Abstract
Due to their large optical cross sections and unique photostability, plasmonic nanostructures are attractive building blocks for next generation image sensors and color detecting or emitting devices with significantly decreased footprints. Plasmonic nanostructures, arrays and metasurfaces provide the ability to encode complex color and polarization patterns. The latter is of particular relevance in information storage[1] and cryptographic applications to generate security tags or steganography[2] with much smaller feature sizes (and thus higher data density)[3] than is possible with conventional holographic techniques. The realization of the full potential of these plasmonic technologies requires the ability to generate “pixels” that can encode a broad range of colors and polarization properties. The conventional approach of generating plasmonic color pixels through top-down patterning is, however, intrinsically limited in compositional and structural variety. We introduce herein a sequential directed self-assembly approach that facilitates the utilization of the strong material- and shape-dependence of colloidal plasmonic nanoparticles (“atoms”) and their assemblies (“molecules”) to generate switchable color and polarization patterns across the entire visible range of the electromagnetic spectrum.
Keywords: plasmon hybridization, directed self-assembly, metasurface, plasmonic printing
Table of contents entry
Nanoparticles of different materials, shapes, and sizes are integrated into plasmonic atoms and molecules of defined shape and location through sequential directed self-assembly following a single patterning step. A rational tuning of the emitted color across the visible range of the electromagnetic spectrum and switchable polarization properties are demonstrated. Self-assembled plasmonic pixels provide tunable, stable, and switchable optical responses.
The current strategies for generating plasmonic colors can be subdivided into two main strategies: structural color[4] and pixel based approaches. The former include wavelength-selective extraordinary transmission through nanohole[5] or nanoslit arrays[6]diffractive coupling in nanoparticle arrays,[7] interference-based shaping of transmission and reflection in guided-mode resonance filters[8] or metal-insulator-metal devices[9]. Structural color is an intrinsic photonic effect and, therefore, still requires feature sizes of a few microns. Due to the large optical cross-sections of metal nanoparticles, much smaller feature sizes are possible using individual nanoparticles or plasmonic molecules as color determining pixels.[2b, 10]
Despite impressive accomplishments,[2b, 2c, 10a] pixel-based plasmonic printing has, so far, been limited to nanostructures generated from top-down nanopatterning of a mask with a typical spatial resolution of > 10 nm and subsequent evaporation or sputtering of one metal (typically gold or aluminum). Although multiple subsequent patterning and evaporation steps can be used to integrate multiple metals, the precise spatial alignment of the individual fabrication steps is challenging and time consuming, if at all possible for intricate patterns. Limitation to only one metal decreases, however, the range and tunability of the accessible color palette. To utilize plasmon coupling[11] within plasmonic molecules as color rending mechanism, it is desirable to generate strongly coupled electromagnetic systems, ideally with interparticle separations below 5 nm. These short interparticle separations are necessary in order to access the full dynamic range available through plasmon hybridization.[12] Even with state-of-the-art direct write electron beam lithography (EBL), it remains challenging to generate these short separations reliably. Furthermore, evaporated or sputtered metal nanostructures often offer inferior optical properties when compared with wet-chemically grown nanoparticles and nanostructures,[13] as the former possess more surface roughness and grain boundaries that increase plasmon damping.[14]
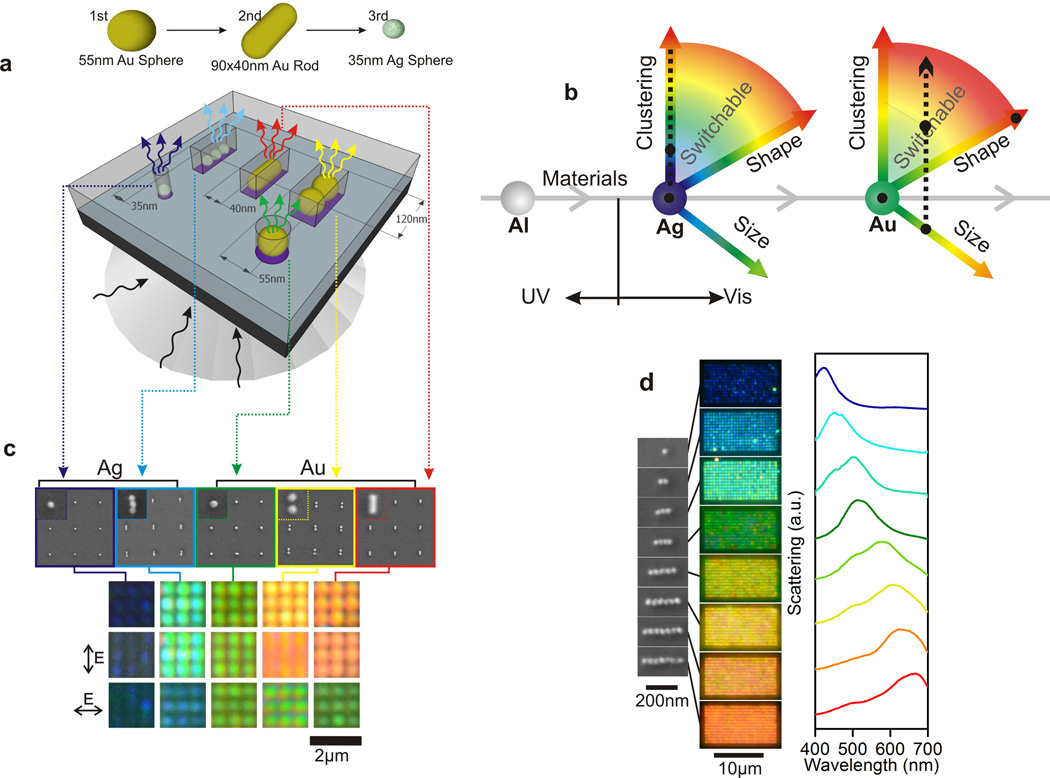
Our approach to address these challenges and to achieve a continuous tuning of the plasmonic pixel response across the visible range of the electromagnetic spectrum is based on a directed assembly approach that generates plasmonic atoms and molecules of well-defined composition, clustering level, and geometry (Figure 1a and 1b). Different from conventional top-down fabrication, which combines a nanopatterning tool, such as EBL, and metal evaporation to write the metal nanostructure of interest, the proposed fabrication strategy uses EBL to define binding sites for the assembly of colloidal nanoparticles into clusters. In this way the ability of self-assembly to generate strongly coupled metal nanoparticles with few nanometer gap separations is combined with the lateral patterning precision of EBL on longer length scales. [7b, 15] We mention in passing that other more favorably scaling top-down methods, such as deep UV lithography or hot embossing, are also applicable for patterning purposes instead of EBL.[16] In this work we use a directed assembly approach to guide multiple plasmonic building blocks, comprising metal nanoparticles of different size, shape and composition, to separate locations and, thus, generate two-dimensional electromagnetic materials of higher complexity. Our approach exploits the fact that the morphology of the binding sites created in the photoresist encodes for a size- and shape-selective immobilization of nanoparticles and nanoparticle clusters at pre-determined locations. Only nanoparticles with the matching size/geometry or smaller dimensions “fit” into a given binding site at one specific location. Our rationale was that by incubating the mask with colloidal nanoparticles in the sequence of decreasing size it is, thus, possible to subsequently fill the available binding sites with different metal nanoparticles.
Figure 1. Controlling colors and polarization properties with site-directed assembly of plasmonic nanoparticles.
a) Plasmonic atoms and molecules of different materials, shapes and sizes are guided to sequentially assemble at pre-determined binding sites that match the geometry of the elementary building blocks. b) Schematic color coordinate system that highlight the degrees of freedom to control the color of plasmonic atoms and molecules. Black dots and dashed lines represent the points demonstrated in this Letter and supporting information. c) SEM and color darkfield images of plasmonic pixels assembled on a same substrate via sequential assembly. d) SEM images, darkfield images and scattering spectra of the longitudinal mode of linear clusters of 35 nm silver nanospheres with increasing cluster size.
The nanoparticle material, size, and shape determine the resonance wavelength of the individual nanoparticle building blocks.[17] In this study we chose gold and silver nanoparticles with approximate diameters of 55 nm and 35 nm, respectively, as well as gold nanorods with an aspect ratio of 2.3 (long axis ≈ 90 nm) as elementary units for generating plasmonic pixels as their resonance wavelengths cover different regions of the visible electromagnetic spectrum (blue, green and red, respectively). We used EBL to pattern a mask in a photoresist, but other methods, such as deep UV lithography or hot embossing, can also be used and facilitate fabrication at a larger scale. After a successful generation of the desired pattern, the binding sites were treated with an aqueous solution of polylysine to charge them positively. All nanoparticles, including the gold nanorods, used in this work were pegylated with HS-(CH2)11-(OC2H4)6-OCH2-COOH and were, therefore, negatively charged at pH = 7.0. Attractive Coulomb interactions between nanoparticles and binding sites were important to facilitate an efficient assembly of nanoparticles into densely packed clusters during a capillary-force-assisted self-assembly.[18] At the same time, repulsive interactions between the nanoparticles prevented the formation of multiple layers on the binding sites.
We experimentally validated the ability of the directed self-assembly approach to generate pixels comprising different plasmonic atoms and molecules of defined shape, orientation, and composition on one chip. We generated five different binding sites (120 nm × 55 nm, 120 nm × 40 nm, 120 nm × 35 nm as well as disk-like binding sites with diameters of approximately 55 nm and 35 nm) and incubated the samples first with 55 nm gold nanospheres, then with gold nanorods, and finally with 35 nm silver nanospheres (Figure 1a). This approach led to the selective formation of gold nanosphere dimers on the 120 nm × 55 nm rectangular sites and monomers on the 55 nm disk-shaped sites. The gold nanorods selectively bound to the vacant 120 nm×40 nm binding sites, while silver nanoparticle trimers and monomers were formed exclusively on the 120 nm × 35 nm rectangular and 35 nm diameter disk-shaped binding sites, respectively. Figure 1c contains SEM and optical darkfield images of the assembled structures for the five investigated binding sites. Silver spheres, gold spheres and gold rods show vivid blue, green and red colors, while the linear silver nanoparticle trimers and gold dimers are cyan and yellow, respectively. Gold and silver nanoparticles show natural size distributions (which is significantly broader in the case of silver). Since the size dependence of the plasmon resonance is weak,[19] the spectral variations of the resonance peak wavelength for the individual building blocks used in this work are relatively modest and lie within a range of < 15 nm.[20] In the future, the size variations of the building blocks, especially in the case of silver, can be further reduced by incorporating recent advancements in NP synthesis.[21] The spacing among the pixels in the array was chosen as 600 nm. Figure 1c shows magnified sections (1.8 µm × 1.8 µm) of fabricated square arrays with total dimensions of 20 µm × 20 µm (Figure S1, Supporting Information). At the chosen periodicity the individual clusters are still clearly recognized as separate spots, ruling out the presence of far-field diffraction (Figure S2, Supporting Information).
The assembly yields were determined by optical inspection of large arrays of assembled nanopixels containing hundreds of individual pixels with sufficiently large separations to avoid diffractive coupling. For each pixel the color and intensity in the recorded images are evaluated to determine if none, one or more particles have bound. Table 1 lists the resulting yield achieved by us for each type of pixel using the presented approach as function of the assembly order. The latter defines the sequence in which the different nanoparticle building blocks were added. The yields of assembly are > 95% for gold nanosphere based pixels, > 93% for gold nanorod based pixels, and > 80% for silver nanosphere based pixels. The major source of imperfection in all cases was incomplete binding resulting in empty or only partially occupied binding sites. Another source of error is the misbinding of nanoparticles (nanoparticle immobilization on wrong binding site) whose occurrence increases with increasing particle size distribution. The yields in Table 1 decrease in the assembly order. We attribute this effect to a decrease in the binding affinity between nanoparticle and binding site caused by a gradual removal of the electro-statically absorbed poly-lysine polymers in subsequent washing steps.
Table 1.
Pixel yields after each assembly step.
| Assembly Order |
1 | 1 | 2 | 3 | 3 |
|---|---|---|---|---|---|
| Pixel | Au Monomer | Au Cluster | Au Rod | Ag Monomer | Ag Cluster |
| Yield | 97.0% | 95.2% | 93.7% | 90.7% | 80.8% |
The assembly of nanoparticles into molecules, in which the plasmons of the individual nanoparticle hybridize, makes it possible to red-shift the plasmon resonances of the respective building blocks and, if anisotropic plasmonic molecules are generated, also to induce and enhance light polarizing properties. Figure 1c demonstrates the distinct polarization dependence of the scattering signal for one-dimensional silver and gold nanoparticle clusters as well as for gold nanorods. If the polarization direction of the detected light is rotated from pointing along the long to pointing along the short axis of the scatterers, the detected color switches from green to blue (silver trimer), from orange to green (gold dimer) and from red to green (gold nanorod). In contrast, the optical responses of the isotropic gold and silver spheres are unpolarized and, therefore, independent of the analyzer orientation.
The broad spectral tunability of plasmonic cluster pixels via the size and aspect ratio of the contained plasmonic molecules is demonstrated for one-dimensional nanoparticle chains in Figure 1d. By variation of the binding site length, Land thus average cluster size, nof 35 nm diameter silver nanoparticles, the plasmon resonance can be systematically shifted. The peak resonance wavelength increases continuously with the length of the binding site in the investigated range between L ≈ 35 – 250 nm (Figure S3a). Plasmon coupling also results in a strong increase of the scattering intensity with growing cluster size (Figure S3b). Interestingly, disk-shaped clusters of silver nanospheres and gold nanospheres can generate a broad range of similar colors (Figure S4a and S4b, Supporting Information), but only the linear nanoparticle chains achieve an efficient polarization of the scattered light. Consequently, the ability to pattern nanoparticles through directed self-assembly creates unique opportunities for tuning the resonance wavelength and light polarization independently from each other. Utilizing the different polarization responses of anisotropic and isotropic plasmonic atoms and molecules, color cryptographic images can be constructed where the information is hidden under unpolarized light but revealed with polarized light.
Plasmonic atoms and molecules represent the smallest possible plasmonic pixels. The range of colors enabled by them can be further broadened if multiple plasmonic atoms and/or molecules are integrated in one sub-diffraction spot, so that the colors of the individual elements mix.[10a] The process of plasmonic color mixing is illustrated in Figure 2a and 2bwhich shows scattering and SEM images of two overlapping arrays of gold nanorods and nanospheres assembled using the sequential directed self-assembly approach. In the overlap region of the two arrays the center to center distance between the two different building blocks is 150 nm, which is below the diffraction limit (Figure 2b inset). Consequently, we effectively generated new pixels with multiple components in the overlap region whose color is determined by the mixing of the light emitted by the colocalized components in the far-field. For the particular example in Figure 2a mixing of the emitted red and green light generates an overall yellow impression.
Figure 2. Color mixing by optical colocalization of plasmonic atoms.
a) Darkfield image of a red gold nanorod array (upper-left) that overlaps with a second array containing green 55 nm gold nanospheres (lower-right) to create a yellow impression in the overlap region. b) SEM image of overlapping gold nanorod and nanosphere arrays. The inset contains a magnified view of an individual pixel containing both gold nanorod and nanosphere in one sub-wavelength spot. c) Digital camera pictures (top) of complex pixels containing different ratios of silver and gold spheres and gold rods. The corresponding SEM images are shown below. The individual nanoparticles were colored as they appear under darkfield illumination.
The generation of colors through mixing is a common approach in all modern display techniques in which the color of each pixel is usually determined by the relative intensities of blue, green and red sub-pixels. Applying the RGB color model to our plasmonic nanopixels, a broader range of colors including non-spectral colors such as magenta can be achieved by implementing more complex pixels that integrate multiple nanoparticles and clusters in a sub-diffraction area. This is demonstrated in Figure 2c where each pixel is a complex of four building blocks. By systematically changing the ratio of blue (silver nanoparticle), green (gold nanoparticle) and red (gold rod) building blocks within each pixel, we have achieved secondary and tertiary colors around the color wheel within the visible range of the electromagnetic spectrum. If we treat the individual binding steps as independent events, the estimated yields can be as high as 89% for complex pixels composed of four gold nanospheres and as low as 66% for pixels composed of four Ag nanospheres.
One property that makes plasmonic printing particularly interesting for security tagging is that anisotropic nanoparticles or nanoparticle clusters allow the spatial encoding of complex, wavelength dependent polarization responses that are easily detectable. To demonstrate this property, we assembled a BU-PHO-NANO logo in which different components provided different resonance wavelength and polarization properties (Figure 3a). The letters “BU” are composed of 90×40 nm gold nanorods, “PHO” of 55 nm gold nanospheres dimers, and “NANO” of 35 nm silver horizontally aligned dimers and trimers (Figure 3b, 3c, 3d and Figure S5, Supporting Information). The spacing between the individual pixels was 600 nm and, thus, close to the diffraction limit. Polarization dependent color switching of all three parts is observed by rotating a linear analyzer (Movie Logo.gif, Supporting Information). The anisotropic nanoparticle clusters and rods show different polarization responses and allow a switching of the color as function of polarization. Using both gold and silver as building blocks, we succeeded in generating switchable anisotropic molecules across the entire visible range. The gold rods switch between green and red, whereas the gold dimers change from orange to green-yellow. The silver trimer, finally, changes from a deep blue to a vivid blue-green. The orientation of the long axis of the rod or nanoparticle cluster determines the polarization direction associated with the low energy longitudinal plasmon resonance, whereas the orthogonal polarization is associated with the higher energy vertical resonance.[11b]
Figure 3. Polarization-dependent color switching through active multicolor pixels.
a) Darkfield image of a logo observed without analyzer (top), and with a linear analyzer with vertical orientation (middle), or horizontal orientation (bottom). b–d) SEM images of the letter “B”, “H” and first “N” in the logo.
The multicolor logo created with a single patterning step contains some fabrication imperfections but the switchable color and polarization response is clearly demonstrated. In the future, the assembly-based plasmonic printing process can be further improved through optimization of the patterning process and sharpening of the nanoparticle size distributions.
In summary, we have demonstrated that directed self-assembly facilitates the integration of colloidal nanoparticles into sub-diffraction limit color pixels for plasmonic printing. The directed self-assembly is unique in that it facilitates the integration of different materials (gold vs. silver) and nanoparticle shapes (spheres vs. rods) as individual nanoparticles or near-field coupled clusters with a single top-down patterning step. While previous studies relied on size and shape of the nanoparticles as control parameters to generate plasmonic colors on the nanoscale,[10a, 22] the template guided self-assembly approach augments the existing capabilities with additional degrees of freedom for fine-tuning plasmonic colors by providing control over the chemical composition of the nanoparticles and the plasmon coupling between them. Guided self-assembly also allows the spatial encoding of polarization dependent spectral responses across the entire visible range of the electromagnetic spectrum using anisotropic shaped clusters and particles. Plasmonic atoms and molecules represent the smallest possible color units in plasmonic printing. Rational assembly strategies for these structures that facilitate color and light polarization manipulation on deeply sub-wavelength scales will advance the field of plasmonic nanoprinting and its applications, for instance, in future high density optical data storage, encryption and display technologies Last but not least, we would also like to emphasize that plasmonic pixels based on plasmon coupling generate electromagnetic hot spots in the gaps between the nanoparticles. The ability to pattern hot-spots with colors across the visible range of the electromagnetic spectrum and beyond at well-defined locations provides new opportunities for surface enhanced and non-linear spectroscopies.
Experimental
Materials
Silver nanospheres and negative charged gold nanorods with nominal size of 90 × 40 nm were purchased from Nanopartz Inc. The silver nanospheres had a Z-average diameter of 36.3 ± 1.0nm with a polydispersity index (PDI) of 0.330 as determined by Dynamic Light Scattering. Gold nanospheres were obtained via a standard Turkevich synthesis and had a Z-average diameter of 54.6 ± 0.8nm with a PDI of 0.061.
Electron-beam lithography
950 PMMA A3 was diluted with anisole in a ratio of 2:1 (v:v). Diluted PMMA solution was spin coated onto a 1.2 cm × 1.2 cm ITO glass substrate (Sigma-Aldrich) with spin speed of 2000 rpm for 45 seconds and baked in 170°C oven for 20 min. The PMMA layer thickness was around 100 nm. Thinner PMMA templates increased the nanoparticle binding efficiency. EBL was performed with a Zeiss SUPRA 40VP SEM equipped with a Raith beam blanker and a nanopattern generation system (NPGS). Considering the sensitive dependence of the cluster configuration on the binding site size, dosage ramping was performed for all experiments to achieve optimized selectivity. The patterned substrates were developed in a solution mixture of 1:3 Methyl isobutyl ketone (MIBK) : 2-proponal (v:v) for 70 seconds followed by extensive rinsing in 2-proponal and blow dry.
Directed self-assembly
5 µL of 2% poly-lysine aqueous solution (w/v) was applied directly onto the developed EBL pattern. The ITO-glass substrate with poly-lysine solution covering the pattern was placed in a humidified environment for 1 hour. Prolonged incubation with poly-lysine solution was avoided to prevent loss of adhesion of the PMMA layer to the ITO surface. After incubation, the substrate was washed extensively in water and 2-proponal to remove poly-lysine molecules that were non-specifically attached to the PMMA.
All nanoparticles were passivized by thiol-alkyl-PEG (HS-C11H22-EG6-COOH, EG=OCH2CH2) (NANOCS Inc.) through overnight incubation with a PEG : NP ratio of 106: 1. All particles were washed at least five times by centrifugation in water to remove excess free PEG molecules and then concentrated to a minimum concentration of 30 nM. The nanoparticles are negatively charged with zeta potential of −28.5±0.5mV. 2 µL of each concentrated nanoparticle solution were applied sequentially onto the patterned PMMA mask in the sequence from biggest/widest to smallest/narrowest. To increase the binding efficiency, colloidal solutions on the substrate were blown back and forth across the pattern for 30~50 times using a nitrogen stream. This approach utilized capillary force at the air-liquid interface to push the nanoparticles into the binding site wells as well as facilitate uniform closed packing within clusters. After the binding of nanoparticles, a low adhesion tape (1310TB100-P5D Ultratape Inc.) was gently pressed onto the PMMA surface and then slowly peeled off to remove any nanoparticles that are unspecifically sticking to the PMMA top surface.
Optical Measurements
The ITO-glass substrates are placed onto a water droplet on a coverslide with the pattern facing downward. The spectrum and digital camera pictures were recorded on an Olympus IX71 inverted microscope equipped with an imaging spectrometer (Andor Shamrock with Andor Newton EMCCD). All digital pictures were taken with a Nikon D3100 SLR through a microscope adaptor. The samples were illuminated at oblique angles from the unpatterned substrate side using an oil-immersion DF condenser (Olympus U-DCW, NA = 1.2 ~ 1.4). A xenon lamp (λ = 380–720 nm, Agilent Polychrome 3000) was used as illumination source. The light scattered from the sample was collected with a 60× oil objective lens. A polarizer was placed after the objective to record polarization dependent scattering spectra or images.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (NCI) through grant 5R01CA138509 to BMR. ((Supporting Information is available online from Wiley InterScience or from the author)).
References
- 1.Myers CJ, Celebrano M, Krishnan M. Nat. Nanotechnol. 2015;10:886. doi: 10.1038/nnano.2015.173. [DOI] [PubMed] [Google Scholar]
- 2.a) Chen WT, Yang K-Y, Wang C-M, Huang Y-W, Sun G, Chiang ID, Liao CY, Hsu W-L, Lin HT, Sun S, Zhou L, Liu AQ, Tsai DP. Nano Lett. 2014;14:225. doi: 10.1021/nl403811d. [DOI] [PubMed] [Google Scholar]; b) Montelongo Y, Tenorio-Pearl JO, Williams C, Zhang S, Milne WI, Wilkinson TD. Proc. Natl. Acad. Sci. USA. 2014;111:12679. doi: 10.1073/pnas.1405262111. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Goh XM, Zheng Y, Tan SJ, Zhang L, Kumar K, Qiu C-W, Yang JKW. Nat. Commun. 2014;5:5361. doi: 10.1038/ncomms6361. [DOI] [PubMed] [Google Scholar]
- 3.a) Ni X, Kildishev AV, Shalaev VM. Nat. Commun. 2013;4:2807. [Google Scholar]; b) Zhou F, Liu Y, Cai W. Opt. Express. 2013;21:4348. doi: 10.1364/OE.21.004348. [DOI] [PubMed] [Google Scholar]
- 4.Xu T, Shi H, Wu YK, Kaplan AF, Ok JG, Guo LJ. Small. 2011;7:3128. doi: 10.1002/smll.201101068. [DOI] [PubMed] [Google Scholar]
- 5.a) Inoue D, Miura A, Nomura T, Fujikawa H, Sato K, Ikeda N, Tsuya D, Sugimoto Y, Koide Y. Appl. Phys. Lett. 2011;98:093113. [Google Scholar]; b) Chen Q, Cumming DRS. Opt. Express. 2010;18:14056. doi: 10.1364/OE.18.014056. [DOI] [PubMed] [Google Scholar]; c) Laux E, Genet C, Skauli T, Ebbesen TW. Nature Photon. 2008;2:161. [Google Scholar]; d) Yokogawa S, Burgos SP, Atwater HA. Nano Lett. 2012;12:4349. doi: 10.1021/nl302110z. [DOI] [PubMed] [Google Scholar]
- 6.Zhou J, Guo J. Sci. Rep. 2013;4:3614. doi: 10.1038/srep03614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.a) Gopinath A, Boriskina SV, Ning-Ning F, Reinhard BM, Dal Negro L. Nano Letters. 2008;8:2423. doi: 10.1021/nl8013692. [DOI] [PubMed] [Google Scholar]; b) Hong Y, Qiu Y, Chen T, Reinhard BM. Adv. Funct. Mater. 2014;24:739. doi: 10.1002/adfm.201301837. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Auguie B, Barnes WL. Phys. Rev. Lett. 2008;101:143902. doi: 10.1103/PhysRevLett.101.143902. [DOI] [PubMed] [Google Scholar]; d) Olson J, Manjavacas A, Kiu L, Chang W-S, Foerster B, King NS, Knight MW, Nordlander P, Halas NJ, Link S. Proc. Natl. Acad. Sci. USA. 2014;111:14348. doi: 10.1073/pnas.1415970111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.a) Reader-Harris P, Ricciardi A, Krauss TD, Di Falco A. Opt. Express. 2013;21:1002. doi: 10.1364/OE.21.001002. [DOI] [PubMed] [Google Scholar]; b) Nguyen-Huu N, Lo Y-L, Chen Y-B. Opt. Commun. 2011;284:2473. [Google Scholar]
- 9.a) Diest K, Dionne JA, Spain M, Atwater HA. Nano Lett. 2009;9:2579. doi: 10.1021/nl900755b. [DOI] [PubMed] [Google Scholar]; b) Xu T, Wu YK, Luo X, Guo LJ. Nat. Commun. 2010;1:59. doi: 10.1038/ncomms1058. [DOI] [PubMed] [Google Scholar]
- 10.a) Kumar K, Duan H, Hedge RS, Koh SCW, Wei JN, Yang JKW. Nat. Nanotechnol. 2012;7:557–561. doi: 10.1038/nnano.2012.128. [DOI] [PubMed] [Google Scholar]; b) Tan SJ, Zhang L, Zhu D, Goh XM, Wang YM, Kumar K, Qiu C-W, Yang JKW. Nano Lett. 2014;14:4023. doi: 10.1021/nl501460x. [DOI] [PubMed] [Google Scholar]
- 11.a) Wu L, Reinhard BM. Chem. Soc. Rev. 2014;43:3884. doi: 10.1039/c3cs60340g. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Halas NJ, Lal S, Chang W-S, Link S, Nordlander P. Chem. Rev. 2011;111:3913. doi: 10.1021/cr200061k. [DOI] [PubMed] [Google Scholar]
- 12.a) Jain PK, Huang W, El-Sayed MA. Nano Lett. 2007;7:2080. doi: 10.1021/nl071813p. [DOI] [PubMed] [Google Scholar]; b) Reinhard BM, Siu M, Agarwal H, Alivisatos AP, Liphardt J. Nano Lett. 2005;5:2246. doi: 10.1021/nl051592s. [DOI] [PubMed] [Google Scholar]; c) Yang L, Wang H, Yan B, Reinhard BM. J. Phys. Chem. C. 2010;114:4901. doi: 10.1021/jp911858v. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Nordlander P, Oubre C, Prodan E, Li K, Stockman MI. Nano Lett. 2004;4:899. [Google Scholar]
- 13.Huang J-S, Callegari V, Geisler P, Bruening C, Kern J, Prangsma JC, Wu X, Feichtner T, Ziegler J, Weinmann P, Kamp M, Forchel A, Biagioni P, Sennhauser U, Hecht B. Nat. Commun. 2010;1:150. doi: 10.1038/ncomms1143. [DOI] [PubMed] [Google Scholar]
- 14.a) Chen K-P, Drachev VP, Borneman JD, Kildishev AV, Shalaev VM. Nano Lett. 2010;10:916. doi: 10.1021/nl9037246. [DOI] [PubMed] [Google Scholar]; b) Sambles JR. Solid State Commun. 1984;49:343. [Google Scholar]
- 15.a) Henzie J, Andrews SC, Ling XY, Li Z, Yang P. Proc. Natl. Acad. Sci. USA. 2013;110:6640. doi: 10.1073/pnas.1218616110. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Chen T, Pourmand M, Feizpour A, Cushman B, Reinhard BM. J. Phys. Chem. Lett. 2013;4:2147. doi: 10.1021/jz401066g. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Thibaut T, Zheng Y, Ng SH, Mudie S, Altissimo M, Bach U. Angew. Chem. Intern. Ed. 2012;51:8732. doi: 10.1002/anie.201204609. [DOI] [PubMed] [Google Scholar]; d) Kuemin C, Nowack L, Bozano L, Spencer ND, Wolf H. Adv. Funct. Mater. 2012;22:702. [Google Scholar]; e) Yan B, Thubagere A, Premasiri WR, Ziegler LD, Dal Negro L, Reinhard BM. Acs Nano. 2009;3:1190. doi: 10.1021/nn800836f. [DOI] [PubMed] [Google Scholar]; f) Yang L, Yan B, Premasiri WR, Ziegler LD, Dal Negro L, Reinhard BM. Adv. Funct. Mater. 2010;20:2619. [Google Scholar]
- 16.a) Dhawan A, Duval A, Nakkach M, Barbillon G, Moreau J, Canva M, Vo-Dinh T. Nanotechnology. 2011;22:165301. doi: 10.1088/0957-4484/22/16/165301. [DOI] [PubMed] [Google Scholar]; b) Ding T, Sigle DO, Herrmann LO, Wolverson D, Baumberg JJ. ACS Appl. Mater. Interfaces. 2014;6:17358. doi: 10.1021/am505511v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kelly KL, Coronado E, Zhao LL, Schatz GC. J. Phys. Chem. B. 2003;107:668. [Google Scholar]
- 18.Kralchevsky PA, Nagayama K. Langmuir. 1994;10:23. [Google Scholar]
- 19.Kreibig U, Vollmer M. Optical Properties of Metal Clusters. Berlin: Springer; 1995. [Google Scholar]
- 20.a) Jain PK, Lee KS, El-Sayed IH, El-Sayed MA. J. Phys. Chem. B. 2006;110:7238. doi: 10.1021/jp057170o. [DOI] [PubMed] [Google Scholar]; b) Saion E, Gharibshahi E, Naghavi K. Int. J. Mol. Sci. 2013;14:7880. doi: 10.3390/ijms14047880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.a) Tao AR, Habas S, Yang P. Small. 2008;4:310–325. [Google Scholar]; b) Sun Y, Xia Y. Science. 2002;298:2176. doi: 10.1126/science.1077229. [DOI] [PubMed] [Google Scholar]
- 22.Montelongo Y, Tenorio-Pearl JO, Williams C, Zhang S, Milne WI, Wilkinson TD. Proc. Natl. Acad. Sci. USA. 2014;111:12679. doi: 10.1073/pnas.1405262111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.