent molecular modeling study [62] predicted five possible genistein binding sites in NBDs (Fig. 4B). Among them, sites 1a and 1b were located close to residue W401 and Y1219 important for stable biding of ATP to ABP1 and ABP2. Sites 2a and 2b were located almost symmetrically on NBD1 and NBD2, but not at the dimer interface. Site 3 was located in the middle of NBD1–NBD2 interface. Sites 1a and 1b are suitable for explaining the experimental results that genistein inhibits CFTR competitively with ATP in the higher concentration range [56, 57, 59]. On the other hand, sites 2a, 2b and 3 are candidates for the potentiation binding site because (i) they involve multiple aromatic amino acids residues possibly provide a higher binding affinity for genistein and (ii) they are physically close to the highly conserved glutamine residue in the Q-loop of each NBD (Q493 for NBD1 and Q1291 for NBD2), which interacts with the ABC signature motif, thus accounting for the effects of genistein seen in the CF associated G551D and G1349D mutations in the signature motif [54, 55, 63]. For site 3, genistein can bind to the NBD dimer interface and directly interact with F494 of NBD1 and F1294 and H1348 of NBD2 to stabilize the NBD dimer. On the other hand,, in sites 2a and 2b, genistein may also stabilize the NBD dimer by interacting with the interface residues F494 and F1294 or by inducing NBDs’ conformational changes. Further studies are required to verify these predictions.
CURCUMIN AND ITS EFFECTS ON CFTR
Curcuminoids, the main components in Asian spice turmeric, a yellow compound isolated from Curcuma longa. The major constituents of Curcuma longa include curcumin (Fig. 5A), demethoxycurcumin and bisdemethoxycurcumin (Fig. 5B). They share a common unsaturated alkyl-linked biphenyl structural feature (Fig. 5) responsible for their major pharmacological effects. Also curcumin has two hydrophobic phenyl domains connected by a flexible linker (Fig. 5) that allows curcumin to adopt many different conformations suitable for maximizing hydrophobic contacts with the protein. Most natural anti-oxidants can be classified into two types of compounds involving phenolic and β-diketone moiety [64]. The unique structure of curcumin that two phenols are connected with an enol form of a β-diketone (Fig. 5A) might underlie its strong anti-oxidant activity leading to the anti-inflammatory properties [65]. In addition, curcumin inhibits the metabolism of arachidonic acid, activities of lipoxygenase, cyclooxygenase, cytokines (TNF-α, IL-1β and IL-6) and NF-κB, and also release of steroids [65]. These biological properties may account for curcumin’s wide medical effects such as wound-healing [66], antiviral, anti-HIV [67], anti-amyloidogenic [68] and anti-cancer [69] effects [70].
Fig. (5). Structures of (A) curcumin and (B) major curcumin analogues.
Note that curcumin is tautomeric keto-enol mixture whereas the enol form is predominant. Commercial available “curcumin” compound contains approximately 77% curcumin, 17% demethoxycurcumin and 3% bisdemethoxycurcumin [70].
It has been suggested that such widely varied effects are conferred through direct bindings to other macromolecules by the α, β-unsaturated β-diketone moiety with keto–enol tautomerism, carbonyl and enolic groups of the β-diketone moiety, methoxy and phenolic hydroxyl groups, and the phenyl rings [65, 71] (Fig. 5).
For CFTR, Egan et al. [58] reported that oral administration of curcumin to ΔF508-CFTR mice could correct its trafficking defects although these results remain controversial (e.g., [72]). Importantly, curcumin was reported to potentiate wild type (WT)-,ΔF508- [46] and G551D-CFTR [73].
Kirk and his colleagues [47, 73] reported that curcumin has two major effects on CFTR: increasing ATP-independent activation of CFTR and promoting oligomer formation of CFTR molecules. Curcumin activates G551D-CFTR mutant (Fig. 6A-a) as well as WT-CFTR even in the absence of ATP [73]. Surprisingly curcumin could activate Δ1198-or W1282-CFTR whose NBD2 is deleted in addition to the absence of ATP (Fig. 6A-b), that is, the curcumin-induced activation does not require the heterodimerization of the two NBDs [73]. This observation indicates that curcumin can affect CFTR gating through a mechanism independent of NBDs, CFTR’s gating machinery. However, it is also worth to note that the curcumin- indicted activation still depended on a prior phosphorylation of RD by protein kinase A, consistent with the notion that the RD plays a permissive role in controlling CFTR function.
Fig. (6). Effects of curcumin on CFTR channel.
(A) Effects of curcumin on channel function of CFTR. Macroscopic currents obtained from insideout excised patches expressing (a) G551D- and (b) Δ1198-CFTR channels. Membrane potential was clamped to a ramp waveform from −80 to +80 mV. The channels were initially phosphorylated by ATP and PKA (control condition). See [73] for more detail. Curcumin strongly potentiated (a) G551DCFTR and also (b) Δ1198-CFTR lacking NBD2 in the absence of ATP. The inset of (b) shows the dose-response cure of the curcumin effects. Remade from [73] with permission.
(B) Curcumin-induced cross-linking of CFTR molecules. Upper panel: Structures of (synthetic) curcumin and BSc3596, a curcumin analog that cannot cross-link CFTRs by being β-diketone reactive group cyclized. (a) SDS-PAGE of Δ1198-CFTR in microsome after 30 min incubation with 30 µM curcumin, synthetic curcumin and BSc3596. (b) SDS-PAGE of WT- and G551D-CFTR after 30 min incubation with 30 µM curcumin or BSc3596 or with 1mM DSS or DMSO only (vehicle). (c) Macroscopic inside-out currents showing a β-diketone-cyclized curcumin analog BSc3596 can strongly potentiate Δ1198-CFTR without cross-linking. See [47] for more detail. Reproduced from [47] with permission.
Curcumin-induced activation of G551D-CFTR and the NBD2 deletion constructs that originally had very low opening rate was due to an increase of the opening rates. Prolonged exposure of curcumin induced an irreversible activation with robust activity whereas its short exposure induced a reversible activation [73], suggesting that curcumin might induce an irreversible conformational change of the CFTR molecules.
Kirk et al. [47] also found that curcumin cross-linked CFTR molecules including ΔF505, G551D and Δ1198-CFTR into SDS-resistant oligomers (Fig. 6B-b). Curcumin-induced CFTR cross-linking might depend on its α, β-unsaturated β-diketone moiety because removal of this moiety by cyclization (e.g, Bsc3596) eliminated the cross-linking effect (Fig. 6B-a). However, importantly, the cyclic derivatives could still activate WT-, G551D- and Δ1198-CFTR (Fig. 6B-c), suggesting that the activation mechanism by curcumin is not caused by cross-linking. It is interesting to note that the majority of the curcumin-induced CFTR oligomers seemed to be in a dimeric form (Fig. 6B), consistent with the results that the purified CFTR proteins can be in a dimeric form based on single particle analysis as well as native PAGE electrophoresis [74, 75].
ADDITIVE AND SYNERGISTIC: COMBINED EFFECTS OF GENISTEIN AND CURCUMIN ON G551D-CFTR
In this section, we discuss the combined effects of genistein and curcumin we recently reported [38]. (Fig. 7) shows the effects of genistein and curcumin on G511D-CFTR using the whole-cell clamping technique. The whole cell currents obtained from CHO cells overexpressing G551D-CFTR channels were recorded at 0 mV membrane potential with a regular ramp waveform from −100 to +100 mV. In this condition, the peak-to-peak difference in the whole-cell current traces reflected the CFTR channel activity (see [38] for more detail). Both genistein and curcumin significantly increased G551D-CFTR whole-cell currents activated by the cAMP-PKA stimulation. However the time-courses of the potentiation effect was different between them, that is, genistein acted faster than curcumin in both the on and off processes (Figs. 7AB).
Fig. (7). Effects of genistein or curcumin in sole administration on whole-cell currents of G551D-CFTR channels expressed in CHO cells.
Representative traces for G551D-CFTR whole-cell currents affected by (A) 80 µM genistein and (B) 30 µM curcumin.
(C) The dose response relationship for genistein (●) (n = 11) and curcumin (■) (n = 12). Error bars represent SEM. The fold-increase in the whole cell current (IWC) was calculated by dividing the mean current at 100 mV in the presence of genistein or curcumin by the mean current in control after the leak current subtraction. Reproduced from [38] with a modification.
Genistein showed a biphasic dose-response relationship with an attenuation of the potentiation at a high concentration range, but curcumin showed no inhibitory effect over the range of concentrations tested (Fig. 7C). Curcumin potentiated G551D-CFTR almost reversibly in this experimental condition (Fig. 7B) in contrast to the irreversible activation with a prolonged application reported previously [73]. The potentiation effect by curcumin was smaller than that by genistein up to 60 µM, the highest soluble concentration of curcumin (Fig. 7C).
Importantly, curcumin and genistein showed an additive potentiation effect on G551D-CFTR channels, that is, curcumin further increased G551D-CFTR channel activity maximally potentiated by genistein (Figs. 8A-a and 8B) [38]. These observations suggest that genistein and curcumin affect G551D-CFTR through different mechanisms. This result is consistent with previous studies for the mechanism of actions of genistein and curcumin on CFTR (see above). It is worthy to note that Berger et al. [46] demonstrated that addition of increasing concentrations of curcumin in the presence of genistein “inhibited” WT-CFTR Cl- currents. This suggest that the combined effect of curcumin and genistein strongly depends on the ATP-dependent gating mechanism in the CFTR channel.
Fig. (8). Additive and Synergistic effects of curcumin and genistein on G551D-CFTR whole-cell current.
(A) Representative traces for G551D-CFTR whole-cell currents affected by (a) 80 µM genistein without and with 30 µM curcumin (additive effect) and (b) 10 µM genistein without and with 10 µM curcumin (synergistic effect). G551D-CFTR whole-cell currents were stimulated by 10 µM FSK + 100 µM CPT-cAMP. Membrane potential was clamped to the ramp waveform shown in the inset. (a) Reproduced from [38] with a modification. See [38] for more detail.
(B) Normalized current densities of G551D-CFTR expressed in CHO cells obtained from whole cell currents potentiated by various combinations of curcumin and genistein are indicated. Normalized to mean of the whole-cell current density from WT-CFTR expressed in CHO cells. Error bars represent SEM. Reproduced from [38] with a modification. See [38] for more detail.
More importantly, a combined application of genistein and curcumin in relatively low concentrations produced a significant synergistic effect (Figs. 8A-b and 8B) [38]. Sole application of 10 µM genistein or 5 µM curcumin could potentiate G551D-CFTR channels only by ~ 2–3 fold (Fig. 8B), however, the combined application of 10 µM genistein and 5 µM curcumin produced a much larger (~30-fold) increase in G551D-CFTR currents. This potentiation effect was similar to that obtained with much higher doses of the two compounds (Fig. 7).
Although the mechanisms of the CFTR potentiators described above are still unclear, we can get some insight into the relationship between mechanisms of these two potentiators from the response of CFTR channel activity to the drugs when they are applied together. If CFTR channel maximally potentiated by one drug is further potentiated by another drug (additive effect), it suggested that the two drugs work through different binding site. For example, UCCF-029 and UCCF-853 have been reported to show an additive effect and suggested that they work through different binding sites [43]. No additive effect of two drugs suggests that they might share a common mechanism of action or binding site. Actually, genistein and NS004 [42], genistein and UCCF-029 [41], or genistein and capsaicin [39] have been reported to be the case.
It is worth to note that the amount of potentiation effects of curcumin and genistein were quantitatively different between whole-cell and cell-attached configurations [38]. The dialysis of the intracellular milieu in whole-cell configuration might be one of the most important factors underlying this discrepancy. Also one can suggest differences in the side of access, cytoplasmic versus extracellular, of the drugs, phosphorylation level of RD [56, 59] and ATP concentrations in cytoplasmic side. These might become clues to further elucidate the mechanisms of the CFTR potentiators including curcumin and genistein.
CLINICAL IMPLICATIONS
Recently Accurso et al [14] reported that in human subjects the CFTR potentiator VX-770 which can rescue ~30 – 40 % of the G551D gating defect [12] was associated with within-subject improvements in CFTR and lung function. There is no doubt that this is a very significant milestone in cystic fibrosis drug research. However, except for VX-770, still few of the known CFTR potentiators seem effective enough to restore the activity (Po) of mutants associated with severe CF [9].
Previously, Zegarra-Moran et al [76] reported that 200 µM genistein rescued the gating defect of G551D-CFTR up to 20% of the WT-CFTR level. However, this kind of concentration is too high for a usual blood concentration of drugs. Importantly, low concentration of curcumin showed a synergistic effect of restoring the gating defect of G551D-CFTR up to ~ 50 % of the WT-CFTR level when applied to the channels minimally potentiated by low concentration of genistein (Fig. 8B) [9]. Thus, even if each compound by itself cannot potentiate the channel activity of the CFTR mutants to a level beneficial to CF patients, a combination of the two compounds might succeed in activating the mutant CFTR channel up to a therapeutic level. Furthermore, lowering the dose of each reagent may minimize potential harmful side-effects.
In January 2012, Kalydeco (VX-770) was approved by the FDA as the first and only CFTR potentiator used for the treatment of CF patients age 6 years or older who carry the G551D mutation (Vertex Pharmaceuticals). Now (November 2012) clinical trial of combined application of Kalydeco and VX-809 for patients with at least one copy of the ΔF508 mutation of CF is being performed. The second part of a Phase 2 clinical trial reported that people who received the combination treatment showed improvements in lung function by 5–10 % or more compared with placebos with no serious adverse events (Vertex Pharmaceuticals). Fruitful results from more investigations for the combined effects of multiple CFTR modulators are expected.
Acknowledgments
We thank our laboratory colleagues for valuable discussions. During the preparation of this review and some novel data, Y. Sohma was supported by KAKENHI (JSPS22590212 and MEXT23118714) and Keio Gijuku Academic Development Funds, and T.-C. Hwang by the National Institutes of Health (grant R01DK55835) and a grant (Hwang11P0) from the Cystic Fibrosis Foundation.
Footnotes
CONFLICT OF INTEREST
The authors confirm that this article content has no conflicts of interest.
REFERENCES
- 1.Gadsby DC, Vergani P, Csanady L. The ABC protein turned chloride channel whose failure causes cystic fibrosis. Nature. 2006;440:477–483. doi: 10.1038/nature04712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Riordan JR, Rommens JM, Kerem B, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245:1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- 3.Welsh MJ, Smith AE. Molecular mechanisms of CFTR chloride channel dysfunction in cystic fibrosis. Cell. 1993;73:1251–1254. doi: 10.1016/0092-8674(93)90353-r. [DOI] [PubMed] [Google Scholar]
- 4.Gray MA, Greenwell JR, Argent BE. Secretin-regulated chloride channel on the apical plasma membrane of pancreatic duct cells. J Membr Biol. 1988;105:131–142. doi: 10.1007/BF02009166. [DOI] [PubMed] [Google Scholar]
- 5.Knowles MR, Stutts MJ, Spock A, Fischer N, Gatzy JT, Boucher RC. Abnormal ion permeation through cystic fibrosis respiratory epithelium. Science. 1983;221:1067–1070. doi: 10.1126/science.6308769. [DOI] [PubMed] [Google Scholar]
- 6.Quinton PM. Missing Cl conductance in cystic fibrosis. Am J Physiol. 1986;251:C649–C652. doi: 10.1152/ajpcell.1986.251.4.C649. [DOI] [PubMed] [Google Scholar]
- 7.Welsh MJ, Liedtke CM. Chloride and potassium channels in cystic fibrosis airway epithelia. Nature. 1986;322:467–470. doi: 10.1038/322467a0. [DOI] [PubMed] [Google Scholar]
- 8.Wong PY. CFTR gene and male fertility. Mol Hum Reprod. 1998;4:107–110. doi: 10.1093/molehr/4.2.107. [DOI] [PubMed] [Google Scholar]
- 9.Amaral MD, Kunzelmann K. Molecular targeting of CFTR as a therapeutic approach to cystic fibrosis. Trends Pharmacol Sci. 2007;28:334–341. doi: 10.1016/j.tips.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 10.Verkman AS, Galietta LJ. Chloride channels as drug targets. Nat Rev Drug Discov. 2009;8:153–171. doi: 10.1038/nrd2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pedemonte N, Zegarra-Moran O, Galietta LJ. High-throughput screening of libraries of compounds to identify CFTR modulators. Methods Mol Biol. 2011;741:13–21. doi: 10.1007/978-1-61779-117-8_2. [DOI] [PubMed] [Google Scholar]
- 12.Van Goor F, Hadida S, Grootenhuis PD, et al. Rescue of CF airway epithelial cell function in vitro by a CFTR potentiator, VX-770. Proc Natl Acad Sci USA. 2009;106:18825–18830. doi: 10.1073/pnas.0904709106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Goor F, Hadida S, Grootenhuis PD, et al. Correction of the F508del-CFTR protein processing defect in vitro by the investigational drug VX-809. Proc Natl Acad Sci USA. 2011;108:18843–18848. doi: 10.1073/pnas.1105787108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Accurso FJ, Rowe SM, Clancy JP, et al. Effect of VX-770 in persons with cystic fibrosis and the G551D-CFTR mutation. N Engl J Med. 2010;363:1991–2003. doi: 10.1056/NEJMoa0909825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clancy JP, Rowe SM, Accurso FJ, et al. Results of a phase IIa study of VX-809, an investigational CFTR corrector compound, in subjects with cystic fibrosis homozygous for the F508del-CFTR mutation. Thorax. 2012;67:12–18. doi: 10.1136/thoraxjnl-2011-200393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenberg MF, O'Ryan LP, Hughes G, et al. The cystic fibrosis transmembrane conductance regulator (CFTR): three-dimensional structure and localization of a channel gate. J Biol Chem. 2011;286:42647–42654. doi: 10.1074/jbc.M111.292268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dawson RJ, Locher KP. Structure of a bacterial multidrug ABC transporter. Nature. 2006;443:180–185. doi: 10.1038/nature05155. [DOI] [PubMed] [Google Scholar]
- 18.Aller SG, Yu J, Ward A, et al. Structure of P-glycoprotein reveals a molecular basis for poly-specific drug binding. Science. 2009;323:1718–1722. doi: 10.1126/science.1168750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hwang TC, Sheppard DN. Gating of the CFTR Cl- channel by ATP-driven nucleotide-binding domain dimerisation. J Physiol. 2009;587:2151–2161. doi: 10.1113/jphysiol.2009.171595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsai MF, Li M, Hwang TC. Stable ATP binding mediated by a partial NBD dimer of the CFTR chloride channel. J Gen Physiol. 2010;135:399–414. doi: 10.1085/jgp.201010399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsai MF, Shimizu H, Sohma Y, Li M, Hwang TC. State-dependent modulation of CFTR gating by pyrophosphate. J Gen Physiol. 2009;133:405–419. doi: 10.1085/jgp.200810186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou Z, Wang X, Liu HY, Zou X, Li M, Hwang TC. The two ATP binding sites of cystic fibrosis transmembrane conductance regulator (CFTR) play distinct roles in gating kinetics and energetics. J Gen Physiol. 2006;128:413–422. doi: 10.1085/jgp.200609622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shimizu H, Yu YC, Kono K, et al. A stable ATP binding to the nucleotide binding domain is important for reliable gating cycle in an ABC transporter CFTR. J Physiol Sci. 2010;60:353–362. doi: 10.1007/s12576-010-0102-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng SH, Rich DP, Marshall J, Gregory RJ, Welsh MJ, Smith AE. Phosphorylation of the R domain by cAMP-dependent protein kinase regulates the CFTR chloride channel. Cell. 1991;66:1027–1036. doi: 10.1016/0092-8674(91)90446-6. [DOI] [PubMed] [Google Scholar]
- 25.Mense M, Vergani P, White DM, Altberg G, Nairn AC, Gadsby DC. In vivo phosphorylation of CFTR promotes formation of a nucleotide-binding domain heterodimer. EMBO J. 2006;25:4728–4739. doi: 10.1038/sj.emboj.7601373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baker JM, Hudson RP, Kanelis V, et al. CFTR regulatory region interacts with NBD1 predominantly via multiple transient helices. Nat Struct Mol Biol. 2007;14:738–745. doi: 10.1038/nsmb1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang W, Wu J, Bernard K, et al. ATP-independent CFTR channel gating and allosteric modulation by phosphorylation. Proc Natl Acad Sci USA. 2010;107:3888–3893. doi: 10.1073/pnas.0913001107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vergani P, Lockless SW, Nairn AC, Gadsby DC. CFTR channel opening by ATP-driven tight dimerization of its nucleotide-binding domains. Nature. 2005;433:876–880. doi: 10.1038/nature03313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jih KY, Sohma Y, Li M, Hwang TC. Identification of a novel post-hydrolytic state in CFTR gating. J Gen Physiol. 2012;139:359–370. doi: 10.1085/jgp.201210789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jih KY, Sohma Y, Hwang TC. Nonintegral stoichiometry in CFTR gating revealed by a pore-lining mutation. J Gen Physiol. 2012;140:347–359. doi: 10.1085/jgp.201210834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zielenski J, Tsui LC. Cystic fibrosis: genotypic and phenotypic variations. Annu Rev Genet. 1995;29:777–807. doi: 10.1146/annurev.ge.29.120195.004021. [DOI] [PubMed] [Google Scholar]
- 32.Chang XB, Tabcharani JA, Hou YX, et al. Protein kinase A (PKA) still activates CFTR chloride channel after mutagenesis of all 10 PKA consensus phosphorylation sites. J Biol Chem. 1993;268:11304–11311. [PubMed] [Google Scholar]
- 33.Cutting GR, Kasch LM, Rosenstein BJ, et al. A cluster of cystic fibrosis mutations in the first nucleotide-binding fold of the cystic fibrosis conductance regulator protein. Nature. 1990;346:366–369. doi: 10.1038/346366a0. [DOI] [PubMed] [Google Scholar]
- 34.Li C, Ramjeesingh M, Wang W, et al. ATPase activity of the cystic fibrosis transmembrane conductance regulator. J Biol Chem. 1996;271:28463–28468. doi: 10.1074/jbc.271.45.28463. [DOI] [PubMed] [Google Scholar]
- 35.Bompadre SG, Sohma Y, Li M, Hwang TC. G551D and G1349D, two CF-associated mutations in the signature sequences of CFTR, exhibit distinct gating defects. J Gen Physiol. 2007;129:285–298. doi: 10.1085/jgp.200609667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cai Z, Taddei A, Sheppard DN. Differential sensitivity of the cystic fibrosis (CF)-associated mutants G551D and G1349D to potentiators of the cystic fibrosis transmembrane conductance regulator (CFTR) Cl- channel. J Biol Chem. 2006;281:1970–1977. doi: 10.1074/jbc.M510576200. [DOI] [PubMed] [Google Scholar]
- 37.Illek B, Fischer H. Flavonoids stimulate Cl conductance of human airway epithelium in vitro and in vivo. Am J Physiol. 1998;275:L902–L910. doi: 10.1152/ajplung.1998.275.5.L902. [DOI] [PubMed] [Google Scholar]
- 38.Yu YC, Miki H, Nakamura Y, et al. Curcumin and genistein additively potentiate G551D-CFTR. J Cyst Fibros. 2011;10:243–252. doi: 10.1016/j.jcf.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ai T, Bompadre SG, Wang X, Hu S, Li M, Hwang TC. Capsaicin potentiates wild-type and mutant cystic fibrosis transmembrane conductance regulator chloride-channel currents. Mol Pharmacol. 2004;65:1415–1426. doi: 10.1124/mol.65.6.1415. [DOI] [PubMed] [Google Scholar]
- 40.Pedemonte N, Sonawane ND, Taddei A, et al. Phenylglycine and sulfonamide correctors of defective delta F508 and G551D cystic fibrosis transmembrane conductance regulator chloride-channel gating. Mol Pharmacol. 2005;67:1797–1807. doi: 10.1124/mol.105.010959. [DOI] [PubMed] [Google Scholar]
- 41.Al-Nakkash L, Springsteel MF, Kurth MJ, Nantz MH. Activation of CFTR by UCCF-029 and genistein. Bioorg Med Chem Lett. 2008;18:3874–3877. doi: 10.1016/j.bmcl.2008.06.051. [DOI] [PubMed] [Google Scholar]
- 42.Al-Nakkash L, Hu S, Li M, Hwang TC. A common mechanism for cystic fibrosis transmembrane conductance regulator protein activation by genistein and benzimidazolone analogs. J Pharmacol Exp Ther. 2001;296:464–472. [PubMed] [Google Scholar]
- 43.Caci E, Folli C, Zegarra-Moran O, et al. CFTR activation in human bronchial epithelial cells by novel benzoflavone and benzimidazolone compounds. Am J Physiol Lung Cell Mol Physiol. 2003;285:L180–L188. doi: 10.1152/ajplung.00351.2002. [DOI] [PubMed] [Google Scholar]
- 44.Noel S, Faveau C, Norez C, Rogier C, Mettey Y, Becq F. Discovery of pyrrolo[2,3-b]pyrazines derivatives as submicromolar affinity activators of wild type, G551D, and F508del cystic fibrosis transmembrane conductance regulator chloride channels. J Pharmacol Exp Ther. 2006;319:349–359. doi: 10.1124/jpet.106.104521. [DOI] [PubMed] [Google Scholar]
- 45.Derand R, Bulteau-Pignoux L, Mettey Y, et al. Activation of G551D CFTR channel with MPB-91: regulation by ATPase activity and phosphorylation. Am J Physiol Cell Physiol. 2001;281:C1657–C1666. doi: 10.1152/ajpcell.2001.281.5.C1657. [DOI] [PubMed] [Google Scholar]
- 46.Berger AL, Randak CO, Ostedgaard LS, Karp PH, Vermeer DW, Welsh MJ. Curcumin stimulates cystic fibrosis transmembrane conductance regulator Cl- channel activity. J Biol Chem. 2005;280:5221–5226. doi: 10.1074/jbc.M412972200. [DOI] [PubMed] [Google Scholar]
- 47.Bernard K, Wang W, Narlawar R, Schmidt B, Kirk KL. Curcumin cross-links cystic fibrosis transmembrane conductance regulator (CFTR) polypeptides and potentiates CFTR channel activity by distinct mechanisms. J Biol Chem. 2009;284:30754–30765. doi: 10.1074/jbc.M109.056010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dixon RA, Ferreira D. Genistein. Phytochemistry. 2002;60:205–211. doi: 10.1016/s0031-9422(02)00116-4. [DOI] [PubMed] [Google Scholar]
- 49.Andersson C, Servetnyk Z, Roomans GM. Activation of CFTR by genistein in human airway epithelial cell lines. Biochem Biophys Res Commun. 2003;308:518–522. doi: 10.1016/s0006-291x(03)01436-0. [DOI] [PubMed] [Google Scholar]
- 50.Hwang TC, Wang F, Yang IC, Reenstra WW. Genistein potentiates wild-type and delta F508-CFTR channel activity. Am J Physiol. 1997;273:C988–C998. doi: 10.1152/ajpcell.1997.273.3.C988. [DOI] [PubMed] [Google Scholar]
- 51.Illek B, Fischer H, Santos GF, Widdicombe JH, Machen TE, Reenstra WW. cAMP-independent activation of CFTR Cl channels by the tyrosine kinase inhibitor genistein. Am J Physiol. 1995;268:C886–C893. doi: 10.1152/ajpcell.1995.268.4.C886. [DOI] [PubMed] [Google Scholar]
- 52.Illek B, Zhang L, Lewis NC, Moss RB, Dong JY, Fischer H. Defective function of the cystic fibrosis-causing missense mutation G551D is recovered by genistein. Am J Physiol. 1999;277:C833–C839. doi: 10.1152/ajpcell.1999.277.4.C833. [DOI] [PubMed] [Google Scholar]
- 53.Schmidt A, Hughes LK, Cai Z, et al. Prolonged treatment of cells with genistein modulates the expression and function of the cystic fibrosis transmembrane conductance regulator. Br J Pharmacol. 2008;153:1311–1323. doi: 10.1038/sj.bjp.0707663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Melin P, Thoreau V, Norez C, Bilan F, Kitzis A, Becq F. The cystic fibrosis mutation G1349D within the signature motif LSHGH of NBD2 abolishes the activation of CFTR chloride channels by genistein. Biochem Pharmacol. 2004;67:2187–2196. doi: 10.1016/j.bcp.2004.02.022. [DOI] [PubMed] [Google Scholar]
- 55.Moran O, Galietta LJ, Zegarra-Moran O. Binding site of activators of the cystic fibrosis transmembrane conductance regulator in the nucleotide binding domains. Cell Mol Life Sci. 2005;62:446–460. doi: 10.1007/s00018-004-4422-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang F, Zeltwanger S, Yang IC, Nairn AC, Hwang TC. Actions of genistein on cystic fibrosis transmembrane conductance regulator channel gating. Evidence for two binding sites with opposite effects. J Gen Physiol. 1998;111:477–490. doi: 10.1085/jgp.111.3.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weinreich F, Wood PG, Riordan JR, Nagel G. Direct action of genistein on CFTR. Pflugers Arch. 1997;434:484–491. doi: 10.1007/s004240050424. [DOI] [PubMed] [Google Scholar]
- 58.Egan ME, Pearson M, Weiner SA, et al. Curcumin, a major constituent of turmeric, corrects cystic fibrosis defects. Science. 2004;304:600–602. doi: 10.1126/science.1093941. [DOI] [PubMed] [Google Scholar]
- 59.Lansdell KA, Cai Z, Kidd JF, Sheppard DN. Two mechanisms of genistein inhibition of cystic fibrosis transmembrane conductance regulator Cl− channels expressed in murine cell line. J Physiol. 2000;524(Pt 2):317–330. doi: 10.1111/j.1469-7793.2000.t01-1-00317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Miquel J, Bernd A, Sempere JM, Diaz-Alperi J, Ramirez A. The curcuma antioxidants: pharmacological effects and prospects for future clinical use. A review. Arch Gerontol Geriatr. 2002;34:37–46. doi: 10.1016/s0167-4943(01)00194-7. [DOI] [PubMed] [Google Scholar]
- 61.Hwang TC, Koeppe RE, 2nd, Andersen OS. Genistein can modulate channel function by a phosphorylation-independent mechanism: importance of hydrophobic mismatch and bilayer mechanics. Biochemistry. 2003;42:13646–13658. doi: 10.1021/bi034887y. [DOI] [PubMed] [Google Scholar]
- 62.Huang SY, Bolser D, Liu HY, Hwang TC, Zou X. Molecular modeling of the heterodimer of human CFTR's nucleotide-binding domains using a protein-protein docking approach. J Mol Graph Model. 2009;27:822–828. doi: 10.1016/j.jmgm.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Derand R, Bulteau-Pignoux L, Becq F. The cystic fibrosis mutation G551D alters the non-Michaelis-Menten behavior of the cystic fibrosis transmembrane conductance regulator (CFTR) channel and abolishes the inhibitory Genistein binding site. J Biol Chem. 2002;277:35999–36004. doi: 10.1074/jbc.M206121200. [DOI] [PubMed] [Google Scholar]
- 64.Osawa T, Sugiyama Y, Inayoshi M, Kawakishi S. Antioxidative activity of tetrahydrocurcuminoids. Biosci Biotechnol Biochem. 1995;59:1609–1612. doi: 10.1271/bbb.59.1609. [DOI] [PubMed] [Google Scholar]
- 65.Itokawa H, Shi Q, Akiyama T, Morris-Natschke SL, Lee KH. Recent advances in the investigation of curcuminoids. Chin Med. 2008;3:11. doi: 10.1186/1749-8546-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gopinath D, Ahmed MR, Gomathi K, Chitra K, Sehgal PK, Jayakumar R. Dermal wound healing processes with curcumin incorporated collagen films. Biomaterials. 2004;25:1911–1917. doi: 10.1016/s0142-9612(03)00625-2. [DOI] [PubMed] [Google Scholar]
- 67.Singh RK, Rai D, Yadav D, Bhargava A, Balzarini J, De Clercq E. Synthesis, antibacterial and antiviral properties of curcumin bioconjugates bearing dipeptide, fatty acids and folic acid. Eur J Med Chem. 2010;45:1078–1086. doi: 10.1016/j.ejmech.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ono K, Hasegawa K, Naiki H, Yamada M. Curcumin has potent anti-amyloidogenic effects for Alzheimer's beta-amyloid fibrils in vitro. J Neurosci Res. 2004;75:742–750. doi: 10.1002/jnr.20025. [DOI] [PubMed] [Google Scholar]
- 69.Aggarwal BB, Kumar A, Bharti AC. Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res. 2003;23:363–398. [PubMed] [Google Scholar]
- 70.Zhou H, Beevers CS, Huang S. The targets of curcumin. Curr Drug Targets. 2011;12:332–347. doi: 10.2174/138945011794815356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Grynkiewicz G, Slifirski P. Curcumin and curcuminoids in quest for medicinal status. Acta Biochim Pol. 2012;59:201–212. [PubMed] [Google Scholar]
- 72.Song Y, Sonawane ND, Salinas D, et al. Evidence against the rescue of defective DeltaF508-CFTR cellular processing by curcumin in cell culture and mouse models. J Biol Chem. 2004;279:40629–40633. doi: 10.1074/jbc.M407308200. [DOI] [PubMed] [Google Scholar]
- 73.Wang W, Bernard K, Li G, Kirk KL. Curcumin opens cystic fibrosis transmembrane conductance regulator channels by a novel mechanism that requires neither ATP binding nor dimerization of the nucleotide-binding domains. J Biol Chem. 2007;282:4533–4544. doi: 10.1074/jbc.M609942200. [DOI] [PubMed] [Google Scholar]
- 74.Mio K, Ogura T, Mio M, et al. Three-dimensional reconstruction of human cystic fibrosis transmembrane conductance regulator chloride channel revealed an ellipsoidal structure with orifices beneath the putative transmembrane domain. J Biol Chem. 2008;283:30300–30310. doi: 10.1074/jbc.M803185200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang L, Aleksandrov LA, Zhao Z, Birtley JR, Riordan JR, Ford RC. Architecture of the cystic fibrosis transmembrane conductance regulator protein and structural changes associated with phosphorylation and nucleotide binding. J Struct Biol. 2009;167:242–251. doi: 10.1016/j.jsb.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 76.Zegarra-Moran O, Romio L, Folli C, et al. Correction of G551D-CFTR transport defect in epithelial monolayers by genistein but not by CPX or MPB-07. Br J Pharmacol. 2002;137:504–512. doi: 10.1038/sj.bjp.0704882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hwang TC, Sheppard DN. Molecular pharmacology of the CFTR Cl- channel. Trends Pharmacol Sci. 1999;20:448–453. doi: 10.1016/s0165-6147(99)01386-3. [DOI] [PubMed] [Google Scholar]