Abstract
Chronic biofilm formation by Pseudomonas aeruginosa in cystic fibrosis (CF) lungs is a major cause of morbidity and mortality for patients with CF. To gain insights into effectiveness of novel anti-infective therapies, the inhibitory effects of fosfomycin, tobramycin, and a 4 : 1 (wt/wt) fosfomycin/tobramycin combination (FTI) on Pseudomonas aeruginosa biofilms grown on cultured human CF-derived airway cells (CFBE41o-) were investigated. In preformed biofilms treated for 16 h with antibiotics, P. aeruginosa CFU per mL were reduced 4 log10 units by both FTI and tobramycin at 256 mg L−1, while fosfomycin alone had no effect. Importantly, the FTI treatment contained five times less tobramycin than the tobramycin-alone treatment. Inhibition of initial biofilm formation was achieved at 64 mg L−1 FTI and 16 mg L−1 tobramycin. Fosfomycin (1024 mg L−1) did not inhibit biofilm formation. Cytotoxicity was also determined by measuring lactate dehydrogenase (LDH). Intriguingly, sub-inhibitory concentrations of FTI (16 mg L−1) and tobramycin (4 mg L−1) and high concentrations of fosfomycin (1024 mg L−1) prevented bacterially mediated airway cell toxicity without a corresponding reduction in CFU. Overall, it was observed that FTI and tobramycin demonstrated comparable activity on biofilm formation and disruption. Decreased administration of tobramycin upon treatment with FTI might lead to a decrease in negative side effects of aminoglycosides.
Keywords: Pseudomonas aeruginosa, fosfomycin, biofilm, antibiotic resistance, tobramycin
Introduction
Pseudomonas aeruginosa is one of the leading causes of early mortality for patients with cystic fibrosis (CF) (Gibson et al., 2003). CF is the most common life-threatening genetic disorder among Caucasians, and pathology is caused by mutations in a single gene, encoding the cystic fibrosis transmembrane conductance regulator (CFTR) (Gibson et al., 2003). CFTR transports chloride across the cellular luminal membrane, and mutation of this protein leads to altered chloride transport, thickened mucus secretions in the airways, and decreased mucus clearance from the lungs (Boucher, 2004). Pseudomonas aeruginosa exploits these defects to establish chronic infection in the thickened airway mucus (Gomez & Prince, 2007). As a chronic infection, the bacteria exhibit adaptive behaviors that increase their tolerance to antibiotics, including the expression of genes leading to biofilm formation.
Biofilm formation is a well-established component of P. aeruginosa infection in CF airways (Gomez & Prince, 2007). Biofilms impact several characteristics of the chronic airway infection in CF, including increased tolerance to antipseudomonal antibiotics and failure of antibiotics to eradicate infections. Biofilms are surface-associated microbial communities that can display 10-fold to over 1000-fold increases in antibiotic tolerance compared with planktonic (free swimming) bacteria (Mah & O’Toole, 2001; Patel, 2005). In the CF lung, this tolerance necessitates increased antibiotic usage for maintenance therapy for chronic P. aeruginosa infection, which in turn can lead to greater induced antibiotic resistance. Consequently, existing antibiotic therapies are becoming less effective for treating chronic P. aeruginosa infections because of observed increases in resistance to the drugs used for treatment (Smith et al., 2006; Emerson et al., 2010; Mowat et al., 2011; Plummer & Wildman, 2011). Combinations of antibiotics may prove an important strategy for suppressing or eliminating chronic airway infection.
Fosfomycin/tobramycin for inhalation (FTI) is a novel antibiotic combination with properties that make it an interesting candidate for treating chronic airway infections, such as in CF and non-CF bronchiectasis patients (MacLeod et al., 2009). Fosfomycin is a phosphonic antibiotic that interferes with bacterial cell wall synthesis across a wide range of Gram-positive and Gram-negative bacteria (Shrestha & Tomford, 2001), including anaerobes (Altes Gutierrez & Rodriguez Noriega, 1977; Inouye et al., 1989). Tobramycin is an aminoglycoside antibiotic that is commonly used to treat Gram-negative infections, and as an aerosol, it is commonly used to suppress chronic airway infection in CF (Geller et al., 2002). It is also demonstrated to have a synergistic benefit when given in combination with other antibiotics (MacLeod et al., 2012). Both antibiotics have activity in environments where biofilms are present. Fosfomycin’s high treatment success rates for UTIs may be in part due to its activity under anaerobic conditions (Inouye et al., 1989), which exist throughout the biofilm microenvironment. Tobramycin, the minor component of FTI, is also highly active against P. aeruginosa growing planktonically and in biofilms (Ratjen et al., 2009). Therefore, a combination of fosfomycin and tobramycin formulated for nebulized delivery to the airways may be an attractive alternative to treating P. aeruginosa biofilms infections. FTI maintains high levels of antibacterial activity in mucin, which is the major component of the airway mucus present in elevated amounts in the lungs of chronically infected CF patients. In the presence of mucin, fosfomycin enhances the active uptake of tobramycin into P. aeruginosa resulting in greater inhibition of protein synthesis, enhanced bacterial killing, and ultimately a lower frequency of development of resistance (MacLeod et al., 2012).
In this study, we tested the effects of a novel 4 : 1 (wt/wt) fosfomycin/tobramycin combination on P. aeruginosa biofilms. FTI has demonstrated activity against a range of Gram-positive and Gram-negative pathogens, including antibiotic-resistant strains (MacLeod et al., 2009). However, its activity against bacteria grown in biofilms has not yet been fully characterized. To compare the effectiveness of FTI with the component antibiotics, we utilized a co-culture model in which P. aeruginosa biofilms were grown on CF airway epithelial cells (CFBE41o-) (Anderson et al., 2008; Moreau-Marquis et al., 2010). This model system has been previously used to more effectively model the biofilm that forms within CF airways (Anderson et al., 2008, 2010; Moreau-Marquis et al., 2008, 2009). We tested the effect of antibiotics on these co-culture biofilms. We found that FTI and tobramycin administered individually disrupted biofilms to greater extent than other antibiotics tested. Intriguingly, treatment with subinhibitory concentrations of FTI, fosfomycin, and tobramycin also led to decreased cytotoxicity of the bacteria toward the cultured epithelial cells.
Materials and methods
Bacterial strains and culture conditions
Pseudomonas aeruginosa strain PAO1 was used for all evaluations (Stover et al., 2000). Bacteria were grown in LB broth overnight at 37 °C with shaking prior to experimental procedures.
Antibiotics
Tobramycin sulfate and fosfomycin disodium were obtained from Sigma-Aldrich (St. Louis, MO). All antibiotics were diluted from stock solutions into complete tissue culture medium to the concentrations indicated.
Cell culture
For co-culture experiments, we used CFBE41o-cells (CFBE), which are immortalized airway epithelial cells homozygous for the common ΔF508 CFTR mutation (Cozens et al., 1994; Bruscia et al., 2002; Swiatecka-Urban et al., 2005). CFBE cultures were maintained in 165-cm2 flasks with Feeding Medium [Minimal Essential Medium (MEM) supplemented with 10% fetal bovine serum, 2 mM glutamine, 50 U mL−1 penicillin, and 50 μg mL−1 streptomycin], as previously described (Anderson et al., 2008). Medium was replaced every 2–3 days.
Co-culture assays
In preparation for co-culture experiments, 24-well tissue culture plates were seeded with 2 × 105 CFBE cells in 0.5 mL Feeding Medium per well, as previously described (Anderson et al., 2008). Medium was replaced every 2–3 days. Nearly 1–2 days before bacterial inoculation, the feeding medium was replaced with MEM without phenol red and supplemented with 2 mM glutamine and 10% fetal bovine serum. Plates were considered ready to use for experiments 7–10 day after seeding.
Biofilm disruption
To assess the ability of antibiotics to disrupt preformed P. aeruginosa biofilms, CFBE cells were inoculated with overnight cultures of P. aeruginosa at an MOI (multiplicity of infection) of 30 : 1 in Assay Medium (MEM without phenol red, supplemented with 2 mM glutamine only), as previously described (Anderson et al., 2008). After an hour incubation at 37 °C and 5% CO2, medium was removed and 0.5 mL fresh Assay Medium, supplemented with 0.4% arginine, was added, and the co-culture was incubated at 37 °C and 5% CO2 for an additional 6 h. This method has been shown to result in P. aeruginosa biofilm formation on the cultured epithelial cells within this timeframe (Anderson et al., 2008; Moreau-Marquis et al., 2008, 2010). After this incubation, medium was replaced with 0.5 mL fresh Assay Medium supplemented with FTI, fosfomycin, or tobramycin, and the co-culture was incubated for 16 h at 37 °C and 5% CO2. Biofilm levels were assessed by harvesting remaining bacteria, followed by serial dilution and plating on LB agar plates for CFU determination. Co-cultures were washed three times with 1 mL PBS and then incubated for 10–15 min with 1 mL 0.1% Triton-X 100 to lyse the epithelial cells. Supernatants were harvested, vortexed for 3 min, serially diluted, and plated on LB agar plates, as previously described (Anderson et al., 2008).
Biofilm inhibition
Inhibition of biofilm formation was assayed by inoculation of cells with P. aeruginosa as above in 0.5 mL Assay Medium, 0.4% arginine, and antibiotics as indicated. This assay differed from the biofilm disruption assay described above in that the antibiotics were added at the time of inoculation of the monolayer of airway cells by the bacteria. The assay was incubated for 16 h at 37 °C and 5% CO2 before biofilm levels were assessed as above.
Cytotoxicity
Epithelial cell cytotoxicity was determined in biofilm disruption and inhibition experiments by harvesting 300 μL of supernatant prior to washing the wells with PBS. This supernatant was centrifuged at 16 000 g in a microcentrifuge for 2 min, and 50 μL was assayed in duplicate for release of lactate dehydrogenase (LDH) using the CytoTox96® Non-radioactive Cytotoxicity Assay Kit (G1780, Promega, Madison, WI), according to the manufacturer’s instructions (Anderson et al., 2008). 0% cytotoxicity was determined by measuring spontaneous release of LDH from CFBE cells (untreated); this value became the baseline for the experiment. 100% cytotoxicity was determined by addition of 1% Triton-X 100 to completely lyse the CFBE cells. To assay the toxicity of antibiotics alone, CFBE cells, prepared as above, were incubated with FTI, tobramycin, or fosfomycin for 16 h at 37 °C and 5% CO2.
Statistical analysis
Student’s t-tests were performed to reveal statistical differences between treatments. P values < 0.05 were considered significant.
Results
Disruption of preformed biofilms
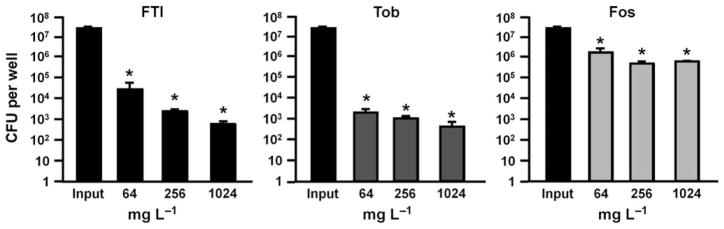
To investigate whether FTI, tobramycin, or fosfomycin could disrupt preformed biofilms, P. aeruginosa strain PAO1 was co-cultured with airway epithelial cells to form biofilms, as described in previous reports of this model (Anderson et al., 2008; Moreau-Marquis et al., 2008, 2010). Biofilm levels before treatment with antibiotics reached approximately 3 × 107 CFU/well. Treatment with FTI and tobramycin appeared to result in dose-dependent effects, but none of the three antibiotics completely eradicated P. aeruginosa (Fig. 1). Treatment with FTI and tobramycin at 256 mg L−1 resulted in an approximate 10 000-fold (4 log10 CFU per well) reduction in P. aeruginosa, while the same concentration of fosfomycin resulted in < 2 log10 killing (Fig. 1). The 256 mg L−1 FTI (containing 51.2 mg L−1 tobramycin) exerted a similar effect on biofilm destruction as 64 mg L−1 tobramycin; both of these treatments contain approximately the same amount of tobramycin (Table 1). Likewise, treatment with 1024 mg L−1 FTI (containing 204.8 mg L−1 tobramycin) and 256 mg L−1 tobramycin resulted in similar CFU values (Fig. 1).
Fig. 1.
Destruction of preformed biofilms. Co-culture biofilms were formed for 7 h. These biofilms were then treated with the indicated antibiotic concentrations for an additional 16 h. Biofilm levels were determined by CFU remaining in the well after treatment. Controls reached approximately 3 × 107 CFU per well before treatment. Tob, tobramycin; Fos, fosfomycin; Input = bacterial level at the start of antibiotic incubation (see text for details). *P < 0.05, compared to Input. Error bars represent standard deviation.
Table 1.
Amounts of fosfomycin and tobramycin in tested concentrations of FTI*
| FTI (total) | 1 | 4 | 16 | 64 | 256 | 1024 |
|---|---|---|---|---|---|---|
| Fosfomycin | 0.8 | 3.2 | 12.8 | 51.2 | 204.8 | 819.2 |
| Tobramycin | 0.2 | 0.8 | 3.2 | 12.8 | 51.2 | 204.8 |
All values are in mg per L.
Inhibition of biofilm formation
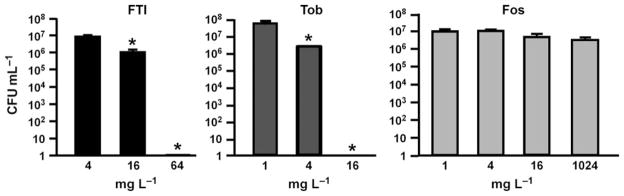
We further tested whether biofilm formation could be inhibited by FTI, fosfomycin, or tobramycin. After 16 h of incubation with antibiotics, complete inhibition of biofilm formation was achieved with 64 mg L−1 FTI and 16 mg L−1 tobramycin (Fig. 2). Fosfomycin did not inhibit biofilm formation, even at the highest concentration evaluated (1024 mg L−1).
Fig. 2.
Inhibition of biofilm formation. Antibiotics were added at the time of inoculation with Pseudomonas aeruginosa. Biofilm levels were determined at 16 h after inoculation. Tob, tobramycin; Fos, fosfomycin. *P < 0.05, compared to lowest antibiotic concentration. Error bars represent standard deviation.
Nonbactericidal effects of antibiotics
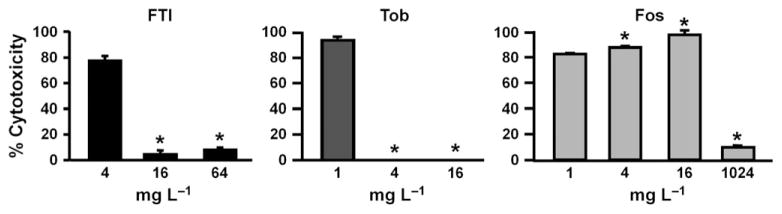
The effects of antibiotics on CFBE cytotoxicity are shown in Fig. 3. Treatment with FTI and tobramycin (64 mg L−1) resulted in < 10% cytotoxicity to CFBE cells (Fig. 3). Moderate cytotoxicity was seen after treatment with 1024 mg L−1 FTI (approximately 12%) and tobramycin (approximately 23%). Fosfomycin treatment (1024 mg L−1) resulted in < 10% toxicity.
Fig. 3.
FTI, tobramycin, and fosfomycin exert little toxic effect on CFBE cells. CFBE cells were incubated with FTI, tobramycin (Tob), or fosfomycin (Fos) for 16 h, after which cytotoxicity was assessed by measuring LDH release. *P < 0.05, compared to lowest antibiotic concentration. Error bars represent standard deviation.
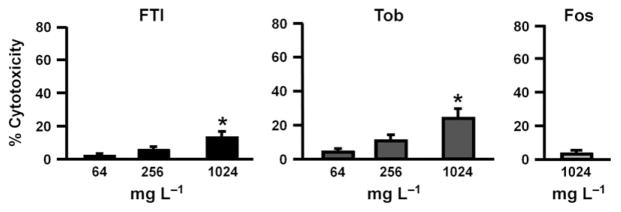
Similar high levels of cytotoxicity (> 70%) were observed for all three drugs in biofilm disruption experiments (Fig. 4); 256 mg L−1 FTI and 1024 mg L−1 FTI contain similar amounts of tobramycin as the 64 mg L−1 tobramycin and 256 mg L−1 tobramycin treatments, respectively (Table 1). We have previously shown that P. aeruginosa biofilms exert a high level of cytotoxicity to CFBE cells by 7 h (Anderson et al., 2008, 2010), and thus, much of the cytotoxicity in our assays might have occurred before antibiotic addition. It is equally likely that extended incubation of biofilms on CFBE cells (23 h total time) further enhanced this cytotoxicity.
Fig. 4.
Cytotoxicity after treatment of preformed biofilms with antibiotics. Conditions correspond to those in Fig. 1. Cytotoxicity was determined by measuring LDH release from the epithelial cells. 100% cytotoxicity was defined by complete lysis of all epithelial cells by 1% Triton-X 100. Tob, tobramycin, Fos, fosfomycin. *P < 0.05, compared to lowest antibiotic concentration. Error bars represent standard deviation.
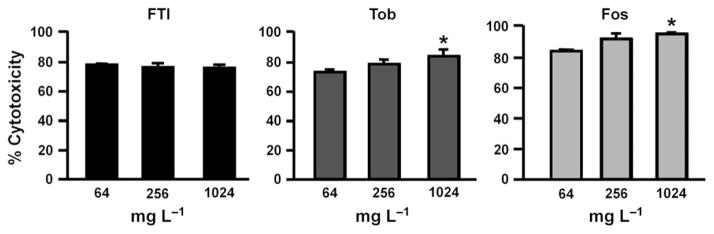
In biofilm inhibition assays (Fig. 5), < 10% cytotoxicity was observed in CFBE cells treated with FTI (16 and 64 mg L−1) or tobramycin (4 and 16 mg L−1). In the case of 16 mg L−1 tobramycin, this reduced cytotoxicity correlated with complete prevention of biofilm formation (Fig. 2). Intriguingly, treatment with 4 mg L−1 tobramycin failed to prevent biofilm formation (Fig. 2), but resulted in minimal CFBE cell toxicity (Fig. 5). For fosfomycin, < 1 log10 reduction in P. aeruginosa CFU was observed after treatment with 1–16 mg L−1 (Fig. 2), which correlated with high cytotoxicity (Fig. 5). Higher fosfomycin concentrations (1024 mg L−1) also failed to inhibit P. aeruginosa biofilm formation (Fig. 2), yet prevented CFBE cell cytotoxicity (Fig. 5). In both biofilm disruption and inhibition experiments, lack of antibiotic treatment resulted in complete bacterial killing of CFBE cells, resulting in essentially 100% cytotoxicity (data not shown).
Fig. 5.
Cytotoxicity after biofilm inhibition studies. Conditions correspond to those in Fig. 2. Cytotoxicity was determined by measuring LDH release from the epithelial cells. Tob, tobramycin, Fos, fosfomycin. *P < 0.05, compared to lowest antibiotic concentration. Error bars represent standard deviation.
Discussion
We demonstrated for the first time that a 4 : 1 (wt/wt) fosfomycin/tobramycin combination eradicated P. aeruginosa biofilms grown on CF-derived airway cells at concentrations that are easily achievable with nebulized delivery (Geller et al., 2002). This finding is relevant because P. aeruginosa, persisting in the CF lung as biofilms, displays increased antibiotic tolerance compared with planktonic (nonbiofilm) counterparts. FTI is a nebulized antibiotic combination that has shown activity toward a wide range of respiratory pathogens including methicillin-resistant S. aureus, P. aeruginosa, nontypeable H. influenzae, and Moraxella catarhalis (MacLeod et al., 2009). In addition, FTI has a superior frequency of resistance profile relative to fosfomycin and tobramycin alone (MacLeod et al., 2009). In this study, we compared the activity of FTI, tobramycin, and fosfomycin treatments in eradicating and preventing P. aeruginosa biofilm formation on CF-derived airway epithelial cells. We believe this biofilm model more closely mimics biofilms established in the airways of patients with CF than previous reports using biofilms established directly on plastic. This model also informs the relationship of bacteria, antibiotics, and airway epithelial cytotoxicity.
The susceptibility of P. aeruginosa to both antibiotics (and their combination) was dramatically less than for previously reported values generated using conventional susceptibility testing methods (MacLeod et al., 2009). FTI and tobramycin demonstrated comparable activity against preformed biofilms (Fig. 1) as well as the inhibition of new biofilms (Fig. 2) on CFBE cells. Fosfomycin exerted very little effect in either type of biofilm assay. Studies have shown that P. aeruginosa exhibits a lower metabolic rate in biofilms (Xu et al., 1998; Walters et al., 2003; Borriello et al., 2004), which may in part explain some of the differences in antimicrobial activity. In addition, fosfomycin acts by inhibition of bacterial cell wall biosynthesis and requires actively growing bacteria for maximal activity, whereas FTI and tobramycin inhibit protein synthesis and are active against slow- and fast-growing bacteria. Combinations consisting of fosfomycin and ofloxacin have also shown activity against P. aeruginosa growing in biofilms on an abiotic surface (Kumon et al., 1995; Monden et al., 2002). We also noticed that treatment of preformed biofilm by 1024 mg L−1 fosfomycin resulted in approximately 106 bacteria remaining (Fig. 1), but in biofilm inhibition experiments, this antibiotic treatment led to biofilms containing 107 CFU (Fig. 2). While the reasons behind this effect are unclear at this time, we speculate that during biofilm inhibition experiments, when P. aeruginosa are incubated from the beginning of the experiment with fosfomycin, there might be an adaptive response to the presence of the antibiotic. It will be interesting in future experiments to explore this phenomenon further.
These studies demonstrate that FTI and tobramycin have comparable activities against P. aeruginosa growing in biofilms. This is surprising because on a weight/weight basis, FTI is composed of 80% fosfomycin and 20% tobramycin (Table 1). Because tobramycin has a much higher molecular weight than fosfomycin (467.5 g mol−1 and 138.1 g mol−1, respectively), the molar ratio is approximately 13 : 1, with fosfomycin being the major component. Furthermore, fosfomycin alone showed no inhibition of P. aeruginosa biofilm growth (Figs 1 and 2), suggesting that fosfomycin is enhancing the activity of tobramycin. These data also strongly suggest that FTI will provide identical levels of bacterial killing of P. aeruginosa growing in biofilms as tobramycin, but with the potential for significantly reduced development of aminoglycoside ototoxicity and nephrotoxicity (Hammett-Stabler & Johns, 1998). In particular, in biofilm disruption experiments, 256 μg mL−1 of both FTI and tobramycin resulted in similar levels of biofilm reduction (Fig. 1), but FTI treatment achieved this result with much less tobramycin (51.2 μg mL−1). Likewise, in biofilm inhibition experiments, similar concentrations of FTI (64 μg mL−1) and tobramycin (16 μg mL−1) completely inhibited biofilm formation on CFBE cells (Fig. 2). Although the difference in tobramycin content between these inhibition treatments was less dramatic (12.8 μg mL−1 tobramycin in 64 μg mL−1 FTI), the FTI treatment still contained less tobramycin. Future in-depth analyses of different FTI and tobramycin concentrations will further identify optimal treatment concentrations for biofilm disruption and biofilm inhibition on CF airway cells.
In the CF lung, a hyperinflammatory immune reaction leads to tissue destruction (Gibson et al., 2003; Griese et al., 2008; Doring et al., 2011), although bacterially mediated tissue destruction also likely occurs. In fact, the P. aeruginosa Type III secretion system (T3SS) secretes potent toxins that have been shown to kill a variety of cells, including airway epithelial and inflammatory cells (Hauser & Engel, 1999; Sato & Frank, 2004; Yahr & Wolfgang, 2006; Anderson et al., 2010). Thus, we felt it is important to measure cytotoxicity of the bacteria toward the epithelial cells in co-culture biofilm assays to determine whether bacterially mediated toxicity could be decreased or averted with antimicrobial therapy. T3SS can be quite active during planktonic existence, but appears to be downregulated in mature biofilms (Goodman et al., 2004; Laskowski et al., 2004; Kuchma et al., 2005; Kulasekara et al., 2005). The early stages of biofilm formation and P. aeruginosa colonization of the CF lung may be crucial for reducing bacterially mediated toxicity during early CF lung infection with P. aeruginosa.
Our results suggest that early treatment with FTI, tobramycin, and fosfomycin might help to reduce cellular toxicity in the CF lung. We observed that while CFBE cytotoxicity levels remained high in the biofilm destruction assays (Fig. 4), addition of FTI or tobramycin could prevent toxicity in inhibition experiments (Fig. 5). Even subinhibitory concentrations of FTI, tobramycin, and fosfomycin could prevent toxicity in biofilm inhibition experiments (Fig. 5). In these instances, even though CFBE cells were exposed to a large number of bacteria, the cellular toxicity remained quite low. These results suggest that antibiotic treatment could influence bacterial gene expression, leading to decreased expression of toxic factors, although it is also possible that the antibiotics affected the epithelial cells directly. Importantly, antibiotic treatments alone exerted little toxic effect on airway cells (Fig. 3). In previous studies, we found that treatment of P. aeruginosa biofilms with tobramycin leads to a decrease in bacterial virulence and that this was related, in part, to regulation of T3SS gene transcription (Anderson et al., 2008, 2010). There are other examples of antibiotics affecting bacterial virulence and inducing host immunomodulatory effects (Wood et al., 2006; Anderson et al., 2008; Lopez-Boado & Rubin, 2008). It is possible that treatment of P. aeruginosa biofilms by fosfomycin also leads to inhibition of T3SS and other virulence factors. Decreased virulence factor production, in turn, could lead to decreased innate immune signaling by airway cells.
The types of analyses herein reported are vital for understanding the complex interaction between bacterial growth, biofilm-mediated antibiotic tolerance, inflammatory response, and epithelial toxicity. Future studies will further elucidate this intricate host–pathogen interaction.
Acknowledgments
We thank B. Coffey and C. Redelman for helpful conversations. Portions of the data were presented the 23rd Annual North American Cystic Fibrosis Conference, October 15–17, 2009, Minneapolis, MN, (abstract 277). This work was supported by RSFG from IUPUI and PRF from Purdue University to G. Anderson. This study was in part supported by Gilead Sciences, Inc. D.L.M., T.F.K., and N.R.H., own stock and options in Gilead Sciences, Inc.
References
- Altes Gutierrez A, Rodriguez Noriega A. In vitro sensitivity of anaerobic bacteria to fosfomycin. Chemotherapy. 1977;23(Suppl 1):51–57. doi: 10.1159/000222026. [DOI] [PubMed] [Google Scholar]
- Anderson GG, Moreau-Marquis S, Stanton BA, O’Toole GA. In vitro analysis of tobramycin-treated Pseudomonas aeruginosa biofilms on cystic fibrosis-derived airway epithelial cells. Infect Immun. 2008;76:1423–1433. doi: 10.1128/IAI.01373-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson GG, Yahr TL, Lovewell RR, O’Toole GA. The Pseudomonas aeruginosa magnesium transporter MgtE inhibits transcription of the type III secretion system. Infect Immun. 2010;78:1239–1249. doi: 10.1128/IAI.00865-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borriello G, Werner E, Roe F, Kim AM, Ehrlich GD, Stewart PS. Oxygen limitation contributes to antibiotic tolerance of Pseudomonas aeruginosa in biofilms. Antimicrob Agents Chemother. 2004;48:2659–2664. doi: 10.1128/AAC.48.7.2659-2664.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher RC. New concepts of the pathogenesis of cystic fibrosis lung disease. Eur Respir J. 2004;23:146–158. doi: 10.1183/09031936.03.00057003. [DOI] [PubMed] [Google Scholar]
- Bruscia E, Sangiuolo F, Sinibaldi P, Goncz KK, Novelli G, Gruenert DC. Isolation of CF cell lines corrected at DeltaF508-CFTR locus by SFHR-mediated targeting. Gene Ther. 2002;9:683–685. doi: 10.1038/sj.gt.3301741. [DOI] [PubMed] [Google Scholar]
- Cozens AL, Yezzi MJ, Chin L, Simon EM, Finkbeiner WE, Wagner JA, Gruenert DC. CFTR expression and chloride secretion in polarized immortal human bronchial epithelial cells. Am J Respir Cell Mol Biol. 1994;10:38–47. doi: 10.1165/ajrcmb.10.1.7507342. [DOI] [PubMed] [Google Scholar]
- Doring G, Parameswaran IG, Murphy TF. Differential adaptation of microbial pathogens to airways of patients with cystic fibrosis and chronic obstructive pulmonary disease. FEMS Microbiol Rev. 2011;35:124–146. doi: 10.1111/j.1574-6976.2010.00237.x. [DOI] [PubMed] [Google Scholar]
- Emerson J, McNamara S, Buccat AM, Worrell K, Burns JL. Changes in cystic fibrosis sputum microbiology in the United States between 1995 and 2008. Pediatr Pulmonol. 2010;45:363–370. doi: 10.1002/ppul.21198. [DOI] [PubMed] [Google Scholar]
- Geller DE, Pitlick WH, Nardella PA, Tracewell WG, Ramsey BW. Pharmacokinetics and bioavailability of aerosolized tobramycin in cystic fibrosis. Chest. 2002;122:219–226. doi: 10.1378/chest.122.1.219. [DOI] [PubMed] [Google Scholar]
- Gibson RL, Burns JL, Ramsey BW. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am J Respir Crit Care Med. 2003;168:918–951. doi: 10.1164/rccm.200304-505SO. [DOI] [PubMed] [Google Scholar]
- Gomez MI, Prince A. Opportunistic infections in lung disease: Pseudomonas infections in cystic fibrosis. Curr Opin Pharmacol. 2007;7:244–251. doi: 10.1016/j.coph.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Goodman AL, Kulasekara B, Rietsch A, Boyd D, Smith RS, Lory S. A signaling network reciprocally regulates genes associated with acute infection and chronic persistence in Pseudomonas aeruginosa. Dev Cell. 2004;7:745–754. doi: 10.1016/j.devcel.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Griese M, Kappler M, Gaggar A, Hartl D. Inhibition of airway proteases in cystic fibrosis lung disease. Eur Respir J. 2008;32:783–795. doi: 10.1183/09031936.00146807. [DOI] [PubMed] [Google Scholar]
- Hammett-Stabler CA, Johns T. Laboratory guidelines for monitoring of antimicrobial drugs. National Academy of Clinical Biochemistry. Clin Chem. 1998;44:1129–1140. [PubMed] [Google Scholar]
- Hauser AR, Engel JN. Pseudomonas aeruginosa induces type-III-secretion-mediated apoptosis of macrophages and epithelial cells. Infect Immun. 1999;67:5530–5537. doi: 10.1128/iai.67.10.5530-5537.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye S, Watanabe T, Tsuruoka T, Kitasato I. An increase in the antimicrobial activity in vitro of fosfomycin under anaerobic conditions. J Antimicrob Chemother. 1989;24:657–666. doi: 10.1093/jac/24.5.657. [DOI] [PubMed] [Google Scholar]
- Kuchma SL, Connolly JP, O’Toole GA. A three-component regulatory system regulates biofilm maturation and type III secretion in Pseudomonas aeruginosa. J Bacteriol. 2005;187:1441–1454. doi: 10.1128/JB.187.4.1441-1454.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulasekara HD, Ventre I, Kulasekara BR, Lazdunski A, Filloux A, Lory S. A novel two-component system controls the expression of Pseudomonas aeruginosa fimbrial cup genes. Mol Microbiol. 2005;55:368–380. doi: 10.1111/j.1365-2958.2004.04402.x. [DOI] [PubMed] [Google Scholar]
- Kumon H, Ono N, Iida M, Nickel JC. Combination effect of fosfomycin and ofloxacin against Pseudomonas aeruginosa growing in a biofilm. Antimicrob Agents Chemother. 1995;39:1038–1044. doi: 10.1128/aac.39.5.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskowski MA, Osborn E, Kazmierczak BI. A novel sensor kinase-response regulator hybrid regulates type III secretion and is required for virulence in Pseudomonas aeruginosa. Mol Microbiol. 2004;54:1090–1103. doi: 10.1111/j.1365-2958.2004.04331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Boado YS, Rubin BK. Macrolides as immunomodulatory medications for the therapy of chronic lung diseases. Curr Opin Pharmacol. 2008;8:286–291. doi: 10.1016/j.coph.2008.01.010. [DOI] [PubMed] [Google Scholar]
- MacLeod DL, Barker LM, Sutherland JL, Moss SC, Gurgel JL, Kenney TF, Burns JL, Baker WR. Antibacterial activities of a fosfomycin/tobramycin combination: a novel inhaled antibiotic for bronchiectasis. J Antimicrob Chemother. 2009;64:829–836. doi: 10.1093/jac/dkp282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod DL, Velayudhan J, Kenney TF, Therrien JH, Sutherland JL, Barker LM, Baker WR. Fosfomycin enhances the active transport of tobramycin in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2012;56:1529–1538. doi: 10.1128/AAC.05958-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mah TF, O’Toole GA. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001;9:34–39. doi: 10.1016/s0966-842x(00)01913-2. [DOI] [PubMed] [Google Scholar]
- Monden K, Ando E, Iida M, Kumon H. Role of fosfomycin in a synergistic combination with ofloxacin against Pseudomonas aeruginosa growing in a biofilm. J Infect Chemother. 2002;8:218–226. doi: 10.1007/s10156-002-0186-6. [DOI] [PubMed] [Google Scholar]
- Moreau-Marquis S, Bomberger JM, Anderson GG, Swiatecka-Urban A, Ye S, O’Toole GA, Stanton BA. The {Delta} F508-CFTR mutation results in increased biofilm formation by Pseudomonas aeruginosa by increasing iron availability. Am J Physiol Lung Cell Mol Physiol. 2008;295:L25–L37. doi: 10.1152/ajplung.00391.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau-Marquis S, O’Toole GA, Stanton BA. Tobramycin and FDA-approved iron chelators eliminate Pseudomonas aeruginosa biofilms on cystic fibrosis cells. Am J Respir Cell Mol Biol. 2009;41:305–313. doi: 10.1165/rcmb.2008-0299OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau-Marquis S, Redelman CV, Stanton BA, Anderson GG. Co-culture models of Pseudomonas aeruginosa biofilms grown on live human airway cells. J Vis Exp. 2010;44:e2186. doi: 10.3791/2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowat E, Paterson S, Fothergill JL, Wright EA, Ledson MJ, Walshaw MJ, Brockhurst MA, Winstanley C. Pseudomonas aeruginosa population diversity and turnover in cystic fibrosis chronic infections. Am J Respir Crit Care Med. 2011;183:1674–1679. doi: 10.1164/rccm.201009-1430OC. [DOI] [PubMed] [Google Scholar]
- Patel R. Biofilms and antimicrobial resistance. Clin Orthop Relat Res. 2005;437:41–47. doi: 10.1097/01.blo.0000175714.68624.74. [DOI] [PubMed] [Google Scholar]
- Plummer A, Wildman M. Duration of intravenous antibiotic therapy in people with cystic fibrosis. Cochrane Database Syst Rev. 2011;1:CD006682. doi: 10.1002/14651858.CD006682.pub3. [DOI] [PubMed] [Google Scholar]
- Ratjen F, Brockhaus F, Angyalosi G. Aminoglycoside therapy against Pseudomonas aeruginosa in cystic fibrosis: a review. J Cyst Fibros. 2009;8:361–369. doi: 10.1016/j.jcf.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Sato H, Frank DW. ExoU is a potent intracellular phospholipase. Mol Microbiol. 2004;53:1279–1290. doi: 10.1111/j.1365-2958.2004.04194.x. [DOI] [PubMed] [Google Scholar]
- Shrestha NK, Tomford JW. Fosfomycin: a review. Infect Dis Clin Pract. 2001;10:255–260. [Google Scholar]
- Smith EE, Buckley DG, Wu Z, et al. Genetic adaptation by Pseudomonas aeruginosa to the airways of cystic fibrosis patients. Proc Natl Acad Sci USA. 2006;103:8487–8492. doi: 10.1073/pnas.0602138103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stover CK, Pham XQ, Erwin AL, et al. Complete genome sequence of Pseudomonas aeruginosa PA01, an opportunistic pathogen. Nature. 2000;406:959–964. doi: 10.1038/35023079. [DOI] [PubMed] [Google Scholar]
- Swiatecka-Urban A, Brown A, Moreau-Marquis S, et al. The short apical membrane half-life of rescued {Delta}F508-cystic fibrosis transmembrane conductance regulator (CFTR) results from accelerated endocytosis of {Delta}F508-CFTR in polarized human airway epithelial cells. J Biol Chem. 2005;280:36762–36772. doi: 10.1074/jbc.M508944200. [DOI] [PubMed] [Google Scholar]
- Walters MC, 3rd, Roe F, Bugnicourt A, Franklin MJ, Stewart PS. Contributions of antibiotic penetration, oxygen limitation, and low metabolic activity to tolerance of Pseudomonas aeruginosa biofilms to ciprofloxacin and tobramycin. Antimicrob Agents Chemother. 2003;47:317–323. doi: 10.1128/AAC.47.1.317-323.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood LF, Leech AJ, Ohman DE. Cell wall-inhibitory antibiotics activate the alginate biosynthesis operon in Pseudomonas aeruginosa: roles of sigma (AlgT) and the AlgW and Prc proteases. Mol Microbiol. 2006;62:412–426. doi: 10.1111/j.1365-2958.2006.05390.x. [DOI] [PubMed] [Google Scholar]
- Xu KD, Stewart PS, Xia F, Huang CT, McFeters GA. Spatial physiological heterogeneity in Pseudomonas aeruginosa biofilm is determined by oxygen availability. Appl Environ Microbiol. 1998;64:4035–4039. doi: 10.1128/aem.64.10.4035-4039.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahr TL, Wolfgang MC. Transcriptional regulation of the Pseudomonas aeruginosa type III secretion system. Mol Microbiol. 2006;62:631–640. doi: 10.1111/j.1365-2958.2006.05412.x. [DOI] [PubMed] [Google Scholar]