Abstract
BACKGROUND
The association of body-mass index (BMI) from adolescence to adulthood with obesity-related diseases in young adults has not been completely delineated.
METHODS
We conducted a prospective study in which we followed 37,674 apparently healthy young men for incident angiography-proven coronary heart disease and diabetes through the Staff Periodic Examination Center of the Israeli Army Medical Corps. The height and weight of participants were measured at regular intervals, with the first measurements taken when they were 17 years of age.
RESULTS
During approximately 650,000 person-years of follow-up (mean follow-up, 17.4 years), we documented 1173 incident cases of type 2 diabetes and 327 of coronary heart disease. In multivariate models adjusted for age, family history, blood pressure, lifestyle factors, and biomarkers in blood, elevated adolescent BMI (the weight in kilograms divided by the square of the height in meters; mean range for the first through last deciles, 17.3 to 27.6) was a significant predictor of both diabetes (hazard ratio for the highest vs. the lowest decile, 2.76; 95% confidence interval [CI], 2.11 to 3.58) and angiography-proven coronary heart disease (hazard ratio, 5.43; 95% CI, 2.77 to 10.62). Further adjustment for BMI at adulthood completely ablated the association of adolescent BMI with diabetes (hazard ratio, 1.01; 95% CI, 0.75 to 1.37) but not the association with coronary heart disease (hazard ratio, 6.85; 95% CI, 3.30 to 14.21). After adjustment of the BMI values as continuous variables in multivariate models, only elevated BMI in adulthood was significantly associated with diabetes (β = 1.115, P = 0.003; P = 0.89 for interaction). In contrast, elevated BMI in both adolescence (β = 1.355, P = 0.004) and adulthood (β = 1.207, P = 0.03) were independently associated with angiography-proven coronary heart disease (P = 0.048 for interaction).
CONCLUSIONS
An elevated BMI in adolescence — one that is well within the range currently considered to be normal — constitutes a substantial risk factor for obesity-related disorders in midlife. Although the risk of diabetes is mainly associated with increased BMI close to the time of diagnosis, the risk of coronary heart disease is associated with an elevated BMI both in adolescence and in adulthood, supporting the hypothesis that the processes causing incident coronary heart disease, particularly atherosclerosis, are more gradual than those resulting in incident diabetes. (Funded by the Chaim Sheba Medical Center and the Israel Defense Forces Medical Corps.)
Although obesity in adulthood is a well-documented risk factor for both type 2 diabetes and coronary heart disease, it remains unclear whether a longer history of relative overweight, starting earlier in life, poses an additional risk. Furthermore, whereas the trajectory of weight and height from birth to adolescence is well documented, the progression of body-mass index (BMI) from adolescence into adulthood is less well described. Obese children probably have higher odds of becoming obese adults.1 Moreover, although an elevated BMI in childhood or adolescence may not necessarily represent adiposity,2–4 childhood obesity is associated with classic cardiometabolic risk factors, as is the case in adults.5–15 Some studies16–20 —though not all5,7,21,22 — have shown that elevated BMI in childhood or adolescence is associated with an increased risk of disease or death later in life. What is lacking is knowledge of how childhood BMI interacts with the particular pathologic mechanisms of obesity-related diseases and whether it does so even within the BMI range that is now considered normal.
Given the current obesity pandemic, it is important to determine whether elevated BMI in childhood, adolescence, or adulthood, or an increase in BMI during the transition from adolescence to adulthood, contributes independently to the risk of disease.23 Increases in BMI in childhood and adolescence may be closely associated with a higher incidence of coronary heart disease and type 2 diabetes mellitus in young adults.24 In fact, the occurrence of these diseases in the third to fifth decades of life may be the result of distinct associations with risk factors that are overlooked when the diseases are studied later in life.
To address these points, we used data from the Metabolic, Lifestyle, and Nutrition Assessment in Young Adults (MELANY)25 study of the Israel Defense Forces (IDF) Medical Corps and followed more than 37,000 apparently healthy young men whose BMI was measured at adolescence and into early adulthood, in order to identify incident cases of type 2 diabetes and angiography-proven coronary heart disease.
METHODS
STUDY POPULATION
The MELANY study25–28 is an ongoing investigation conducted at the IDF Medical Corps Staff Periodic Examination Center (SPEC). All personnel remaining in the army beyond the period of mandatory military service are periodically examined (every 3 to 5 years) at the SPEC, beginning at approximately 25 years of age. At each examination, participants complete a questionnaire to provide demographic and medical information. Weight, height, and blood pressure are measured, and a complete physical examination is performed. Between SPEC visits, primary care for IDF personnel is provided at designated military clinics, and medical information is recorded in a central database, allowing ongoing, close, uniform follow-up, with repeated updates of measurements. For all the cohort participants, the SPEC database also includes data on weight and height measured at adolescence (17 years of age) obtained during an initial medical evaluation before induction into the army.
The study included 37,674 male career personnel whose BMI (calculated as the weight in kilograms divided by the square of the height in meters) at adolescence ranged between 15 and 36. Men with previously diagnosed diabetes (type 1 or 2) or coronary heart disease at adolescence were excluded, as were 135 men with newly diagnosed diabetes presenting with diabetic ketoacidosis and positive autoantibodies (diagnosis of type 1 diabetes). Among the 1173 men in whom incident diabetes developed during the study, 20 (1.7%) were suspected of having type 1 diabetes because of the need for rapid initiation of insulin treatment. Since no data on levels of autoantibodies were available for this subgroup, however, they were not excluded.
The IDF Institutional Review Board approved the study and waved the requirement for informed consent on the basis of the understanding that the anonymity of participants would be preserved.
FOLLOW-UP AND OUTCOMES
Participants were followed prospectively from the time of their first visit to the SPEC (at ≥25 years of age). Their measured height and weight at 17 years of age (adolescence) were tracked retrospectively from the IDF central database. Two cases of diabetes were retrospectively defined as having developed between adolescence and the first SPEC visit. Follow-up ended at the time of diagnosis of diabetes or coronary heart disease, death, or retirement from military service or on December 31, 2007, whichever came first. Mean (±SD) total follow-up was 17.4±7.4 years (median, 16.9) (corresponding to conclusion of follow-up after at least 8 years for 99.99% of participants and after at least 12 years for 96.4%). There were 127 deaths during follow-up.
The diagnosis of 1173 incident cases of diabetes was based on two fasting plasma glucose levels of 126 mg per deciliter (7.0 mmol per liter) or higher, in accordance with criteria established by the American Diabetes Association Expert Committee. To accommodate changes in the diagnostic criteria for diabetes during follow-up, all fasting glucose values were reviewed to identify participants as having type 2 diabetes if their level of fasting plasma glucose was 126 to 140 mg per deciliter (7.0 to 7.8 mmol per liter). End-point determination was made at each sequential SPEC visit by measuring fasting glucose levels. Diagnoses of diabetes made between visits were established by IDF primary care physicians and confirmed by a committee of military physicians.
The outcome definition for coronary heart disease in MELANY27 was angiography-proven stenosis of more than 50% in at least one coronary artery. Before 35 years of age, participants were referred for a diagnostic procedure on the basis of their own report of a specific problem; all participants 35 years of age or older underwent a treadmill exercise test (in accordance with the Bruce protocol) in the presence of a board-certified cardiologist. End points for the exercise test were ST-segment depression of more than 2 mm in two contiguous leads, measured 80 msec after the J point; symptoms of angina; exhaustion; or achievement of the target heart rate. All participants with an abnormal stress test were referred for coronary angiography. When the stress-test findings were borderline or when participants reported angina symptoms without diagnostic changes on electrocardiography, thallium-201 myocardial-perfusion imaging was performed, followed by coronary angiography for participants with an abnormal scan. Those presenting with symptoms of angina or myocardial infarction between SPEC visits were referred for coronary angiography after consultation with a board-certified cardiologist.
LABORATORY METHODS
Glucose levels were determined at an on-site laboratory with the use of fresh blood samples collected in test tubes containing sodium fluoride to inhibit in vitro glycolysis. Biochemical analyses were performed at a laboratory authorized to perform tests according to the international quality standard ISO-9002. Lipids were measured directly, with the exception of low-density lipoproteins, which were calculated. Biochemical markers were measured with an automated analyzer (BM/Hitachi 917, Boehringer Mannheim).
STATISTICAL ANALYSIS
General linear models were used to compare age-adjusted means and proportions for the baseline characteristics of the population across the deciles of mean BMI at adolescence (Table 1). The medians of the deciles were fit as continuous variables to estimate the trend of variables across deciles in a linear regression model. Cox proportional-hazards analysis, stratified according to the interval between SPEC visits, was used to estimate the hazard ratio and 95% confidence interval for the development of type 2 diabetes or coronary heart disease. Repeated assessments of smoking status, physical activity, and BMI in adulthood were time-dependent variables. We controlled for blood biomarkers only at baseline, since these variables might be part of the causal chain. In several steps, we gradually added to the age-adjusted model selected traditional risk factors considered to be potential confounders for the specific diseases, as detailed in Tables 2 and 3. For both diabetes and coronary heart disease, the final model across deciles of adolescent BMI (model 4) was additionally adjusted for BMI in adulthood as a time-dependent, continuous variable. In addition, a linear model was used to assess adolescent BMI as a continuous variable. In a longitudinal growth chart, we depicted repeated BMI measurements from adolescence until data were censored in adulthood. BMI was stratified according to cutoff points established by the Centers for Disease Control and Prevention (CDC), with the use of the 10th, 25th, 50th, 75th, and 90th percentiles of the entire population. The curves corresponding to the 50th percentile for persons in whom coronary heart disease or diabetes developed were also calculated. To assess the interaction between quintiles of BMI at adolescence and in adulthood in relation to the risk of diabetes or coronary heart disease, we cross-classified the study population across 25 groups and excluded two extreme combinations (participants in quintile 1 in adolescence who were in quintile 5 in adulthood, and participants in quintile 5 in adolescence who were in quintile 1 in adulthood), each including less than 0.4% of the study population. All statistical analyses were performed with SPSS statistical software, version 16.0.
Table 1.
Cohort Characteristics across Percentile Increments of Body-Mass Index in Adolescence.*
| Characteristic | Percentile of BMI in Adolescence (N = 37,674) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1–10 | 11–20 | 21–30 | 31–40 | 41–50 | 51–60 | 61–70 | 71–80 | 81–90 | 91–100 | |
| Measurements in adolescence | ||||||||||
|
| ||||||||||
| No. of participants | 3783 | 3690 | 3860 | 3668 | 3856 | 3739 | 3761 | 3852 | 3698 | 3767 |
|
| ||||||||||
| BMI† | ||||||||||
|
| ||||||||||
| Mean | 17.32±0.64 | 18.58±0.25 | 19.35±0.20 | 19.99±0.17 | 20.60±0.18 | 21.24±0.19 | 21.94±0.22 | 22.84±0.31 | 24.16±0.47 | 27.62±2.30 |
|
| ||||||||||
| Range | 15.04–18.11 | 18.12–19.00 | 19.01–19.69 | 19.70–20.28 | 20.29–20.90 | 20.91–21.56 | 21.57–22.34 | 22.35–23.39 | 23.40–25.06 | 25.07–35.99 |
|
| ||||||||||
| Age (yr) | 17.1±0.46 | 17.1±0.45 | 17.1±0.46 | 17.1±0.46 | 17.1±0.47 | 17.1±0.43 | 17.1±0.46 | 17.1±0.46 | 17.1±0.47 | 17.1±0.49 |
|
| ||||||||||
| Weight (kg)† | 51.9±4.6 | 55.9±4.5 | 58.2±4.7 | 60.0±4.7 | 61.8±4.9 | 63.9±4.8 | 66.1±5.2 | 68.9±5.5 | 72.9±6.0 | 83.8±9.6 |
|
| ||||||||||
| Height (cm)† | 173.0±6.9 | 173.3±6.9 | 173.3±6.8 | 173.1±6.6 | 173.1±6.8 | 173.4±6.5 | 173.5±6.8 | 173.5±6.8 | 173.6±6.9 | 174.0±6.9 |
|
| ||||||||||
| Measurements in adulthood | ||||||||||
|
| ||||||||||
| Age (yr) | 30.0±4.9 | 30.5±5.2 | 30.7±5.3 | 30.6±5.3 | 30.8±5.4 | 31.0±5.4 | 30.9±5.5 | 30.7±5.4 | 30.5±5.4 | 30.0±5.2 |
|
| ||||||||||
| BMI† | 21.35±2.56 | 22.67±2.47 | 23.40±2.42 | 24.01±2.52 | 24.63±2.61 | 25.16±2.57 | 25.84±2.69 | 26.67±2.84 | 28.01±3.14 | 30.63±4.27 |
|
| ||||||||||
| Weight (kg)† | 66.8±9.4 | 70.9±9.4 | 73.0±9.1 | 74.7±9.7 | 76.6±9.7 | 78.4±9.8 | 80.6±10.3 | 83.2±10.5 | 87.5±11.7 | 96.4±15.3 |
|
| ||||||||||
| Height (cm)† | 176.8±6.8 | 176.7±6.8 | 176.6±6.8 | 176.3±6.6 | 176.3±6.6 | 176.4±6.4 | 176.5±6.8 | 176.6±6.7 | 176.7±6.8 | 177.3±6.8 |
|
| ||||||||||
| Change in BMI† | 4.03±2.49 | 4.09±2.47 | 4.06±2.41 | 4.03±2.51 | 4.03±2.60 | 3.93±2.57 | 3.90±2.68 | 3.83±2.83 | 3.85±3.11 | 3.01±4.08 |
|
| ||||||||||
| Blood pressure (mm Hg)† | ||||||||||
|
| ||||||||||
| Systolic | 115.0±11.5 | 115.9±11.7 | 116.5±11.7 | 117.0±11.6 | 117.3±11.6 | 117.9±12.0 | 118.5±12.1 | 119.3±12.2 | 120.5±13.1 | 123.3±13.8 |
|
| ||||||||||
| Diastolic | 73.2±8.8 | 73.8±8.9 | 74.4±8.9 | 74.6±9.0 | 74.8±8.9 | 75.4±9.0 | 75.6±9.0 | 76.1±9.2 | 76.9±9.8 | 78.5±10.2 |
|
| ||||||||||
| Heart rate (beats/min)† | 70.7±11.2 | 70.4±11.3 | 70.3±10.7 | 70.2±11.0 | 70.2±10.9 | 70.3±11.1 | 70.6±11.2 | 70.8±11.3 | 71.6±11.8 | 73.3±12.1 |
|
| ||||||||||
| Fasting glucose (mg/dl)† | 89.9±11.0 | 90.2±10.8 | 90.4±11.3 | 90.5±13.5 | 90.6±12.5 | 90.9±12.8 | 91.3±13.6 | 91.0±12.5 | 92.0±16.5 | 93.6±20.4 |
|
| ||||||||||
| Lipid levels† | ||||||||||
|
| ||||||||||
| Cholesterol (mg/dl) | ||||||||||
|
| ||||||||||
| Total | 179.1±36.3 | 181.9±36.5 | 185.1±37.9 | 185.4±37.3 | 185.7±37.4 | 188.2±37.9 | 189.4±38.9 | 188.6±37.5 | 191.6±37.7 | 195.2±38.6 |
|
| ||||||||||
| HDL | 48.3±11.2 | 48.0±11.2 | 47.5±11.4 | 46.9±10.8 | 46.6±10.5 | 46.3±10.7 | 45.6±10.5 | 46.0±10.8 | 44.8±10.9 | 43.6±10.4 |
|
| ||||||||||
| LDL | 107.6±31.4 | 110.8±32.4 | 113.7±34.1 | 113.9±32.9 | 114.5±34.3 | 116.0±34.3 | 118.8±34.9 | 116.2±32.7 | 119.8±33.8 | 120.5±34.3 |
|
| ||||||||||
| Triglycerides | ||||||||||
|
| ||||||||||
| Median | 85 | 91 | 93 | 93 | 96 | 101 | 103 | 105 | 114 | 126 |
|
| ||||||||||
| Interquartile range | 62–125 | 65–131 | 66–135 | 67–134 | 69–141 | 69–146 | 71–151 | 73–155 | 77–167 | 86–191 |
|
| ||||||||||
| Aspartate aminotransferase (U/liter)† | 23.2±6.8 | 21.7±6.4 | 24.4±8.4 | 25.1±9.5 | 25.5±8.3 | 25.5±9.0 | 29.7±8.2 | 31.2±8.4 | 31.6±13.7 | 31.8±14.8 |
|
| ||||||||||
| Alanine aminotransferase (U/liter)† | 26.4±8.9 | 27.4±9.8 | 32.4±10.8 | 27.6±10.2 | 31.4±9.6 | 28.6±8.6 | 32.1±10.6 | 31.1±9.9 | 35.8±11.4 | 39.8±10.4 |
|
| ||||||||||
| Family history† | ||||||||||
|
| ||||||||||
| First-degree relative with diabetes (%) | 17.7 | 18.1 | 18.4 | 18.8 | 18.5 | 18.5 | 21.5 | 19.8 | 21.2 | 24.4 |
|
| ||||||||||
| First-degree relative with CHD (%) | 9.2 | 8.7 | 9 | 9.5 | 9.3 | 10 | 10.4 | 11.2 | 10.3 | 11.5 |
|
| ||||||||||
| Lifestyle† | ||||||||||
|
| ||||||||||
| Current smoker (%) | 22.8 | 23.2 | 23.1 | 21.6 | 24.9 | 22.9 | 22.5 | 23.4 | 24.5 | 27.7 |
|
| ||||||||||
| Physical activity | ||||||||||
|
| ||||||||||
| Mean (min/wk) | 135±80 | 136±77 | 130±75 | 126±76 | 120±74 | 120±74 | 119±71 | 116±74 | 112±71 | 107±71 |
|
| ||||||||||
| <150 min/wk (%) | 21.6 | 23.1 | 23.2 | 24.4 | 24.5 | 25.1 | 23.8 | 24 | 20.9 | 18.7 |
|
| ||||||||||
| ≥150 min/wk (%) | 10.7 | 11.1 | 11.7 | 10.8 | 9.4 | 9.2 | 9 | 8.1 | 7 | 6.2 |
Plus–minus values are means ±SD. Change in BMI (body-mass index, the weight in kilograms divided by the square of the height in meters) indicates the mean difference between BMI measured at 17 years of age (adolescence) and 30 years of age (adulthood). Family history of diabetes was defined as a first-degree relative of any age with diabetes, and family history of coronary heart disease (CHD) was defined as a first-degree male relative younger than 55 years of age with CHD or a first-degree female relative younger than 65 years of age with CHD. Intraassay and interassay coefficients of variation for glucose were both 2% at 6.6 mmol per liter; those for total cholesterol were 0.8% and 1.7%, respectively; those for triglycerides were 1.5% and 1.8%, respectively; and those for high-density lipoprotein (HDL) cholesterol were 2.9% and 3.6%, respectively. To convert the values for cholesterol to millimoles per liter, multiply by 0.02586. To convert the values for glucose to millimoles per liter, multiply by 0.05551. To convert the values for triglycerides to milllimoles per liter, multiply by 0.01129. BMI denotes body-mass index, CHD coronary heart disease, and LDL low-density lipoprotein.
P<0.05 for trend.
Table 2.
Hazard Ratios for Incident Diabetes in Adulthood According to Percentile of BMI in Adolescence.*
| Variable | Percentile of BMI in Adolescence (N = 37,674) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1–10 | 11–20 | 21–30 | 31–40 | 41–50 | 51–60 | 61–70 | 71–80 | 81–90 | 91–100 | |
| Characteristic | ||||||||||
|
| ||||||||||
| BMI range | <18.11 | 18.12–19.00 | 19.01–19.69 | 19.70–20.28 | 20.29–20.90 | 20.91–21.56 | 21.57–22.34 | 22.35–23.39 | 23.40–25.06 | >25.07 |
|
| ||||||||||
| No. of participants | 3,783 | 3,690 | 3,860 | 3,668 | 3,856 | 3,739 | 3,761 | 3,852 | 3,698 | 3,767 |
|
| ||||||||||
| No. of cases of diabetes | 76 | 74 | 81 | 94 | 96 | 93 | 101 | 132 | 162 | 264 |
|
| ||||||||||
| Mean follow-up (yr) | 17.6±7.3 | 17.8±7.4 | 17.6±7.4 | 17.8±7.4 | 17.9±7.4 | 17.9±7.4 | 17.4±7.4 | 17.2±7.3 | 16.2±7.2 | <0.001 |
|
| ||||||||||
| Person-yr of follow-up | 63,994 | 64,644 | 68,419 | 64,295 | 68,620 | 66,979 | 67,066 | 66,691 | 63,181 | 60,354 |
|
| ||||||||||
| Hazard ratio (95% CI) | ||||||||||
|
| ||||||||||
| Model 1 — adjusted for age | 1.00 | 0.93 (0.67–1.28) | 1.07 (0.79–1.45) | 1.09 (0.75–1.37) | 1.11 (0.82–1.50) | 1.11 (0.81–1.49) | 1.21 (0.89–1.61) | 1.69 (1.27–2.24) | 2.26 (1.72–2.97) | 4.18 (3.21–5.40) |
|
| ||||||||||
| Model 2 — adjusted for age and family history of diabetes | 1.00 | 0.93 (0.67–1.27) | 1.06 (0.78–1.44) | 1.01 (0.74–1.39) | 1.10 (0.82–1.49) | 1.11 (0.82–1.50) | 1.15 (0.86–1.55) | 1.67 (1.26–2.21) | 2.21 (1.68–2.90) | 4.02 (3.12–5.19) |
|
| ||||||||||
| Model 3 — adjusted for age, family history of diabetes, blood pressure, physical activity level, fasting glucose level, and triglyceride level† | 1.00 | 0.86 (0.62–1.18) | 1.03 (0.76–1.40) | 1.02 (0.75–1.39) | 1.06 (0.78–1.43) | 1.00 (0.74–1.37) | 1.03 (0.76–1.38) | 1.47 (1.11–1.95) | 1.79 (1.36–2.35) | 2.76 (2.12–3.58) |
|
| ||||||||||
| Model 4 — adjusted for age, family history of diabetes, blood pressure, physical activity level, fasting glucose level, triglyceride level, and BMI in adulthood‡ | 1.00 | 0.75 (0.55–1.04) | 0.86 (0.64–1.17) | 0.76 (0.56–1.05) | 0.74 (0.54–1.01) | 0.69 (0.51–0.94) | 0.63 (0.46–0.86) | 0.84 (0.63–1.13) | 0.89 (0.66–1.19) | 1.01 (0.75–1.37) |
Plus–minus values are means ±SD. BMI denotes body-mass index.
In model 3, we controlled for age as a continuous variable, presence or absence of family history of diabetes, systolic and diastolic blood pressure (systolic, <120 mm Hg; diastolic, <80 mm Hg; systolic, 120 to 129 mm Hg, or diastolic, 80 to 84 mm Hg; systolic, 130 to 139 mm Hg, or diastolic, 85 to 89 mm Hg; and systolic, ≥140 mm Hg, or diastolic, ≥90 mm Hg), physical activity level (nonactive, <150 min per week; active, >150 min per week), fasting glucose level (quintiles), and serum triglyceride level (quintiles).
In model 4, for diabetes, the final model across deciles of BMI at 17 years of age was additionally adjusted for BMI in adulthood as a time-dependent, continuous variable.
Table 3.
Hazard Ratios for Incident Angiography-Proven Coronary Heart Disease in Adulthood According to Percentile of BMI in Adolescence.*
| Variable | Percentile of BMI in Adolescence (N = 37,674) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1–10 | 11–20 | 21–30 | 31–40 | 41–50 | 51–60 | 61–70 | 71–80 | 81–90 | 91–100 | |
| Characteristic | ||||||||||
|
| ||||||||||
| BMI range | 15.04–18.11 | 18.12–19.00 | 19.01–19.69 | 19.70–20.28 | 20.29–20.90 | 20.91–21.56 | 21.57–22.34 | 22.35–23.39 | 23.40–25.06 | 25.07–35.99 |
|
| ||||||||||
| No. of participants | 3783 | 3690 | 3860 | 3668 | 3856 | 3739 | 3761 | 3852 | 3698 | 3767 |
|
| ||||||||||
| No. of cases of CHD | 10 | 18 | 28 | 32 | 28 | 33 | 33 | 35 | 43 | 67 |
|
| ||||||||||
| Person-yr of follow-up | 64,199 | 64,819 | 68,663 | 64,516 | 68,841 | 67,152 | 67,281 | 67,048 | 63,520 | 60,935 |
|
| ||||||||||
| Hazard ratio (95% CI) | ||||||||||
|
| ||||||||||
| Model 1 — adjusted for age | 1.00 | 1.69 (0.78–3.66) | 2.43 (0.87–4.18) | 2.72 (1.43–5.94) | 2.48 (0.94–4.54) | 2.83 (1.39–5.74) | 2.84 (1.40–5.77) | 3.27 (1.62–6.61) | 4.33 (2.17–8.61) | 7.65 (3.93–14.88) |
|
| ||||||||||
| Model 2 — adjusted for age and family history of CHD | 1.00 | 1.72 (0.79–3.72) | 3.06 (1.52–6.16) | 2.92 (1.43–5.94) | 1.61 (0.75–3.47) | 2.80 (1.38–5.69) | 2.83 (1.39–5.74) | 3.23 (1.60–6.52) | 4.34 (2.18–8.64) | 7.53 (3.87–14.65) |
|
| ||||||||||
| Model 3 — adjusted for age, family history of CHD, blood pressure, physical activity level, smoking status, LDL cholesterol level, and HDL cholesterol level† | 1.00 | 1.71 (0.79–3.71) | 3.17 (1.58–6.39) | 2.88 (1.42–5.87) | 1.56 (0.73–3.37) | 2.64 (1.30–5.38) | 2.63 (1.29–5.34) | 2.96 (1.46–6.01) | 3.58 (1.79–7.16) | 5.43 (2.77–10.62) |
|
| ||||||||||
| Model 4 — adjusted for age, family history of CHD, blood pressure, physical activity level, smoking status, LDL cholesterol level, HDL cholesterol level, triglyceride level, and BMI in adulthood‡ | 1.00 | 1.77 (0.82–3.85) | 3.34 (1.65–6.74) | 3.10 (1.51–6.34) | 1.70 (0.79–3.70) | 2.90 (1.42–5.96) | 2.96 (1.43–6.09) | 3.41 (1.65–7.06) | 4.27 (2.07–8.81) | 6.85 (3.30–14.21) |
Cox proportional-hazards analysis, stratified over each visit to the Staff Periodic Examination Center (3-to-5-year intervals), was used to estimate the hazard ratios and 95% confidence intervals for the development of type 2 diabetes or coronary heart disease (CHD). Smoking status, physical activity level, and BMI (body-mass index) in adulthood were time-dependent variables. HDL denotes high-density lipoprotein, and LDL low-density lipoprotein.
In Model 3, we controlled for age, presence or absence of family history of CHD, blood pressure, physical activity level, smoking status (current or noncurrent), LDL cholesterol level (quintiles), HDL cholesterol level (quintiles), and serum triglyceride level (quintiles).
In model 4, for CHD, the final model across deciles of BMI at 17 years of age was additionally adjusted for BMI in adulthood as a time-dependent, continuous variable.
RESULTS
CHARACTERISTICS OF STUDY PARTICIPANTS
Characteristics of the cohort of 37,674 men across increasing deciles of BMI at adolescence are presented in Table 1. The mean age at adolescence was 17.44±0.46 years, and the mean BMI ranged between 17.3 in the bottom decile to 27.6 in the top decile, corresponding to a mean weight ranging from 51.9 to 83.8 kg. The mean age at first assessment in adulthood was 30.59±5.30 years (median, 28.02). The mean BMI decile range in adulthood was 21.4 to 30.6. Systolic and diastolic blood pressure, resting heart rate, and serum levels of fasting glucose, low-density lipoprotein (LDL) cholesterol, triglycerides, and liver enzymes increased progressively across deciles of adolescent BMI, whereas the level of high-density lipoprotein (HDL) cholesterol decreased (P<0.001 for trend for all comparisons). With increasing deciles of adolescent BMI, the percentage of current smokers increased, whereas the mean duration of physical exercise per week significantly decreased. The prevalence of a family history of coronary heart disease and a family history of diabetes increased across BMI deciles (P<0.001 for the trend in both categories).
BMI AND INCIDENCE OF DISEASE
During approximately 650,000 person-years of follow-up (mean follow-up, 17.4±7.4 years), we documented 1173 incident cases of type 2 diabetes and 327 incident cases of angiography-proven coronary heart disease, diagnosed between 25 and 45 years of age (i.e., in young adulthood). In a multivariate model adjusted for age, presence or absence of a family history of diabetes, blood pressure, physical activity, and glucose and triglyceride levels, adolescent BMI was a predictor of incident diabetes, with a significantly increased risk observed for the three highest BMI deciles (hazard ratio observed in the highest decile vs. the lowest decile, 2.76; 95% confidence interval [CI], 2.11 to 3.58 [model 3]) (Table 2). When adolescent BMI was modeled as a continuous variable in a multivariate model, the risk of diabetes increased by 9.8% for each increment in 1 BMI unit (hazard ratio, 1.10; 95% CI, 1.08 to 1.12).
In a multivariate model adjusted for age, presence or absence of a family history of coronary heart disease, blood pressure, physical activity, smoking status, and levels of LDL cholesterol, HDL cholesterol, and triglycerides, elevated adolescent BMI was a significant predictor of the incidence of angiography-proven coronary heart disease in young men across the entire BMI range (hazard ratio, 5.43; 95% CI, 2.77 to 10.62 between extreme deciles [model 3]) (Table 3). When adolescent BMI was modeled as a continuous variable in a multivariate model, the risk of coronary heart disease increased by 12.0% for each increment in 1 BMI unit (hazard ratio, 1.12; 95% CI, 1.067 to 1.18).
TRAJECTORY OF BMI
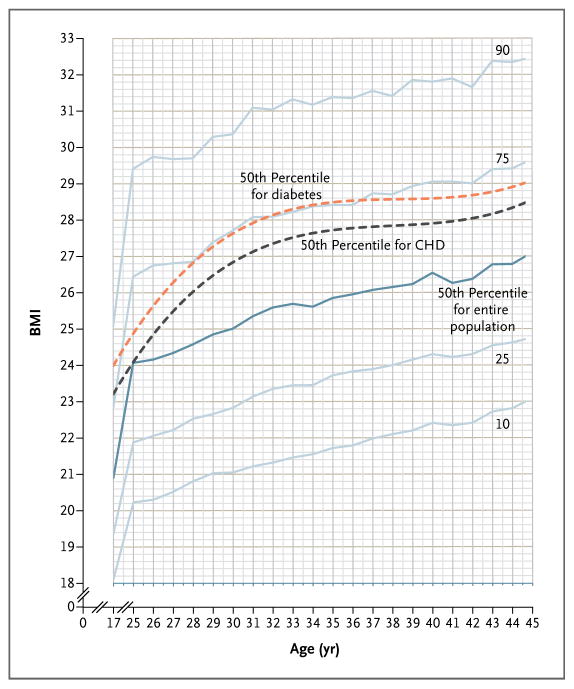
The trajectory of BMI between adolescence and young adulthood was studied longitudinally with the use of common CDC categories for growth charts (Fig. 1). BMI progression lines increased in parallel with the transition from adolescence into young adulthood. Mean annual increase in BMI was 0.3 BMI units between the ages of 17 and 30 years (increasing by approximately 15 kg, or 4 BMI units), and 0.2 BMI units per year thereafter. The 50th percentile for the group of patients in whom either coronary heart disease or diabetes eventually developed deviated from the corresponding curve in the entire study population. (A complementary approach to describing BMI trajectory is shown in Fig. 1 in the Supplementary Appendix, available with the full text of this article at NEJM.org.) For the entire study population, the correlation of adolescent BMI with adult BMI was r = 0.68.
Figure 1. Trajectory of BMI from Adolescence to Young Adulthood.
The figure depicts the trajectory of body-mass index (BMI, the weight in kilograms divided by the square of the height in meters) between adolescence and young adulthood (solid lines), as determined on the basis of repeated measurements of BMI among 37,674 men. It also shows the 50th percentile curves for the group of men in whom coronary heart disease (CHD) or diabetes eventually developed (dashed lines). BMI progression lines increased roughly in parallel during the transition from adolescence to young adulthood. The mean annual rate of rise in BMI between the ages of 17 and 30 years was 0.3 BMI units per year, corresponding to a total of approximately 15 kg, or 4 BMI units.
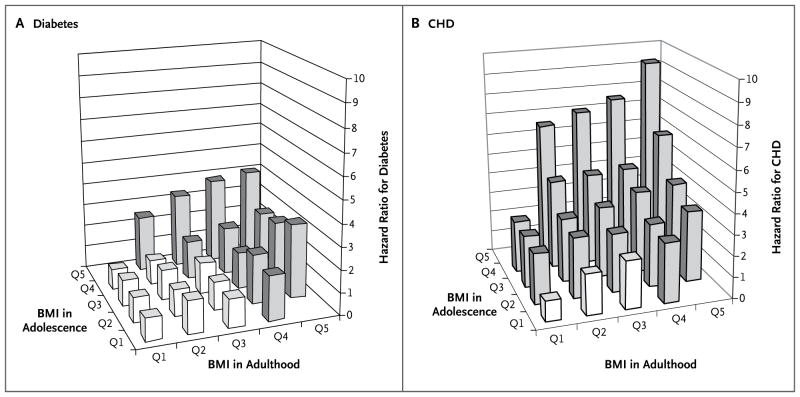
We assessed whether diabetes and coronary heart disease are more closely associated with BMI in adolescence than in early adulthood. In a multivariate Cox model adjusted for age, presence or absence of a family history of diabetes, triglyceride levels, and fasting glucose levels, only BMI in adulthood was significantly associated with the risk of diabetes (β = 1.115, P = 0.003; P = 0.89 for interaction). In contrast, in a model adjusted for age, smoking status, presence or absence of a family history of coronary heart disease, and levels of HDL cholesterol, LDL cholesterol, and triglycerides, both the BMI in adolescence (β = 1.355, P = 0.004) and that in adulthood (β = 1.207, P = 0.03) were significantly and independently associated with the risk of coronary heart disease (P = 0.048 for interaction). These joint associations are depicted in Figures 2A and 2B. Furthermore, the addition of the repeated BMI measurements in adulthood to model 3 for diabetes (model 4, Table 2) completely attenuated the association between BMI in adolescence and the risk of diabetes, whereas for coronary heart disease (model 4, Table 3), adolescent BMI remained a risk factor that was independent of adult BMI.
Figure 2. Hazard Ratios for the Risk of Diabetes and Coronary Heart Disease among Apparently Healthy Young Adults, According to BMI in Adolescence and in Adulthood.
The joint association of body-mass index (BMI) at 17 years of age (adolescence) and 30 years of age (adulthood) is shown for the incidence of diabetes (Panel A) and the incidence of angiography-proven coronary heart disease (CHD) (Panel B), as calculated with the use of multivariate Cox proportional-hazards models. The gray columns denote significantly elevated hazard ratios as compared with those in the lowest BMI quintile in both adolescence and adulthood (reference group). When BMI was analyzed as a continuous variable with the use of a Cox regression model adjusted for age, triglyceride level, presence or absence of a family history of diabetes, and fasting glucose level, only BMI in adulthood was significantly associated with the risk of diabetes (β = 1.115, P = 0.003; P = 0.89 for interaction with BMI in adolescence). In contrast, in a regression model adjusted for age, triglyceride level, smoking status, presence or absence of a family history of CHD, and levels of high-density lipoprotein cholesterol and low-density lipoprotein cholesterol, BMI in both adolescence and adulthood was significantly and independently associated with the risk of CHD (BMI in adolescence, β = 1.355, P=0.004; BMI in adulthood, β = 1.207, P = 0.03; P < 0.05 for interaction).
To assess the importance of weight gain between adolescence and early adulthood in predicting subsequent incident diabetes or coronary heart disease, we calculated the change in BMI between the ages of 17 and 30 years and used it as a continuous variable. When added to the multivariate model (model 3 for each disease), adolescent BMI remained an independent predictor of both diabetes and coronary heart disease. The incremental change in BMI unit was an additional independent predictor of diabetes (hazard ratio, 1.083; 95% CI, 1.063 to 1.105), but not of coronary heart disease (hazard ratio, 1.006; 95% CI, 0.969 to 1.045). The change in BMI was lower among adolescents in the highest decile for BMI than among those in lower deciles (Table 1), which may have led to an underestimation of the added deleterious effect of weight gain on the risk of coronary heart disease.
DISCUSSION
This large-scale, long-term follow-up study suggests that elevated BMI in adolescence has distinctive relationships with type 2 diabetes and coronary heart disease in young adulthood. Diabetes is influenced mainly by recent BMI and weight gain, whereas for coronary heart disease both elevated BMI in adolescence and recent BMI are independent risk factors — that is, the natural history of coronary heart disease (in contrast with that of diabetes) is probably the consequence of gradually increasing atherosclerosis during adolescence and early adulthood that leads to clinically important disease in midlife. It is noteworthy that these conclusions were deduced with BMI values well within the range that is now defined as normal. Adolescent BMIs corresponding with the CDC growth charts at the percentiles of 49.3 (z score, −0.02) and 69.3 (z score, +0.51) were already associated with an increased risk of coronary heart disease and diabetes, respectively. These conclusions highlight the clinical importance of considering BMI history when assessing the risk of coronary heart disease versus the risk of diabetes in overweight or obese young adults. In terms of public health, this study supports concerns about the association between increasing cardiometabolic morbidity in early adulthood and the increase in BMI in adolescence, and the findings may suggest specific age-related considerations for the design of diabetes prevention programs that are distinct from those needed to prevent coronary heart disease. Finally, aided by the growth chart for young adults presented in Figure 1, our study may help to redefine what constitutes a “normal” or “healthy” BMI in adolescence and to highlight the role of elevated BMI at different ages in the pathogenesis of different diseases.
Our study has certain limitations. By the time they are 17 years old, not all boys will have completed puberty or reached their final height; thus, body composition at this age is not equivalent to that of adults, mainly because muscle mass is lower than that of adults. Indeed, increases in height between 17 and 30 years of age were greater on average in the lower deciles of BMI at adolescence; BMI increased by 4 units in the lower deciles and by 3 units in the 10th decile. Intriguingly, both early pubertal development and early adiposity rebound in childhood (the age at which BMI begins to rise after its physiologic nadir at 5 to 7 years of age) were implicated in the development of obesity. Thus, being placed in a lower decile for BMI during adolescence may be associated with having a lower risk of coronary heart disease or type 2 diabetes in early adulthood, reflecting a later onset of puberty or later increase in BMI.
A second consideration is waist circumference, a measure that would have been of value7 in our study but that was not available to us. Nevertheless, the use of BMI, a ratiometric measure, may partly diminish the variation in height over weight. Third, generalization of the study’s conclusions may be limited by the nature of the cohort — army personnel. However, the characteristics of our cohort are similar to those of other cohorts of young adults (as we discussed previously).25 The exclusion of women precludes our ability to determine sex-based differences in the association between BMI and the risk of diabetes or heart disease; we recently addressed this concern as it relates to the incidence of hypertension.26 The strengths of our follow-up study include well-documented outcomes based on strict diagnostic criteria, uniform and systematic follow-up assessments, a high rate of adherence during follow-up, standardized direct (rather than reported) measurements of weight and height, repeated measurements of many confounders, and the large size of the cohort.
Some studies have not shown an association between childhood or adolescent BMI and coronary heart disease in adulthood.22,29,30 However, our study suggests that adolescent BMI predicts angiography-proven coronary heart disease in young adulthood, a finding that is consistent with the results of the Norwegian Health Survey,17 which showed that the risk of coronary heart disease in adulthood is increased by a factor of approximately three among adolescent boys whose BMI is in the 86th percentile or higher. The present analyses show that an elevated BMI in adolescence predicts coronary heart disease in early adulthood, even independently of the BMI at a point in time that is closer to the diagnosis, with a risk for the upper decile of adolescent BMI that is nearly seven times as high as that for the lowest decile. In fact, such a relationship already exists within the normal BMI range as defined by the CDC, which is lower than the cutoffs at the 85th percentile that are normally used in population surveys. Thus, obesity in adolescence is probably only the tip of an iceberg of BMI-related increases in the risk of coronary heart disease that is relevant to approximately 50% of adolescent boys.
Remarkably, for type 2 diabetes, a significantly elevated hazard ratio was observed only in the 80th percentile of adolescent BMI and higher (>22.4), translating into a risk of disease that is nearly three times as high as that for those whose adolescent BMI is in the lowest decile. Moreover, adjustment for BMI in adulthood completely attenuated the effect, suggesting that BMI in adolescence has a more reversible or shorter term effect on the risk of diabetes as compared with its effect on the risk of coronary heart disease. Notably, however, those in the lowest decile for adolescent BMI tended to have a higher risk of future diabetes than those in the higher deciles, particularly when the analysis was adjusted for BMI in adulthood.
Our results might be explained by the fact that diabetes represents a more functional pathomechanism than coronary heart disease, which relies on anatomical changes (atherosclerosis). Such an explanation is consistent with the fact that even clinically established diabetes is readily reversible in response to changes in lifestyle or surgical interventions, whereas atherosclerosis, for example, is responsive to dietary intervention31 only if the intervention takes place before the “clinical horizon” of the disease has been reached.
In conclusion, BMI at the age of 17 years is an independent predictor of coronary heart disease in young adulthood, even when it is well within what is now defined as the normal range of BMI, suggesting that body mass has long-term consequences. Although adolescent BMI is also a predictor of the risk of type 2 diabetes, the risk is not independent of that predicted by BMI in adulthood.
Acknowledgments
Supported by a grant from the Talpiot Medical Leadership Program, Chaim Sheba Medical Center, Tel-Hashomer, Israel (to Dr. Tirosh), and by the Israel Defense Forces Medical Corps.
Footnotes
No potential conflict of interest relevant to this article was reported.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Deshmukh-Taskar P, Nicklas TA, Morales M, Yang SJ, Zakeri I, Berenson GS. Tracking of overweight status from childhood to young adulthood: the Bogalusa Heart Study. Eur J Clin Nutr. 2006;60:48–57. doi: 10.1038/sj.ejcn.1602266. [DOI] [PubMed] [Google Scholar]
- 2.Maynard LM, Wisemandle W, Roche AF, Chumlea WC, Guo SS, Siervogel RM. Childhood body composition in relation to body mass index. Pediatrics. 2001;107:344–50. doi: 10.1542/peds.107.2.344. [DOI] [PubMed] [Google Scholar]
- 3.Rodríguez G, Moreno LA, Blay MG, et al. Body composition in adolescents: measurements and metabolic aspects. Int J Obes Relat Metab Disord. 2004;28(Suppl 3):S54–S58. doi: 10.1038/sj.ijo.0802805. [DOI] [PubMed] [Google Scholar]
- 4.Rolland-Cachera MF, Deheeger M, Bellisle F, Sempé M, Guilloud-Bataille M, Patois E. Adiposity rebound in children: a simple indicator for predicting obesity. Am J Clin Nutr. 1984;39:129–35. doi: 10.1093/ajcn/39.1.129. [DOI] [PubMed] [Google Scholar]
- 5.Eriksson JG, Forsén T, Tuomilehto J, Osmond C, Barker DJ. Early growth and coronary heart disease in later life: longitudinal study. BMJ. 2001;322:949–53. doi: 10.1136/bmj.322.7292.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Field AE, Cook NR, Gillman MW. Weight status in childhood as a predictor of becoming overweight or hypertensive in early adulthood. Obes Res. 2005;13:163–9. doi: 10.1038/oby.2005.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forsén T, Eriksson JG, Tuomilehto J, Osmond C, Barker DJ. Growth in utero and during childhood among women who develop coronary heart disease: longitudinal study. BMJ. 1999;319:1403–7. doi: 10.1136/bmj.319.7222.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oren A, Vos LE, Uiterwaal CS, Gorissen WH, Grobbee DE, Bots ML. Change in body mass index from adolescence to young adulthood and increased carotid intima-media thickness at 28 years of age: the Atherosclerosis Risk in Young Adults study. Int J Obes Relat Metab Disord. 2003;27:1383–90. doi: 10.1038/sj.ijo.0802404. [DOI] [PubMed] [Google Scholar]
- 9.Sinha R, Fisch G, Teague B, et al. Prevalence of impaired glucose tolerance among children and adolescents with marked obesity. N Engl J Med. 2002;346:802–10. doi: 10.1056/NEJMoa012578. [Erratum, N Engl J Med 2002;346: 1756.] [DOI] [PubMed] [Google Scholar]
- 10.Srinivasan SR, Bao W, Wattigney WA, Berenson GS. Adolescent overweight is associated with adult overweight and related multiple cardiovascular risk factors: the Bogalusa Heart Study. Metabolism. 1996;45:235–40. doi: 10.1016/s0026-0495(96)90060-8. [DOI] [PubMed] [Google Scholar]
- 11.Srinivasan SR, Myers L, Berenson GS. Predictability of childhood adiposity and insulin for developing insulin resistance syndrome (syndrome X) in young adulthood: the Bogalusa Heart Study. Diabetes. 2002;51:204–9. doi: 10.2337/diabetes.51.1.204. [DOI] [PubMed] [Google Scholar]
- 12.Thompson DR, Obarzanek E, Franko DL, et al. Childhood overweight and cardiovascular disease risk factors: the National Heart, Lung, and Blood Institute Growth and Health Study. J Pediatr. 2007;150:18–25. doi: 10.1016/j.jpeds.2006.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tounian P, Aggoun Y, Dubern B, et al. Presence of increased stiffness of the common carotid artery and endothelial dysfunction in severely obese children: a prospective study. Lancet. 2001;358:1400–4. doi: 10.1016/S0140-6736(01)06525-4. [DOI] [PubMed] [Google Scholar]
- 14.Viner RM, Segal TY, Lichtarowicz-Krynska E, Hindmarsh P. Prevalence of the insulin resistance syndrome in obesity. Arch Dis Child. 2005;90:10–4. doi: 10.1136/adc.2003.036467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weiss R, Dziura J, Burgert TS, et al. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med. 2004;350:2362–74. doi: 10.1056/NEJMoa031049. [DOI] [PubMed] [Google Scholar]
- 16.Abraham S, Collins G, Nordsieck M. Relationship of childhood weight status to morbidity in adults. HSMHA Health Rep. 1971;86:273–84. [PMC free article] [PubMed] [Google Scholar]
- 17.Baker JL, Olsen LW, Sørensen TIA. Childhood body-mass index and the risk of coronary heart disease in adulthood. N Engl J Med. 2007;357:2329–37. doi: 10.1056/NEJMoa072515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bjørge T, Engeland A, Tverdal A, Smith GD. Body mass index in adolescence in relation to cause-specific mortality: a follow-up of 230,000 Norwegian adolescents. Am J Epidemiol. 2008;168:30–7. doi: 10.1093/aje/kwn096. [DOI] [PubMed] [Google Scholar]
- 19.Mossberg HO. 40-Year follow-up of overweight children. Lancet. 1989;2:491–3. doi: 10.1016/s0140-6736(89)92098-9. [DOI] [PubMed] [Google Scholar]
- 20.Franks PW, Hanson RL, Knowler WC, Sievers ML, Bennett PH, Looker HC. Childhood obesity, other cardiovascular risk factors, and premature death. N Engl J Med. 2010;362:485–93. doi: 10.1056/NEJMoa0904130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lawlor DA, Leon DA. Association of body mass index and obesity measured in early childhood with risk of coronary heart disease and stroke in middle age: findings from the Aberdeen children of the 1950s prospective cohort study. Circulation. 2005;111:1891–6. doi: 10.1161/01.CIR.0000161798.45728.4D. [DOI] [PubMed] [Google Scholar]
- 22.Lawlor DA, Martin RM, Gunnell D, et al. Association of body mass index measured in childhood, adolescence, and young adulthood with risk of ischemic heart disease and stroke: findings from 3 historical cohort studies. Am J Clin Nutr. 2006;83:767–73. doi: 10.1093/ajcn/83.4.767. [DOI] [PubMed] [Google Scholar]
- 23.Popkin BM, Conde W, Hou N, Monteiro C. Is there a lag globally in overweight trends for children compared with adults? Obesity (Silver Spring) 2006;14:846–53. doi: 10.1038/oby.2006.213. [DOI] [PubMed] [Google Scholar]
- 24.Steinberger J, Daniels SR, Eckel RH, et al. Progress and challenges in metabolic syndrome in children and adolescents: a scientific statement from the American Heart Association Atherosclerosis, Hypertension, and Obesity in the Young Committee of the Council on Cardiovascular Disease in the Young; Council on Cardiovascular Nursing; and Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2009;119:628–47. doi: 10.1161/CIRCULATIONAHA.108.191394. [DOI] [PubMed] [Google Scholar]
- 25.Tirosh A, Shai I, Tekes-Manova D, et al. Normal fasting plasma glucose levels and type 2 diabetes in young men. N Engl J Med. 2005;353:1454–62. doi: 10.1056/NEJMoa050080. [Erratum, N Engl J Med 2006;354:2401.] [DOI] [PubMed] [Google Scholar]
- 26.Tirosh A, Afek A, Rudich A, et al. Progression of normotensive adolescents to hypertensive adults: a study of 26,980 teenagers. Hypertension. 2010;56:203–9. doi: 10.1161/HYPERTENSIONAHA.109.146415. [DOI] [PubMed] [Google Scholar]
- 27.Tirosh A, Rudich A, Shochat T, et al. Changes in triglyceride levels and risk for coronary heart disease in young men. Ann Intern Med. 2007;147:377–85. doi: 10.7326/0003-4819-147-6-200709180-00007. [DOI] [PubMed] [Google Scholar]
- 28.Tirosh A, Shai I, Bitzur R, et al. Changes in triglyceride levels over time and risk of type 2 diabetes in young men. Diabetes Care. 2008;31:2032–7. doi: 10.2337/dc08-0825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Osler M, Lund R, Kriegbaum M, Andersen AM. The influence of birth weight and body mass in early adulthood on early coronary heart disease risk among Danish men born in 1953. Eur J Epidemiol. 2009;24:57–61. doi: 10.1007/s10654-008-9301-z. [DOI] [PubMed] [Google Scholar]
- 30.Must A, Jacques PF, Dallal GE, Bajema CJ, Dietz WH. Long-term morbidity and mortality of overweight adolescents: a follow-up of the Harvard Growth Study of 1922 to 1935. N Engl J Med. 1992;327:1350–5. doi: 10.1056/NEJM199211053271904. [DOI] [PubMed] [Google Scholar]
- 31.Shai I, Spence JD, Schwarzfuchs D, et al. Dietary intervention to reverse carotid atherosclerosis. Circulation. 2010;121:1200–8. doi: 10.1161/CIRCULATIONAHA.109.879254. [DOI] [PubMed] [Google Scholar]