Abstract
The current study examined the association between affect and self-reported alcohol intoxication in women with bulimia nervosa (BN; N = 133). Participants completed a two-week ecological momentary assessment protocol. Momentary global positive affect (PA) and negative affect (NA), as well as the facets of NA (fear, guilt, hostility and sadness), were measured. Forty-five participants endorsed that they “got drunk” during the study period. Daily mean and variability of global PA and NA were compared between days with self-reported alcohol intoxication and days without self-reported alcohol intoxication. Trajectories of affect were modeled prior to and following episodes of self-reported alcohol intoxication. There were no differences in the mean or variability of PA or NA on days characterized by self-reported alcohol intoxication compared to days with no self-reported alcohol intoxication (ps > 0.05). PA decreased significantly prior to self-reported alcohol intoxication and remained stable afterwards. There were no changes in global NA before or after self-reported alcohol intoxication, but an examination of the facets of NA showed that sadness increased following episodes of self-reported alcohol intoxication. These findings showed only partial support for a negative reinforcement model of alcohol use in women with BN.
Keywords: Bulimia Nervosa, Alcohol Use, Ecological Momentary Assessment
1. Introduction
Bulimia nervosa (BN) involves recurrent binge eating episodes, compensatory behaviors such as vomiting and laxative use, and a self-concept dominated by shape and weight (American Psychiatric Association, 2013). The health consequences of BN can be severe and BN is marked by increased mortality (Arcelus et al., 2011). Individuals with BN frequently have comorbid alcohol use disorders (AUD) with comorbidity rates ranging from 30 to 50% (Bulik et al., 1997; Dansky et al., 2000; Holderness et al., 1994; Mitchell et al., 1985). The high comorbidity of BN and AUD is particularly concerning as it is associated with an increased prevalence of both major depressive disorder and suicide attempts (Duncan et al., 2006).
Several hypotheses have been posited to explain the high co-occurrence of eating disorders and substance use disorders, which focus on either shared or causal etiological conceptualizations (Wolfe and Maisto, 2000). In terms of a shared etiological conceptualization, a shared genetic liability to develop both disorders has been the primary hypothesis investigated. There is evidence of shared genetic factors between bulimic behaviors and alcohol misuse (Baker et al., 2010; Munn-Chernoff et al., 2013; Slane et al., 2012; Trace et al., 2013); however, one study has shown this shared genetic liability to be small (Kendler et al., 1995). More of the research to date has focused on exploring possible causal etiologies, typically based on the eating disorder preceding the substance use disorder (Wolfe and Maisto, 2000). Longitudinal data support this trajectory, as adolescents with BN or purging behavior are more than twice as likely to develop binge drinking behavior as non-eating disorder peers (Field et al., 2012). Self-medication and tension-reduction are the two causal conceptualizations that have received the most research attention (Wolfe and Maisto, 2000) and are based on the hypotheses that individuals with eating disorders use substances to alleviate depression (self-medication) or anxiety (tension-reduction), highlighting the role of affect in the development of substance use in individuals with eating disorders. There is also clinical utility in focusing on mechanisms that maintain AUD in individuals with BN, as these findings may be more useful in designing targeted treatment and prevention efforts.
One such maintenance process involves the idea that bulimic behaviors as well as alcohol use serve as strategies to regulate emotions (Dansky et al., 2000). Emotion regulation deficits have been demonstrated separately in both BN (Engel et al., 2007) and AUD samples (Berking et al., 2011). Further, both BN and AUD have each been associated with high and comparable levels of negative urgency, a personality trait defined as the tendency to act rashly in response to negative affect (Fischer et al., 2012). Therefore, one hypothesis for the high comorbidity of BN and AUD is that individuals with emotion regulation difficulties use both eating disorder behaviors and alcohol to regulate negative affect (NA). These behaviors may then be maintained through negative reinforcement. Additionally, alcohol use has been posited to increase positive affect (PA) and thus be maintained through positive reinforcement (Sher and Grekin, 2007). Given that many individuals with BN experience anhedonia (Tchanturia et al., 2012), momentary increases in positive affect may be particularly reinforcing in this population.
One critique of the literature exploring the comorbidity of eating disorders and substance use has been the reliance on prevalence data rather than behavioral data (Wolfe and Maisto, 2000). Ecological momentary assessment (EMA) measures behavioral and psychological variables in “real time” and thus is an ideal methodology to explore behaviors in relation to affect (Stone and Shiffman, 1994; Stone and Shiffman, 2002). Previous research using EMA in BN has shown that NA, particularly guilt, increases prior to behaviors such as binge eating and/or purging, and decreases significantly after, indicating that these behaviors may be maintained through negative reinforcement (Berg et al., 2013; Smyth et al., 2007). In non-eating disorder samples, EMA data reveal that the time period prior to substance use is marked by high NA, specifically nervousness and anger (Swendsen et al., 2000; Todd et al., 2009). Although most of the EMA research in alcohol use has not monitored affect directly after the drinking episode, the anxiolytic properties of alcohol have been shown to decrease feelings of NA (Baker et al., 2004). Thus, the maintenance mechanism of alcohol use may also be negative reinforcement (Baker et al., 2004). The hypothesis that individuals with BN also use alcohol to regulate negative emotions is supported by studies using self-report measures, as individuals with BN are more likely to endorse drinking to cope with negative emotions than individuals with no eating disorder (Luce et al., 2007). However, retrospective recall of motives to drink are limited by recall bias and do not allow for a momentary, functional assessment of the association between alcohol consumption and affect.
Although there is evidence that binge eating/purging and alcohol use function to mitigate negative affect in BN and AUD samples, respectively, little is known about the function of alcohol use in individuals with BN in the context of momentary emotion regulation. The primary aim of the present study was therefore to examine the association between affect and self-reported alcohol intoxication in a sample of women with BN using EMA. Three specific research questions were investigated: 1) Does mean positive affect (PA) and NA on days characterized by self-reported alcohol intoxication differ from days with no self-reported alcohol intoxication in a sample of women with BN? We hypothesized that mean PA would be lower and mean NA would be higher on days with self-reported alcohol intoxication than days with no self-reported alcohol intoxication; 2) Is there more variability in PA and NA on days characterized by self-reported alcohol intoxication than days with no self-reported alcohol intoxication? We hypothesized that days characterized by self-reported alcohol intoxication would have higher variability in both PA and NA than days with no self-reported alcohol intoxication; and 3) Is self-reported alcohol intoxication preceded by decreased PA and increased NA and reinforced by increases in PA and decreases in NA following these episodes? We hypothesized that PA would decrease and NA would increase prior to the episode of self-reported alcohol intoxication and that PA would increase and NA would decrease following these episodes. We also included an exploratory aim to examine the effect of self-reported alcohol intoxication on four facets of NA, specifically guilt, fear, hostility, and sadness.
2. Material and Methods
2.1 Participants
Participants were 133 adult women who met Diagnostic and Statistical Manual of Mental Disorders (4th ed., text rev.; American Psychiatric Association, 2000) criteria for BN. Participants ranged in age from 18 to 55 years, with a mean age of 25.3 (SD = 7.6 years). Most participants were Caucasian (95.5%), currently employed (73.3%), and had never been married (63.9%). Lifetime rates of Axis I disorders were 87.0% for mood disorders and 59.5% for anxiety disorders. All participants were at least 85% of ideal body weight (mean body mass index [BMI] 23.9, SD = 5.2). Detailed descriptions of participants' demographic data, symptom severity, and rates of co-occurring psychopathology have been previously reported (Crosby et al., 2009; Smyth et al., 2007).
2.2 Procedure
This study was approved by the Institutional Review Boards of the University of North Dakota and MeritCare Hospital (Fargo, ND) and was carried out in accordance with the latest version of the Declaration of Helsinki. Participants were recruited through clinical, community, and campus advertisements. Interested participants were initially screened over the phone for inclusion and exclusion criteria. Eligible participants were scheduled for an informational meeting during which they received information about the study, had the opportunity to ask questions about their participation, and then provided written informed consent. Participants completed two assessment visits during which they completed a battery of assessments including semi-structured interviews, self-report questionnaires, and an electrolyte screening to ensure medical stability.
After baseline assessments, eligible participants were given palm-top computers to complete EMA assessments over the course of the next two weeks. The EMA assessment protocol implemented three types of daily self-report methods: 1) signal-contingent recording; 2) interval-contingent recording; and 3) event-contingent recording. With regard to the signal-contingent recording, participants were signaled by the palm-top computer to complete EMA assessment ratings at six semi-random times throughout the day that were all within 20 min of each of six “anchor” times distributed evenly throughout the day: 8:30 a.m., 11:10 a.m., 1:50 p.m., 4:30 p.m., 7:10 p.m., and 9:50 p.m. With regard to interval-contingent recording, participants were instructed to complete EMA assessment ratings at the end of each day. Participants were instructed to complete an event contingent recording immediately following binge eating or purging. During each recording, participants completed two questionnaires, described below. Participants received $200 for completing the two-week assessment period and were given a $50 bonus for completing at least 85% of assessments within 45 min of the palmtop signal. For additional detail regarding the procedure, please refer to Smyth et al. (2007)
2.3 Baseline Measures
2.3.1. Structured Clinical Interview for DSM–IV Axis I Disorders, Patient Edition (SCID– I/P)
The SCID–I/P (First et al., 2002) is a semi-structured interview that measures Axis I psychopathology. The SCID–I/P was administered by a trained doctoral-level psychologist and was used to establish lifetime history of Axis I disorders. The SCID–I/P was used to determine whether participants met current DSM–IV criteria for BN, lifetime criteria for an alcohol use disorder, and lifetime criteria for any other substance use disorder (except nicotine abuse or dependence). All interviews were audiotaped, and inter-rater reliability was calculated on 25 cases from the sample. The kappa coefficient for current DSM–IV BN diagnosis was 1.0.
2.3.2. Eating Disorder Examination (EDE; Fairburn and Cooper, 1993; Fairburn et al., 2008)
The EDE is a widely-used clinician-administered interview comprised of four subscales (Restraint, Eating Concern, Shape Concern, & Weight Concern) reflecting the severity of specific dimensions of eating disorder psychopathology, as well as a Global score. This measure exhibits adequate reliability and demonstrates validity for the assessment of eating disorder symptoms (Berg et al., 2012; Fairburn et al., 2008). The Global score was used in the current study.
2.3.3. Beck Depression Inventory (BDI)
The BDI is a 21-item self-report questionnaire that assesses the severity of current depressive symptoms. The reliability and validity of the BDI have been well documented (Beck et al., 1988). Coefficient alpha in the present study was 0.90.
2.4 Momentary EMA Measures
2.4.1. Positive and Negative Affect States (PANAS)
The PANAS (Watson, 1988) measures two general dimensions of affect (i.e., positive and negative) as well as facets of affect (e.g., fear, guilt). A subset of items was administered and those items were chosen based on their factor loadings (Smyth, et al., 2007). Thirteen items were selected for PA: alert, attentive, calm, cheerful, concentration, confident, determined, energetic, enthusiastic, happy, proud, strong, and relaxed. Eleven items from the PANAS were chosen to assess momentary NA: afraid, lonely, irritable, ashamed, angry, disgusted, nervous, dissatisfied with self, jittery, sad, and angry with self. Participants were asked to rate the extent to which they currently felt these emotions on a 5-point Likert scale, ranging from 1 (not at all) to 5 (extremely). The internal consistency of the abbreviated PA scale (0.87) and NA scale (0.92) was consistent with the internal consistency of the full scales when assessed at the momentary level (range of .85–.91; Watson, 1988). A confirmatory factor analysis of the NA scale using the data from the present sample derived a four factor solution that replicated the results of the original factor analysis (Watson, 1988) with the exception that disgust loaded onto the hostility factor (Berg et al., 2013). The Cronbach's alphas of the four abbreviated lower-order NA subscales were 0.80 (fear), 0.89 (guilt), 0.79 (hostility), and 0.81 (sadness), demonstrating good internal consistency (Berg et al., 2013).
2.4.2. Self-reported Alcohol Intoxication
Items from several scales of eating disorder and self-destructive behavior were used to create a 19-item checklist of momentary behaviors. Only the alcohol related question (“I got drunk”) was used in the analyses. Participants were asked to indicate whether or not they had engaged in a behavior since the previous assessment point.
2.4.3. Eating Disorder Checklist
Participants were asked to indicate whenever they engaged in binge eating, self-induced vomiting, laxative misuse, or diuretic misuse. These behaviors could be recorded either immediately after they occurred or during the next signaled recording. In the current analyses, self-induced vomiting, laxative misuse, and diuretic misuse were combined to form a single variable representing purging.
2.5 Statistical Analyses
2.5.1. Demographics
T-tests and chi-square analyses were conducted to determine whether there were any differences between those who did and did not endorse an episode of self-reported alcohol intoxication on demographic variables and psychopathology.
2.5.2. Between-day analyses
Chi-square analyses were conducted to determine whether there was a difference in prevalence of binge eating and purging on days characterized by self-reported alcohol intoxication compared to days without self-reported alcohol intoxication. Multilevel models were conducted to assess differences in daily level and variability of PA and NA between days with and without self-reported alcohol intoxication. These models were based on a general linear model. Data were aggregated across repeated assessments within days so that mean PA and NA scores could be calculated for each participant for each day of data collection. Variability in PA and NA was calculated with mean squared successive difference (MSSD) statistics to determine the average degree of variability in affect over time. MSSD values symbolized the variation in PA and NA each day in relation to the squared difference across successive time points and the distance between the measured time points (Witte et al., 2005). Mixed model analyses were used to analyze levels of daily affect and variability in affect (level 1) nested within subjects (level 2). The mixed models included a random effect for subjects and fixed effects for type of day (i.e., self-reported alcohol intoxication reported or not).
2.5.3 Within-day analyses
To examine the temporal relationship between both PA and NA and self-reported alcohol intoxication, we modeled the pre- and post- event trajectories of PA, NA, and each facet of NA separately using piecewise linear, quadratic, and cubic functions centered on the time at which the self-reported alcohol intoxication event was reported. Multilevel models included linear functions (i.e., hours prior to event, hours following event), which reflected the rate of change in affect prior to and following self-reported alcohol intoxication; quadratic functions (i.e., [hours prior to event]2, [hours following event]2) which reflected the acceleration in rate of affect change prior to and following self-reported alcohol intoxication; and cubic functions (i.e., [hours prior to event]3, [hours following event]3), which reflected either further acceleration or dampening of the acceleration in rate of affect change. When more than one episode of self-reported alcohol intoxication was reported in a single day (n =10), only the first behavior was used to avoid confounding the relationship between antecedent and consequent mood ratings in relation to the multiple self-reported alcohol intoxication behaviors that occurred throughout any one day.
3. Results
3.1 Description of sample
Of the 133 participants, 45 (33.8%) reported at least one episode of self-reported alcohol intoxication during the study time period. A total of 134 episodes of self-reported alcohol intoxication were recorded across these 45 participants (range: 1-12 episodes) and used for the current study analyses. Comparisons between the individuals who self-reported any episodes of alcohol intoxication and those who did not on measures of demographic variables and baseline comorbid psychopathology are presented in Table 1. There were no significant demographic differences between those who self-reported alcohol intoxication and those who did not. In terms of baseline psychopathology, the only differences were that those who self-reported alcohol intoxication during the study time period were more likely to have a lifetime alcohol use disorder (p = 0.047) but were less likely to have a lifetime diagnosis of another substance use disorder (p = 0.017). An examination of severity of eating disorder symptoms over the course of the two-week EMA protocol found that there were no differences in the mean total number of binge eating and purging episodes reported per day between the group who self-reported alcohol intoxication (mean = 1.30, SD = 0.98) and those who did not self-report alcohol intoxication (mean = 1.22, SD = 0.93; t(131) = 0.46, p = 0.65).
Table 1. Comparisons between those who had at least one episode of self-reported alcohol intoxication (endorsed “I got drunk”) and those with no episodes of self-reported alcohol intoxication during the two-week ecological momentary assessment protocol.
| Did not endorse “I got drunk” (n = 88) | Endorsed “I got drunk” (n = 45) | Comparison | |
|---|---|---|---|
| Age (mean, SD) | 25.37 (7.95) | 25.30 (7.01) | t(129) = 0.05, p = 0.95 |
| BMI (mean, SD) | 23.45 (5.33) | 24.65 (4.94) | t(131) = -1.16, p = 0.97 |
| Caucasian (%, n) | 98.8 (n = 85) | 93.3 (n = 42) | χ2(3) = 4.13, p = 0.25 |
| EDE Global (mean, SD) | 3.18 (1.26) | 3.51 (0.80) | t(124.55) = -1.87, p = 0.07 |
| BDI (mean, SD) | 18.50 (10.01) | 19.78 (10.31) | t(131) = -0.69, p = 0.79 |
| Lifetime AUD (%, n) | 48.86 (n = 43) | 71.11 (n = 32) | χ2(2) = 6.10, p = 0.047 |
| Lifetime SUD (%, n) | 51.14 (n = 45) | 28.89 (n = 13) | χ2(1) = 5.99, p = 0.017 |
3.2 Between-Day Results
There was no difference in prevalence of binge eating on days with and without self-reported alcohol intoxication (χ2 (1) = 0.240, p = 0.63). Purging was more prevalent on days characterized by self-reported alcohol intoxication (58.3%) than days with no self-reported alcohol intoxication (47.7%; χ2 (1) = 0.5136, p = 0.023).
There was no difference in mean daily level of PA on self-reported alcohol intoxication days (mean, 33.96; SE, 0.86) than non-self-reported alcohol intoxication days (mean, 34.01; SE, 0.74; F1787.64 = 0.008; p = 0.93). Differences in MSSD for PA were also not observed (F1883.79 = 0.08; p = 0.78). There was no difference in mean daily level of NA on self-reported alcohol intoxication days (mean, 24.52; SE, 0.83) than the non-self-reported alcohol intoxication days (mean, 24.01; SE, 0.72; F1787.05 = 1.23, p = 0.27). Differences in MSSD for NA were also not found (F1882.57 = 0.01; p = 0.96).
3.3 Within-Day Results
The results of the within-day analyses for PA and NA are provided in Table 2 and illustrated in Figure 1 using data estimated by the linear, quadratic, and cubic coefficients. PA was found to decrease until the time of self-reported alcohol intoxication, with significant linear (p = 0.023), quadratic (p < 0.001), and cubic coefficients (p = 0.002). Results from the linear function indicated that there was no significant change in PA following episodes of self-reported alcohol intoxication (p > 0.05). There was no significant change in overall NA prior to or after episodes of self-reported alcohol intoxication (all ps > 0.05).
Table 2. Within-Day Multilevel Models for Self-Reported Alcohol Intoxication Events.
| Variable | PA | NA | ||||
|---|---|---|---|---|---|---|
| Est. | SE | t | Est. | SE | t | |
| Intercept | 31.764 | 0.635 | 49.990*** | 30.187 | 1.171 | 25.775*** |
| Hours prior to event | -0.305 | 0.134 | -2.275** | -0.024 | 0.126 | -0.188 |
| (Hours prior to event)2 | 0.054 | 0.012 | 4.384*** | -0.021 | 0.012 | -1.725 |
| (Hours prior to event)3 | -0.001 | 0.001 | -3.121** | 0.001 | 0.001 | 1.424 |
| Hours following event | 0.251 | 0.186 | 1.352 | 0.171 | 0.167 | 1.029 |
| (Hours following event)2 | -0.061 | 0.013 | -4.616*** | 0.023 | 0.013 | 1.768 |
| (Hours following event)3 | 0.002 | 0.001 | 3.147*** | -0.001 | 0.001 | -1.77 |
Note: These analyses are based on 45 individuals and 134 episodes of binge drinking. PA (positive affect) and NA (negative affect) derived from a modified version of the Positive and Negative Affect Scale.
p < 0.01
p < 0.001
Figure 1. Levels of positive and negative affect over time in relation to self-reported alcohol intoxication.
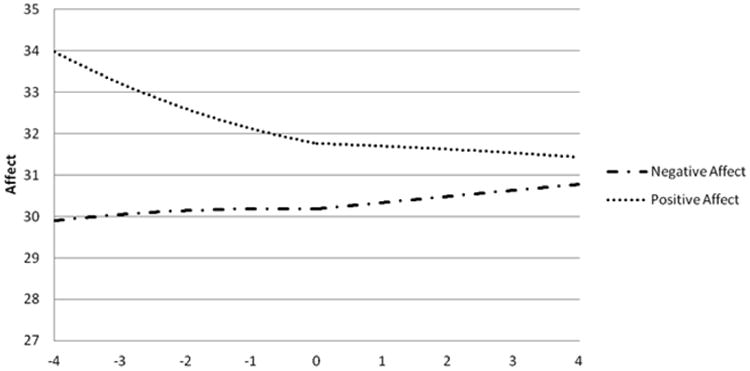
Time in Hours Relative to Self-Reported Alcohol Intoxication
Note: Affect derived from a modified version of the Positive and Negative Affect Scale with higher scores indicating greater affect. The figure is centered on the time of reported alcohol intoxication (0) and presents the affect for the four hours prior (-4:0) and four hours following (0:4) alcohol intoxication.
The results of the within-day analyses for each facet of NA are provided in Table 3 and illustrated in Figure 2 using data estimated by the linear, quadratic, and cubic coefficients. Examination of the facets of NA revealed that there was no change in sadness prior to self-reported alcohol intoxication but that sadness increased following the event (p = 0.038). There were no significant changes in trajectories for fear, guilt, or hostility prior to or after self-reported alcohol intoxication (ps > 0.05). To further examine the unique effect of sadness, we repeated the within-day analyses described above, examining sadness using the other three facets of NA as covariates. This post-hoc analysis revealed that there was still no change in sadness prior to self-reported alcohol intoxication but that sadness still increased following the event (p = 0.027)
Table 3. Within-day multilevel models for facets of negative affect for self-reported alcohol intoxication events.
| Fear | Guilt | Hostility | Sadness | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Est. | SE | t | Est. | SE | t | Est. | SE | t | Est. | SE | t |
| Intercept | 2.232 | 0.117 | 19.011*** | 3.063 | 0.117 | 26.213*** | 2.453 | 0.127 | 19.362*** | 2.802 | 0.125 | 22.337*** |
| Hours prior to event | -0.004 | 0.013 | -0.313 | -0.001 | 0.014 | -0.073 | 0.010 | 0.014 | 0.724 | -0.015 | 0.015 | -1.015 |
| (Hours prior to event)2 | -0.001 | 0.001 | -0.984 | -0.003 | 0.001 | -2.041* | -0.002 | 0.001 | -1.246 | -0.002 | 0.001 | -1.103 |
| (Hours prior to event)3 | 0.000 | 0.000 | 1.033 | 0.000 | 0.000 | 1.209 | 0.000 | 0.000 | 0.596 | 0.000 | 0.000 | 1.670 |
| Hours following event | 0.004 | 0.017 | 0.237 | 0.022 | 0.019 | 1.209 | -0.015 | 0.019 | -0.774 | 0.041 | 0.020 | 2.082* |
| (Hours following event)2 | 0.002 | 0.001 | 1.533 | 0.003 | 0.001 | 1.834 | 0.001 | 0.001 | 1.012 | 0.002 | 0.002 | 1.131 |
| (Hours following event)3 | 0.000 | 0.000 | -0.678 | 0.000 | 0.000 | -2.234* | 0.000 | 0.000 | -0.443 | 0.000 | 0.001 | -2.109* |
Note: These analyses are based on 45 individuals and 134 episodes of binge drinking. Facets of negative affect derived from a modified version of the Positive and Negative Affect Scale.
p < 0.05
p < 0.01
p < 0.001
Figure 2. Levels of facets of negative affect over time in relation to self-reported alcohol intoxication.
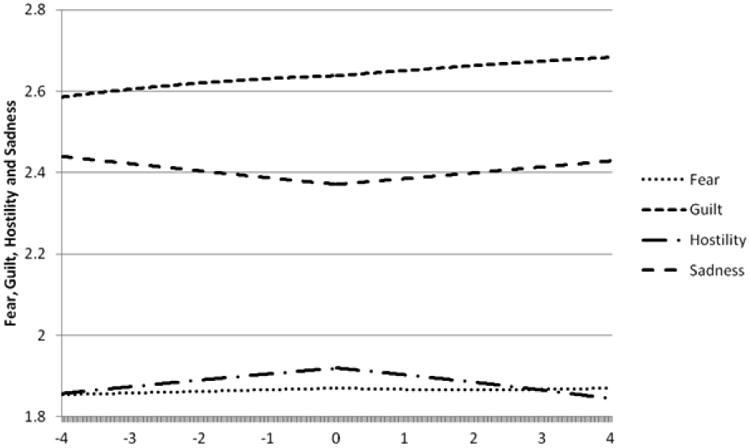
Time in Hours Relative to Self-Reported Alcohol Intoxication
Note: Affect derived from a modified version of the Positive and Negative Affect Scale with higher scores indicating greater affect. The figure is centered on the time of reported alcohol intoxication (0) and presents the affect for the four hours prior (-4:0) and four hours following (0:4) alcohol intoxication.
4. Discussion
BN is often comorbid with AUD, and both disorders are characterized by difficulties in emotion regulation. The present study is the first to examine the association between affect and self-reported alcohol intoxication using EMA data in a sample of women with BN. Contrary to the hypotheses, the between-day results indicated that there were no differences in mean PA or NA or in the variability of PA or NA on days characterized by self-reported alcohol intoxication compared to days without self-reported alcohol intoxication. Within-day results indicated that, consistent with our hypotheses, PA decreased prior to episodes of self-reported alcohol intoxication but, contrary to hypotheses, remained stable for the four hours after. Additionally, there was no change in global NA before or after episodes of self-reported alcohol intoxication. However, exploratory analyses of the facets of NA revealed that sadness was stable prior to episodes of self-reported alcohol intoxication but increased in the hours following these episodes. There were no changes in fear, guilt, or hostility prior to or after self-reported alcohol intoxication. Overall, these results failed to fully support the negative reinforcement model of self-reported alcohol intoxication in women with BN but suggest that reductions in PA may be an important precipitant.
In the present study, self-reported alcohol intoxication was preceded by decreasing PA in the hours prior to the episode but was not associated with lower daily mean PA or variability of PA. This finding is particularly interesting as individuals who drink to enhance PA have been found to drink more heavily than individuals who drink to regulate NA (Cooper, 1994; Cooper et al., 1992). Therefore, given that we were assessing only self-reported alcohol intoxication (i.e., “getting drunk”), we may have captured individuals who were drinking relatively heavily to increase PA and missed episodes of more moderate drinking that might not result in “getting drunk” and that could be used to regulate NA. Additionally, self-reported alcohol intoxication tends to be more social in nature than more moderate levels of drinking, and in those social contexts, drinking is hypothesized to be specifically influenced by a motivation to increase PA (Christiansen et al., 2002). Thus, a possible explanation of our findings is that individuals with BN may be getting drunk in response to decreased PA with the goals of increasing PA and possibly fostering social engagement. Indeed, individuals with BN have been shown to have increased social anhedonia in comparison to controls (Tchanturia et al., 2012), and as such, difficulty enjoying social encounters may be one important motivator for getting intoxicated in individuals with BN. As the context of drinking was not assessed in our protocol, we were unable to test this hypothesis and future research is needed to examine the impact of environmental conditions. Although there was no increase in PA after self-reported alcohol intoxication, PA did not decrease any further following the episode. Thus, getting drunk may have served to stabilize decreasing PA.
Self-reported alcohol intoxication was not associated with either daily high mean NA or increasing NA in the hours before the episode. Thus, data from the present study do not support the hypothesis that individuals with BN consumed alcohol to down-regulate NA in the moment. This finding is particularly unexpected as days characterized by self-reported alcohol intoxication had a higher prevalence of purging, and purging days have been shown to have greater mean and variability of negative affect (Smyth et al., 2007). However, while the difference in prevalence of purging was statistically significant, the difference was likely not clinically significant as purging occurred on slightly over half (58.3%) of days characterized by self-reported alcohol intoxication compared to slightly under half (47.7%) of days with no self-reported alcohol intoxication. There was no difference in prevalence of binge eating, which has also been shown to be associated with high negative affect, on days characterized by self-reported alcohol intoxication compared to days with no self-reported alcohol intoxication (Smyth et al., 2007).
Using alcohol to cope with NA is a function of both the availability of alcohol and alternative coping strategies (Abrams & Niaura, 1987). As both binge eating and purging have been shown to regulate NA in individuals with BN (Berg et al., 2013; Smyth et al., 2007), and food is typically much more readily available than alcohol, alcohol use may not be the preferred momentary strategy for coping with NA among individuals with BN. Additionally, proximal (e.g., same day) NA may not lead to immediate alcohol use, as alcohol use may result in cognitive and physical impairments. For example, an individual may not attempt to regulate NA related to a pending work deadline with immediate alcohol use as this might impact ability to complete the assignment, but instead delay alcohol consumption for the weekend once the work is complete. Indeed, exerting self-control to cope with stress over a period of time may lead to decreased self-control at a later time (Muraven and Baumeister, 2000). Thus, getting through a challenging time without engaging in alcohol use may result in decreased self-control at a later point, potentially leading to getting drunk without proximal high or increasing NA (Muraven and Baumeister, 2000).
Our finding that sadness increased following episodes of self-reported alcohol intoxication was not surprising, given the depressive effects of alcohol. However, it is noteworthy that increased sadness was the only consequence of self-reported alcohol intoxication that was observed in our sample. Increasing sadness should theoretically serve as punishment for the behavior, rather than a reinforcement or maintenance mechanism. However, if self-reported alcohol intoxication is occurring primarily in social situations, as hypothesized above, the sadness may be attributed to loneliness at the end of a social interaction in addition to or instead of the increased sadness being related to the alcohol use itself. Although we were unable to examine the context of the drinking episodes, given that the sadness scale used in the study was comprised of the items “sad” and “lonely,” this hypothesis is plausible. Thus, the ability to remain in and engage in a social situation as well as the stabilization of PA may have served as an immediate reinforcement for alcohol use, even if a longer-term increase in sadness was also experienced.
One methodological issue is particularly important in considering the results from this study. As this study was originally designed primarily to assess affect surrounding bulimic behaviors, the decision was made to assess only episodes in which the individual endorsed “I got drunk” rather than all episodes of alcohol use. Therefore, we were unable to center our within-day analyses on time of the initial drink, but rather, on the time at which the self-reported alcohol intoxication was reported—which could have been once the alcohol use episode was already either well underway or even completed. Thus, we may have missed changes in affect that could have occurred immediately prior to and following the first drink. Inconsistency is notable in methodologies reported in the alcohol use EMA literature, with some studies examining first alcohol drink only, some examining each drink separately, and others examining drinking episodes as a whole, as was done in our study (for a review, see Shiffman, 2009). Therefore, although we were able to examine trajectories of affect around the time intoxication was reported, we were unable to examine affect around the first drink of the drinking episode or episodes of moderate alcohol use.
The current study had several strengths. This study is the first to examine the association between affect and self-reported alcohol intoxication using EMA in a BN sample. The time stamping of assessments in EMA allows for improved reliability and validity of the data compared to other methods of self-report or self-monitoring. However, several limitations should be noted. Despite the use of time stamping and EMA methodology, the data are still self-report in nature. We were unable to assess the reliability of the item “got drunk” in this sample and we did not collect data on the number of servings of alcohol. Thus, it is possible that participants reported that they “got drunk” when the number of alcoholic drinks consumed would not have been substantial enough to result in intoxication. Additionally, as described above, the use of the item “I got drunk” may mean that the affective response to the first alcohol drink was not captured. We did not assess the context in which the individual was drinking and thus were not able to examine our hypothesis about drinking in social situations. Finally, the number of episodes of self-reported alcohol intoxication was rather small, including only 134 episodes, which may have impacted power.
5. Conclusion
This study provided an initial exploration of the role of affect in episodes of self-reported alcohol intoxication in women with BN using EMA methodology. Overall, our findings did not support the hypothesis that self-reported alcohol intoxication in individuals with BN is reinforced by decreases in NA following the drinking episode. Rather, these episodes appear to be preceded by decreases in PA and followed by increases in sadness. Therefore, alcohol use may have a different maintenance mechanism in women with BN than their eating disorder symptoms. Further research is needed to identify situations in which individuals with BN may be at highest risk of alcohol intoxication. Additionally, further research should examine affect surrounding more moderate episodes of alcohol use in a BN population. Understanding the function of moderate alcohol use as well as getting drunk in a population at high risk for comorbidity will serve to better inform prevention and treatment efforts.
Highlights.
We modeled affect around episodes of intoxication in women with bulimia nervosa
No differences in mean affect on days characterized by intoxication
No differences in variability of affect on days characterized by intoxication
Positive affect decreased prior to episodes of intoxication
Sadness increased following episodes of intoxication
Acknowledgments
Research reported in this publication was supported by Grants R01 MH59674 and T32 MH082761 from the National Institute of Mental Health and the National Center for Advancing Translational Sciences of the National Institutes of Health Award Number UL1TR000114. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th, text rev. American Psychiatric Press; Washington, DC: 2000. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fifth. American Psychiatric Publishing; Arlington, VA: 2013. [Google Scholar]
- Arcelus J, Mitchell AJ, Wales J, Nielsen S. Mortality rates in patients with anorexia nervosa and other eating disorders: a meta-analysis of 36 studies. Archives Gen Psychiatry. 2011;68(7):724–731. doi: 10.1001/archgenpsychiatry.2011.74. [DOI] [PubMed] [Google Scholar]
- Baker JH, Mitchell KS, Neale MC, Kendler KS. Eating disorder symptomatology and substance use disorders: prevalence and shared risk in a population based twin sample. Int J Eat Disord. 2010;43(7):648–658. doi: 10.1002/eat.20856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychol Rev. 2004;111(1):33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Beck A, Epstein N, Brown G, Steer R. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56:893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- Berg KC, Crosby RD, Cao L, Peterson CB, Engel SG, Mitchell JE, Wonderlich SA. Facets of negative affect prior to and following binge-only, purge-only, and binge/purge events in women with bulimia nervosa. J Abnorm Psychol. 2013;122(1):111–118. doi: 10.1037/a0029703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg KC, Peterson CB, Frazier P, Crow SJ. Psychometric evaluation of the eating disorder examination and eating disorder examination-questionnaire: a systematic review of the literature. Int J Eat Disord. 2012;45(3):428–438. doi: 10.1002/eat.20931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berking M, Margraf M, Ebert D, Wupperman P, Hofmann SG, Junghanns K. Deficits in emotion-regulation skills predict alcohol use during and after cognitive-behavioral therapy for alcohol dependence. J Consult Clin Psychol. 2011;79(3):307–318. doi: 10.1037/a0023421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulik C, Sullivan P, Joyce P, Carter F. Lifetime comorbidity of alcohol dependence in women with bulimia nervosa. Addictive Behaviors. 1997;22:437–446. doi: 10.1016/s0306-4603(96)00053-6. [DOI] [PubMed] [Google Scholar]
- Christiansen M, Vik PW, Jarchow A. College student heavy drinking in social contexts versus alone. Addict Behav. 2002;27(3):393–404. doi: 10.1016/s0306-4603(01)00180-0. [DOI] [PubMed] [Google Scholar]
- Cooper ML. Motivations for alcohol use among adolescents: Development and validation of a four-factor model. Psychol Assessment. 1994;6(2):117. [Google Scholar]
- Cooper ML, Russell M, Skinner JB, Windle M. Development and validation of a three-dimensional measure of drinking motives. Psychol Assessment. 1992;4(2):123. [Google Scholar]
- Crosby RD, Wonderlich SA, Engel SG, Simonich H, Smyth J, Mitchell JE. Daily mood patterns and bulimic behaviors in the natural environment. Behav Res Ther. 2009;47(3):181–188. doi: 10.1016/j.brat.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dansky B, Brewerton T, Kilpatrick D. Comorbidity of bulimia nervosa and alcohol use disorders: Results from the national women's study. nt J Eat Disord. 2000;27:180–190. doi: 10.1002/(sici)1098-108x(200003)27:2<180::aid-eat6>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Duncan AE, Neuman RJ, Kramer JR, Kuperman S, Hesselbrock VM, Bucholz KK. Lifetime psychiatric comorbidity of alcohol dependence and bulimia nervosa in women. Drug Alcohol Depend. 2006;84(1):122–132. doi: 10.1016/j.drugalcdep.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Engel SG, Boseck JJ, Crosby RD, Wonderlich SA, Mitchell JE, Smyth J, Miltenberger R, Steiger H. The relationship of momentary anger and impulsivity to bulimic behavior. Behav Res Ther. 2007;45(3):437–447. doi: 10.1016/j.brat.2006.03.014. [DOI] [PubMed] [Google Scholar]
- Fairburn C, Cooper Z. The Eating Disorders Examination. In: Fairburn C, Wilson G, editors. Binge-Eating: Nature, Assessment and Treatment. 12th. Guilford Press; New York: 1993. pp. 317–360. [Google Scholar]
- Fairburn CG, Cooper Z, O'Conner M. Eating disorder examination. In: Fairburn CG, editor. Cognitive behavior therapy and eating disorders. 16. Guilford Press; New York, NY: 2008. pp. 265–303. [Google Scholar]
- Field AE, Sonneville KR, Micali N, Crosby RD, Swanson SA, Laird NM, Treasure J, Solmi F, Horton NJ. Prospective association of common eating disorders and adverse outcomes. Pediatrics. 2012;130(2):e289–295. doi: 10.1542/peds.2011-3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer R, Gibbon M, Williams JB. Biometrics Research. New York State Psychiatric Institute; New York: 2002. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. (SCID-I/P) [Google Scholar]
- Fischer S, Settles R, Collins B, Gunn R, Smith GT. The role of negative urgency and expectancies in problem drinking and disordered eating: testing a model of comorbidity in pathological and at-risk samples. Psychol Addict Behav. 2012;26(1):112–123. doi: 10.1037/a0023460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holderness C, Brooks-Gunn J, Warren M. Co-morbidity of eating disorders and substance abuse. Review of the literature. nt J Eat Disord. 1994;16:1–35. doi: 10.1002/1098-108x(199407)16:1<1::aid-eat2260160102>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Walters EE, Neale MC, Kessler RC, Heath AC, Eaves LJ. The structure of the genetic and environmental risk factors for six major psychiatric disorders in women: phobia, generalized anxiety disorder, panic disorder, bulimia, major depression and alcoholism. Archives Gen Psychiatry. 1995;52:374–383. doi: 10.1001/archpsyc.1995.03950170048007. [DOI] [PubMed] [Google Scholar]
- Luce KH, Engler PA, Crowther JH. Eating disorders and alcohol use: group differences in consumption rates and drinking motives. Eat Behav. 2007;8(2):177–184. doi: 10.1016/j.eatbeh.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Mitchell J, Hatsukami D, Eckert E, Pyle R. Characteristics of 275 patients with bulimia. Am J Psych. 1985;142:482–485. doi: 10.1176/ajp.142.4.482. [DOI] [PubMed] [Google Scholar]
- Munn-Chernoff MA, Duncan AE, Grant JD, Wade TD, Agrawal A, Bucholz KK, Madden PA, Martin NG, Heath AC. A twin study of alcohol dependence, binge eating, and compensatory behaviors. J Stud Alcohol Drugs. 2013;74(5):664–673. doi: 10.15288/jsad.2013.74.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraven M, Baumeister RF. Self-regulation and depletion of limited resources: Does self-control resemble a muscle? Psychol Bull. 2000;126(2):247. doi: 10.1037/0033-2909.126.2.247. [DOI] [PubMed] [Google Scholar]
- Sher KJ, Grekin ER. Alcohol and Affect Regulation. In: Gross JJ, editor. Handbook of emotion regulation. Guilford Press; New York, NY: 2007. pp. 560–580. [Google Scholar]
- Shiffman S. Ecological momentary assessment (EMA) in studies of substance use. Psychol Assess. 2009;21(4):486–497. doi: 10.1037/a0017074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slane JD, Burt SA, Klump KL. Bulimic behaviors and alcohol use: shared genetic influences. Behav Genet. 2012;42(4):603–613. doi: 10.1007/s10519-012-9525-2. [DOI] [PubMed] [Google Scholar]
- Smyth JM, Wonderlich SA, Heron KE, Sliwinski MJ, Crosby RD, Mitchell JE, Engel SG. Daily and momentary mood and stress are associated with binge eating and vomiting in bulimia nervosa patients in the natural environment. J Consult Clin Psychol. 2007;75(4):629–638. doi: 10.1037/0022-006X.75.4.629. [DOI] [PubMed] [Google Scholar]
- Stone A, Shiffman S. Ecological Momentary Assessment (EMA) in behavioral medicine. Ann Behav Med. 1994;16:199–202. [Google Scholar]
- Stone AA, Shiffman S. Capturing momentary, self-report data: a proposal for reporting guidelines. Ann Behav Med. 2002;24(3):236–243. doi: 10.1207/S15324796ABM2403_09. [DOI] [PubMed] [Google Scholar]
- Swendsen JD, Tennen H, Carney MA, Affleck G, Willard A, Hromi A. Mood and alcohol consumption: an experience sampling test of the self-medication hypothesis. J Abnorm Psychol. 2000;109(2):198–204. [PubMed] [Google Scholar]
- Tchanturia K, Davies H, Harrison A, Fox JR, Treasure J, Schmidt U. Altered social hedonic processing in eating disorders. Int J Eat Disord. 2012;45(8):962–969. doi: 10.1002/eat.22032. [DOI] [PubMed] [Google Scholar]
- Todd M, Armeli S, Tennen H. Interpersonal problems and negative mood as predictors of within-day time to drinking. Psychol Addict Behav. 2009;23(2):205–215. doi: 10.1037/a0014792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trace SE, Thornton LM, Baker JH, Root TL, Janson LE, Lichtenstein P, Pedersen NL, Bulik CM. A behavioral-genetic investigation of bulimia nervosa and its relationship with alcohol use disorder. Psychiatry Res. 2013;208(3):232–237. doi: 10.1016/j.psychres.2013.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, C LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54(6):1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Witte TK, Fitzpatrick KK, Joiner TE, Jr, Schmidt NB. Variability in suicidal ideation: a better predictor of suicide attempts than intensity or duration of ideation? J Affect Disord. 2005;88(2):131–136. doi: 10.1016/j.jad.2005.05.019. [DOI] [PubMed] [Google Scholar]
- Wolfe WL, Maisto SA. The relationship between eating disorders and substance use: moving beyond co-prevalence research. Clin Psychol Rev. 2000;20(5):617–631. doi: 10.1016/s0272-7358(99)00009-4. [DOI] [PubMed] [Google Scholar]


