Abstract
Myoepithelial carcinoma (MECA) is an underrecognized rare tumor with a diverse clinical behavior. The histologic features of this tumor are not well characterized, much less its grading, which is controversial. The objective of this study is to provide a better characterization of MECA and its prognostic factors. A total of 48 cases were retrieved from the pathology files. The cases were subjected to a detailed histopathologic, immunohistochemical, statistical, and clinical analysis. Tumors were classified as de novo MECA in 22 cases (46%) and carcinoma ex-pleomorphic adenoma (CA ex-PA) in 26 cases (54%). Tumor necrosis, high mitotic count (≥6/10 high-power fields), and severe pleomorphism were identified in 38%, 33%, and 21%, respectively. Perineural invasion, vascular invasion, and positive margins were noted in 10%, 12%, and 47%, respectively. Median follow-up was 38 months. Four patients had lymph node metastasis at presentation, 9 developed local recurrences, and 12 had distant metastases with the lung being the most common site (83%). The presence of CA ex-PA, necrosis, and vascular invasion correlated significantly with disease-free survival (P = 0.02, 0.01, 0.03, respectively). No distant recurrence was noted in all 23 patients lacking necrosis in their neoplasms (median follow-up: 44 mo). MECA is a relatively aggressive tumor that is associated with a high rate of distant metastasis (27%). Compared with de novo MECA, CA ex-PA correlates with worse clinical outcome. A grading system based on the presence of tumor necrosis should be used to identify high-grade MECA and predict its clinical behavior.
Keywords: myoepithelial carcinoma, carcinoma ex-pleomorphic adenoma, necrosis
Myoepithelial carcinoma (MECA) is a rare malignant tumor, comprising <2% of all salivary gland carcinomas.1 However, this tumor is underrecognized and might not be as rare as has been reported. The entity was first described in 1970s,1–5 and was added to the second edition of the World Health Organization classification of salivary gland tumors in 1991.6 Histologically, MECA is defined as a neoplasm composed almost exclusively of myoepithelial cells and characterized by an infiltrative growth pattern.1,3,7 However, this tumor shows a wide variety of cell types and architectural patterns, and its histologic features are not well delineated. MECA may arise de novo, but about 50% may develop within a preexisting pleomorphic adenoma (carcinoma ex-pleomorphic adenoma [CA ex-PA]).1,7 The difference in clinical outcome between de novo MECA and CA ex-PA, MECA type, is somewhat unclear.1,8,9 One confounding issue regarding diagnosing MECA has been tumor grading, especially given the variable and unpredictable reported clinical behavior of this tumor.3,9 Interestingly, cytologically bland MECAs with minimal mitotic activity have been reported to recur and cause death.9–11 However, tumors with aggressive histologic features (cytologic atypia, increased mitotic activity, and necrosis) have been reported to occasionally behave in a relatively indolent manner.9,12,13 To further provide a better characterization of MECA of salivary gland and its prognostic factors, we performed a morphologic analysis of 48 MECAs and correlated the various histopathologic parameters with clinical outcome to establish a grading system that will improve patient outcome stratification.
MATERIALS AND METHODS
Samples Selection
After obtaining Institutional Review Board approval, the database of the pathology department at Memorial Sloan-Kettering Cancer Center (MSKCC) was searched for all MECAs diagnosed and surgically resected at MSKCC between 1990 and 2013. Hematoxylin and eosin slides were available on 48 MECAs of major and minor salivary glands with adequate material (defined as at least 1 tumor section per 1 cm of the tumor).
Histopathologic Analysis and Immunohistochemistry
The 48 MECA cases were reviewed independently by a head and neck pathologist (N.K.) without knowledge of the patients’ clinical outcome. MECA was defined as a neoplasm that almost exclusively manifests myoepithelial differentiation and is characterized by infiltrative growth pattern, according to the criteria described in the World Health Organization book on head and neck tumors and the Armed Forces Institute of Pathology fascicle.1,3 Tumor size was assessed on the basis of the gross examination and/or microscopic analysis of the primary tumors. The tumors were evaluated for the following histologic parameters: evidence of coexisting pleomorphic adenoma, growth patterns, cell types, extracellular matrix characteristics, perineural invasion, and vascular invasion. The tumor borders were classified as multinodular, encapsulated, or infiltrative. Mitotic rate in the carcinoma was determined by counting mitotic figures in 10 contiguous high-power fields (HPF, × 400) in the areas of greatest concentrations of mitoses using an Olympus microscope (U-DO model BX-40; Olympus America Inc., Melville, NY). Using that microscope type, these 10HPFs correspond to 2.4mm2. The presence or absence of atypical mitotic figures, nuclear pleomorphism, apoptosis, and tumor necrosis was recorded. Microscopic resection margins were reported as positive (tumor at the margin) or negative (tumor not present at the margin). The presence or absence of regional lymph node (LN) and distant metastasis was noted. Immunohistochemical studies were performed on 4-µmthick sections obtained from paraffin-embedded blocks in 38 cases. The antibody manufacturers and dilutions are as follows: AE1/AE3: Dako, Carpinteria, CA, 1:1600; Cam5.2: Becton Dickinson, Burlington, NC, 1:50; p63: Ventana, Tucson, AZ, ready to use from vendor; S100: Dako, 1:8000; Smooth muscle actin: Vector, Burlingame, CA, 1:50; Calponin: Ventana, ready to use from the vendor; high–molecular weight cytokeratin: Ventana, ready to use from vendor; EMA: Ventana, ready to use from vendor. All antibodies were monoclonal except S100, which was polyclonal. Appropriate positive and negative controls were included. The 10 MECAs that were not tested for immunohistochemistry showed the typical histomorphology of MECA.
Follow-up
Clinical characteristics were obtained from the electronic medical records. The patients’ clinical data were reviewed for age at the time of diagnosis, sex, tumor site, adjuvant treatment, and tumor recurrence. The tumors were staged using the AJCC Cancer Staging Manual, seventh edition.14 Follow-up (FU) data were not available on 4 patients who were not ultimately treated postoperatively at MSKCC. Recurrence was determined on the basis of clinical examination or imaging studies. Dates of the initial surgery, first recurrence, last FU, and death were analyzed. The status at the last FU was classified as follows: no evidence of disease, alive with disease, dead of disease, or dead of other causes.
Statistical Analysis
For disease-free survival (DFS) analysis, all time points were calculated from the date of the surgery to first disease recurrence or to last FU date in patients with no recurrent tumor. The survival rates were estimated using the Kaplan-Meier method, and univariate analysis was performed on 41 cases using the log-rank test. Three cases were excluded because patients presented with metastasis at the time of surgery. The following variables were examined: presence of pleomorphic adenoma, tumor site (major vs. minor salivary glands), tumor size (5 cm or higher), mitotic rate ≥6/10 HPF, atypical mitosis, apoptosis, tumor necrosis, severe nuclear pleomorphism, vascular invasion, perineural invasion, positive margins, adjuvant treatment, nodal metastasis, and distant metastasis. In addition, stratification of pleomorphic adenoma presence and tumor necrosis into 4 patient groups was performed to examine the relationship between the 2 variables and DFS.
RESULTS
Clinical Features
The clinical features, anatomic sites, and TNM stage are displayed in Table 1. The patients’ age varied from 25 to 90 years (median 59 y). Twenty-five patients were female, and 23 were male. The tumors were located in the parotid gland in 35 cases (73%), submandibular gland in 7 (14%), and minor salivary glands in 6 (12%). The tumor size ranged from 0.9 to 9.5 cm (median 3.05 cm). Median size of CA ex-PA was 3.5 versus 3.0 cm for de novo carcinoma. One patient with CA ex-PA had a prior pleomorphic adenoma in the same site. Twenty-nine of 45 (64%) patients had postoperative radiation therapy. Five of 44 (11%) patients received chemotherapy.
TABLE 1.
Clinicopathologic Features of 48 MECAs
| N (%) | |
|---|---|
| Age median (range) (y) | 59 (25–90) |
| Female/male | 25/23 |
| Tumor site | |
| Parotid | 35 (73) |
| Submandibular gland | 7 (14.5) |
| Minor salivary gland | 6 (12.5) |
| Tumor size, median (range) (cm) | 3.05 (0.9–9.5) |
| CA ex-PA | 22 (46) |
| De novo carcinoma | 26 (54) |
| Severe pleomorphism | 10 (21) |
| Mitosis (≥ 6/10 HPF) | 16 (33) |
| Atypical mitosis | 4 (8) |
| Necrosis | 22 (38) |
| Apoptosis | 30 (62) |
| Positive margin | 21/45* (47) |
| Vascular invasion | 6 (12) |
| Perineural invasion | 5 (10) |
| Adjuvant radiation therapy | 29/45† (64) |
| Adjuvant chemotherapy | 5/44† (11) |
| LN metastasis | 4/40‡ (10) |
| Distant metastasis | 12/44§ (27) |
| TNM stage | |
| I | 10/47‖ (21) |
| II | 18/47‖ (38) |
| III | 13/47‖ (28) |
| IV | 6/47‖ (13) |
Margins status could not be assessed in 3 cases.
Radiotherapy and chemotherapy status were unknown in 3 and 4 cases, respectively.
LN dissection was performed in 40 cases.
FU was available on 44 cases.
Tumor staging was available on 47 cases.
Histopathologic Features
Architecture and Growth Patterns
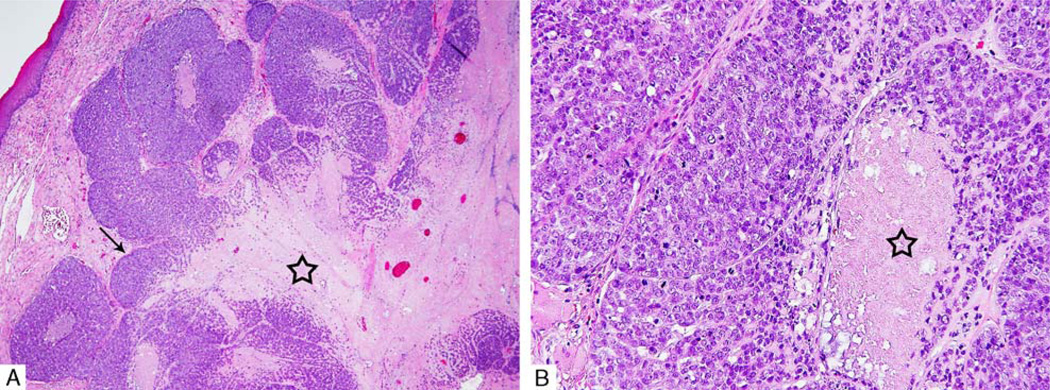
Histologically, all tumors showed invasive borders. The vast majority (44 cases, 92%) had multinodular architecture either in the form of a single nodule with lobulated borders (Fig. 1A) or separate nodules divided by fibrous bands. A sheet-like arrangement was noted in only 3 tumors, whereas 1 tumor consisted of an encapsulated nodule with capsular invasion. In 46% of cases, the tumor cells had a zonal arrangement with a hypercellular peripheral rim surrounding a hypocellular center (Fig. 1A). The hypocellular central areas were usually myxoid and/or necrotic. The tumor cells are arranged mostly in solid and trabecular growth patterns. In addition, the following growth patterns were identified: cribriform (usually focal), thin cords, small clusters, and tubule-like with luminal formations. Tumor-related duct formations were noted only in 4 de novo MECAs. These were focal, identified in <10% of the tumor.
FIGURE 1.
A, Multinodular architecture with lobulated borders and zonal cellular arrangement with a hypercellular peripheral rim (arrow) surrounding a hypocellular center (star). B, High-power view showing tumor necrosis (star).
Cell Types
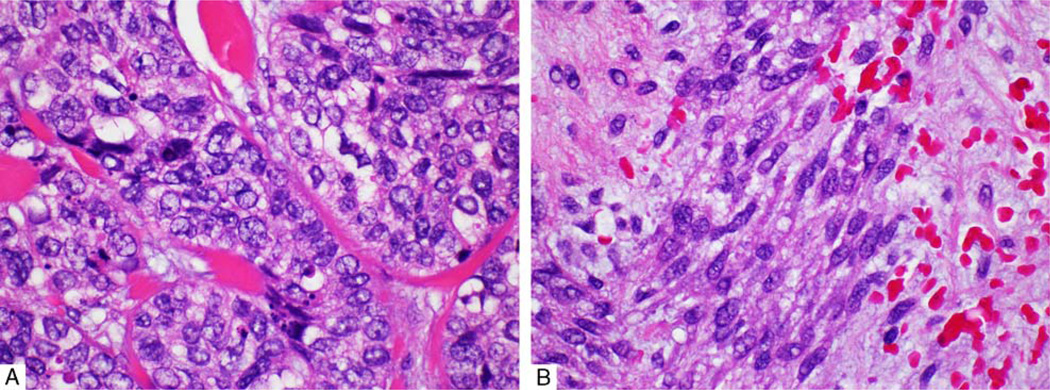
The MECAs were composed of a wide variety of cell types. Although, most tumors showed a mixture of several cell types, 1 type often dominated. The epithelioid cell was the most commonly predominant type (38 cases, 79%), followed by the spindle cell (9 cases, 19%). Epithelioid cells (Fig. 2A) were polygonal, characterized by eosinophilic cytoplasm and ovoid nuclei. The spindle cells (Fig. 2B) demonstrated elongated nuclei with light eosinophilic cytoplasm. In 7 cases (15%), tumor cells showed basaloid morphology with scant cytoplasm and high nuclear to cytoplasmic ratio. The latter morphology was mostly associated with high-grade features (ie, increased mitoses and tumor necrosis). Plasmacytoid cells were found in 7 cases (15%). Four carcinomas (8%) displayed clear cells with 2 showing diffuse involvement by clear cells.
FIGURE 2.
A, Epithelioid cells: polygonal cells with eosinophilic cytoplasm and inconspicuous nucleoli. B, Spindle cells showing elongated nuclei and light eosinophilic cytoplasm.
Extracellular Matrix and Metaplasia in the MECA
Two main extracellular types of matrix were noted in MECA: myxoid and hyalinized. These were found alone or in combination within the same tumor. Myxoid stroma was seen in 39 tumors (81%), ranging from diffuse to focal involvement. Myxochondroid stroma was noted in 4 cases. Two tumors showed extracellular cartilage. Hyalinized stroma was seen in 20 tumors (42%). Squamous metaplasia was identified in 18 (37%) tumors, 1 of which had extensive metaplasia.
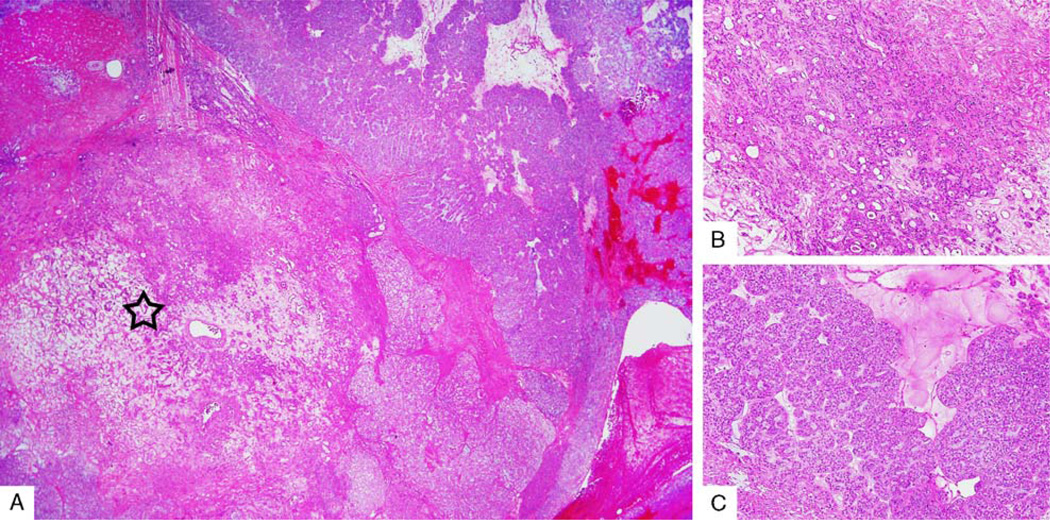
CA ex-PA
Tumors were classified as de novo MECAs in 22 cases (46%) and CA ex-PA in 26 cases (54%) (Figs. 3A–C). Twenty-four of 26 CA ex-PA cases were widely invasive, and 2 were noninvasive. The benign PA component comprised a variable proportion of the entire neoplasm, ranging from <5% to 70%. The PA component was found either as a well-demarcated area from the malignant component or intermixed with the malignant component. The PA in all cases comprised ducts and myoepithelial cells. Stromal hyalinization was noted in 67% (16/24) of the PA components. Among these, 8 showed extensive hyalinization with occasional benign ducts.
FIGURE 3.
A, Low-power view of myoepithelial CA ex-PA with a discrete central pleomorphic adenoma component (star). B, High-power view of the pleomorphic adenoma component showing benign ductal structures. C, High-power view of the malignant MECA component.
Nuclear Atypia and Proliferative Features
Tumor cells showed a wide spectrum of nuclear atypia ranging from bland nuclei to severe pleomorphism, which was noted in 10 tumors (21%). In 1 case, tumor cells displayed endocrine-like atypia, showing giant bizarre nuclei and low nuclear to cytoplasmic ratio.
Mitotic activities ranged from 0 to 25/10HPF (Fig. 1B). The mean overall mitotic activity was 5.8/ 10HPF, with 16 of 48 (33%) tumors showing ≥6 mitoses/10HPF. Apoptosis was found in 30 tumors (62%). Tumor necrosis was noted in 22 carcinomas (38%) (Fig. 1A). Tumors with necrosis showed a variety of cell types including epithelioid and spindle. Atypical mitoses were seen in 4 tumors, all of which had tumor necrosis.
Other Histologic Parameters
MECAs showed perineural invasion in 5 of 48 cases (10%), vascular invasion in 6 of 48 (12%), and positive margins in 21 of 47 (47%). Margin status could not be assessed in 3 tumors.
Immunohistochemical Features
Immunohistochemical stains were performed on 38 cases (79%). All tested tumors were positive for a keratin and at least for 1 of the myoepithelial markers (S100, calponin, p63, and SMA) (Table 2). The majority of the positive S100 MECAs (82%) showed diffuse immunostaining in the tumor cells.
TABLE 2.
Immunohistochemical Features of MECA
| IHC | N (%) |
|---|---|
| Keratin | 38/38 (100) |
| S100 | 34/38 (89) |
| Calponin | 25/33 (76) |
| P63 | 21/24 (87.5) |
| SMA | 23/36 (64) |
IHC indicates immunohistochemical stains; SMA, smooth muscle actin.
Clinical Outcome
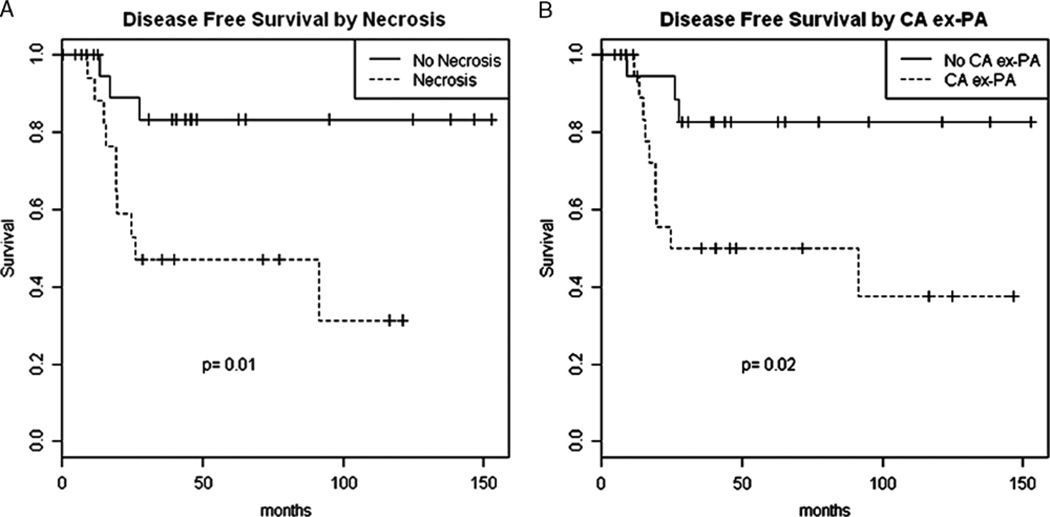
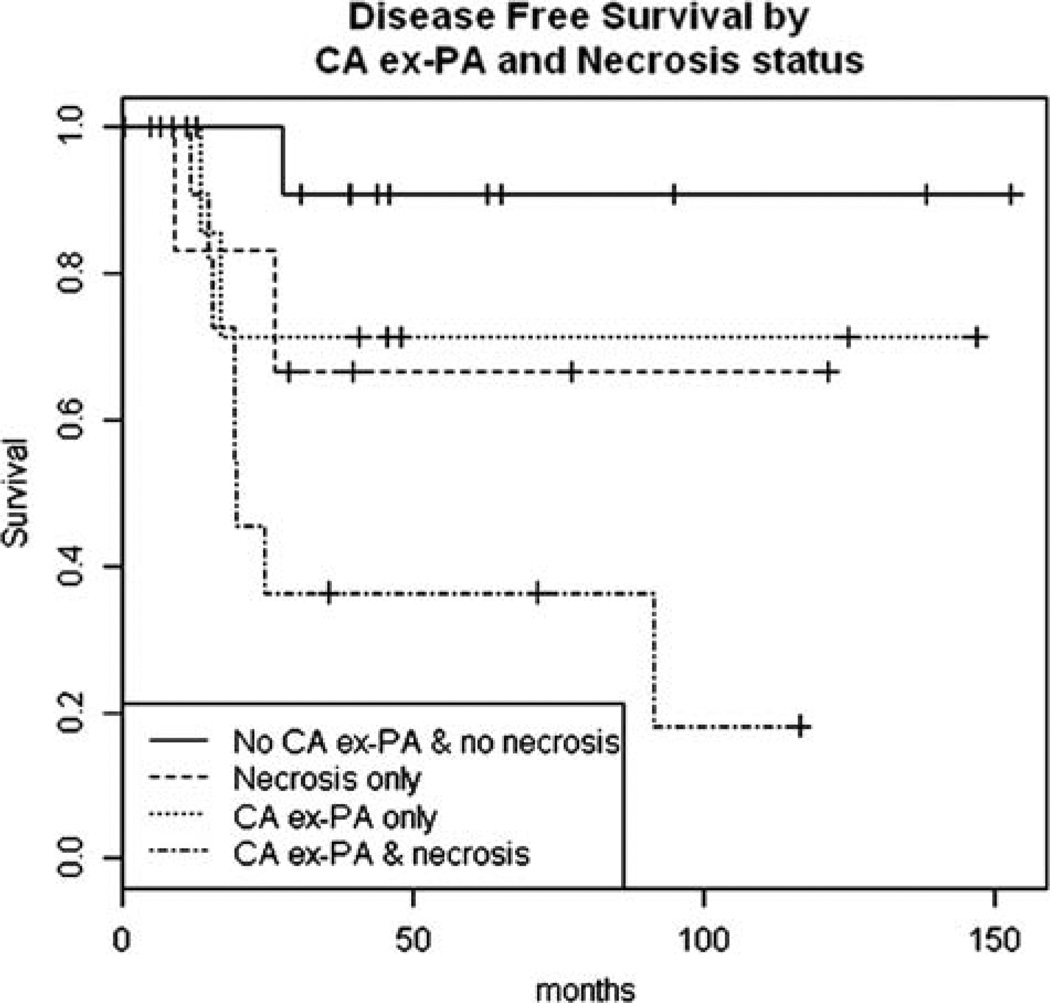
Adequate FU was available on 44 cases. Four of 40 patients (10%) had LN metastasis at presentation, and 9 of 44 patients (20%) developed local recurrence. Distant metastases to lung, brain, bone, and soft tissue were identified in 12 of 44 patients (27%). Lung was the most common metastatic site, occurring in 10 (83%) of the 12 patients with distant disease. Three patients developed distant metastases in multiple sites. However, because distant metastases at presentation were present in 3 cases, univariate survival analysis was performed on 41 patients only (Table 3). The median FU for these 41 cases was 38 months. No distant recurrence was noted in any of the 23 patients lacking tumor necrosis (median FU: 44 mo). The presence of PA component (CA ex-PA), tumor necrosis, and vascular invasion correlated significantly with DFS (P=0.02, 0.01, 0.03, respectively) (Figs. 4A, B). Moreover, qualitative stratification analysis suggested that CA ex-PA and necrosis may have independent effects on outcome (Fig. 5) (Table 4). The following covariates did not correlate with DFS: increased mitosis, atypical mitosis, apoptosis, severe pleomorphism, perineural invasion, positive LNs, and positive margin.
TABLE 3.
Association Between Clinicopathologic Parameters and DFS (41 Cases)
| Clinicopathologic Parameters | 5-y DFS Rate (%) | P* |
|---|---|---|
| Tumor size (cm) | ||
| < 5 | 73 | 0.07 |
| ≥ 5 | 34 | |
| Site | 0.69 | |
| Major salivary gland | 65 | |
| Minor salivary gland | 75 | |
| Severe pleomorphism | 0.26 | |
| Present | 50 | |
| Absent | 71 | |
| Atypical mitoses | 0.79 | |
| Present | 75 | |
| Absent | 65 | |
| Mitosis per 10 HPF | 0.24 | |
| < 6 | 72 | |
| ≥ 6 | 57 | |
| Necrosis | 0.01 | |
| Focal/ > focal | 47 | |
| No | 83 | |
| Apoptosis | 0.30 | |
| Yes | 61 | |
| No | 75 | |
| CA ex-PA | 0.02 | |
| Present | 50 | |
| Absent | 83 | |
| LNs metastasis | 0.70 | |
| Positive | 75 | |
| Negative | 61 | |
| Margin status | 0.13 | |
| Positive | 58 | |
| Negative | 71 | |
| Perineural invasion | 0.71 | |
| Present | 60 | |
| Absent | 67 | |
| Vascular invasion | 0.03 | |
| Present | 33 | |
| Absent | 73 | |
| Radiotherapy | 0.21 | |
| Present | 60 | |
| Absent | 80 | |
| Chemotherapy | 0.12 | |
| Present | 33 | |
| Absent | 70 |
Significant P-values are in bold.
CI indicates confidence interval.
FIGURE 4.
Correlation between recurrence and the presence of tumor necrosis (A) and the presence of pleomorphic adenoma component (CA ex-PA) (B).
FIGURE 5.
Qualitative stratification analysis suggesting that CA ex-PA and necrosis may have independent effects on clinical outcome (P = 0.018).
TABLE 4.
Association Between CA ex-PA/Necrosis Stratification and Recurrence
| Combined CA ex-PA and Tumor Necrosis Status |
DFS (95% CI) | P |
|---|---|---|
| CA ex-PA | Necrosis | 0.37 (0.17, 0.80) | 0.018 |
| CA ex-PA | No necrosis | 0.71 (0.45, 1.00) | |
| No CA ex-PA | Necrosis | 0.67 (0.38, 1.00) | |
| No CA ex-PA | No necrosis | 0.91 (0.75, 1.00) |
CI indicates confidence interval.
DISCUSSION
In this study we examined the clinicopathologic features of 48 MECAs and correlated the various histopathologic parameters with clinical outcome to identify pathologic covariates associated with DFS. To the best of our knowledge, this is the largest reported series of MECAs with adequate clinical FU data.
Although MECA was described as an entity >40 years ago,15 it remains underrecognized, and its diagnostic criteria as well as its prognostic factors are still not well delineated. Given its morphologic heterogeneity, MECA may have been misdiagnosed in the past as various salivary gland tumors or even misclassified as “malignant mixed tumor.” Therefore, many of the reported CA ex-PA/malignant mixed tumors or adenocarcinoma not otherwise specified might actually represent MECAs with or without a PA component. In accordance with previous studies,1,9 our data showed that about half of MECAs developed in a preexisting PA (CA ex-PA). Moreover, MECA has been reported to be the second most common histologic type of CA ex-PA after salivary duct carcinoma.3,16
Histologically, the most characteristic feature of MECA (CA ex-PA and de novo) is its multinodular architecture and its zonal cellular arrangement. The latter consists of a hypercellular peripheral rim surrounding a hypocellular sometimes necrotic center. These 2 features help differentiate MECA from benign tumors like pleomorphic adenoma and myoepithelioma. Morphologic heterogeneity is another typical histologic feature of MECA, with tumors mostly displaying a mixture of different cell types and growth patterns. In the current study, focal luminal formations were observed in de novo MECAs; however, true ductal formations were rare and identified only in 4 cases, all of which had <10% duct formations. Allowing a minimal amount of ductal differentiation in MECA is a subject of debate.3 In our opinion, limited foci of ductal differentiation should not preclude the diagnosis of de novo MECA if the tumor is otherwise typical. If there is more than focal duct formation, the diagnosis of epithelial-MECA seems appropriate in the de novo carcinoma. In contrast, in CA ex- PA, finding more than focal ducts should not automatically lead to a misdiagnosis of epithelial-MECA, as many of these ducts could be benign and belong to the PA component. This is a particular diagnostic issue when the PA is intermixed with the MECA. Another important pitfall is the misclassification of the tumor as mucoepidermoid carcinoma because of the presence of squamous metaplasia in MECA. We have encountered a few cases in these series and our practice in which this mistake occurred. In some cases, determination of myoepithelial differentiation on the sole basis of routine morphology might not be sufficient.3,7 In these cases in which the morphology is suggestive but not definitive of MECA, reactivity for a cytokeratin and at least 1 of the myoepithelial markers, including S100, smooth muscle actin, calponin, and p63, is required. In this study, stained tumors were positive for a keratin and at least 1 of the myoepithelial markers. In keeping with the study of Kane and Bagwan, S100 was positive in the majority of MECAs.17 However, we found tumors to show a variable staining pattern for myoepithelial markers including S100; therefore, we believe that a panel of myoepithelial markers should be performed when the diagnosis of MECA is histologically suspected.
In accordance with previously reported studies, we found the clinical behavior of MECA to be relatively aggressive.1,3,9,13,15,17 Approximately, one third of the patients developed distant metastases, with lung being the most common site. In addition, 4 patients (10%) had LN metastasis at presentation, 9 (27%) developed local recurrences, and 1 died of the disease.
In view of its diverse reported behavior, 1 confounding issue regarding diagnosing MECA has been the assessment of tumor grading. Previous studies have shown no clear correlation between different histologic features of MECA and its clinical behavior.9,10,13,17,18 In a study by Nagao et al, 3 of 10 patients had MECAs with marked cytologic atypia, increased mitoses, and necrosis; among these, 1 died of his disease, but 2 had no evidence of disease after 8 and 11 years of FU.13 Savera et al9 classified MECAs as high grade when they showed marked cytologic atypia and nuclear pleomorphism and as low grade when they displayed relatively uniform small to intermediate-sized nuclei with fine chromatin. In that study, 2 of 15 patients with low-grade tumors developed distant metastases, and, in addition, 1 died of his disease.9 Our data also showed no correlation between nuclear atypia and DFS and in our opinion MECAs should not be graded on the basis of their nuclear appearance. In a recent study by Kane and colleagues, adequate FU was available on 18 patients, of which 12 had recurrences and 2 had distant metastases. The authors used a 3-tiered grading system instead of a 2-tiered one. They found no significant prognostic value for cytologic atypia, mitosis, and necrosis.17 In contrast to prior reported studies, our data showed that the presence of tumor necrosis correlated significantly with worse DFS. The discrepancy between our finding and the previous published data may be related to the fact that we analyzed a larger number of patients with FU. The absence of tumor necrosis appears to identify a group at lower risk for distant recurrence. Indeed, no distant recurrence was noted in all 23 patients lacking tumor necrosis with a median FU of almost 4 years. Therefore, we suggest defining high-grade MECA on the basis of tumor necrosis. This would also render the grading of MECA an easier and more reproducible exercise for pathologists, as the identification of tumor necrosis is a relatively objective histologic criterion. Although a high mitotic rate failed to correlate significantly with patients’ outcome in this study, this could be related to the number of cases analyzed in this cohort. Larger studies might be helpful to further clarify the prognostic significance of increased mitoses.
In addition to tumor necrosis, our study showed that CA ex-PA correlated with a worse clinical behavior compared with de novo MECA. Moreover, stratification analysis suggested that CA ex-PA and tumor necrosis may have independent effects. Previously, there has been a controversy regarding the difference in clinical outcome with regard to the presence or absence of a pleomorphic adenoma component. For example, a study by Di Palma and Guzzo8 has considered MECA to be high grade when it arises de novo and low grade when it is associated with a pleomorphic adenoma. However, both Nagao and colleagues and Savera and colleagues have shown similar clinical behavior in de novo carcinoma compared with CA ex-PA.1,9,13 In contrast, we found that CA ex-PA correlated significantly with worse DFS compared with de novo MECA. This does not seem to be related to tumor size, as the median sizes of both CA ex-PA and de novo MECA were somewhat similar (3.5 vs. 3.0 cm). At the molecular level, PLAG1 and HMGA2 rearrangements have been reported to be the most common genetic events in CA ex-PA including MECA type.19,20 Unlike CA ex- PA, de novo MECA lacks abnormalities in PLAG1 or HMGA2 genes.21 Therefore, using fluorescence in situ hybridization for PLAG1 and HMGA2 can distinguish CA ex-PA from its de novo counterparts and help improve tumor stratification. With regard to the molecular profile of MECA, EWSR1 gene rearrangements, which have been reported in myoepithelial tumors arising outside of the salivary gland and in hyalinizing clear cell carcinoma,22,23 were found to be absent in MECA of the salivary gland (de novo and CA ex-PA).21,23
In summary, MECA is an underrecognized tumor with a broad spectrum of morphologies. It is a relatively aggressive tumor that is associated with a high rate of distant metastasis. Compared with de novo MECA, CA ex-PA correlates with poorer clinical behavior. In addition, the presence of tumor necrosis correlates with worse DFS, and no distant recurrence is noted in patients with tumors lacking necrosis. Therefore, we suggest using a grading system based on the presence of tumor necrosis and a thorough search for a residual PA in the tumor. This may help predict behavior and better stratify patients for current and novel therapies.
Footnotes
Conflicts of Interest and Source of Funding: The authors have disclosed that they have no significant relationships with, or financial interest in, any commercial companies pertaining to this article.
REFERENCES
- 1.Barnes LEJ, Reichart P, Sidransky D. Pathology and Genetics Head and Neck Tumours. Lyon: IARC Press; 2005. pp. 240–241. [Google Scholar]
- 2.Stromeyer FW, Haggitt RC, Nelson JF, et al. Myoepithelioma of minor salivary gland origin. Light and electron microscopical study. Arch Pathol. 1975;99:242–245. [PubMed] [Google Scholar]
- 3.Ellis GL, Auclair PL. Tumors of the Salivary Glands Vol Fascicle 9. Washington, DC: ARP Press; 2008. [Google Scholar]
- 4.Kahn LB, Schoub L. Myoepithelioma of the palate. Histochemical and ultrastructural observations. Arch Pathol. 1973;95:209–212. [PubMed] [Google Scholar]
- 5.Leifer C, Miller AS, Putong PB, et al. Myoepithelioma of the parotid gland. Arch Pathol. 1974;98:312–319. [PubMed] [Google Scholar]
- 6.Seifert G, Sobin L. Histologic Typing of Salivary Gland Tumours. 2nd. Berlin: Springer-Verlag; 1991. [Google Scholar]
- 7.Seethala RR. An update on grading of salivary gland carcinomas. Head Neck Pathol. 2009;3:69–77. doi: 10.1007/s12105-009-0102-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Palma S, Guzzo M. Malignant myoepithelioma of salivary glands: clinicopathological features of ten cases. Virchows Arch A Pathol Anat Histopathol. 1993;423:389–396. doi: 10.1007/BF01607152. [DOI] [PubMed] [Google Scholar]
- 9.Savera AT, Sloman A, Huvos AG, et al. Myoepithelial carcinoma of the salivary glands: a clinicopathologic study of 25 patients. Am J Surg Pathol. 2000;24:761–774. doi: 10.1097/00000478-200006000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Ibrahim R, Bird DJ, Sieler MW. Malignant myoepithelioma of the larynx with massive metastatic spread to the liver: an ultrastructural and immunocytochemical study. Ultrastruct Pathol. 1991;15:69–76. doi: 10.3109/01913129109021305. [DOI] [PubMed] [Google Scholar]
- 11.Michal M, Skalova A, Simpson RH, et al. Clear cell malignant myoepithelioma of the salivary glands. Histopathology. 1996;28:309–315. doi: 10.1046/j.1365-2559.1996.d01-439.x. [DOI] [PubMed] [Google Scholar]
- 12.Dardick I. Myoepithelioma: definitions and diagnostic criteria. Ultrastruct Pathol. 1995;19:335–345. doi: 10.3109/01913129509021906. [DOI] [PubMed] [Google Scholar]
- 13.Nagao T, Sugano I, Ishida Y, et al. Salivary gland malignant myoepithelioma: a clinicopathologic and immunohistochemical study of ten cases. Cancer. 1998;83:1292–1299. doi: 10.1002/(sici)1097-0142(19981001)83:7<1292::aid-cncr4>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 14.Edge SB, Byrd DR, Compton CC, et al. American Joint Committee on Cancer, Cancer Staging Manual. 7. Chicago, IL: Springer; 2010. pp. 83–86. [Google Scholar]
- 15.Barnes L. Surgical Pathology of the Head and Neck. 3rd. Vol. 1. New York: Informa Healthcare; 2009. [Google Scholar]
- 16.Katabi N, Gomez D, Klimstra DS, et al. Prognostic factors of recurrence in salivary carcinoma ex pleomorphic adenoma, with emphasis on the carcinoma histologic subtype: a clinicopathologic study of 43 cases. Hum Pathol. 2010;41:927–934. doi: 10.1016/j.humpath.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 17.Kane SV, Bagwan IN. Myoepithelial carcinoma of the salivary glands: a clinicopathologic study of 51 cases in a tertiary cancer center. Arch Otolaryngol Head Neck Surg. 2010;136:702–712. doi: 10.1001/archoto.2010.104. [DOI] [PubMed] [Google Scholar]
- 18.Hsiao CH, Cheng CJ, Yeh KL. Immunohistochemical and ultrastructural study of malignant plasmacytoid myoepithelioma of the maxillary sinus. J Formos Med Assoc. 1997;96:209–212. [PubMed] [Google Scholar]
- 19.Bahrami A, Dalton JD, Shivakumar B, et al. PLAG1 alteration in carcinoma ex pleomorphic adenoma: immunohistochemical and fluorescence in situ hybridization studies of 22 cases. Head Neck Pathol. 2012;6:328–335. doi: 10.1007/s12105-012-0353-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martins C, Fonseca I, Roque L, et al. PLAG1 gene alterations in salivary gland pleomorphic adenoma and carcinoma ex-pleomorphic adenoma: a combined study using chromosome banding, in situ hybridization and immunocytochemistry. Mod Pathol. 2005;18:1048–1055. doi: 10.1038/modpathol.3800386. [DOI] [PubMed] [Google Scholar]
- 21.Katabi N, Ghossein R, Dogan S, et al. A PLAG1 and HMGA2 abnormalities in differential diagnosis of carcinoma ex-pleomorphic adenoma and de-novo counterparts. Mod Pathol. 2014;27(suppl 2):320A. doi: 10.1016/j.humpath.2014.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Antonescu CR, Katabi N, Zhang L, et al. EWSR1-ATF1 fusion is a novel and consistent finding in hyalinizing clear-cell carcinoma of salivary gland. Genes Chromosomes Cancer. 2011;50:559–570. doi: 10.1002/gcc.20881. [DOI] [PubMed] [Google Scholar]
- 23.Antonescu CR, Zhang L, Chang NE, et al. EWSR1-POU5F1 fusion in soft tissue myoepithelial tumors. A molecular analysis of sixty-six cases, including soft tissue, bone, and visceral lesions, showing common involvement of the EWSR1 gene. Genes Chromosomes Cancer. 2010;49:1114–1124. doi: 10.1002/gcc.20819. [DOI] [PMC free article] [PubMed] [Google Scholar]