Abstract
During Xenopus gastrulation, mesendodermal cells are internalized and display different movements. Head mesoderm migrates along the blastocoel roof, while trunk mesoderm undergoes convergent extension (C&E). Different signals are implicated in these processes. Our previous studies reveal that signals through ErbB receptor tyrosine kinases modulate Xenopus gastrulation, but the mechanisms employed are not understood. Here we report that ErbB signals control both C&E and head mesoderm migration. Inhibition of ErbB pathway blocks elongation of dorsal marginal zone explants and activin-treated animal caps without removing mesodermal gene expression. Bipolar cell shape and cell mixing in the dorsal region are impaired. Inhibition of ErbB signaling also interferes with migration of prechordal mesoderm on fibronectin. Cell–cell and cell–matrix interaction and cell spreading are reduced when ErbB signaling is blocked. Using antisense morpholino oligonucleotides, we show that ErbB4 is involved in Xenopus gastrulation morphogenesis, and it partially regulates cell movements through modulation of cell adhesion and membrane protrusions. Our results reveal for the first time that vertebrate ErbB signaling modulates gastrulation movements, thus providing a novel pathway, in addition to non-canonical Wnt and FGF signals, that controls gastrulation. We further demonstrate that regulation of cell adhesive properties and cell morphology may underlie the functions of ErbBs in gastrulation.
Keywords: ErbB signaling, Gastrulation, Convergent extension, Migration, Adhesion, Membrane protrusion
Introduction
Vertebrate body plan is established during the process of gastrulation during which cells from prospective germ layers are brought to their proper locations by concerted cell movements, so that ectoderm covers the embryos, endoderm lines the internal cavity, and mesoderm lies in between. In Xenopus, different cell behaviors in gastrulation have been described (Keller, 1991; Winklbauer et al., 1996; Keller et al., 2003; Solnica-Krezel, 2005). In the animal region, cells undergo radial intercalation to form fewer layers of cells that spread vegetally in a process called epiboly. Within the marginal region, mesodermal and endodermal cells involute through the blastopore to internalize. Following involution, head mesoderm migrates animally along the blastocoel roof (BCR), while trunk mesoderm undertakes mediolateral intercalation to converge towards the midline and extend in an anterior–posterior direction. This convergent extension (C&E) movement is critical for blastopore closure and narrowing and elongation of body axis. In the vegetal region, endodermal cells initiate vegetal rotation movements prior to and extending into gastrulation, providing an essential force for gastrulation. The highly orchestrated cell movements in different regions of the embryos require dynamic and integrated regulation of cell–cell and cell–matrix interactions, and multiple signaling pathways have been implicated in modulation of various aspects of cell movements during gastrulation.
The best studied gastrulation movement is C&E (Wallingford et al., 2002). Both non-canonical Wnt and fibroblast growth factor (FGF) signals are shown to regulate C&E. The Wnt/Planar Cell Polarity (PCP) pathway modulates polarized membrane protrusion in chordamesoderm through the cytoplasmic proteins Dishevelled and Rho and Rac GTPases (Sokol, 1996; Wallingford et al., 2000; Habas et al., 2001, 2003; Tahinci and Symes, 2003; Kwan and Kirschner, 2005); while the Wnt/Ca2+ pathway regulates C&E through Cdc42 (Choi and Han, 2002; Penzo-Mendez et al., 2003). Stringent control of non-canonical Wnt signal levels is important, as both elevation and inhibition of the signaling lead to impaired C&E. FGF modulates C&E via different downstream signals. By activat ing mitogen-activated protein kinase (MAPK) pathway, FGF stimulates expression of its direct target Brachyury, which mediates mesodermal induction as well as transcription of Wnt1—a crucial component in the non-canonical Wnt pathway. FGF thus controls C&E indirectly this way (Amaya et al., 1991; Conlon and Smith, 1999; Tada and Smith, 2000; Kwan and Kirschner, 2003; Yokota et al., 2003). FGF also activates protein kinase C (PKC) and Ca2+ pathway to impact on morphogenesis directly without an effect on cell fate, and this branch of FGF signals can be modulated by the negative feedback regulator Sprouty2 (Nutt et al., 2001; Sivak et al., 2005).
Migration of head mesoderm along the BCR seems to be controlled by distinct signals. Platelet-derived growth factor (PDGF) pathway is implicated in this process. PDGF-A ligand is expressed in the animal cells, and PDGF receptor-α is expressed in the mesodermal cells (Jones et al., 1993). PDGF signaling is not required for C&E or head mesoderm migration per se, but is essential for providing guidance cue for directional migration of prechordal mesoderm towards the animal pole (Ataliotis et al., 1995; Symes and Mercola, 1996; Nagel et al., 2004). The transcription factor Brachyury, which is necessary for C&E in trunk mesoderm, inhibits migration of head mesoderm (Kwan and Kirschner, 2003). It is thus likely that the two types of cell movements are coordinated.
In our previous studies, we showed that a different growth factor signaling, the ErbB pathway, is also involved in Xenopus gastrulation (Nie and Chang, 2006). ErbBs are receptor tyrosine kinases that mediate signals from epidermal growth factor (EGF)-like cytokines. Upon binding to ligands, ErbBs are tyrosine-phosphorylated and can activate multiple downstream signaling cascades, including the MAPK, the phosphatidylino sitol-3 kinase (PI3K), and the phospholipase C gamma (PLCγ)/PKC pathways (Olayioye et al., 2000; Yarden and Sliwkowski, 2001; Carpenter, 2003; Citri et al., 2003). ErbBs regulate diverse cellular functions, such as proliferation, migration, differentiation and survival/death, and participate in various developmental processes during both invertebrate and vertebrate early embryogenesis (Yarden and Sliwkowski, 2001; Moghal and Sternberg, 2003; Shilo, 2003). Our previous work uncovered that all ErbBs were expressed in early Xenopus embryos at gastrula stages, and inhibition of ErbB signaling by dominant negative ErbB receptors (DN-ErbBs) induced gastrulation defects (Nie and Chang, 2006). However, the mechanisms underlying the functions of ErbBs in gastrulation are not understood.
In this study, we used both the truncated ErbB receptors and ErbB4-specific antisense morpholino oligonucleotides (MO) to investigate the roles of ErbBs in gastrulation. We demonstrate that ErbBs control both C&E and head mesoderm migration, thus identifying ErbB signaling as a unique regulator of different gastrulation movements. We further show that ErbBs regulate gastrulation at least partially through modulation of cell adhesion and cell shape changes, providing a mechanism for the actions of ErbBs during gastrulation.
Materials and methods
Embryo manipulations, morpholino oligonucleotides and plasmid construction
Embryos were obtained, maintained and microinjected with capped RNAs or ErbB4-MO as described (Chang et al., 1997). A standard control MO (Gene Tools Inc.) and the ErbB4-MO containing the sequence 5′-TTCCCTCCAAAAACTCTGGATCTCC-3′, which hybridizes to −29 to −5 position relative to the translational start site of XErbB4 (Genbank accession no. DQ646916), were used. The membrane-tethered EGFP and tdTomato plasmids were constructed by a PCR-based strategy to add the CAAX sequence of Ras to the C-termini of these proteins. The tdTomato template was kindly provided by Dr. Roger Tsien (UAB university-wide license through Dr. Bradley Yoder).
Head mesoderm migration assay
Anterior DMZ explants were dissected from stage 10.5–11 embryos and incubated in tissue culture dishes coated with 20 μg/ml human fibronectin (Sigma) overnight at 4°C (Winklbauer, 1990). The migratory behaviors of the cells were followed at 1-h intervals for 6 h. The farthest distance of coherent cell migration was measured. The average distances in injected explants were calculated and compared with that in control explants, and the significance of the differences was assessed by Student t-test.
Cell intercalation assay
RNAs encoding membrane-tethered fluorescent proteins EGFP and tdTomato were injected separately into different dorsal blastomeres of two- to four-cell stage embryos, alone or with RNAs encoding DN-ErbBs (2 ng). Openface explants were made by dissecting dorsal explants and peeling out the ectoderm at late gastrula stages (~stage 12). The explants were plated on fibronectin-coated coverslip with the mesoderm layer facing the matrix. The explants were then examined with Olympus IX70 inverted fluorescence microscope at neurula stages.
Cell adhesion and membrane protrusion assays
DMZ explants were dissected at early gastrula stages and dissociated for an hour in calcium- and magnesium-free buffer (CMFB). For cell–cell adhesion assay, the dissociated cells were plated on agarose-coated dishes in a buffer containing calcium and magnesium (MBSH) and allowed to reaggregate by orbital horizontal shaking for 3 h. For cell–matrix adhesion assay, the dissociated cells were plated on fibronectin-coated coverslips in MBSH for an hour. Loose, non-adherent cells were then gently washed away and pictures were taken before and after the washes. For cell spreading assay, cells adhering to fibronectin-coated coverslips were examined by phase-contrast microscopy. For observation of membrane protrusions, dissociated DMZ cells from embryos injected with membrane-tethered EGFP with or without ErbB4-MO were plated on FN-coated coverslip for an hour to allow adhesion, and the behaviors of adherent cells were recorded by time-lapse movies (3′-4′ per movie with 3″ frame intervals) using Olympus IX70 inverted fluorescence microscope. Different types of protrusions were counted for each cell and the numbers in control and injected samples were compared.
In situ hybridization (ISH), whole mount immunohistochemistry (WMIHC), βGal and Nile Blue staining and TUNEL assay
ISH and WMIHC were performed as described (Chang et al., 1997). Fibronectin antibody (Sigma) was used at 1:200 dilution and the secondary antibody (Jackson Laboratories) was used at 1:500–1:1000 dilutions. For lineage tracing with nuclear βGal (nβGal), the embryos were injected with 100 pg nβGal RNA and 2 ng RNAs encoding DN-ErbBs or 20 ng ErbB4-MO and incubated to late gastrula and neurula stages before examined by staining with the Red Gal substrate (Research Organics). For cell death analysis of the cells shed from the DMZ explants, cells were collected and stained with 1% Nile Blue Sulfate for 15–30 min. TUNEL assay was performed as described by Hensey and Gautier (1998).
Results
Inhibition of ErbB signaling leads to gastrulation defects in early Xenopus embryos
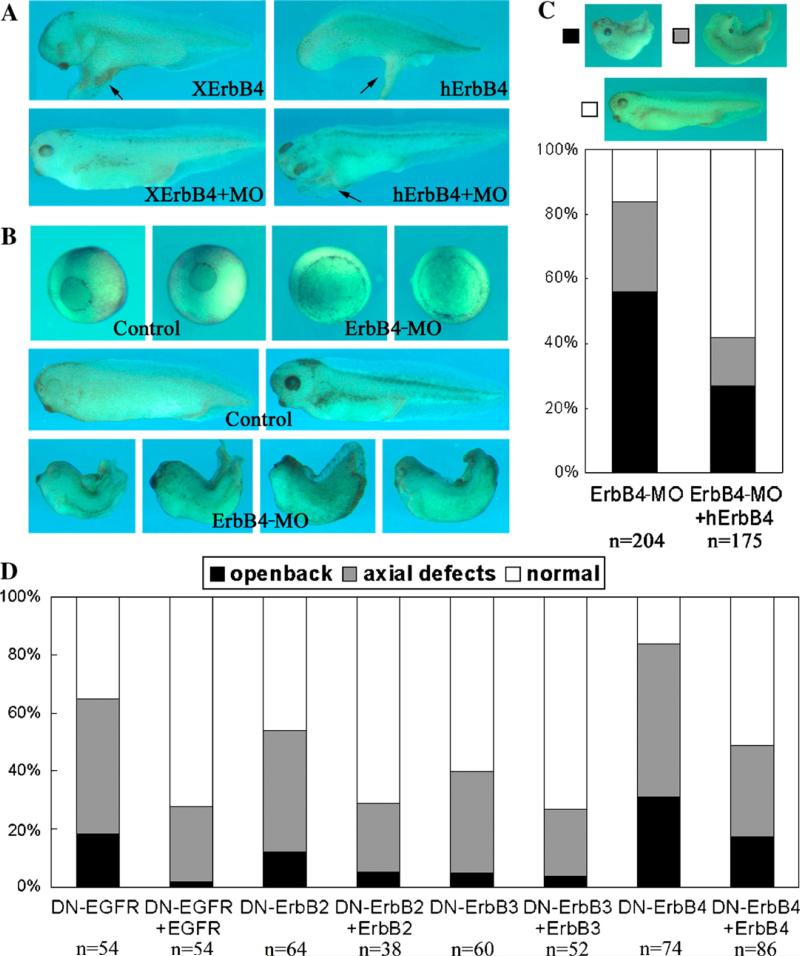
Previously, we showed that blocking ErbB pathway with truncated ErbB receptors (DN-ErbBs) that lacked the tyrosine kinase domain and the C-terminal tails resulted in delayed or impaired blastopore closure, indicating that ErbBs might regulate gastrulation (Nie and Chang, 2006). To further examine the specificity of this phenotype and the particular ErbB receptors involved in this process, we analyzed the effect of depleting one ErbB, ErbB4, on Xenopus gastrulation and performed rescue experiments. As shown in Fig. 1A, an ErbB4-specific MO blocked induction of ectopic structures by Xenopus, but not human, ErbB4; and the MO did not inhibit EGFR or ErbB2 (not shown). When injected into early Xenopus embryos, ErbB4-MO induced gastrulation defects. The morphants displayed impaired blastopore closure and showed open back phenotype as well as reduced head structures and shortened body axis (Fig. 1B). By contrast, embryos injected with a control MO in parallel developed normally (not shown). The defects could be partially rescued by coinjected human ErbB4 (Fig. 1C), demonstrating that the effect of ErbB4-MO on Xenopus gastrulation is specific. Similarly, impaired gastrulation induced by DN-ErbBs was partially rescued by coinjected wild-type receptors (Fig. 1D).
Fig. 1.
ErbB signaling regulates Xenopus gastrulation. (A) ErbB4-specific MO blocks induction of ectopic structures by Xenopus, but not by human, ErbB4. RNAs encoding ErbB4 (1–2 ng), with or without 20 ng ErbB4-MO, were injected into the animal region of two-cell stage embryos. (B) Injection of ErbB4-MO (20 ng) into dorsal marginal zone (DMZ) of four-cell stage embryos induced gastrulation defects. Morphants showed delayed blastopore closure, open back, and axial and head defects. (C) Human ErbB4 (5–8 pg) partially rescued gastrulation defects induced by ErbB4-MO. (D) Rescue of gastrulation defects induced by DN-ErbBs (2–4 ng) with wild-type ErbBs (0.2 ng). In panels C and D, embryos with different phenotypes were counted and summarized in the bar graph.
To see whether the gastrulation defects were due to defective dorsal mesodermal specification or a direct effect on cell movements, we assayed for expression of marker genes by whole mount in situ hybridization. Transcription of the mesodermal gene Brachyury (Xbra), the dorsal mesodermal marker chordin and the head mesodermal gene goosecoid was not inhibited by loss of ErbB signaling at gastrula stages (Fig. 2A). The pattern of chordin and goosecoid expression, however, was altered. While chordin domain was more restricted in the dorsal region in control embryos, the expression was spread into more lateral territories in embryos injected with DN-ErbB RNAs or ErbB4-MO. Similarly, goosecoid-expressing cells migrated away from the blastopore during gastrulation in wild-type embryos, but they remained around the blastopore in embryos in which ErbB signaling was blocked (Fig. 2A). At neurula stages, both Xbra and chordin were detected in the notochord as elongated bands, but their expression domains were shortened and widened in embryos where ErbB signaling was impaired (Fig. 2B). In addition, Sox3, a neural marker, showed the similar expression pattern of reduced length but increased width in embryos where ErbB signaling was compromised (Fig. 2B). Our data suggest that inhibition of ErbB signals does not interfere with dorsal mesoderm formation, but the movements of mesodermal cells during gastrulation may be perturbed.
Fig. 2.
Inhibition of ErbB signaling did not interfere with mesodermal induction in early frog embryos. (A) Expression of the mesodermal marker Brachyury (Xbra), the dorsal mesodermal marker Chordin and the head mesodermal marker Goosecoid was not blocked by loss of ErbB signaling in frog gastrulae. (B) At neurula stages, transcription of the notochord markers Xbra and chordin as well as the neural marker Sox3 was not impaired. The pattern of dorsal gene expression was wider and shorter in ErbB-inhibited embryos compared with that in control embryos.
ErbB signaling regulates convergent extension movements
Defects in different gastrulation movements can lead to impaired blastopore closure. Blocking FGF or Wnt signaling, for example, results in impaired C&E and failure of blastopore to close (Wallingford et al., 2002). Inhibition of PDGF signaling, in contrast, prevents directional migration of head mesoderm along the BCR, which also results in defective blastopore closure (Ataliotis et al., 1995; Nagel et al., 2004). To understand how ErbBs regulate gastrulation, we first examined whether ErbB signaling is required for C&E movements.
One way to assess C&E is the dorsal marginal zone (DMZ) explant experiment. Tissues taken from DMZ at gastrula stages contain axial and paraxial mesoderm and undergo C&E to form elongated explants. To see whether ErbB signals regulate this process, we dissected DMZ explants at early gastrula stages (stage 10) and incubated them to mid-neurula stages (stage 17) for examination. Control DMZ explants displayed characteristic narrowing and elongation, but explants from DN-ErbB-expressing or ErbB4 morphant embryos showed dramatic decrease in elongation (Figs. 3A, C). Comparing the effects of blocking ErbB and FGFR signals showed that truncated FGFRs inhibited C&E more efficiently, resulting in DMZ explants with round shape (Fig. 3A). To further exclude the possibility that ErbBs may regulate mesodermal cell fate which then affects C&E secondarily, we analyzed gene expression in these explants by RT-PCR. Expression of multiple markers at both gastrula (stage 11) and early tailbud (stage 22) stages was not significantly inhibited by DN-ErbBs, though there were slight variations among samples in different experiments (Fig. 3B). This was in sharp contrast with the explants expressing truncated FGFRs, where most of the markers were almost completely eliminated (Fig. 3B). To further test the specificity of the phenotype, we again performed rescue experiments. Elongation of DMZ explants was nicely rescued by coexpression of wild-type ErbBs with truncated receptors or human ErbB4 with ErbB4-MO (Fig. 3C). Our data indicate that ErbB signaling regulates C&E directly without affecting mesodermal cell fate, and that ErbBs and FGFRs may modulate C&E movements through distinct mechanisms.
Fig. 3.
ErbB signaling regulated C&E in DMZ explants. (A) DN-ErbBs blocked DMZ explants elongation. 2 ng RNAs encoding DN-ErbBs or DN-FGFRs were injected into the DMZ regions of four-cell stage embryos. DMZ explants were dissected from gastrula (stage 10) embryos and cultured till neurula stages (stage 17). (B) Unlike DN-FGFRs, DN-ErbBs did not significantly inhibit mesodermal marker expression in DMZ explants. (C) Similarly to DN-ErbBs, ErbB4-MO blocked C&E. The C&E movements were rescued by coexpression of human ErbB4 with ErbB4-MO, or wild-type ErbBs with DN-ErbBs.
One interesting phenomenon we observed was that many mesodermal cells dissociated from the explants at late neurula to tailbud stages when ErbB signaling was inhibited by DN-ErbBs or when ErbB4 was depleted in the explants. No shedding was observed in explants expressing DN-FGFRs (Suppl. Fig. 1 and data not shown). Analyses of cell death with Nile blue staining revealed that most cells dissociated from the explants were still alive, suggesting that blocking ErbB signaling did not significantly affect cell survival. Our results imply that ErbBs may regulate cell–cell adhesion. This possibility will be addressed later (see below).
Other ways to assess C&E include the animal cap assay. Animal caps, which normally form atypical epidermis, can be induced by activin to express mesendodermal markers. These induced explants undergo C&E movements similar to those exhibited by endogenous dorsal mesodermal cells. To see whether ErbBs participate in C&E in induced animal caps, we examined the morphology of and marker expression in activin- treated caps. We showed that similar to that in DMZ explants, inhibition of ErbB signaling in animal caps blocked activin-induced cap elongation without altering mesendodermal gene expression (Suppl. Fig. 2). This result confirms that ErbBs regulate cell movements without affecting cell fate.
To see whether ErbBs influence C&E in whole embryos, we next analyzed C&E in vivo by labeling dorsal cells with a lineage tracer, the nuclear β-galactosidase (nβGal). RNA encoding nβGal was injected alone or with DN-ErbB RNAs or ErbB4-MO into the marginal region of two dorsal blastomeres of four-cell stage embryos. The embryos were examined for βGal staining at gastrula or neurula stages. Labeled dorsal cells in control embryos or embryos expressing a control MO converged toward the dorsal midline as development proceeded, so that by the end of neurulation only a narrow stripe of labeled cells along the midline was visible. In embryos injected with DN-ErbB RNAs or ErbB4-MO, stained cells were distributed in a much wider area and the anterior–posterior (AP) extension of the labeled domain was also shorter (Fig. 4). As the staining in some of the samples was weaker, we also addressed whether cell death could be a main reason for impaired C&E. We thus performed TdT-mediated dUTP digoxigenin nick end labeling (TUNEL) assay to examine apoptosis. We observed a slight increase in apoptotic cells in some embryos injected with DN-ErbBs or ErbB4-MO, but the overall effect on cell death was mild (Suppl. Fig. 3). This implies that apoptosis may not be a major contributor for the observed phenotype. Our data illustrate that when ErbB signaling was blocked, cells did not converge in a mediolateral direction efficiently and the extension of axial/paraxial mesoderm along the AP direction was also impaired.
Fig. 4.
Inhibition of ErbB signaling blocked C&E in early Xenopus embryos. RNA encoding nuclear βGal (0.1 ng) was coinjected with DN-ErbB RNAs (2 ng) or 20 ng control-MO or ErbB4-MO into DMZ of four-cell stage embryos. The pattern of dorsal cell distribution was examined by staining of the injected embryos at gastrula or neurula stages with the Red Gal substrate. While the labeled cells formed a long and narrow stripe along the dorsal midline in control or control-MO-injected embryos, cells from DN-ErbB- or ErbB4-MO-expressing embryos showed a much wider and shorter distribution.
Regulation of cell shape and intercalation by ErbB signaling
During C&E, dorsal mesodermal cells display bipolar elongated shape and move along mediolateral direction toward the midline. To investigate whether this mediolateral cell intercalation behavior is influenced by altered ErbB signaling, we carried out a cell tracing experiment using membrane-tethered fluorescent proteins EGFP and tdTomato. RNAs encoding these fluorescent proteins were injected separately into different dorsal blastomeres of two- to four-cell stage embryos, with or without RNAs encoding DN-ErbBs. Dorsal explants were dissected from injected embryos at gastrula stages and plated on fibronectin-coated coverslip. The morphology and the locations of labeled cells were then observed at neurula stages under fluorescence microscope. In control embryos, labeled cells adopted bipolar shape and moved across the midline, so that cells with different labeling intermixed in the dorsal region (Fig. 5). When DN-ErbBs were coinjected with the fluorescent proteins, cells in two halves of the embryos respected their boundary and did not mix much along the midline. In addition, most cells displayed rounded or rectan gular morphology and did not take the spindle shape. Some of the rectangular cells elongated in the mediolateral direction, but they failed to intercalate efficiently (Fig. 5). Our results suggest that ErbB signaling controls cell morphological changes required for C&E, and blocking ErbB pathway prevents mediolateral cell intercalation.
Fig. 5.
Blocking ErbB signaling interfered with bipolar cell morphology and inhibited mediolateral cell intercalation. The membrane-tethered fluorescent proteins EGFP and tdTomato were injected separately into different dorsal blastomeres of two- to four-cell stage embryos, alone or with RNAs encoding DN-ErbBs (2 ng). Dorsal explants were dissected at late gastrula stages (~stage 12) and plated on fibronectin-coated coverslip. The explants were then examined at neurula stages. DN-ErbBs blocked bipolar spindle cell shape and cell mixing in the dorsal region.
Inhibition of ErbB signaling interferes with head mesoderm migration
In addition to C&E, directional migration of anterior mesendoderm along the BCR is also critical for proper gastrulation morphogenesis; blocking this movement (e.g., by truncated PDGFR) can lead to impaired blastopore closure (Ataliotis et al., 1995). To see whether ErbB signaling also participates in regulation of head mesoderm migration, we performed an in vitro explant assay. Anterior DMZ explants encompassing the head mesoderm were dissected from stage 10.5 embryos and plated on fibronectin-coated dishes. Migration of the explants was followed at 1-h intervals for 6 h. In control explants, cells migrating away from the central tissue were already visible after 1-h incubation; by the end of 6 h, a sheet of cells moving out of the explants was seen clearly in culture dishes (Figs. 6A, C). Quantification of the distances the furthest cells migrated indicated that head mesoderm traveled on average as far as 0.32–0.38 mm (Figs. 6B, D). Explants from DN-ErbB injected embryos still attached to the fibronectin-coated dishes, but fewer explants migrated. Compared with 84–86% control explants with migratory cells, only 34–43% explants expressing DN-ErbBs displayed cell migration (Fig. 6). Instead of a continuous sheet of cells spreading out from the explants, tissues expressing DN-ErbBs contained mainly individual migratory cells without much interconnection (Fig. 6A), indicating that cell–cell interaction might be compromised. For the cells that did move out of DN-ErbB-expressing explants, the distances they traveled were much shorter than those in control explants. The average distances of furthest cell migration were 0.15 mm, 0.15 mm, 0.17 mm and 0.13 mm for DN-EGFR, DN-ErbB2, DN-ErbB3 and DN-ErbB4 explants, respectively, about half of the distance the control head mesoderm migrated (Fig. 6B). Student t-test indicated that the differences between the control and the injected samples were significant, with the p-values less than 0.0001 for all DN-ErbBs (Fig. 6B). When comparing DN-ErbB-expressing explants with those from ErbB4 morphant embryos, we found that a higher percentage of head mesoderm migrated in the morphants (63% vs. 34–43%), and the average distances the cells traveled were also further than those in DN-ErbB explants (0.2 mm, with the p-value of 1.3E-6 when compared with control explants; Fig. 6D). Despite this, cells displayed similar defects in adhesion behaviors so that individual cells were seen spreading around the core tissues after 6 h (Fig. 6C). Expression of human ErbB4 with ErbB4-MO effectively rescued the cell dissociation phenotype (Figs. 6C, D). Our data reveal that in addition to trunk mesoderm C&E, ErbB signaling also regulates head mesoderm migration, and this may be achieved partially through modulation of cell adhesion.
Fig. 6.
Inhibition of ErbB signaling impaired head mesoderm migration on fibronectin (FN) substratum. (A) Anterior DMZ explants were dissected from stage 10.5–11 embryos and plated on FN-coated dish. Migration of head mesoderm was recorded at 1-h intervals for 6 h. A continuous sheet of cells was seen to migrate away from the control explants, but cells from DN-ErbB-expressing explants did not migrate efficiently and often moved as unconnected individual cells. (B) Quantification of anterior mesendoderm migration. The number and percentage of explants with migratory cells were listed under each sample, and the distances of furthest cell migration in explants containing migratory cells were shown in the bar graph. Student t-test suggested that the differences between the control and the injected samples were significant, with the p-values less than 0.0001. (C) Migration of head mesoderm from ErbB4 morphant embryos was less affected than those from DN-ErbB-expressing explants, but cells dissociated during the course of migration. The cell adhesion defects were rescued by human ErbB4. (D) Quantification of head mesoderm migration from ErbB4 morphant embryos. The percentage of explants maintaining cell adhesion was calculated among the migrated explants. Student t-test indicated that the difference in average migration distances between the control and the ErbB4-MO explants was significant, with the p-value of 1.3E-6.
ErbB signaling is not required for fibronectin deposition
During gastrulation, head mesoderm migrates on fibronectin substratum deposited at the inner surface of the BCR by ectodermal cells. The fibronectin meshwork is required for both adhesion and migration of head mesoderm. Since the neuregulin ligand and all four ErbBs are expressed in the ectoderm, it is possible that the ectodermal ErbB signaling controls the deposition of fibronectin matrix. To test this hypothesis, we examined the expression of fibronectin at gastrula stages by immunohistochemistry studies. Both wild-type and DN-ErbB-expressing gastrulae displayed a thin layer of fibronectin matrix lining the inner surface of the BCR (Suppl. Fig. 4), indicating that blocking ErbB signaling does not prevent synthesis or deposition of fibronectin. It is currently unknown whether the organization of fibronectin fibrils is also unaffected.
ErbB signaling regulates cell–cell and cell–matrix adhesion and cell spreading
Cells change their adhesive properties dynamically during morphogenesis. Alterations in cell–cell and/or cell–matrix adhesion can have deleterious effect on cell movements, leading to gastrulation defects. In our experiments, we noticed that inhibition of ErbB signaling resulted in cell shedding in DMZ explants and individual cell migration in head mesoderm, implying that ErbBs might modulate cell–cell adhesion. To examine this possibility more directly, we performed cell dissociation and reaggression assay. DMZ explants were dissected from early gastrula (stage 10) embryos and dissociated in calcium- and magnesium-free buffer (CMFB) for 1 h. The dissociated cells were then reaggregated in buffer containing calcium and magnesium (MBSH) for 3 h with orbital horizontal shaking. Cells from control DMZ explants formed large clusters after 3 h (Fig. 7). Cells from DMZ explants expressing DN-ErbBs (Fig. 7A) or ErbB4-MO (Fig. 7B) still reaggregated; however, the clusters they formed were often smaller and looser. The data demonstrate that ErbB signaling influences mesendodermal cell–cell adhesion.
Fig. 7.
Blocking ErbB signaling affected cell–cell adhesion. DMZ explants were dissected at early gastrula stages and dissociated in CMFB for an hour before reaggregation in MBSH with horizontal shaking. Cells from control explants reaggregated to form large clusters. Cells expressing DN-ErbBs (A) or ErbB4-MO (B) still reaggregated, but they formed smaller and looser clusters.
To see whether ErbB signaling also regulates cell–matrix adhesion, we next plated dissociated DMZ cells on fibronectin-coated coverslip in MBSH buffer. After 1-h incubation to allow cells to bind to fibronectin, unattached cells were washed away gently. Comparing the cells left on the coverslip before and after the wash suggested that there was a reduction in the number of the cells retained on the coverslip when ErbB signaling was inhibited. While about 92% of cells from control DMZ adhered to the coverslip, 82%, 70%, 65%, 64% and 64% of cells from samples injected with DN-ErbBs or ErbB4-MO remained on the coverslip. Coexpression of human ErbB4 with ErbB4-MO rescued cell adhesion to 89% of adherent cells (Fig. 8). A closer look at the morphology of the cells that retained on the coverslip indicated that about 58% of control cells underwent flattening and spreading, but only 27–35% of cells from embryos expressing DN-ErbBs or ErbB4-MO could spread and assume multipolar cell shape (Fig. 9). The effect of ErbB4-MO on cell spreading on fibronectin was nicely rescued by coexpressed human ErbB4 (Fig. 9), suggesting that the effect was specific. Our results show that ErbB signaling modulates cell–matrix interaction and the subsequent cell shape changes in response to matrix signals.
Fig. 8.
ErbB signaling modulated adhesion of mesodermal cells to fibronectin. Dissociated DMZ cells were plated on FN-coated coverslip for an hour before loose, non-adherent cells were removed with gentle wash. Pictures were taken before and after the wash and the number of the cells on the coverslip was counted. While about 92% of cells from control explants remained attached to the coverslip, 64–82% of cells from explants injected with DN-ErbBs or ErbB4-MO adhered to the glass after the wash. The effect of ErbB4-MO on cell adhesion was rescued by coexpression of human ErbB4 (89% of adherent cells).
Fig. 9.
ErbB signaling controlled cell spreading on fibronectin. (A) Cells from control DMZ explants spread upon binding to FN, but cells from explants injected with DN-ErbBs or ErbB4-MO showed reduced ability to spread. (B) Quantification of cell spreading on FN-coated coverslip. Control samples showed 58% of cells spreading on FN, but 35%, 34%, 27% and 33% of cells expressing DN-EGFR, DN-ErbB2, DN-ErbB3 and DN-ErbB4 spread on FN respectively. 30% of cells expressing ErbB4-MO spread, and the number increased to 52% when human ErbB4 was coexpressed with the MO.
Regulation of membrane protrusions by ErbB signaling
During morphogenesis, cells modify their membrane protrusions dynamically to fulfill the needs for polarization, shape changes and movements. Defects in membrane protrusive activities can account for impaired cell movements. To comprehend whether ErbB signaling regulates cell protrusions, we made time-lapse movies of individual mesendodermal cells labeled with membrane-tethered EGFP at gastrula stages. We observed that control cells extended and retracted lamellipodia and filopodia animatedly, but cells expressing ErbB4-MO showed much reduced and static membrane protrusions. These cells, however, had increased membrane blebs, which actively changed their locations within the cells. The alteration in membrane protrusions caused by depletion of ErbB4 was rescued with coexpressed human ErbB4, so that the formation of dynamic lamellipodia and filopodia was restored (Fig. 10, and Supplementary movies). The data demonstrate that ErbB signaling regulates the dynamics of membrane protrusions in mesendodermal cells during gastrulation.
Fig. 10.
ErbB signaling influenced formation of membrane protrusions. Embryos were injected with membrane-tethered EGFP with or without ErbB4-MO and human ErbB4. DMZ explants were dissected and dissociated in CMFB for an hour, and dissociated cells were plated on FN-coated coverslip in MBSH for an hour to allow cell adhesion. Time-lapse movies were made to record the membrane protrusive activities. (A) Control cells showed dynamic lamellipodia and filopodia formation, whereas cells expressing ErbB4-MO did not form these protrusions efficiently. Instead, they had dynamic membrane blebs. The defects in protrusive activities were rescued when ErbB4-MO was coexpressed with human ErbB4 RNA. (B) Quantification of membrane protrusions in different samples. Lamellipodia, filopodia and membrane blebs were counted separately and summarized in the bar graph. An average of 1.3 lamellipodia, 1.7 filopodia and 0.3 membrane blebs per minute were observed in control cells. Expression of ErbB4-MO decreased both lamellipodia and filopodia to 0.3 times per minute, but increased membrane blebs to 0.7 times per minute. Coinjection of human ErbB4 shifted the frequencies of these membrane protrusions back to 0.9, 1.3 and 0.3 times per minute respectively. Student t-test indicated that the differences in protrusive activities between ErbB-MO and control cells were significant (p-values of 2.4E-24, 1.3E-9 and 1.7E-2 for the three different protrusions), while the differences between control and the rescued samples were not significant (p-values of 0.08, 0.19 and 0.35 for the three protrusions). The total numbers of cells counted were given under the bar graph.
Discussion
ErbB signaling controls diverse cellular functions, including cell migration in Drosophila and cell contraction in C. elegans (Lehmann, 2001; Moghal and Sternberg, 2003). In vertebrate, ErbBs are shown to modulate cell motility under pathological conditions during cancer cell invasion and metastasis (Holbro et al., 2003). However, it is unclear whether ErbBs also regulate cell movements under normal physiological conditions, such as during early vertebrate development. In this study, we provide first evidence that ErbB signaling is essential for Xenopus gastrulation morphogenesis by controlling both convergent extension and head mesoderm migration. We show that ErbBs modulate cell adhesion, spreading, and membrane protrusions, indicating that an important mechanism underlying the activities of ErbBs in gastrulation is to modify cell adhesive properties and cell morphology. Our work thus suggests that vertebrate ErbB signaling regulates cell motility during early embryogenesis.
Convergent extension
Currently, two signaling pathways, those of non-canonical Wnt and FGF, are shown to regulate convergent extension. In the Wnt/PCP pathway, Wnt5A and Wnt11 signal through Frizzled to activate Dishevelled, which recruits Daam1 to activate Rho and Rho effectors ROKα and JNK and recruits Rac1 to activate JNK (Moon et al., 1993; Sokol, 1996; Djiane et al., 2000; Medina et al., 2000; Tada and Smith, 2000; Wallingford et al., 2000; Habas et al., 2001, 2003; Yamanaka et al., 2002; Kinoshita et al., 2003; Tahinci and Symes, 2003; Kim and Han, 2005; Kwan and Kirschner, 2005). This pathway can be inhibited by protein kinase A (Park et al., 2006). In the Wnt/Ca2+ pathway, G proteins are activated downstream of Frizzled to mobilize intracellular calcium and stimulate PKC to activate Cdc42 (Choi and Han, 2002; Penzo-Mendez et al., 2003). The activated Rho/Rac/Cdc42 GTPases regulate cytos keleton reorganization to control dynamics and polarization of cellular protrusions—lamellipodia and filopodia, thus affecting cell behaviors (Wallingford et al., 2000; Settleman, 2001; Tahinci and Symes, 2003; Jaffe and Hall, 2005; Ren et al., 2006). FGF signaling, in contrast, regulates both mesoderm induction and C&E movements. FGF stimulates MAPK pathway to induce mesodermal genes, including Brachyury, a gene required for C&E. FGF also activates PKC and Ca2+ signals to regulate morphogenesis directly without affecting mesodermal fate (Amaya et al., 1991; Conlon and Smith, 1999; Nutt et al., 2001; Sivak et al., 2005). FGF regulates C&E at least partially through its downstream target NRH/NRH1, which activates Rho family GTPases and controls filopodia formation and cell intercalation in dorsal cells (Sasai et al., 2004; Chung et al., 2005).
In this study, we show that a different signaling pathway, which is mediated by ErbB receptors, also regulates convergent extension. We used two different means to disrupt ErbB signaling. The truncated ErbB receptors that lacked the tyrosine kinase domain and the C-terminal tails could not phosphorylate downstream targets or provide docking sites for downstream effectors, but they could still bind ligands and form various heterodimers with other ErbBs. They thus promiscuously blocked endogenous signals through all ErbB receptors with varied efficiency. By contrast, ErbB4-MO specifically blocked translation of Xenopus ErbB4, but not other ErbBs; it therefore only interfered with signaling through receptor dimers containing ErbB4. Using these two loss-of-function strategies, we find that ErbB signaling influences cell movements without interrupting mesodermal cell fate, even though overexpression of ErbBs can induce mesodermal markers in animal caps (Nie and Chang, 2006). This suggests that the primary role of ErbB signaling in early Xenopus development is not to modulate mesoderm formation, but to control the behaviors of the mesodermal cells. When ErbB signaling is disrupted, dorsal mesodermal cells fail to adopt bipolar spindle cell shape and lose their ability to intercalate mediolaterally. ErbBs may thus influence dynamic cytoskeleton reorganization that is essential for correct cell morphology and movement during C&E. It will be important in future to determine the effect of ErbB signaling on formation and polarization of membrane protrusions as well as actin organization in trunk mesodermal cells undergoing C&E. Several downstream signals may mediate the actions of ErbBs during C&E. The PKC/Ca2+ pathway, MAPK, PI3K, Src family kinases, c-Abl tyrosine kinase, and Rho family GTPases can all be activated by ErbBs in mammalian cells to regulate cancer cell migration and invasion (Fanger et al., 1997; Adam et al., 1998; Kassis et al., 1999; Olayioye et al., 2000; Spencer et al., 2000; Dittmar et al., 2002; Holbro et al., 2003; Woodring et al., 2003; Bromann et al., 2004; Playford and Schaller, 2004; Sewell et al., 2005). Some of these downstream components overlap with those stimulated by non-canonical Wnt and FGF pathways; while others, such as the Src family members and PI3K, are shown to play a role in gastrulation in Xenopus and/or zebrafish (Carballada et al., 2001; Denoyelle et al., 2001; Jopling and den Hertog, 2005). It is possible that non-canonical Wnt, FGF and ErbB signals regulate overlapping and distinct aspects of cell behaviors, such as formation or polarization of membrane protrusions; collectively, the three signaling pathways control cell morphology, adhesion and motility during convergent extension.
Prechordal mesoderm migration
In addition to C&E, ErbBs also regulate head mesoderm migration. Disruption of ErbB signaling leads to reduced ability of prechordal mesoderm to migrate on fibronectin substratum. Several defects may account for this phenomenon. First, the cells show reduced adhesion to each other, so that instead of forming an intact cell sheet on fibronectin, many cells lose contact with their neighbors and migrate as individual cells. This results in randomization of cell movements (Winklbauer et al., 1992), and consequently reduced overall distance of migration. Second, the cells also show reduced adhesion to fibronectin as well as impaired ability to spread on this matrix. Close inspection of the cells that do adhere to fibronectin demonstrates that cells do not form lamellipodia and filopodia efficiently; instead they form dynamic membrane blebs that unable to exert traction for cell locomotion. ErbB signaling thus influences cytoskeleton reorganization in both head mesoderm and trunk mesoderm to regulate different movements. This is unique among signaling pathways involved in gastrulation morphogenesis. The only other signal that participates in regulation of cell behaviors in both mesodermal populations is the Fizzled-PKC pathway, and its function in head mesoderm migration is to separate involuted mesoderm from the overlying ectoderm at the blastocoel roof to maintain a stable interface (Wacker et al., 2000; Winklbauer et al., 2001). In comparison, PDGF signaling regulates head mesoderm migration without affecting convergent extension, and it modulates directionality rather than motility of the head mesoderm (Symes and Mercola, 1996; Nagel et al., 2004). In addition, transcription factors Goosecoid and Brachyury are expressed in the prechordal and chordamesoderm, respectively. They repress each other's transcription and control different adhesive and polarized protrusive activities (Wacker et al., 1998; Latinkic and Smith, 1999; Kwan and Kirschner, 2003). This ensures that the head and the trunk mesoderm display different protrusive and motile behaviors (Shih and Keller, 1992; Winklbauer and Selchow, 1992). It is currently unclear and will be important to understand how ErbB signaling crosstalks with these regional specific factors/signals to control distinct cell behaviors in the head and the trunk mesoderm.
ErbB signaling and cell adhesion
One common cell property regulated by ErbB signaling in both head and trunk mesoderm is cell adhesion. Trunk DMZ explants expressing DN-ErbBs or ErbB4-MO display cell shedding from mid-neurula stages onward (Suppl. Fig. 1); while anterior DMZ explants with impaired ErbB signaling dissociate during the course of migration (Fig. 6). These data indicate that contacts between neighboring cells are compromised in the absence of ErbB signaling. One way ErbBs may regulate cell–cell interaction is through modulation of cell surface adhesion molecules cadherin family members. In mammalian cells, EGFR has been shown to bind to cadherin as well as catenin family members to influence the assembly of cadherin–catenin complex and to impact on cell adhesion during cancer cell invasion and metastasis (Hoschuetzky et al., 1994; Kanai et al., 1995; Al Moustafa et al., 2002; Hirohashi and Kanai, 2003; Brunton et al., 2004; Qian et al., 2004). Though other ErbB members have not been examined in detail, it is possible that most, if not all, ErbBs may regulate activities of particular cadherin members to alter cell adhesive behaviors in gastrulation. Changes in cadherin expression and/or activities are associated with gastrulation defects in Xenopus, zebrafish and mouse (Kuhl and Wedlich, 1996; Babb and Marrs, 2004; Gumbiner, 2005; Lecuit, 2005; Montero et al., 2005; Shimizu et al., 2005; Ulrich et al., 2005; Zohn et al., 2006), highlighting the importance of cadherin-mediated cell adhesion in this morphogenetic process. In Xenopus, different cadherins may participate in different cell movements, so that disruption of E-cadherin leads to ectodermal lesion (Levine et al., 1994), activation as well as inhibition of C-cadherin prevents convergent extension (Lee and Gumbiner, 1995; Zhong et al., 1999), while XB/U-cadherin is required for head mesoderm migration (Winklbauer et al., 1992; Kuhl et al., 1996). ErbBs may potentially cooperate with different cadherins in different regions to modulate cell–cell adhesion, and this may be critical for coherent and coordinated cell movements in both the head and the trunk mesoderm.
Cell–matrix interaction is also subjected to regulation by ErbB signaling, as mentioned above, and this may be achieved via modification of matrix organization and/or activities of integrin family of matrix receptors. In Xenopus, both fibronectin and its receptor integrin are implicated in different movements during gastrulation, including radial cell intercalation during epiboly, mesodermal cell migration along the blastocoel roof, and convergent extension (Smith et al., 1990; Winklbauer and Nagel, 1991; DeSimone, 1994; Ramos et al., 1996; Winklbauer and Keller, 1996; Nagel and Winklbauer, 1999; Marsden and DeSimone, 2001, 2003; Davidson et al., 2002, 2004, 2006; Goto et al., 2005). Extracellular signals, such as activin, FGF, and the non-canonical Wnt components Frizzled, Strabismus and Prickled, can control the assembly of fibronectin fibrillar meshwork and affect cellular behaviors during morphogenesis (Nagel and Winklbauer, 1999; Goto et al., 2005). Although ErbBs do not seem to influence fibronectin synthesis and deposition (Suppl. Fig. 4), it is possible that they may modulate the assembly of fibronectin into organized fibrils. In addition, ErbBs may directly regulate activities of integrin. In mammalian cells, EGFR and ErbB2 associate with several integrins to modulate tumor progression, invasion and would healing (Falcioni et al., 1997; Adelsman et al., 1999; Gambaletta et al., 2000; Hintermann et al., 2001, 2005; Mariotti et al., 2001; Guo et al., 2006). It is conceivable that ErbBs may utilize a similar strategy in gastrulation to interact with and modify the functions of integrins. The consequence of this interaction may affect not only adhesion of mesodermal cells to the fibronectin matrix, but also formation and polarization of membrane protrusions in mesodermal cells in response to matrix signals.
In summary, we report here that ErbB signaling regulates both mesoderm migration and convergent extension during Xenopus gastrulation, and ErbB4 may participate in both processes. Knockdown of ErbB4 with specific MO mimics most aspects of the phenotypes induced by using truncated ErbB receptors, though migration of head mesoderm is less affected. This may reflect a partially redundant action of other ErbBs during head mesoderm migration. ErbBs may control gastrulation movements through modulation of cell adhesion, morphology and membrane protrusions. Our studies leave several key questions unanswered. For example, it is not known how ErbBs utilize various downstream signals to modulate different cell behaviors and movements, whether ErbBs regulate activities of cadherins and integrins directly, and how ErbBs may interact with other signaling pathways to control cell adhesion, polarity and motility during gastrulation. These questions need to be investigated further in future.
Supplementary Material
Acknowledgments
We thank Dr. Rudolf Winklbauer for kindly providing us with a detailed protocol for head mesoderm migration assay and Dr. Ray Keller for patiently explaining gastrulation and expertly demonstrating all necessary techniques to us at the MBL embryology course in Woods Hole, MA. We also thank Drs. Roger Tsien, Courtney Haycraft and Bradley Yoder for providing the constructs encoding fluorescent proteins to us. This work is supported by NIH grant.
Abbreviations
- C&E
convergence and extension
- BCR
blastocoel roof
Footnotes
Uncited reference
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.ydbio.2006.10.039.
References
- Adam L, Vadlamudi R, Kondapaka SB, Chernoff J, Mendelsohn J, Kumar R. Heregulin regulates cytoskeletal reorganization and cell migration through the p21-activated kinase-1 via phosphatidylinositol-3 kinase. J. Biol. Chem. 1998;273:28238–28246. doi: 10.1074/jbc.273.43.28238. [DOI] [PubMed] [Google Scholar]
- Adelsman MA, McCarthy JB, Shimizu Y. Stimulation of β1-integrin function by epidermal growth factor and Heregulin-β has distinct requirements for erbB2 but a similar dependence on phosphoinositide 3-OH kinase. Mol. Biol. Cell. 1999;10:2861–2878. doi: 10.1091/mbc.10.9.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Moustafa AE, Yen L, Benlimame N, Alaoui-Jamali MA. Regulation of E-cadherin/catenin complex patterns by epidermal growth factor receptor modulation in human lung cancer cells. Lung Cancer. 2002;37:49–56. doi: 10.1016/s0169-5002(02)00025-9. [DOI] [PubMed] [Google Scholar]
- Amaya E, Musci TJ, Kirschner MW. Expression of a dominant negative mutant of the FGF receptor disrupts mesoderm formation in Xenopus embryos. Cell. 1991;66:257–270. doi: 10.1016/0092-8674(91)90616-7. [DOI] [PubMed] [Google Scholar]
- Ataliotis P, Symes K, Chou MM, Ho L, Mercola M. PDGF signalling is required for gastrulation of Xenopus laevis. Development. 1995;121:3099–3110. doi: 10.1242/dev.121.9.3099. [DOI] [PubMed] [Google Scholar]
- Babb SG, Marrs JA. E-cadherin regulates cell movements and tissue formation in early zebrafish embryos. Dev. Dyn. 2004;230:263–277. doi: 10.1002/dvdy.20057. [DOI] [PubMed] [Google Scholar]
- Bromann PA, Korkaya H, Courtneidge SA. The interplay between Src family kinases and receptor tyrosine kinases. Oncogene. 2004;23:7957–7968. doi: 10.1038/sj.onc.1208079. [DOI] [PubMed] [Google Scholar]
- Brunton VG, MacPherson IR, Frame MC. Cell adhesion receptors, tyrosine kinases and actin modulators: a complex three-way circuitry. Biochim. Biophys. Acta. 2004;1692:121–144. doi: 10.1016/j.bbamcr.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Carballada R, Yasuo H, Lemaire P. Phosphatidylinositol-3 kinase acts in parallel to the ERK MAP kinase in the FGF pathway during Xenopus mesoderm induction. Development. 2001;128:35–44. doi: 10.1242/dev.128.1.35. [DOI] [PubMed] [Google Scholar]
- Carpenter G. ErbB4: mechanism of action ad biology. Exp. Cell Res. 2003;284:66–77. doi: 10.1016/s0014-4827(02)00100-3. [DOI] [PubMed] [Google Scholar]
- Casci T, Freeman M. Control of EGF receptor signalling: lessons from fruitflies. Cancer Metastasis Rev. 1999;18:181–201. doi: 10.1023/a:1006313122373. [DOI] [PubMed] [Google Scholar]
- Chang C, Wilson PA, Mathews LS, Hemmati-Brivanlou A. A Xenopus type I activin receptor mediates mesodermal but not neural specification during embryogenesis. Development. 1997;124:827–837. doi: 10.1242/dev.124.4.827. [DOI] [PubMed] [Google Scholar]
- Choi SC, Han JK. Xenopus Cdc42 regulates convergent extension movements during gastrulation through Wnt/Ca2+ signaling pathway. Dev. Biol. 2002;244:342–357. doi: 10.1006/dbio.2002.0602. [DOI] [PubMed] [Google Scholar]
- Chung HA, Hyodo-Miura J, Nagamune T, Ueno N. FGF signal regulates gastrulation cell movements and morphology through its target NRH. Dev. Biol. 2005;282:95–110. doi: 10.1016/j.ydbio.2005.02.030. [DOI] [PubMed] [Google Scholar]
- Citri A, Skaria KB, Yarden Y. The deaf and the dumb: the biology of ErbB-2 and ErbB-3. Exp. Cell Res. 2003;284:54–65. doi: 10.1016/s0014-4827(02)00101-5. [DOI] [PubMed] [Google Scholar]
- Conlon FL, Smith JC. Interference with Brachyury function inhibits convergent extension, causes apoptosis, and reveals separate requirements in the FGF and activin signalling pathways. Dev. Biol. 1999;213:85–100. doi: 10.1006/dbio.1999.9330. [DOI] [PubMed] [Google Scholar]
- Davidson LA, Hoffstrom BG, Keller R, DeSimone DW. Mesendoderm extension and mantle closure in Xenopus laevis gastrulation: combined roles for integrin α5β1, fibronectin, and tissue geometry. Dev. Biol. 2002;242:109–129. doi: 10.1006/dbio.2002.0537. [DOI] [PubMed] [Google Scholar]
- Davidson LA, Keller R, DeSimone DW. Assembly and remodeling of the fibrillar fibronectin extracellular matrix during gastrulation and neurulation in Xenopus laevis. Dev. Dyn. 2004;231:888–895. doi: 10.1002/dvdy.20217. [DOI] [PubMed] [Google Scholar]
- Davidson LA, Marsden M, Keller R, DeSimone DW. Integrin α5β1 and fibronectin regulate polarized cell protrusions required for Xenopus convergence and extension. Curr. Biol. 2006;16:833–844. doi: 10.1016/j.cub.2006.03.038. [DOI] [PubMed] [Google Scholar]
- Denoyelle M, Valles AM, Lentz D, Thiery JP, Boyer B. Mesoderm-independent regulation of gastrulation movements by the Src tyrosine kinase in Xenopus embryo. Differentiation. 2001;69:38–48. doi: 10.1046/j.1432-0436.2001.690104.x. [DOI] [PubMed] [Google Scholar]
- DeSimone DW. Adhesion and matrix in vertebrate development. Curr. Opin. Cell Biol. 1994;6:747–751. doi: 10.1016/0955-0674(94)90103-1. [DOI] [PubMed] [Google Scholar]
- Dittmar T, Husemann A, Schewe Y, Nofer JR, Niggemann B, Zanker KS, Brandt BH. Induction of cancer cell migration by epidermal growth factor is initiated by specific phosphorylation of tyrosine 1248 of c-erbB-2 receptor via EGFR. FASEB J. 2002;16:1823–1825. doi: 10.1096/fj.02-0096fje. [DOI] [PubMed] [Google Scholar]
- Djiane A, Riou JF, Umbhauer M, Boucaut JC, Shi DL. Role of frizzled 7 in the regulation of convergent extension movements during gastrulation in Xenopus laevis. Development. 2000;127:3091–3100. doi: 10.1242/dev.127.14.3091. [DOI] [PubMed] [Google Scholar]
- Falcioni R, Antonini A, Nistico P, Di Stefano S, Crescenzi M, Natali PG, Sacchi A. α6β4 and α6β1 integrins associate with ErbB-2 in human carcinoma cell lines. Exp. Cell Res. 1997;236:76–85. doi: 10.1006/excr.1997.3695. [DOI] [PubMed] [Google Scholar]
- Fanger GR, Johnson NL, Johnson GL. MEK kinases are regulated by EGF and selectively interact with Rac/Cdc42. EMBO J. 1997;16:4961–4972. doi: 10.1093/emboj/16.16.4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambaletta D, Marchetti A, Benedetti L, Mercurio AM, Sacchi A, Falcioni R. Cooperative signaling between α6β4 integrin and ErbB-2 receptor is required to promote phosphatidylinositol 3-kinase-dependent invasion. J. Biol. Chem. 2000;275:10604–10610. doi: 10.1074/jbc.275.14.10604. [DOI] [PubMed] [Google Scholar]
- Goto T, Davidson L, Asashima M, Keller R. Planar cell polarity genes regulate polarized extracellular matrix deposition during frog gastrulation. Curr. Biol. 2005;15:787–793. doi: 10.1016/j.cub.2005.03.040. [DOI] [PubMed] [Google Scholar]
- Gumbiner BM. Regulation of cadherin-mediated adhesion in morpho-genesis. Nat. Rev., Mol. Cell Biol. 2005;6:622–634. doi: 10.1038/nrm1699. [DOI] [PubMed] [Google Scholar]
- Guo W, Pylayeva Y, Pepe A, Yoshioka T, Muller WJ, Inghirami G, Giancotti F. β4 integrin amplifies ErbB2 signaling to promote mammary tumorigenesis. Cell. 2006;126:489–502. doi: 10.1016/j.cell.2006.05.047. [DOI] [PubMed] [Google Scholar]
- Habas R, Kato Y, He X. Wnt/Frizzled activation of Rho regulates vertebrate gastrulation and requires a novel Formin homology protein Daam1. Cell. 2001;107:843–854. doi: 10.1016/s0092-8674(01)00614-6. [DOI] [PubMed] [Google Scholar]
- Habas R, Dawid IB, He X. Coactivation of Rac and Rho by Wnt/Frizzled signaling is required for vertebrate gastrulation. Genes Dev. 2003;17:295–309. doi: 10.1101/gad.1022203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensey C, Gautier J. Programmed cell death during Xenopus development: a spatio-temporal analysis. Dev. Biol. 1998;203:36–48. doi: 10.1006/dbio.1998.9028. [DOI] [PubMed] [Google Scholar]
- Hintermann E, Bilban M, Sharabi A, Quaranta V. Inhibitory role of α6β4-associated erbB-2 and phosphoinositide 3-kinase in keratinocyte hapto-tactic migration dependent on α3β1 integrin. J. Cell Biol. 2001;153:465–478. doi: 10.1083/jcb.153.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hintermann E, Yang N, O'Sullivan D, Higgins JMG, Quaranta V. Integrin α6β4-erbB2 complex inhibits haptotaxis by up-regulating E-cadherin cell–cell junctions in keratinocytes. J. Biol. Chem. 2005;280:8004–8015. doi: 10.1074/jbc.M406301200. [DOI] [PubMed] [Google Scholar]
- Hirohashi S, Kanai Y. Cell adhesion system and human cancer morphogenesis. Cancer Sci. 2003;94:575–581. doi: 10.1111/j.1349-7006.2003.tb01485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbro T, Civenni G, Hynes NE. The ErbB receptors and their role in cancer progression. Exp. Cell Res. 2003;284:99–110. doi: 10.1016/s0014-4827(02)00099-x. [DOI] [PubMed] [Google Scholar]
- Hoschuetzky H, Aberle H, Kemler R. β-Catenin mediates the interaction of the cadherin–catenin complex with epidermal growth factor receptor. J. Cell Biol. 1994;127:1375–1380. doi: 10.1083/jcb.127.5.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu. Rev. Cell Dev. Biol. 2005;21:247–269. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- Jones SD, Ho L, Smith JC, Yordan C, Stiles CD, Mercola M. The Xenopus platelet-derived growth factor α receptor: cDNA cloning and demonstration that mesoderm induction establishes the lineage-specific pattern of ligand and receptor gene expression. Dev. Genet. 1993;14:185–193. doi: 10.1002/dvg.1020140305. [DOI] [PubMed] [Google Scholar]
- Jopling C, den Hertog J. Fyn/Yes and non-canonical Wnt signalling converge on RhoA in vertebrate gastrulation cell movements. EMBO Rep. 2005;6:426–431. doi: 10.1038/sj.embor.7400386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai Y, Ochiai A, Shibata T, Oyama T, Ushijima S, Akimoto S, Hirohashi S. c-erbB-2 gene product directly associates with β-catenin and plakoglobin. Biochem. Biophys. Res. Commun. 1995;208:1067–1072. doi: 10.1006/bbrc.1995.1443. [DOI] [PubMed] [Google Scholar]
- Kassis J, Moellinger J, Lo H, Greenberg NM, Kim HG, Wells A. A role for phospholipase C-γ-mediated signaling in tumor cell invasion. Clin. Cancer Res. 1999;5:2251–2260. [PubMed] [Google Scholar]
- Keller R. Early embryonic development of Xenopus laevis. Methods Cell Biol. 1991;36:61–113. doi: 10.1016/s0091-679x(08)60273-3. [DOI] [PubMed] [Google Scholar]
- Keller R, Davidson LA, Shook DR. How we are shaped: the biomechanics of gastrulation. Differentiation. 2003;71:171–205. doi: 10.1046/j.1432-0436.2003.710301.x. [DOI] [PubMed] [Google Scholar]
- Kim GH, Han JK. JNK and ROKα function in the noncanonical Wnt/ RhoA signaling pathway to regulate Xenopus convergent extension movements. Dev. Dyn. 2005;232:958–968. doi: 10.1002/dvdy.20262. [DOI] [PubMed] [Google Scholar]
- Kinoshita N, Iioka H, Miyakoshi A, Ueno N. PKCδ is essential for Dishevelled function in a noncanonical Wnt pathway that regulates Xenopus convergent extension movements. Genes Dev. 2003;17:1663–1676. doi: 10.1101/gad.1101303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl M, Wedlich D. Xenopus cadherins: sorting out types and functions in embryogenesis. Dev. Dyn. 1996;207:121–134. doi: 10.1002/(SICI)1097-0177(199610)207:2<121::AID-AJA1>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Kuhl M, Finnemann S, Binder O, Wedlich D. Dominant negative expression of a cytoplasmically deleted mutant of XB/U-cadherin disturbs mesoderm migration during gastrulation in Xenopus laevis. Mech. Dev. 1996;54:71–82. doi: 10.1016/0925-4773(95)00462-9. [DOI] [PubMed] [Google Scholar]
- Kwan KM, Kirschner MW. Xbra functions as a switch between cell migration and convergent extension in the Xenopus gastrula. Development. 2003;130:1961–1972. doi: 10.1242/dev.00412. [DOI] [PubMed] [Google Scholar]
- Kwan KM, Kirschner MW. A microtubule-binding Rho-GEF controls cell morphology during convergent extension of Xenopus laevis. Develop ment. 2005;132:4599–4610. doi: 10.1242/dev.02041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latinkic BV, Smith JC. Goosecoid and Mix.1 repress Brachyury expression and are required for head formation in Xenopus. Development. 1999;126:1769–1779. doi: 10.1242/dev.126.8.1769. [DOI] [PubMed] [Google Scholar]
- Lecuit T. Adhesion remodeling underlying tissue morphogenesis. Trends Cell Biol. 2005;15:34–42. doi: 10.1016/j.tcb.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Lee CH, Gumbiner BM. Disruption of gastrulation movements in Xenopus by a dominant-negative mutant for C-cadherin. Dev. Biol. 1995;171:363–373. doi: 10.1006/dbio.1995.1288. [DOI] [PubMed] [Google Scholar]
- Lehmann R. Cell migration in invertebrates: clues from border and distal tip cells. Curr. Opin. Genet. Dev. 2001;11:457–463. doi: 10.1016/s0959-437x(00)00217-3. [DOI] [PubMed] [Google Scholar]
- Levine E, Lee CH, Kintner C, Gumbiner BM. Selective disruption of E-cadherin function in early Xenopus embryos by a dominant negative mutant. Development. 1994;120:901–909. doi: 10.1242/dev.120.4.901. [DOI] [PubMed] [Google Scholar]
- Mariotti A, Kedeshian PA, Dans M, Curatola AM, Gagnoux-Palacios L, Giancotti FG. EGF-R signaling through Fyn kinase disrupts the function of integrin α6β4 at hemidesmosomes: role in epithelial cell migration and carcinoma invasion. J. Cell Biol. 2001;155:44–457. doi: 10.1083/jcb.200105017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden M, DeSimone DW. Regulation of cell polarity, radial intercalation and epiboly in Xenopus: novel roles for integrin and fibronectin. Development. 2001;128:3635–3647. doi: 10.1242/dev.128.18.3635. [DOI] [PubMed] [Google Scholar]
- Marsden M, DeSimone D. Integrin–ECM interactions regulate cadherin-dependent cell adhesion and are required for convergent extension in Xenopus. Curr. Biol. 2003;13:1182–1191. doi: 10.1016/s0960-9822(03)00433-0. [DOI] [PubMed] [Google Scholar]
- Medina A, Reintsch W, Steinbeisser H. Xenopus frizzled 7 can act in canonical and non-canonical Wnt signaling pathways: implications on early patterning and morphogenesis. Mech. Dev. 2000;92:227–237. doi: 10.1016/s0925-4773(00)00240-9. [DOI] [PubMed] [Google Scholar]
- Moghal N, Sternberg PW. The epidermal growth factor system in Caenorhabditis elegans. Exp. Cell Res. 2003;284:150–159. doi: 10.1016/s0014-4827(02)00097-6. [DOI] [PubMed] [Google Scholar]
- Montero JA, Carvalho L, Wilsch-Brauninger M, Kilian B, Mustafa C, Heisenberg CP. Shield formation at the onset of zebrafish gastrulation. Development. 2005;132:1187–1198. doi: 10.1242/dev.01667. [DOI] [PubMed] [Google Scholar]
- Moon RT, Campbell RM, Christian JL, McGrew LL, Shih J, Fraser S. Xwnt-5A: a maternal Wnt that affects morphogenetic movements after overexpression in embryos of Xenopus laevis. Development. 1993;119:97–111. doi: 10.1242/dev.119.1.97. [DOI] [PubMed] [Google Scholar]
- Nagel M, Winklbauer R. Establishment of substratum polarity in the blastocoel roof of the Xenopus embryo. Development. 1999;126:1975–1984. doi: 10.1242/dev.126.9.1975. [DOI] [PubMed] [Google Scholar]
- Nagel M, Tahinci E, Symes K, Winklbauer R. Guidance of mesoderm cell migration in the Xenopus gastrula requires PDGF signaling. Development. 2004;131:2727–2736. doi: 10.1242/dev.01141. [DOI] [PubMed] [Google Scholar]
- Nie S, Chang C. Regulation of early Xenopus development by ErbB signaling. Dev. Dyn. 2006;235:301–314. doi: 10.1002/dvdy.20623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt SL, Dingwell KS, Holt CE, Amaya E. Xenopus Sprouty2 inhibits FGF-mediated gastrulation movements but does not affect mesoderm induction and patterning. Genes Dev. 2001;15:1152–1166. doi: 10.1101/gad.191301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olayioye MA, Neve RM, Lane HA, Hynes NE. The ErbB signaling network: receptor heterodimerization in development and cancer. EMBO J. 2000;19:3159–3167. doi: 10.1093/emboj/19.13.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park E, Kim GH, Choi SC, Han JK. Role of PKA as a negative regulator of PCP signaling pathway during Xenopus gastrulation move ments. Dev. Biol. 2006;292:344–357. doi: 10.1016/j.ydbio.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Penzo-Mendez A, Umbhauer M, Djiane A, Boucaut JC, Riou JF. Activation of Gβγ signaling downstream of Wnt-11/Xfz-7 regulates Cdc42 activity during Xenopus gastrulation. Dev. Biol. 2003;257:302–314. doi: 10.1016/s0012-1606(03)00067-8. [DOI] [PubMed] [Google Scholar]
- Playford MP, Schaller MD. The interplay between Src and integrin in normal and tumor biology. Oncogene. 2004;23:7928–7946. doi: 10.1038/sj.onc.1208080. [DOI] [PubMed] [Google Scholar]
- Qian X, Karpova T, Sheppard AM, McNally J, Lowy DR. E-cadherin-mediated adhesion inhibits ligand-dependent activation of diverse receptor tyrosine kinases. EMBO J. 2004;23:1739–1748. doi: 10.1038/sj.emboj.7600136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos JW, Whittaker CA, DeSimone DW. Integrin-dependent adhesive activity is spatially controlled by inductive signals at gastrulation. Development. 1996;122:2873–2883. doi: 10.1242/dev.122.9.2873. [DOI] [PubMed] [Google Scholar]
- Ren R, Nagel M, Tahinci E, Winklbauer R, Symes K. Migrating anterior mesoderm cells and intercalating trunk mesoderm cells have distinct responses to Rho and Rac during Xenopus gastrulation. Dev. Dyn. 2006;235:1090–1099. doi: 10.1002/dvdy.20711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasai N, Nakazawa Y, Haraguchi T, Sasai Y. The neurotrophin-receptor-related protein NRH1 is essential for convergent extension movements. Nat. Cell Biol. 2004;6:741–748. doi: 10.1038/ncb1158. [DOI] [PubMed] [Google Scholar]
- Settleman J. Rac 'n Rho: the music that shapes a developing embryo. Dev. Cell. 2001;1:321–331. doi: 10.1016/s1534-5807(01)00053-3. [DOI] [PubMed] [Google Scholar]
- Sewell JM, Smyth JF, Langdon SP. Role of TGFα stimulation of the ERK, PI3 kinase and PLCγ pathways in ovarian cancer growth and migration. Exp. Cell Res. 2005;304:305–316. doi: 10.1016/j.yexcr.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Shih J, Keller R. Cell motility driving mediolateral intercalation in explants of Xenopus laevis. Development. 1992;116:901–914. doi: 10.1242/dev.116.4.901. [DOI] [PubMed] [Google Scholar]
- Shilo BZ. Signaling by the Drosophila epidermal growth factor receptor pathway during development. Exp. Cell Res. 2003;284:140–149. doi: 10.1016/s0014-4827(02)00094-0. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Yabe T, Muraoka O, Yonemura S, Aramaki S, Hatta K, Bae YK, Nojima H, Hibi M. E-cadherin is required for gastrulation cell movements in zebrafish. Mech. Dev. 2005;122:747–763. doi: 10.1016/j.mod.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Sivak JM, Petersen LF, Amaya E. FGF signal interpretation is directed by Sprouty and Spred proteins during mesoderm formation. Dev. Cell. 2005;8:689–701. doi: 10.1016/j.devcel.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Smith JC, Symes K, Hynes RO, DeSimone DW. Mesoderm induction and the control of gastrulation in Xenopus laevis: the roles of fibronectin and integrins. Development. 1990;108:229–238. doi: 10.1242/dev.108.2.229. [DOI] [PubMed] [Google Scholar]
- Solnica-Krezel L. Conserved patterns of cell movements during vertebrate gastrulation. Curr. Biol. 2005;15:R213–R228. doi: 10.1016/j.cub.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Sokol SY. Analysis of Dishevelled signaling pathways during Xenopus development. Curr. Biol. 1996;6:1456–1467. doi: 10.1016/s0960-9822(96)00750-6. [DOI] [PubMed] [Google Scholar]
- Spencer KSR, Graus-Porta D, Leng J, Hynes NE, Klemke RL. ErbB2 is necessary for induction of carcinoma cell invasion by ErbB family receptor tyrosine kinases. J. Cell Biol. 2000;148:385–397. doi: 10.1083/jcb.148.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symes K, Mercola M. Embryonic mesoderm cells spread in response to platelet-derived growth factor and signaling by phosphatidylinositol 3-kinase. Proc. Natl. Acad. Sci. U. S. A. 1996;93:9641–9644. doi: 10.1073/pnas.93.18.9641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada M, Smith JC. Xwnt11 is a target of Xenopus Brachyury: regulation of gastrulation movements via Dishevelled, but not through the canonical Wnt pathway. Development. 2000;127:2227–2238. doi: 10.1242/dev.127.10.2227. [DOI] [PubMed] [Google Scholar]
- Tahinci E, Symes K. Distinct functions of Rho and Rac are required for convergent extension during Xenopus gastrulation. Dev. Biol. 2003;259:318–335. doi: 10.1016/s0012-1606(03)00206-9. [DOI] [PubMed] [Google Scholar]
- Ulrich F, Krieg M, Schotz EM, Link V, Castanon I, Schnabel V, Taubenberger A, Mueller D, Puech PH, Heisenberg CP. Wnt11 functions in gastrulation by controlling cell cohesion through Rab5c and E-cadherin. Dev. Cell. 2005;9:555–564. doi: 10.1016/j.devcel.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Wacker S, Brodbeck A, Lemaire P, Niehrs C, Winklbauer R. Patterns and control of cell motility in the Xenopus gastrula. Development. 1998;125:1931–1942. doi: 10.1242/dev.125.10.1931. [DOI] [PubMed] [Google Scholar]
- Wacker S, Grimm K, Joos T, Winklbauer R. Development and control of tissue separation at gastrulation in Xenopus. Dev. Biol. 2000;224:428–439. doi: 10.1006/dbio.2000.9794. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Rowning BA, Vogeli KM, Rothbacher U, Fraser SE, Harland RM. Dishevelled controls cell polarity during Xenopus gastrulation. Nature. 2000;405:81–85. doi: 10.1038/35011077. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Fraser SE, Harland RM. Convergent extension: the molecular control of polarized cell movement during embryonic develop ment. Dev. Cell. 2002;2:695–706. doi: 10.1016/s1534-5807(02)00197-1. [DOI] [PubMed] [Google Scholar]
- Winklbauer R. Mesodermal cell migration during Xenopus gastrulation. Dev. Biol. 1990;142:155–168. doi: 10.1016/0012-1606(90)90159-g. [DOI] [PubMed] [Google Scholar]
- Winklbauer R, Keller RE. Fibronectin, mesoderm migration, and gastrulation in Xenopus. Dev. Biol. 1996;177:413–426. doi: 10.1006/dbio.1996.0174. [DOI] [PubMed] [Google Scholar]
- Winklbauer R, Nagel M. Directional mesodermal cell migration in the Xenopus gastrula. Dev. Biol. 1991;148:573–589. doi: 10.1016/0012-1606(91)90275-8. [DOI] [PubMed] [Google Scholar]
- Winklbauer R, Selchow A. Motile behavior and protrusive activity of migratory mesoderm cells from the Xenopus gastrula. Dev. Biol. 1992;150:335–351. doi: 10.1016/0012-1606(92)90246-d. [DOI] [PubMed] [Google Scholar]
- Winklbauer R, Selchow A, Nagel M, Angres B. Cell interaction and its role in mesoderm cell migration during Xenopus gastrulation. Dev. Dyn. 1992;195:290–302. doi: 10.1002/aja.1001950407. [DOI] [PubMed] [Google Scholar]
- Winklbauer R, Nagel M, Selchow A, Wacker S. Mesoderm migration in the Xenopus gastrula. Int. J. Dev. Biol. 1996;40:305–311. [PubMed] [Google Scholar]
- Winklbauer R, Medina A, Swain RK, Steinbeisser H. Frizzled-7 signalling controls tissue separation during Xenopus gastrulation. Nature. 2001;413:856–860. doi: 10.1038/35101621. [DOI] [PubMed] [Google Scholar]
- Woodring PJ, Hunter T, Wang JYJ. Regulation of F-actin-dependent processes by the Abl family tyrosine kinases. J. Cell Sci. 2003;116:2613–2626. doi: 10.1242/jcs.00622. [DOI] [PubMed] [Google Scholar]
- Yamanaka H, Moriguchi T, Masuyama N, Kusakabe M, Hanafusa H, Takada R, Takada S, Nishida E. JNK functions in the non-canonical Wnt pathway to regulate convergent extension movements in vertebrates. EMBO Rep. 2002;3:69–75. doi: 10.1093/embo-reports/kvf008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarden Y, Sliwkowski MX. Untangling the ErbB signaling network. Nat. Rev., Mol. Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- Yokota C, Kofron M, Zuck M, Houston DW, Isaacs H, Asashima M, Wylie CC, Heasman J. A novel role for a nodal-related protein: Xnr3 regulates convergent extension movements via the FGF receptor. Development. 2003;130:2199–2212. doi: 10.1242/dev.00434. [DOI] [PubMed] [Google Scholar]
- Zhong Y, Brieher WM, Gumbiner BM. Analysis of C-cadherin regulation during tissue morphogenesis with an activating antibody. J. Cell Biol. 1999;144:351–359. doi: 10.1083/jcb.144.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zohn IE, Li Y, Skolnik EY, Anderson KV, Han J, Niswander L. p38 and a p38-interacting protein are critical for downregulation of E-cadherin during mouse gastrulation. Cell. 2006;125:957–969. doi: 10.1016/j.cell.2006.03.048. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.