Abstract
Natural killer (NK) cell activation is regulated by the integration of signals from inhibitory and activating cell surface receptors. Both NKG2A and NKG2C, pair with CD94 to form inhibitory and activating receptors specific for the HLA-E-canonical peptide complex. HLA-E is a nonclassical MHC Class Ib molecule with limited polymorphism. It preferentially binds to and presents leader sequence peptides derived from classical MHC class I molecules. Wilson Liao and colleagues have identified an association between NKG2C deficiency and psoriasis. They have also discovered an HLA-C-dependent association between HLA-E and psoriasis. Their research highlights the importance of NK cells in the pathophysiology of psoriasis. Herein we propose two different models to explain the association between NKG2C, HLA-E, and psoriasis. In the first model we hypothesize that NKG2C deficiency and/or HLA-E O1:01 can inhibit the ability of NK cells to regulate autoreactive T cells, predisposing to psoriasis. The second model proposes that HLA-E 01:03 can disrupt the presentation of the psoriasis-inducing self-determinant by HLA-C, thereby protecting against psoriasis.
Natural Killer (NK) cells, NK T cells, and T cells Bearing NK receptors
NK cells are innate lymphocytes that share some properties with cells of the adaptive immune system. They are well known for their cytotoxic and cytokine-producing effector functions. NK cells have the capacity to distinguish diseased cells (virally or bacterially infected, cancerous or otherwise stressed cells) from healthy cells. They also have the ability to proliferate in response to a viral infection and to form a long-lived memory-like population to protect against future pathogen encounters (1). More recently, it has been demonstrated that NK cells can act as immune regulatory cells (2). Specifically, they can promote inflammation by secreting IFN-γ, tumor necrosis factor (TNF), and other cytokines. The NK-secreted cytokines can also indirectly affect NK cell function by inducing antigen-presenting cells (APCs) to mature and secrete cytokines (e.g. IL-12), which directly act on NK cells (4-6). In contrast, NK cells can also down-regulate inflammatory responses by killing APCs and activated T cells (7, 8)(9)(10). Because of their strong influence over the immune system, it has been suggested that NK cells act as immunologic “rheostats” (11). Fortunately, the same biologic medications designed to target Th1 and Th17 cell responses also target the pathologic NK cytokine cascades (12, 13).
How an NK cell will respond to an encounter with an autoreactive T cell will depend on a variety of factors. Under normal physiologic conditions, a NK cell's response is tightly regulated by the integration of signals from its activating and inhibitory cell surface receptors. These receptors include killer cell Ig-like receptors (KIRs), and NKG2x receptors (x=2A, 2C, and 2D) (14, 15). Of the different KIR molecules, KIR2DL1 and KIR2DS1 are unique in that they bind to the psoriasis-linked HLA-C molecule via its C2 epitope. KIR2DS1 is a NK activating receptor, which, like HLA-Cw*0602, is associated with psoriasis (16, 17). Wilson and colleagues chose to further explore the relationship between NK cells and psoriasis by searching for additional associations between NK receptors, their ligands, and psoriasis. The NKG2 receptors, NKG2A and NKG2C, pair with CD94 to form inhibitory and activating receptors, respectively (Figure 1). In this issue of Experimental Dermatology, they find an association between NKG2C-deficiency and psoriasis (18). CD94/NKG2A and CD94/NKG2C are different from other NK receptors in that they both recognize the nonclassical MHC Class Ib molecule, HLA-E (19, 20). Unlike classical HLA molecules, HLA-E is characterized by a limited polymorphism and a conserved peptide-binding groove. It preferentially binds and presents 9-mer peptides derived from the leader sequence of other MHC class I molecules. Leader sequence peptides for HLA-E-binding are generated by the signal peptide peptidase and further proteasome processing (Figure 2) (21). Importantly, the two HLA-E alleles, 01:01 and 01:03, differ in their cell surface expression and their peptide binding affinities.
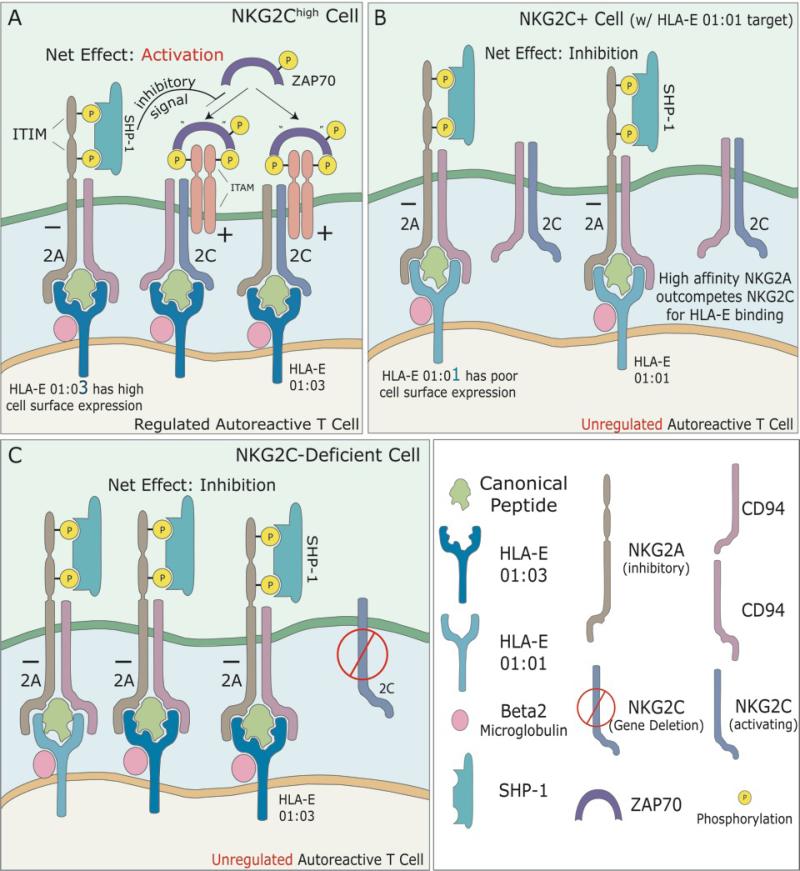
Figure 1. Inhibition of regulatory NK cells (or NK-like T cells) links NKG2C-deficiency and HLA-E01:01 to psoriasis.
The successful regulation of autoreactive T-cells by NK cells (or NK-like T cells) is dependent upon the integration of signals obtained from the NKG2A and NKG2C inhibitory and activating receptors. Peptide-bound HLA-E complexes found on the surface of autoreactive T-cells bind to CD94/NK2x (x=A, C) receptors on NK-regulatory cells. When bound to an HLA-E peptide complex, the immunoreceptor tyrosine-based inhibition motifs (ITIM) of the inhibitory receptor, CD94/NKG2A, become phosphorylated, allowing for SHP-1 protein to bind, and dephosphorylate ZAP70. Dephosphorylated ZAP70, is unable to bind to immunoreceptor tyrosine-based activation motifs (ITAMs), thus, inhibiting activation of the NK cell. CD94/NKG2C is an activating receptor. When bound to an HLA-E peptide complex this receptor interacts with ZAP70, via its ITAMs, to transmit an activating signal. Activated NK cells then function to regulate autoreactive T-cells.
A.) NKG2Chigh Regulatory Cell. High expression of CD94/NKG2C receptors, results in a net positive activation signal of the corresponding NK cell, allowing for regulation of autoreactive T-cells. HLA-E 01:03 has a higher affinity for antigen peptides and a higher cell surface expression compared to HLA-E 01:01. CD94/NKG2C is protective against psoriasis.
B.) Homozygous for HLA-E 01:01. Homozygosity for HLA-E 01:01 results in expression of fewer cell surface HLA-E molecules. CD94/NKG2A has a 6-fold higher affinity for HLA-E 01:01 and outcompetes CD94/NKG2C. This results in a net inhibition of NK cells, and thus unregulated autoreactive T-cells, predisposing to psoriasis.
C.) Deletion of NKG2C. Deletion of the activating NKG2C receptors favors inhibition of NK cells, and subsequently, unregulated autoreactive T-cells, predisposing to psoriasis.
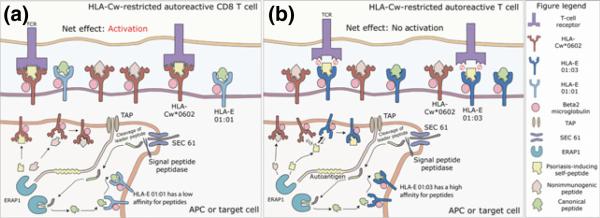
Figure 2. Determinant capture as an alternative model to explain the link between HLA-E and psoriasis.
Endoplasmic reticulum aminopeptidase 1 (ERAP1) functions to process peptides within the endoplamic reticulum, which are then presented by HLA molecules on antigen presenting cells or target cells to autoreactive CD8+ T-cells.
A.) HLA-Cw*0602 Restricted Autoreactive CD8+ T-cells. HLA-E 01:01 has a lower affinity for peptides compared to HLA-Cw*0602. It presents mainly canonical peptides derived from the leader sequence of classical HLA class one molecules. Psoriasis-inducing self peptides presented by HLA-Cw*0602 on the surface of an APC or target cells are recognized by autoreactive T-cells, resulting in activation of pathogenic CD8+ T-cells, predisposing to psoriasis.
B.) HLA-E 01:03 captures the psoriasis-inducing self-peptide from HLA-C. The increased peptide-binding affinity of HLA-E 01:03 allows it to outcompete HLA-Cw*0602 for binding to the ERAP1-processed psoriasis-inducing self-peptide. The HLA-E 01:03-presented self-peptide is not recognized by autoreactive CD8+ T cells due to incompatible binding sites between the TCR and HLA-E MHC molecule. As a result, activation of the autoreactive CD8+ T-cell is prevented. HLA-E 01:03 is protective against psoriasis in HLA-Cw*0602 positive patients.
Some T cells can express a variety of NK molecules, including the characteristic NK activating and inhibitory receptors. For example, nearly all human CD8+ T cells express the NKG2D activating receptor and a smaller fraction express NKG2A and NKG2C. There is also a specialized population of innate-like lymphocytes referred to as NK T cells. These are specialized glycolipid-specific T cells that are restricted to the non-polymorphic MHC class I-like molecule, CD1d. Human NK T cells that utilize a semi-invariant Vα24-Jα18:Vβ11 T cell receptor (TCR) to recognize CD1d-bound glycolipid antigens are referred to as type I or invariant NK T cells (iNK T cells). Like NK cells, NK T cells and the T cells bearing NK receptors can partake in similar immune regulatory activities. In atopic dermatitis IL-22 producing NK-like cells are known as “Th17/Th22 cytokine-producing innate cells” (3) and in allergic contact dermatitis (ACD), iNK T cells function as effector cells by producing cytotoxic granules (22). We do not limit the mechanisms described herein to any one particular NK molecule-bearing cell type.
HLA-E, NKG2C deficiency, and the development of psoriasis
NKG2C high cells are known to expand in response to viral pathogens and remain elevated longitudinally (1). With respect to their ability to prevent psoriasis, cells expressing elevated levels of the activating NKG2C receptor are more likely to become activated in response to an encounter with an autoreactive T cell (Figure 1A). The activated NKG2C-expressing cell can then kill the autoreactve T cell, thereby preventing psoriasis. Of note psoriasis plaques apparently have fewer NKG2C positive cells when compared to NKG2A (23). This may limit the regulatory function of the NK compartment in patients with psoriasis.
When coupled to CD94, both NKG2A and NKG2C can bind to HLA-E molecules presenting canonical peptides derived from the leader sequence of classical MHC class I molecules. However, when compared to NKG2C, NKG2A posses higher affinity for HLA-E/peptide complexes (Figure 1B). Thus, the poor cell surface expression of HLA-E 01:01 creates a competitive state where the higher affinity NKG2A outcompetes NKG2C for HLA-E/peptide binding resulting in net inhibition of the NK cell. In this scenario, the autoreactive T cell remains unregulated and psoriasis is favored. Similarly, if someone is deficient in NKG2C due to a gene deletion, the inhibitory signal of NKG2A is not counterbalanced by an activating signal from NKG2C (Figure 1C). The outcome is again a lack of NK cell activation, resulting in an unregulated autoreactive T cell.
Determinant Capture may explain the link between HLA-E and psoriasis
Above we hypothesized a link between HLA-E and psoriasis based upon preferential binding of the inhibitory receptor, NKG2A, to HLA-E. However, this model does not explain the HLA-C-dependent association between HLA-E and psoriasis. We thus propose the following as an alternative model.
HLA-E is known to bind preferentially to the leader sequence of MHC class I molecules (Figure 2). However, it can also bind to other ERAP 1-processed peptides and elicit HLA-E-restricted αβ T cell responses (24), similar to a classical MHC molecule. The model of antigen processing proposed by Sercarz and colleagues dictates that the cell surface expression of a particular peptide/MHC complex is the result of a competitive process in which different MHC molecules compete for binding to the same antigenic peptide (25). Since psoriasis is strongly associated with HLA-Cw*0602, it is likely that HLA-C binds to and presents a, yet to be identified, psoriasis-inducing self-peptide. If this psoriasis-inducing self-peptide also binds well to HLA-E, which has five characteristic hydrophobic pockets, then it is possible that HLA-E can compete with HLA-C for binding to the auto-antigen. In this model, HLA-E does not compete with HLA-C for binding to all peptides, just the relatively few that have MHC-binding motifs for both HLA-E and HLA-C. The high peptide-binding affinity of HLA-E 01:03 protects against psoriasis because HLA-E 01:03 can “capture” the psoriasis-inducing self-peptide from HLA-C (Figure 2). Contrariwise, the low peptide-binding affinity of HLA-E 01:01 predisposes individuals to the development of psoriasis because presentation of the psoriasis-inducing self-peptide by HLA-C remains unopposed (Figure 2).
Acknowledgments
Funding Sources: EM was supported by career awards from the HHMI and the BWF. This work was supported by the NIH DP2OD008752.
Footnotes
Conflict of interest disclosures: None Reported
Authorship Acknowledgements:
The authors, Forum Patel, Alina I Marusina, Christopher Duong, Iannis E Adamopoulos, and Emanual Maverakis, all contributed equally to the designing, drafting, and revising of this manuscript for submission.
References
- 1.Foley B, Cooley S, Verneris MR, et al. Cytomegalovirus reactivation after allogeneic transplantation promotes a lasting increase in educated NKG2C+ natural killer cells with potent function. Blood. 2012;119:2665–2674. doi: 10.1182/blood-2011-10-386995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vivier E, Tomasello E, Baratin M, et al. Functions of natural killer cells. Nat Immunol. 2008;9:503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 3.Park CO, Noh S, Jin S, et al. Insight into newly discovered innate immune modulation in atopic dermatitis. Experimental dermatology. 2013;22:6–9. doi: 10.1111/exd.12034. [DOI] [PubMed] [Google Scholar]
- 4.Walzer T, Dalod M, Robbins SH, et al. Natural-killer cells and dendritic cells: “l'union fait la force”. Blood. 2005;106:2252–2258. doi: 10.1182/blood-2005-03-1154. [DOI] [PubMed] [Google Scholar]
- 5.Moretta L, Ferlazzo G, Bottino C, et al. Effector and regulatory events during natural killer-dendritic cell interactions. Immunol Rev. 2006;214:219–228. doi: 10.1111/j.1600-065X.2006.00450.x. [DOI] [PubMed] [Google Scholar]
- 6.Degli-Esposti MA, Smyth MJ. Close encounters of different kinds: dendritic cells and NK cells take centre stage. Nat Rev Immunol. 2005;5:112–124. doi: 10.1038/nri1549. [DOI] [PubMed] [Google Scholar]
- 7.Long EO, Kim HS, Liu D, et al. Controlling natural killer cell responses: integration of signals for activation and inhibition. Annu Rev Immunol. 2013;31:227–258. doi: 10.1146/annurev-immunol-020711-075005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Orange JS. Formation and function of the lytic NK-cell immunological synapse. Nat Rev Immunol. 2008;8:713–725. doi: 10.1038/nri2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lang PA, Lang KS, Xu HC, et al. Natural killer cell activation enhances immune pathology and promotes chronic infection by limiting CD8+ T-cell immunity. Proc Natl Acad Sci U S A. 2012;109:1210–1215. doi: 10.1073/pnas.1118834109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andrews DM, Estcourt MJ, Andoniou CE, et al. Innate immunity defines the capacity of antiviral T cells to limit persistent infection. J Exp Med. 2010;207:1333–1343. doi: 10.1084/jem.20091193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Waggoner SN, Cornberg M, Selin LK, et al. Natural killer cells act as rheostats modulating antiviral T cells. Nature. 2012;481:394–398. doi: 10.1038/nature10624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ottaviani C, Nasorri F, Bedini C, et al. CD56brightCD16(−) NK cells accumulate in psoriatic skin in response to CXCL10 and CCL5 and exacerbate skin inflammation. Eur J Immunol. 2006;36:118–128. doi: 10.1002/eji.200535243. [DOI] [PubMed] [Google Scholar]
- 13.Sivamani RK, Goodarzi H, Garcia MS, et al. Biologic therapies in the treatment of psoriasis: a comprehensive evidence-based basic science and clinical review and a practical guide to tuberculosis monitoring. Clin Rev Allergy Immunol. 2013;44:121–140. doi: 10.1007/s12016-012-8301-7. [DOI] [PubMed] [Google Scholar]
- 14.Bryceson YT, March ME, Ljunggren HG, et al. Activation, coactivation, and costimulation of resting human natural killer cells. Immunol Rev. 2006;214:73–91. doi: 10.1111/j.1600-065X.2006.00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol. 2008;9:495–502. doi: 10.1038/ni1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luszczek W, Manczak M, Cislo M, et al. Gene for the activating natural killer cell receptor, KIR2DS1, is associated with susceptibility to psoriasis vulgaris. Hum Immunol. 2004;65:758–766. doi: 10.1016/j.humimm.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 17.Holm SJ, Sakuraba K, Mallbris L, et al. Distinct HLA-C/KIR genotype profile associates with guttate psoriasis. J Invest Dermatol. 2005;125:721–730. doi: 10.1111/j.0022-202X.2005.23879.x. [DOI] [PubMed] [Google Scholar]
- 18.Zeng X, Chen H, Gupta R, et al. Deletion of the activating NKG2C receptor and a functional polymorphism in its ligand HLA-E in psoriasis susceptibility. Exp Dermatol. 2013;22:679–681. doi: 10.1111/exd.12233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Braud VM, Allan DS, O'Callaghan CA, et al. HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C. Nature. 1998;391:795–799. doi: 10.1038/35869. [DOI] [PubMed] [Google Scholar]
- 20.Vance RE, Kraft JR, Altman JD, et al. Mouse CD94/NKG2A is a natural killer cell receptor for the nonclassical major histocompatibility complex (MHC) class I molecule Qa-1(b). J Exp Med. 1998;188:1841–1848. doi: 10.1084/jem.188.10.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bland FA, Lemberg MK, McMichael AJ, et al. Requirement of the proteasome for the trimming of signal peptide-derived epitopes presented by the nonclassical major histocompatibility complex class I molecule HLA-E. J Biol Chem. 2003;278:33747–33752. doi: 10.1074/jbc.M305593200. [DOI] [PubMed] [Google Scholar]
- 22.Balato A, Zhao Y, Harberts E, et al. CD1d-dependent, iNKT-cell cytotoxicity against keratinocytes in allergic contact dermatitis. Experimental dermatology. 2012;21:915–920. doi: 10.1111/exd.12036. [DOI] [PubMed] [Google Scholar]
- 23.Batista MD, Ho EL, Kuebler PJ, et al. Skewed distribution of natural killer cells in psoriasis skin lesions. Exp Dermatol. 2013;22:64–66. doi: 10.1111/exd.12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pietra G, Romagnani C, Manzini C, et al. The emerging role of HLA-E-restricted CD8+ T lymphocytes in the adaptive immune response to pathogens and tumors. Journal of biomedicine & biotechnology. 2010;2010:907092. doi: 10.1155/2010/907092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sercarz EE, Maverakis E. Mhc-guided processing: binding of large antigen fragments. Nat Rev Immunol. 2003;3:621–629. doi: 10.1038/nri1149. [DOI] [PubMed] [Google Scholar]