Abstract
Previous data have demonstrated that administration of inflammatory cytokines or their inducers leads to altered basal ganglia function associated with reduced psychomotor speed. Decreased psychomotor speed, referred to clinically as psychomotor retardation, is a cardinal symptom of major depressive disorder (MDD) and has been associated with poor antidepressant treatment response. We therefore examined the association between plasma inflammatory markers and psychomotor speed in ninety-three un-medicated patients with MDD. Psychomotor speed was assessed by a range of neuropsychological tests from purely motor tasks (e.g. movement latency and finger tapping) to those that involved motor activity with increasing cognitive demand and cortical participation (e.g. Trails A and Digit Symbol Substitution Task (DSST)). Linear regression analyses were performed to determine the relationship of inflammatory markers and psychomotor task performance controlling for age, race, sex, education, body mass index, and severity of depression. MDD patients exhibited decreased psychomotor speed on all tasks relative to normative standards. Increased IL-6 was associated with decreased performance on simple and choice movement time tasks, whereas MCP-1 was associated with decreased performance on the finger tapping task and DSST. IL-10 was associated with increased performance on the DSST. In an exploratory principle component analysis including all psychomotor tasks, IL-6 was associated with the psychomotor speed factor. Taken together, the data indicate that a peripheral inflammatory profile including increased IL-6 and MCP-1 is consistently associated with psychomotor speed in MDD. These data are consistent with data demonstrating that inflammation can affect basal ganglia function, and indicate that psychomotor speed may be a viable outcome variable for anti-inflammatory therapies in depression and other neuropsychiatric disorders with increased inflammation.
Keywords: Depression, Cytokines, Chemokines, Inflammation, Cognition, Psychomotor Speed
1. Introduction
Psychomotor retardation is a clinical symptom that is used to make the diagnosis of major depressive disorder (MDD) and is objectively measured by neurocognitive assessments of psychomotor speed. Psychomotor retardation has been reliably reported in patients with MDD (American Psychiatric Association, 2013; Ravnkilde et al., 2002), and has been associated with a poor response to antidepressant treatment (Bruder et al., 2014; Taylor et al., 2006). Alterations in psychomotor performance in MDD are believed to involve dysfunction in dopamine-rich regions of the basal ganglia including the dorsal striatum (Ebert et al., 1996; Martinot et al., 2001).
One pathway that may contribute to psychomotor retardation and psychomotor slowing in depression is inflammation (Carvalho et al., 2014). Markers of inflammation including inflammatory cytokines and their soluble receptors, acute phase reactants (e.g. c-reactive protein (CRP), and chemokines (e.g. monocyte chemotactic protein (MCP-1)) and adhesion molecules have been found to be increased in the peripheral blood and cerebrospinal fluid of a subgroup of patients with MDD (Dowlati et al., 2010; Goldsmith et al., 2016; Miller and Raison, 2015; Zunszain et al., 2013). Moreover, administration of inflammatory cytokines, such as interferon (IFN)-alpha, or cytokine inducers such as endotoxin and typhoid vaccination have been shown to lead to depressive symptoms including psychomotor retardation as well as objective measures of psychomotor slowing (Brydon et al., 2008; Capuron et al., 2002; Musselman et al., 2001; Reichenberg et al., 2001; Yirmiya et al., 2000). Indeed, patients administered IFN-alpha demonstrated significantly decreased psychomotor speed as reflected by slower choice movement time as measured by the Cambridge Neuropsychological Test Automated Battery (CANTAB) (Haroon et al., 2015; Majer et al., 2008; Raison et al., 2010). Decreased psychomotor speed following IFN-alpha was in turn correlated with symptoms of depression and fatigue assessed by the Montgomery-Asberg Depression Rating Scale (MADRS; (Montgomery and Asberg, 1979) and the Multidimensional Fatigue Inventory (MFI)(Smets et al., 1995), respectively. Interestingly, psychomotor retardation was recently found to predict the development of depression during IFN-alpha therapy (Whale et al., 2015). Laboratory animals also exhibit decreased locomotor activity after exposure to inflammatory cytokines or inflammatory stimuli (e.g. IL-6 or lipopolysaccharide (LPS))(Frenois et al., 2007; Lenczowski et al., 1999).
Relevant to the mechanism by which inflammation may influence psychomotor performance, inflammation has been shown to alter neural activity and dopamine metabolism in basal ganglia regions including the dorsal striatum as assessed by a variety of neuroimaging strategies following several inflammatory stimuli including IFN-alpha, typhoid vaccination and endotoxin (Brydon et al., 2008; Capuron et al., 2012; Eisenberger et al., 2010; Felger et al., 2015b; Harrison et al., 2009).
Inflammatory markers have also been associated with reduced psychomotor speed in healthy elderly subjects (Palta et al., 2015). However, few studies have examined the relationship between inflammatory markers and tests of psychomotor speed in patients with MDD. One study found a relationship between increased CRP and slower finger tapping frequency in MDD patients both before and after treatment with conventional antidepressants, and another study found increased CRP to be related to decreased Trails A performance (Chang et al., 2012; Krogh et al., 2014). Nevertheless, in both studies, only CRP and/or IL-6 were assessed and the psychometric battery assessing psychomotor performance was limited.
Based on these findings, we endeavored to examine the relationship between an array of both pro- and anti-inflammatory markers and an extensive battery of neurocognitive tests of psychomotor speed in a large sample of un-medicated patients with MDD. Our approach was designed not only to establish the reproducibility of previous findings, but also to extend these findings by using an expanded group of inflammatory markers and a more nuanced battery of psychomotor tasks in a large sample of un-medicated, medically-stable, depressed patients. The psychomotor battery ranged from purely motor tasks (e.g. movement latencies and finger tapping) to those that involved motor activity with increasing cognitive demand and cortical participation (e.g. Trails A and Digit Symbol Substitution Task; DSST). These neurocognitive assessments of psychomotor performance were selected based on the availability of normative standards to allow comparison of our sample to normative means. We hypothesized that decreased psychomotor speed would be correlated with specific plasma markers of inflammation in MDD patients, thereby providing a greater understanding of the relationship between cytokines and psychomotor retardation. Moreover, these data were developed in order to further inform relative immune and neurocognitive endpoints relevant to the testing of anti-inflammatory treatments in patients with MDD and other psychiatric disorders with increased inflammation and impaired psychomotor performance, such as schizophrenia (Aas et al., 2014; Bortolato et al., 2015).
2. Methods and Materials
2.1 Participants
Ninety-three participants (age 21-65) with a primary diagnosis of MDD or bipolar disorder, current episode depressed as determined by Structured Clinical Interview for Diagnostic and Statistical Manual-IV-TR (SCID-IV) were enrolled. Subjects were excluded for a number of medical conditions that might confound study interpretation as confirmed by medical history, laboratory testing, electrocardiogram and physical exam. Patients were excluded for uncontrolled cardiovascular, endocrinologic, hematologic, hepatic, renal, or neurologic disease, autoimmune conditions (i.e. rheumatoid arthritis, inflammatory bowel disease, multiple sclerosis, lupus), chronic infection (i.e. HIV, hepatitis B or C), history of liver abnormalities, or evidence of infection within one month of screening that required antibiotic or antiviral therapy. Participants were also excluded for a history of cancer, pregnancy or lactation; a history of schizophrenia (determined by SCID-IV); active psychotic symptoms of any type; substance abuse and/or dependence within the past six months (determined by SCID-IV); an active eating disorder; obsessive compulsive disorder; active suicidal ideation determined by a score of 3 or higher on item #3 of the 17-item HAM-D; and/or a score of less than 28 on the Mini-Mental State Examination. Subjects were free of all psychotropic medications (e.g. antidepressants, mood stabilizers, antipsychotics, stimulants, sedative hypnotics and benzodiazepines) for at least 4 weeks (8 weeks for fluoxetine) prior to study participation. No patients were withdrawn from psychotropic medications for the purposes of this study. Patients were also free of medications known to affect the immune system including nonsteroidal or steroidal anti-inflammatory medications, statins or angiotensin 2 receptor inhibitors, and were tested for drugs of abuse at screening and on the day of neurocognitive testing and blood sampling. Medications for other medical conditions were allowed as dictated by the patients’ treating physicians. Participants with evidence of active infections were excluded until medically stable. Subjects were recruited from a parent study on phenotyping depressed patients with increased inflammation (ClinicalTrials.gov NCT01426997). All procedures were approved a priori by the Institutional Review Board of Emory University. All participants provided written informed consent.
2.2 Behavioral and neurocognitive assessments
Depression severity was assessed using the 17-item HAM-D. Psychomotor retardation was assessed using objective measures of psychomotor speed on standardized neurocognitive tasks, which has been well-established by the work of our group and others (Capuron et al., 2002; Chang et al., 2012; Krogh et al., 2014; Majer et al., 2008; Raison et al., 2010). Psychomotor performance was measured by 1) the Reaction Time Task of the Cambridge Neuropsychological Test Automated Battery (CANTAB), that includes simple and five-choice reaction time segments and provides distinctions between movement latencies (simple and choice movement time – SMT and CMT, respectively) and reaction time (simple and choice reaction time – SRT and CRT, respectively) measured in milliseconds; 2) the Finger Tapping Test (FTT), a task of pure motor function that requires patients to tap with the index finger of the dominant hand as fast as possible for 10 second intervals resulting in a mean number of taps per 10 seconds; 3) the Digit Symbol Substitution Test (DSST) of the Wechsler Adult Intelligence Scale that measures psychomotor processing speed in which patients are presented with numbers and a corresponding blank box and are instructed to fill in as many boxes in 90 seconds with a matching symbol (available in a legend) yielding a measure of the numbers of boxes successfully completed within 90 seconds; and 4) Trail Making Test Part A, a task where subjects are instructed to draw a line between non-adjoining numbers in consecutive order yielding the mean number of seconds required to complete the task. In addition to offering a rich characterization of multiple aspects of psychomotor performance, these tasks were selected on the basis of having established normed reference sets (normative standards), allowing us to better characterize the psychomotor performance in our depressed sample. Neurocognitive testing was conducted in the afternoon between 3:00pm and 6:00pm on the same day of blood sampling.
2.3 Immune biomarkers
In order to limit variability in sleep-wake patterns, subjects were admitted to the hospital the day prior to blood sampling and neurocognitive assessments. All subjects slept in a single-room with lights out at 11:00 pm and a wake-up time of 7:30 am. To control for circadian variations in immune biomarkers, blood was drawn between 8-10 am the morning of neurocognitive testing. Samples were obtained in chilled EDTA-coated tubes and spun at 1000g for 15 minutes at 4°C, and plasma was collected and stored at −80°C for later batched analysis of CRP and the inflammatory cytokines interleukin (IL)-6, IL-1beta and tumor necrosis factor (TNF) and their soluble receptors as well as MCP-1, all of which have been found to be elevated in patients with major depression (Dowlati et al., 2010; Piletz et al., 2009; Raison et al., 2006). The immunoturbidometric method was used to measure high sensitivity CRP concentrations with a Beckman AU480 chemistry analyzer and Ultra WR CRP kit (Sekisui Diagnostics). Concentrations of cytokines and their soluble receptors as well as the chemokine MCP-1 were assessed in duplicate using high sensitivity multiplex bead-based assays (R& D Systems) and analyzed on a MAGPIX CCD imager (Luminex) as previously described (Felger et al., 2015b; Moieni et al., 2015). Mean inter- and intra-assay coefficients of variation (CV) were reliably <10% (See Supplementary Table 1 for inter- and intra-assay CVs and method detection limits for each of the immune markers). Consistent with previous analyses, cytokine, cytokine receptor and chemokine values were natural log transformed to achieve normality for statistical modeling. No immune variables were below the limits of assay detection. Due to the possibility that time in storage at −80°C may have an effect on measured concentrations of immune markers, the amount of time (days) individual plasma samples were stored in the −80°C freezer was entered as a covariate in each of the regression models described below. This covariate was not significant in any of the models, and adding it to the models did not significantly change the results presented.
2.4 Statistical Analyses
Descriptive statistics including means and standard deviations for the independent and dependent variables were calculated for the sample and are provided in Tables 1 and 2. Pearson correlation coefficients were calculated for the relationship among inflammatory markers and between inflammatory markers and psychomotor task performance. To determine which inflammatory measures predicted psychomotor task performance, stepwise, backward linear regression analyses were performed including all inflammatory markers as independent variables in the models. Each of the psychomotor tasks was analyzed separately to determine similarities and differences in the contribution of inflammatory markers to each of these tasks. Because the inflammatory and neurocognitive data were not normally distributed, all statistical analyses on inflammatory markers and scores on neurocognitive tests were conducted using natural log transformed values. Age, education, sex, race, and body mass index (BMI) were included in all regression models as covariates. Depression as measured by the 17-item HAM-D was also included as a covariate in these analyses, although the item measuring psychomotor retardation was excluded to avoid confounding of the depression measure with the dependent variables. Due to the possible collinearity of peripheral inflammatory markers, the variable inflation factor (VIF) was calculated for each variable in the regression models.
Table 1. Sociodemographic, Clinical and Immune Characteristics of the Study Sample (N=93).
| Variable | |
|---|---|
| Age (years) (±SD) | 39.4 (11.5) |
| Race (% white) | 43.0% |
| Education (% with college degree) | 46.2% |
| BMI (±SD) | 31.5 (8.3) |
| 17-item Ham-D (±SD) | 23.2 (3.3) |
| hsCRP (mg/L) (±SD) | 2.7 (3.1) |
| IL-6 (pg/ml) (±SD) | 1.7 (1.2) |
| TNF alpha (pg/ml) (±SD) | 5.7 (2.2) |
| IL-1 beta (pg/ml) (±SD) | 0.4 (0.2) |
| IL-10 (pg/ml) (±SD) | 0.6 (0.5) |
| sTNFR2 (ng/ml) (±SD) | 2.5 (0.8) |
| sIL-6R (ng/ml) (±SD) | 15.8 (2.8) |
| IL-1RA (ng/ml) (±SD) | 1.9 (2.6) |
| MCP-1 (pg/ml) (±SD) | 129.9 (46.6) |
SD: standard deviation; BMI: body mass index; Ham-D: 17 item Hamilton Depression Rating Scale; SD: standard deviation; hsCRP: high sensitivity C-reactive protein; IL-6: interleukin 6; TNF alpha: tumor necrosis factor alpha; IL-1 beta: interleukin 1 beta; IL-10: interleukin 10; sTNFR2: soluble tumor necrosis factor receptor 2; sIL-6R: soluble interleukin 6 receptor; IL-1RA: interleukin 1 receptor antagonist; MCP-1: monocyte chemoattractant protein 1
Table 2. Mean (±SD) Measures of Psychomotor Speed in the Study Sample (N=93).
| Variable | Comparative Normative Standard |
|
|---|---|---|
| SMT | 576.4 (214.9) | −0.49* |
| CMT | 526.9 (133.3) | −0.33* |
| SRT | 365.9 (73.3) | −0.48* |
| CRT | 384.2 (66.8) | −0.34* |
| FTT | 40.9 (9.3) | 50.5 (8.4) |
| DSST | 53.2 (10.9) | 70.0 (16.3) |
| Trails A | 32.7 (13.2) | 25.5 (6.0) |
SD: standard deviation; SMT: Simple Choice Movement Time (milliseconds); SRT: Simple Choice Reaction Time (milliseconds); CMT: Five Choice Movement Time (milliseconds); CRT: Five Choice Reaction Time (milliseconds); DSST: Digit Symbol Substitution Task (# of filled boxes correct); FTT: Finger Tapping Task (mean # of taps); Trails A (seconds);
Normative standards for Movement and Reaction Time values are presented as Z scores relative to the normative mean
In exploratory analyses, we placed all tasks of psychomotor speed into a principle component analysis (PCA) to yield a single factor for psychomotor performance. The resulting factor was then used in linear regression to determine those inflammatory variables that were significantly associated with the resulting factor, including all previously noted covariates.
Inflammatory marker values and values of neurocognitive task performance determined to be outliers by Grubbs test were eliminated from analyses (Grubbs, 1969). The number of values eliminated did not exceed 1 for any one variable. In order to control for multiple comparisons, a Benjamini and Hochberg approach (Hochberg and Benjamini, 1990) was employed. This approach provides better control of type I error rates when conducting multiple hypothesis tests as compared to more conservative approaches (Benjamini and Hochberg, 1995; Holm, 1979). As indicated in Table 3, we corrected for 17 individual predictors. Where sex was a significant predictor in the model (i.e. CMT, SRT, FTT), we performed exploratory analyses including independent sample t-tests to compare psychomotor performance between men and women. In addition, regression analyses were conducted to determine inflammatory predictors of relevant tasks in males and females independently. All analyses were conducted using SPSS version 23.0 (IBM SPSS Statistics for Macintosh, Version 23.0. Armonk, NY: IBM Corp.).
Table 3. Significant Predictors of Psychomotor Speed in Patients with MDD.
| Adjusted R2 |
beta |
Uncorrected
p |
Corrected
p |
||
|---|---|---|---|---|---|
| SMT | 0.16 | IL-6 | 0.28 | 0.008 | 0.012 |
| HamD | 0.24 | 0.018 | 0.021 | ||
| CMT | 0.18 | IL-6 | 0.26 | 0.021 | 0.032 |
| Sex | 0.27 | 0.012 | 0.015 | ||
| Age | 0.24 | 0.019 | 0.027 | ||
| SRT | 0.19 | Sex | 0.25 | 0.018 | 0.024 |
| CRT | 0.17 | BMI | 0.31 | 0.005 | 0.003** |
| Age | 0.23 | 0.025 | 0.044 | ||
| FTT | 0.18 | MCP-1 | −0.26 | 0.015 | 0.018 |
| sTNFR2 | 0.27 | 0.020 | 0.029 | ||
| BMI | −0.33 | 0.006 | 0.006** | ||
| Sex | −0.21 | 0.045 | 0.050 | ||
| DSST | 0.15 | IL-10 | 0.28 | 0.007 | 0.009 |
| MCP-1 | −0.23 | 0.027 | 0.047 | ||
| Education | 0.24 | 0.022 | 0.038 | ||
| Trails A | 0.11 | BMI | 0.24 | 0.021 | 0.035 |
| Education | −0.24 | 0.023 | 0.041 | ||
| Factor Score* | 0.26 | IL-6 | 0.25 | 0.025 | |
| BMI | 0.341 | 0.004 | |||
| Age | 0.21 | 0.029 |
SMT: Simple Choice Movement Time; CMT: Five Choice Movement Time; SRT: Simple Choice Reaction Time; CRT: Five Choice Reaction Time; FTT: DSST: Digit Symbol Substitution Task; Finger Tapping Task; HamD: Hamilton Depression Rating Scale; BMI: Body Mass Index; hsCRP: high sensitivity C-reactive protein; IL-6: interleukin 6; TNF alpha: tumor necrosis factor alpha; IL-1 beta: interleukin 1 beta; IL-10: interleukin 10; sTNFR2: soluble tumor necrosis factor receptor 2; sIL-6R: soluble interleukin 6 receptor; IL-1RA: interleukin 1 receptor antagonist; MCP-1: monocyte chemoattractant protein 1; All results are expressed as the natural log (Ln) of the indicated variables, except for the Factor Score, which was calculated from the Ln of the individual psychomotor tasks; Beta denotes the standardized estimate;
p values uncorrected; Corrected p values based on Benjamini Hochberg <0.05;
Corrected p value not significant based on Benjamini Hochberg procedure for determination of significance
3. Results
3.1 Sociodemographic, Clinical, Neurocognitive and Inflammatory Characteristics of the Sample
Ninety-three un-medicated depressed subjects were included in the analysis. Sociodemographic, clinical and immune data for the sample are shown in Tables 1 and 2. Of note, many inflammatory markers were inter-correlated (see Supplementary Table 2), however, the VIF for all the inflammatory predictors in the regression models were <2, indicating that multi-collinearity among inflammatory predictors did not bias the results. Psychomotor performance of the sample relative to normative standards is provided in Table 2. Average scores for the patients with MDD were at least 1 standard deviation below normative standards on the FTT, DSST and Trails A (Wisdom et al., 2012; Yeudall et al., 1987) and approximately 0.5 standard deviations below the normative mean on the CANTAB tasks.
3.2 Relationship between inflammatory markers and measures of psychomotor speed
Simple correlations between each of the inflammatory markers and measures of psychomotor speed are shown in Supplementary Table 3. Correlations between inflammatory markers and measures of psychomotor speed in females and males for those tasks that showed sex effects are displayed in Supplementary Table 4. Except where noted, all inflammatory markers that were significantly associated with psychomotor performance in linear regression were also significant in parametric correlational analyses.
Simple and Choice Movement Task
IL-6 significantly predicted performance on both SMT and CMT, with higher concentrations of IL-6 predicting slower movement times for each task (Table 3, Figure 1). Significant additional predictors for the SMT included HAM-D scores, while sex and age were significant predictors for CMT performance. Exploratory analyses of the effects of sex on CMT revealed that females exhibited significantly slower movement times than males (553.7ms SD 125.5 versus 470.6ms SD 133.8, respectively; t = −2.872, p = 0.005). IL-10 predicted faster times on the CMT in females (beta = −0.27, p = 0.035), whereas IL-1RA predicted faster times on the CMT in males (beta = −0.50, p = 0.007).
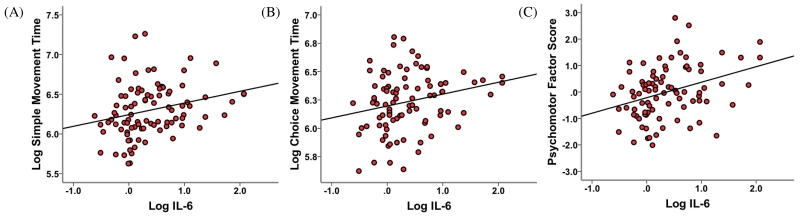
Figure 1. Relationship between Interleukin 6 and (a) Simple Movement Time (b) Choice Movement Time and (c) Psychomotor Factor Score in the group as a whole.
Interleukin 6 (IL-6) was positively associated with performance on (A) Simple Movement Time (SMT), (B) Choice Movement Time, and (C) the Psychomotor Factor Score in linear regression analyses after controlling for sex, age, race, education, body mass index (BMI), and depression (as measured by the 17-item Hamilton Rating Scale for Depression (HAM-D) minus the psychomotor retardation item). (A) IL-6 concentration and time on the SMT were positively correlated (r = 0.25, p = 0.015). (B) IL-6 concentration and time on the CMT were positively correlated (r = 0.24, p = 0.026). (C) IL-6 concentration and the psychomotor factor score were positively correlated (r = 0.34, p = 0.001). All results are expressed as the natural log (Log) of the indicated variables, except for the Factor Score, which was calculated from the Log of the individual psychomotor tasks.
Simple and Choice Reaction Task
There were no significant inflammatory predictors of SRT or CRT (Table 3). Significant additional predictors for SRT included sex. Exploratory analyses of the effects of sex on SRT revealed that females exhibited significantly slower performance compared to males (381.6ms SD 77.4 versus 332.4ms SD 50.1; t = −3.13, p = 0.002). IL-6 predicted worse SRT performance in females (beta = 0.31, p = 0.02), whereas sTNFR2 predicted better SRT performance in males (beta = 0.64, p = 0.001). Significant additional predictors of CRT included BMI and age.
Finger Tapping Task
MCP-1 and sTNFR2 significantly predicted performance on the FTT, with higher concentrations of MCP-1, and lower concentrations of sTNFR2, predicting a lower frequency of finger tapping (Table 3, Figure 2). Of note, sTNFR2 was not significantly correlated with FTT in simple correlational analysis (see Supplementary Table 3). Significant additional predictors of FTT performance included BMI and sex. Exploratory analyses of the effects of sex on FTT revealed that females exhibited significantly lower frequency of finger tapping compared to males (39.4 SD 9.5 versus 44.2 SD 8.0; t = 2.33, p = 0.022). No inflammatory markers predicted performance in females, whereas MCP-1 (beta = −0.54, p = 0.002) and TNF alpha (beta = −0.43, p = 0.012) predicted lower frequency of finger tapping in males. sIL6r predicted a higher frequency of finger tapping in males (beta = 0.41, p = 0.018).
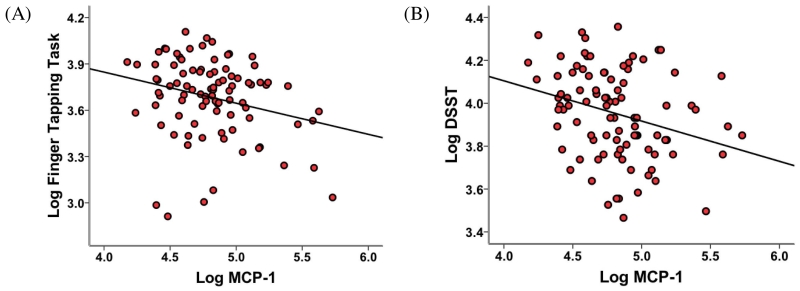
Figure 2. Relationship between Monocyte Chemotactic Protein 1 and (A) Finger Tapping Task and (B) Digit Symbol Substitution Task.
Monocyte Chemotactic Protein 1 (MCP-1) was negatively associated with performance on the (A) Finger Tapping Task (FTT) and (B) the Digit Symbol Substitution Task (DSST). (A) MCP-1 concentration and mean number of taps on the FTT were negatively correlated (r = −0.26, p = 0.015). (B) MCP-1 concentration and mean number of boxes correct on the DSST were negatively correlated (r = −0.28, p = 0.006). All results are expressed as the natural log (Log) of the indicated variables.
Digit Symbol Substitution Task and Trails A
IL-10 and MCP-1 significantly predicted performance on the DSST, with IL-10 predicting better performance and MCP-1 predicting worse performance on this task (Table 3, Figures 2 and 3). Additional significant predictors of DSST performance included education.
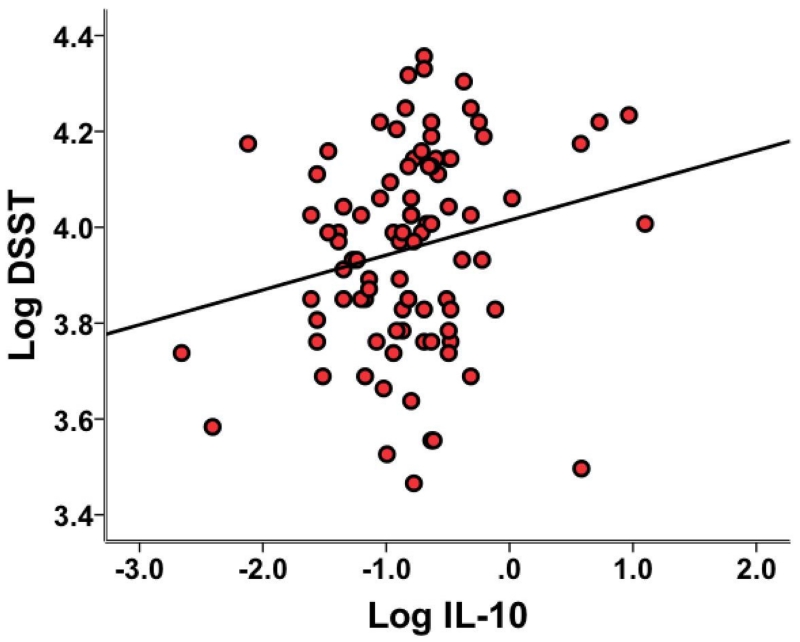
Figure 3. Relationship between Interleukin 10 and Digit Symbol Substitution Task.
Interleukin 10 (IL-10) was positively associated with performance on the Digit Symbol Substitution Task (DSST) in linear regression analyses after controlling for age, race, education, body mass index (BMI), and depression (as measured by the 17-item Hamilton Rating Scale for Depression (HAM-D) minus the psychomotor retardation item). IL-10 concentration and mean number of boxes correct on the DSST were positively correlated (r = 0.21, p = 0.046). All results are expressed as the natural log (Log) of the indicated variables.
There were no significant inflammatory predictors of Trails A performance (Table 3). Significant additional predictors of Trails performance in the sample as a whole included BMI and education.
3.3 Principle Component Analysis
All seven neurocognitive tasks were forced onto one factor with a range of individual loadings from 0.47 to 0.75. A regression factor score was calculated for each subject, and this regression factor score was placed in a linear regression analysis using the same inflammatory variables and covariates as noted above. IL-6 was the only inflammatory variable that significantly predicted this regression factor score. Additional predictors of this factor included age and BMI.
4. Discussion
A significant association between peripheral inflammatory markers and psychomotor speed was found in a sample of un-medicated patients with MDD who exhibited reduced performance on multiple psychomotor tasks compared to normative standards. Increased IL-6 was associated with slower simple and choice movement times, whereas increased MCP-1 predicted worse performance on the FTT and DSST. IL-10 was associated with better performance on the DSST. Finally, the association between IL-6 with psychomotor slowing across all 7 tasks was confirmed using PCA. Taken together, the results indicate that both peripheral pro- and anti-inflammatory markers are associated with psychomotor performance in MDD, and therefore psychomotor performance may serve as an important outcome variable for anti-inflammatory treatment trials in MDD and other neuropsychiatric disorders.
The reported results are consistent with previous literature that has shown associations between psychomotor slowing and inflammation in the context of the administration of cytokines or cytokine inducers as well as in patients with MDD. Nevertheless, whereas previous studies in MDD have explored individual tasks of psychomotor speed and/or individual inflammatory markers, we examined a variety of tasks assessing various degrees of motor control as well as a full panel of inflammatory cytokines and their receptors in addition to the chemokine, MCP-1, previously shown to be elevated in MDD subjects (Piletz et al., 2009; Simon et al., 2008; Sutcigil et al., 2007).
Regarding the specificity of the relationship between individual cytokines and psychomotor performance, plasma IL-6 was reliably found to exhibit associations with movement latencies on the CANTAB as well as with the PCA component of all of the psychomotor tasks. These data are consistent with findings from healthy human volunteers given typhoid vaccination, where higher concentrations of plasma IL-6 predicted slower mean key press reaction times (involving both decision and movement latencies) on a Stroop task in both the congruent and incongruent conditions (Brydon et al., 2008). Of note, increased fMRI activation in the left substantia nigra during the task was also shown to be associated with both higher IL-6 concentrations as well as reduced psychomotor speed. In addition, peripheral blood IL-6 has been shown to predict early declines in psychomotor speed, but not memory or executive function, in a cohort of healthy elderly women (Palta et al., 2015). Studies in laboratory animals are also consistent with a role for IL-6 in psychomotor activity with IL-6 knockout mice exhibiting less motor slowing when exposed to inflammatory stimuli including LPS compared to wild type mice (Bluthe et al., 2000)). Similarly, IL-6 antibodies have been shown to reverse cytokine-induced psychomotor slowing in rodents (Harden et al., 2006; Pang et al., 2006).
Decreased concentrations of plasma IL-10, an anti-inflammatory cytokine, were also associated with psychomotor slowing in our study. Although IL-10 has been correlated with performance on a variety of neurocognitive tasks, relevant to psychomotor function, decreased IL-10 has been associated with worse performance on the DSST and Trails task in patients with co-infection of human immunodeficiency virus (HIV) and hepatitis C virus (Cohen et al., 2011). In addition, IL-10 deficient mice have been shown to exhibit greater LPS-induced impairments in motor performance as well as a shorter latency to fatigue compared to wild type animals (Krzyszton et al., 2008).
We also found plasma MCP-1 (or C-C motif ligand 2 (CCL2)) to be associated with psychomotor slowing. MCP-1 is a chemokine with chemotactic properties on various inflammatory cells including monocytes/macrophages, T lymphocytes, and dendritic cells. MCP-1 has also been shown to attract monocytes to the brain during stress and has exhibited increased expression in the brains of suicide victims (Torres-Platas et al., 2014). Although no study has reported a significant relationship between MCP-1 and psychomotor slowing, it has been associated with cognitive impairment in multiple clinical populations including first episode psychosis (Martinez-Cengotitabengoa et al., 2012), mild cognitive impairment and early stage Alzheimer’s Disease (Galimberti et al., 2006; Westin et al., 2012), HIV-associated cognitive deficits (Yuan et al., 2015), and neurocognitive deficits post carotid endarterectomy (Mack et al., 2008).
In the current study, we found significant results for IL-6, IL-10, and MCP-1. Although these and other inflammatory markers have been associated with changes in locomotor activity and psychomotor speed in this and other studies, it remains unclear whether there are specific relationships between these immune markers and psychomotor activity or whether they represent reliable proxies for another more nuanced profile of inflammatory responses that relate to psychomotor performance. Future studies examining inflammatory signaling pathways in humans and animal models may shed more light on these considerations. Moreover, it is interesting to note that there were no relationships between CRP and psychomotor performance, especially in light of previous literature using similar tasks (Chang et al., 2012; Krogh et al., 2014). Work from our own group indicates that individuals with high baseline CRP showed decreased psychomotor retardation (as measured by item 8, asking about psychomotor retardation, on the HAM-D Scale) following treatment with infliximab versus placebo (Raison et al., 2013). Based on the results of the current study, individual inflammatory cytokines or chemokines (e.g., plasma IL-6 which highly correlated with CRP) and not CRP may serve as a better proxy for the peripheral immune profile that is associated with impaired psychomotor performance in MDD.
We also observed a significant effect of sex in three of the seven tasks. Based on previous work showing that women may respond differentially to inflammatory stimuli (Eisenberger et al., 2009; Moieni et al., 2015), we performed exploratory statistical analyses on males and females separately for these tasks. Men performed significantly faster than women on the CMT and had a higher mean number of taps on the FTT. Men were also significantly faster than women on the SRT task. Sex differences have been shown previously in motor tasks, with men showing faster performance on the FTT (Heaton, 2004; Iverson et al., 2014). Unfortunately, due to the small number of women and especially men in our sample, it is difficult to interpret differential relationships between inflammatory markers and psychomotor performance as a function of sex. Nevertheless, based on the results of our study, the effect of sex on cytokine-induced psychomotor slowing in depression merits further investigation. Future studies examining estrogen and progesterone and their relative interaction with inflammatory cytokines to influence psychomotor speed in MDD may be especially relevant in this regard.
The neurocognitive tests explored in this study have consistently been shown to involve the basal ganglia. For example, increased binding of the dopamine D2/3 ligand 123I-IZBM in the striatum, potentially reflecting reduced dopamine transmission, has been correlated with motor slowing in the CANTAB Reaction Time Task in depressed patients (Shah et al., 1997). In addition, the FTT has been shown to be altered in individuals with basal ganglia disorders (Aparicio et al., 2005), and performance on the DSST has been found to correlate with subcortical atrophy (especially as measured by the bi-caudate ratio) in disorders involving the basal ganglia including Huntington’s Disease and multiple sclerosis (Bermel et al., 2002; Starkstein et al., 1988).
Inflammatory cytokines have consistently been shown to affect the basal ganglia. PET studies of glucose metabolism using 18F-fluorodeoxyglucose in IFN-alpha-treated patients showed increased glucose metabolism in basal ganglia regions believed to be secondary to impaired dopamine-mediated inhibition of oscillatory burst activity in basal ganglia nuclei (Capuron et al., 2007; Juengling et al., 2000). IFN-alpha treatment has also been shown to lead to Parkinson’s-like symptoms, which were relieved by levodopa (Bersano et al., 2008). Similarly, healthy individuals given typhoid vaccination showed increased activity in the basal ganglia using fMRI, which was associated with both increases in peripheral blood IL-6 and psychomotor slowing (Brydon et al., 2008). Moreover, recent work using an advanced microstructure neuroimaging technique has demonstrated that IFN-alpha’s effect on the striatum to induce fatigue preceded mood symptoms by approximately four weeks. These data suggest that the effect of IFN-alpha on the striatum may be specific to locomotor activity (including psychomotor slowing) and not other symptoms of depression (Dowell et al., 2016).
One potential consequence of inflammation effects on the basal ganglia leading to psychomotor slowing may be altered brain connectivity. Recent work has demonstrated that increased inflammation (as measured by CRP) was associated with decreased functional connectivity between the dorsal striatum and ventromedial prefrontal cortex (vmPFC) as well as the presupplementary motor area. Decreased connectivity between these regions was in turn correlated with decreased psychomotor speed as assessed by the FTT and the Trails A task. Furthermore, decreased connectivity between the striatum and the vmPFC was associated with increased plasma cytokine concentrations including IL-6 and IL-1RA (Felger et al., 2015b). Decreased connectivity may be mediated in part by inflammation-induced decreases in dopamine. Studies in both humans and non-human primates have indicated decreased dopamine release following IFN-alpha. Moreover, IFN-alpha-induced changes in dopamine release were reversed by administration of the dopamine precursor levodopa (Felger et al., 2015a; Felger et al., 2013). Furthermore, both the ventral striatum and the vmPFC receive significant dopaminergic innervation (Felger and Miller, 2012; Russo and Nestler, 2013).
Another mechanism by which inflammation may impact psychomotor speed may be through modulation of glutamate in the basal ganglia. IFN-alpha administration has been shown to increase the glutamate to creatine (Glu/Cr) ratio in the left basal ganglia as measured by magnetic resonance spectroscopy (MRS), while also altering the glutamine to glutamate ratio in the anterior cingulate cortex (Haroon et al., 2015; Haroon et al., 2014; Taylor et al., 2014). In addition, increased glutamate in the basal ganglia has been found in association with increased inflammation (as measured by plasma CRP) in patients with major depression where it was associated with psychomotor slowing as assessed by the FTT, DSST and the simple reaction time task on the CANTAB (Haroon et al., 2015).
In terms of relevance to future clinical studies, based on Table 3, the factor score that included all psychomotor tasks had the largest effect size estimate, with IL-6 accounting for 26% of the variance along with the covariates of age and BMI. Based on this analysis, the complete battery of tasks might be the most relevant outcome variable for future clinical trials inhibiting inflammation. Moreover, in individual correlational analyses (see Supplementary Table 3), IL-6 showed the strongest individual association with the combined tasks, accounting for approximately 10% of the variance, with a moderate to large effect size (Cohen’s d = 0.73). Thus, using the full battery and focusing on IL-6 may be most relevant for future clinical trial design.
There are several strengths and weaknesses of the current study that warrant consideration. The study included a well-characterized group of medically-stable depressed patients off psychotropic medications in addition to drugs that may impact the immune system. In addition, an array of inflammatory markers were assessed along with a range of neurocognitive tasks focused on psychomotor speed. In terms of limitations, no control group was included. Nevertheless, the study benefitted from the availability of normative data for all tasks, and in each case, the depressed population exhibited the expected reduced psychomotor performance that has been previously described (Sobin and Sackeim, 1997). In addition, aside from assessments of reaction time components of the Reaction Time Task on the CANTAB, we did not explore other cognitive domains including memory and executive function, which may also be impaired in depression as a function of inflammation. We also only examined the plasma concentrations of the proteins of the indicated cytokines, cytokine receptors and the chemokine and not their biological activity. This may lead to an over or under-estimation of the relationship of the various markers and their relationship with psychomotor performance. Finally, it should be noted that the data is correlational in nature and as such, cause and effect relationships cannot be determined. Future studies involving an interventional component would be required to investigate the directionality of the effects of the immune system on psychomotor speed in depression.
In summary, the results of this study provide further evidence that peripheral inflammatory markers in patients with MDD may contribute to psychomotor slowing which is a primary symptom of the disorder and has been associated with treatment non-response. These data are also consistent with increasing data suggesting that cytokines affect the basal ganglia and dopaminergic pathways that regulate motor activity. Moreover, we found evidence of sex differences in a number of the tasks examined, which are consistent with previous studies that the effects of cytokines on behavior may differ between males and females. Taken together, these data indicate that psychomotor speed may serve as a relevant outcome variable for future studies targeting the immune system, especially IL-6, to treat neuropsychiatric disorders characterized by increased inflammation.
Supplementary Material
Highlights.
-
-
Depressed patients performed below normative standards on tasks of psychomotor speed
-
-
Peripheral inflammatory markers predicted psychomotor speed in depressed patients
-
-
IL-6 and MCP-1 were consistently associated with decreased psychomotor speed
-
-
Psychomotor speed may represent a target for anti-inflammatory therapy in depression
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aas M, Dazzan P, Mondelli V, Melle I, Murray RM, Pariante CM. A systematic review of cognitive function in first-episode psychosis, including a discussion on childhood trauma, stress, and inflammation. Frontiers in psychiatry. 2014;4:182. doi: 10.3389/fpsyt.2013.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: 2013. [Google Scholar]
- Aparicio P, Diedrichsen J, Ivry RB. Effects of focal basal ganglia lesions on timing and force control. Brain and cognition. 2005;58:62–74. doi: 10.1016/j.bandc.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society. Series B (Methodological) 1995;57:289–300. [Google Scholar]
- Bermel RA, Bakshi R, Tjoa C, Puli SR, Jacobs L. Bicaudate ratio as a magnetic resonance imaging marker of brain atrophy in multiple sclerosis. Archives of neurology. 2002;59:275–280. doi: 10.1001/archneur.59.2.275. [DOI] [PubMed] [Google Scholar]
- Bersano A, Aghemo A, Rumi MG, Ballabio E, Candelise L, Colombo M. Recovery after L-DOPA treatment in peginterferon and ribavirin induced parkinsonism. European journal of internal medicine. 2008;19:370–371. doi: 10.1016/j.ejim.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Bluthe RM, Michaud B, Poli V, Dantzer R. Role of IL-6 in cytokine-induced sickness behavior: a study with IL-6 deficient mice. Physiology & behavior. 2000;70:367–373. doi: 10.1016/s0031-9384(00)00269-9. [DOI] [PubMed] [Google Scholar]
- Bortolato B, Carvalho AF, Soczynska JK, Perini GI, McIntyre RS. The Involvement of TNF-alpha in Cognitive Dysfunction Associated with Major Depressive Disorder: An Opportunity for Domain Specific Treatments. Current neuropharmacology. 2015;13:558–576. doi: 10.2174/1570159X13666150630171433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruder GE, Alvarenga JE, Alschuler D, Abraham K, Keilp JG, Hellerstein DJ, Stewart JW, McGrath PJ. Neurocognitive predictors of antidepressant clinical response. Journal of affective disorders. 2014;166:108–114. doi: 10.1016/j.jad.2014.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brydon L, Harrison NA, Walker C, Steptoe A, Critchley HD. Peripheral inflammation is associated with altered substantia nigra activity and psychomotor slowing in humans. Biological psychiatry. 2008;63:1022–1029. doi: 10.1016/j.biopsych.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuron L, Gumnick JF, Musselman DL, Lawson DH, Reemsnyder A, Nemeroff CB, Miller AH. Neurobehavioral effects of interferon-alpha in cancer patients: phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2002;26:643–652. doi: 10.1016/S0893-133X(01)00407-9. [DOI] [PubMed] [Google Scholar]
- Capuron L, Pagnoni G, Demetrashvili MF, Lawson DH, Fornwalt FB, Woolwine B, Berns GS, Nemeroff CB, Miller AH. Basal ganglia hypermetabolism and symptoms of fatigue during interferon-alpha therapy. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2007;32:2384–2392. doi: 10.1038/sj.npp.1301362. [DOI] [PubMed] [Google Scholar]
- Capuron L, Pagnoni G, Drake DF, Woolwine BJ, Spivey JR, Crowe RJ, Votaw JR, Goodman MM, Miller AH. Dopaminergic mechanisms of reduced basal ganglia responses to hedonic reward during interferon alfa administration. Archives of general psychiatry. 2012;69:1044–1053. doi: 10.1001/archgenpsychiatry.2011.2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho AF, Miskowiak KK, Hyphantis TN, Kohler CA, Alves GS, Bortolato B, PM GS, Machado-Vieira R, Berk M, McIntyre RS. Cognitive dysfunction in depression - pathophysiology and novel targets. CNS & neurological disorders drug targets. 2014;13:1819–1835. doi: 10.2174/1871527313666141130203627. [DOI] [PubMed] [Google Scholar]
- Chang HH, Lee IH, Gean PW, Lee SY, Chi MH, Yang YK, Lu RB, Chen PS. Treatment response and cognitive impairment in major depression: association with C-reactive protein. Brain, behavior, and immunity. 2012;26:90–95. doi: 10.1016/j.bbi.2011.07.239. [DOI] [PubMed] [Google Scholar]
- Cohen RA, de la Monte S, Gongvatana A, Ombao H, Gonzalez B, Devlin KN, Navia B, Tashima KT. Plasma cytokine concentrations associated with HIV/hepatitis C coinfection are related to attention, executive and psychomotor functioning. Journal of neuroimmunology. 2011;233:204–210. doi: 10.1016/j.jneuroim.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowell NG, Cooper EA, Tibble J, Voon V, Critchley HD, Cercignani M, Harrison NA. Acute Changes in Striatal Microstructure Predict the Development of Interferon-Alpha Induced Fatigue. Biological psychiatry. 2016;79:320–328. doi: 10.1016/j.biopsych.2015.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctot KL. A meta-analysis of cytokines in major depression. Biological psychiatry. 2010;67:446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- Ebert D, Feistel H, Loew T, Pirner A. Dopamine and depression--striatal dopamine D2 receptor SPECT before and after antidepressant therapy. Psychopharmacology. 1996;126:91–94. doi: 10.1007/BF02246416. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Berkman ET, Inagaki TK, Rameson LT, Mashal NM, Irwin MR. Inflammation-induced anhedonia: endotoxin reduces ventral striatum responses to reward. Biological psychiatry. 2010;68:748–754. doi: 10.1016/j.biopsych.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Inagaki TK, Rameson LT, Mashal NM, Irwin MR. An fMRI study of cytokine-induced depressed mood and social pain: the role of sex differences. NeuroImage. 2009;47:881–890. doi: 10.1016/j.neuroimage.2009.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, Hernandez CR, Miller AH. Levodopa reverses cytokine-induced reductions in striatal dopamine release. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum (CINP) 2015a;18 doi: 10.1093/ijnp/pyu084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, Li Z, Haroon E. Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression. 2015b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, Miller AH. Cytokine effects on the basal ganglia and dopamine function: the subcortical source of inflammatory malaise. Frontiers in neuroendocrinology. 2012;33:315–327. doi: 10.1016/j.yfrne.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, Mun J, Kimmel HL, Nye JA, Drake DF, Hernandez CR, Freeman AA, Rye DB, Goodman MM, Howell LL, Miller AH. Chronic interferon-alpha decreases dopamine 2 receptor binding and striatal dopamine release in association with anhedonia-like behavior in nonhuman primates. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2013;38:2179–2187. doi: 10.1038/npp.2013.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenois F, Moreau M, O’Connor J, Lawson M, Micon C, Lestage J, Kelley KW, Dantzer R, Castanon N. Lipopolysaccharide induces delayed FosB/DeltaFosB immunostaining within the mouse extended amygdala, hippocampus and hypothalamus, that parallel the expression of depressive-like behavior. Psychoneuroendocrinology. 2007;32:516–531. doi: 10.1016/j.psyneuen.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galimberti D, Fenoglio C, Lovati C, Venturelli E, Guidi I, Corra B, Scalabrini D, Clerici F, Mariani C, Bresolin N, Scarpini E. Serum MCP-1 levels are increased in mild cognitive impairment and mild Alzheimer’s disease. Neurobiology of aging. 2006;27:1763–1768. doi: 10.1016/j.neurobiolaging.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Goldsmith DR, Rapaport MH, Miller BJ. A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Molecular psychiatry. 2016 doi: 10.1038/mp.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubbs F. Procedures for Detecting Outlying Observations in Samples. Technometrics. 1969:1–21. [Google Scholar]
- Harden LM, du Plessis I, Poole S, Laburn HP. Interleukin-6 and leptin mediate lipopolysaccharide-induced fever and sickness behavior. Physiology & behavior. 2006;89:146–155. doi: 10.1016/j.physbeh.2006.05.016. [DOI] [PubMed] [Google Scholar]
- Haroon E, Felger JC, Woolwine BJ, Chen X, Parekh S, Spivey JR, Hu XP, Miller AH. Age-related increases in basal ganglia glutamate are associated with TNF, reduced motivation and decreased psychomotor speed during IFN-alpha treatment: Preliminary findings. Brain, behavior, and immunity. 2015;46:17–22. doi: 10.1016/j.bbi.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haroon E, Woolwine BJ, Chen X, Pace TW, Parekh S, Spivey JR, Hu XP, Miller AH. IFN-alpha-induced cortical and subcortical glutamate changes assessed by magnetic resonance spectroscopy. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2014;39:1777–1785. doi: 10.1038/npp.2014.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, Critchley HD. Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biological psychiatry. 2009;66:407–414. doi: 10.1016/j.biopsych.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Miller SW, Taylor MJ, Grant I. Revised comprehensive norms for an expanded Halstead-Reitan battery: demographically adjusted neuropsychological norms for African American and Caucasian adults. Psychological Assessment Resources; Lutz, FL: 2004. [Google Scholar]
- Hochberg Y, Benjamini Y. More powerful procedures for multiple significance testing. Stat Med. 1990;9:811–818. doi: 10.1002/sim.4780090710. [DOI] [PubMed] [Google Scholar]
- Holm S. A Simple Sequentially Rejective Multiple Test Procedure. Scandinavian Journal of Statistics. 1979;6:65–70. [Google Scholar]
- Iverson GL, Brooks BL, Ashton Rennison VL. Minimal gender differences on the CNS vital signs computerized neurocognitive battery. Applied neuropsychology. Adult. 2014;21:36–42. doi: 10.1080/09084282.2012.721149. [DOI] [PubMed] [Google Scholar]
- Juengling FD, Ebert D, Gut O, Engelbrecht MA, Rasenack J, Nitzsche EU, Bauer J, Lieb K. Prefrontal cortical hypometabolism during low-dose interferon alpha treatment. Psychopharmacology. 2000;152:383–389. doi: 10.1007/s002130000549. [DOI] [PubMed] [Google Scholar]
- Krogh J, Benros ME, Jorgensen MB, Vesterager L, Elfving B, Nordentoft M. The association between depressive symptoms, cognitive function, and inflammation in major depression. Brain, behavior, and immunity. 2014;35:70–76. doi: 10.1016/j.bbi.2013.08.014. [DOI] [PubMed] [Google Scholar]
- Krzyszton CP, Sparkman NL, Grant RW, Buchanan JB, Broussard SR, Woods J, Johnson RW. Exacerbated fatigue and motor deficits in interleukin-10-deficient mice after peripheral immune stimulation. American journal of physiology. Regulatory, integrative and comparative physiology. 2008;295:R1109–1114. doi: 10.1152/ajpregu.90302.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenczowski MJ, Bluthe RM, Roth J, Rees GS, Rushforth DA, van Dam AM, Tilders FJ, Dantzer R, Rothwell NJ, Luheshi GN. Central administration of rat IL-6 induces HPA activation and fever but not sickness behavior in rats. The American journal of physiology. 1999;276:R652–658. doi: 10.1152/ajpregu.1999.276.3.R652. [DOI] [PubMed] [Google Scholar]
- Mack WJ, Ducruet AF, Hickman ZL, Zurica J, Starke RM, Garrett MC, Komotar RJ, Quest DO, Solomon RA, Heyer EJ, Sander Connolly E. Elevation of monocyte chemoattractant protein-1 in patients experiencing neurocognitive decline following carotid endarterectomy. Acta neurochirurgica. 2008;150:779–784. doi: 10.1007/s00701-008-1618-6. discussion 784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majer M, Welberg LA, Capuron L, Pagnoni G, Raison CL, Miller AH. IFN-alpha-induced motor slowing is associated with increased depression and fatigue in patients with chronic hepatitis C. Brain, behavior, and immunity. 2008;22:870–880. doi: 10.1016/j.bbi.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Cengotitabengoa M, Mac-Dowell KS, Leza JC, Mico JA, Fernandez M, Echevarria E, Sanjuan J, Elorza J, Gonzalez-Pinto A. Cognitive impairment is related to oxidative stress and chemokine levels in first psychotic episodes. Schizophrenia research. 2012;137:66–72. doi: 10.1016/j.schres.2012.03.004. [DOI] [PubMed] [Google Scholar]
- Martinot M, Bragulat V, Artiges E, Dolle F, Hinnen F, Jouvent R, Martinot J. Decreased presynaptic dopamine function in the left caudate of depressed patients with affective flattening and psychomotor retardation. The American journal of psychiatry. 2001;158:314–316. doi: 10.1176/appi.ajp.158.2.314. [DOI] [PubMed] [Google Scholar]
- Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nature reviews. Immunology. 2015;16:22–34. doi: 10.1038/nri.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moieni M, Irwin MR, Jevtic I, Olmstead R, Breen EC, Eisenberger NI. Sex differences in depressive and socioemotional responses to an inflammatory challenge: implications for sex differences in depression. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2015;40:1709–1716. doi: 10.1038/npp.2015.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. The British journal of psychiatry: the journal of mental science. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Musselman DL, Miller AH, Porter MR, Manatunga A, Gao F, Penna S, Pearce BD, Landry J, Glover S, McDaniel JS, Nemeroff CB. Higher than normal plasma interleukin-6 concentrations in cancer patients with depression: preliminary findings. The American journal of psychiatry. 2001;158:1252–1257. doi: 10.1176/appi.ajp.158.8.1252. [DOI] [PubMed] [Google Scholar]
- Palta P, Xue QL, Deal JA, Fried LP, Walston JD, Carlson MC. Interleukin-6 and C-Reactive Protein Levels and 9-Year Cognitive Decline in Community-Dwelling Older Women: The Women’s Health and Aging Study II. The journals of gerontology. Series A, Biological sciences and medical sciences. 2015;70:873–878. doi: 10.1093/gerona/glu132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang Y, Fan LW, Zheng B, Cai Z, Rhodes PG. Role of interleukin-6 in lipopolysaccharide-induced brain injury and behavioral dysfunction in neonatal rats. Neuroscience. 2006;141:745–755. doi: 10.1016/j.neuroscience.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Piletz JE, Halaris A, Iqbal O, Hoppensteadt D, Fareed J, Zhu H, Sinacore J, Devane CL. Pro-inflammatory biomakers in depression: treatment with venlafaxine. The world journal of biological psychiatry: the official journal of the World Federation of Societies of Biological Psychiatry. 2009;10:313–323. doi: 10.3109/15622970802573246. [DOI] [PubMed] [Google Scholar]
- Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends in immunology. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF, Haroon E, Miller AH. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA psychiatry. 2013;70:31–41. doi: 10.1001/2013.jamapsychiatry.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Rye DB, Woolwine BJ, Vogt GJ, Bautista BM, Spivey JR, Miller AH. Chronic interferon-alpha administration disrupts sleep continuity and depth in patients with hepatitis C: association with fatigue, motor slowing, and increased evening cortisol. Biological psychiatry. 2010;68:942–949. doi: 10.1016/j.biopsych.2010.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravnkilde B, Videbech P, Clemmensen K, Egander A, Rasmussen NA, Rosenberg R. Cognitive deficits in major depression. Scandinavian journal of psychology. 2002;43:239–251. doi: 10.1111/1467-9450.00292. [DOI] [PubMed] [Google Scholar]
- Reichenberg A, Yirmiya R, Schuld A, Kraus T, Haack M, Morag A, Pollmacher T. Cytokine-associated emotional and cognitive disturbances in humans. Arch Gen Psychiatry. 2001;58:445–452. doi: 10.1001/archpsyc.58.5.445. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Nestler EJ. The brain reward circuitry in mood disorders. Nature reviews. Neuroscience. 2013;14:609–625. doi: 10.1038/nrn3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah PJ, Ogilvie AD, Goodwin GM, Ebmeier KP. Clinical and psychometric correlates of dopamine D2 binding in depression. Psychological medicine. 1997;27:1247–1256. doi: 10.1017/s0033291797005382. [DOI] [PubMed] [Google Scholar]
- Simon NM, McNamara K, Chow CW, Maser RS, Papakostas GI, Pollack MH, Nierenberg AA, Fava M, Wong KK. A detailed examination of cytokine abnormalities in Major Depressive Disorder. European neuropsychopharmacology: the journal of the European College of Neuropsychopharmacology. 2008;18:230–233. doi: 10.1016/j.euroneuro.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smets EM, Garssen B, Bonke B, De Haes JC. The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. Journal of psychosomatic research. 1995;39:315–325. doi: 10.1016/0022-3999(94)00125-o. [DOI] [PubMed] [Google Scholar]
- Sobin C, Sackeim HA. Psychomotor symptoms of depression. The American journal of psychiatry. 1997;154:4–17. doi: 10.1176/ajp.154.1.4. [DOI] [PubMed] [Google Scholar]
- Starkstein SE, Brandt J, Folstein S, Strauss M, Berthier ML, Pearlson GD, Wong D, McDonnell A, Folstein M. Neuropsychological and neuroradiological correlates in Huntington’s disease. Journal of neurology, neurosurgery, and psychiatry. 1988;51:1259–1263. doi: 10.1136/jnnp.51.10.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcigil L, Oktenli C, Musabak U, Bozkurt A, Cansever A, Uzun O, Sanisoglu SY, Yesilova Z, Ozmenler N, Ozsahin A, Sengul A. Pro- and anti-inflammatory cytokine balance in major depression: effect of sertraline therapy. Clinical & developmental immunology. 2007;2007:76396. doi: 10.1155/2007/76396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor BP, Bruder GE, Stewart JW, McGrath PJ, Halperin J, Ehrlichman H, Quitkin FM. Psychomotor slowing as a predictor of fluoxetine nonresponse in depressed outpatients. The American journal of psychiatry. 2006;163:73–78. doi: 10.1176/appi.ajp.163.1.73. [DOI] [PubMed] [Google Scholar]
- Taylor MJ, Godlewska B, Near J, Christmas D, Potokar J, Collier J, Klenerman P, Barnes E, Cowen PJ. Effect of interferon-alpha on cortical glutamate in patients with hepatitis C: a proton magnetic resonance spectroscopy study. Psychological medicine. 2014;44:789–795. doi: 10.1017/S0033291713001062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Platas SG, Cruceanu C, Chen GG, Turecki G, Mechawar N. Evidence for increased microglial priming and macrophage recruitment in the dorsal anterior cingulate white matter of depressed suicides. Brain, behavior, and immunity. 2014;42:50–59. doi: 10.1016/j.bbi.2014.05.007. [DOI] [PubMed] [Google Scholar]
- Westin K, Buchhave P, Nielsen H, Minthon L, Janciauskiene S, Hansson O. CCL2 is associated with a faster rate of cognitive decline during early stages of Alzheimer’s disease. PloS one. 2012;7:e30525. doi: 10.1371/journal.pone.0030525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whale R, Fialho R, Rolt M, Eccles J, Pereira M, Keller M, File A, Haq I, Tibble J. Psychomotor retardation and vulnerability to interferon alpha induced major depressive disorder: Prospective study of a chronic hepatitis C cohort. Journal of psychosomatic research. 2015;79:640–645. doi: 10.1016/j.jpsychores.2015.06.003. [DOI] [PubMed] [Google Scholar]
- Wisdom NM, Mignogna J, Collins RL. Variability in Wechsler Adult Intelligence Scale-IV subtest performance across age. Archives of clinical neuropsychology: the official journal of the National Academy of Neuropsychologists. 2012;27:389–397. doi: 10.1093/arclin/acs041. [DOI] [PubMed] [Google Scholar]
- Yeudall LT, Reddon JR, Gill DM, Stefanyk WO. Normative data for the Halstead-Reitan neuropsychological tests stratified by age and sex. Journal of clinical psychology. 1987;43:346–367. doi: 10.1002/1097-4679(198705)43:3<346::aid-jclp2270430308>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Yirmiya R, Pollak Y, Morag M, Reichenberg A, Barak O, Avitsur R, Shavit Y, Ovadia H, Weidenfeld J, Morag A, Newman ME, Pollmacher T. Illness, cytokines, and depression. Annals of the New York Academy of Sciences. 2000;917:478–487. doi: 10.1111/j.1749-6632.2000.tb05412.x. [DOI] [PubMed] [Google Scholar]
- Yuan L, Liu A, Qiao L, Sheng B, Xu M, Li W, Chen D. The relationship of CSF and plasma cytokine levels in HIV infected patients with neurocognitive impairment. BioMed research international. 2015;2015:506872. doi: 10.1155/2015/506872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zunszain PA, Hepgul N, Pariante CM. Inflammation and depression. Current topics in behavioral neurosciences. 2013;14:135–151. doi: 10.1007/7854_2012_211. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.