Abstract
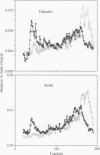
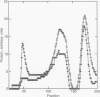
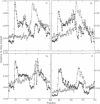
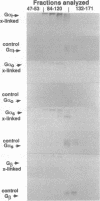
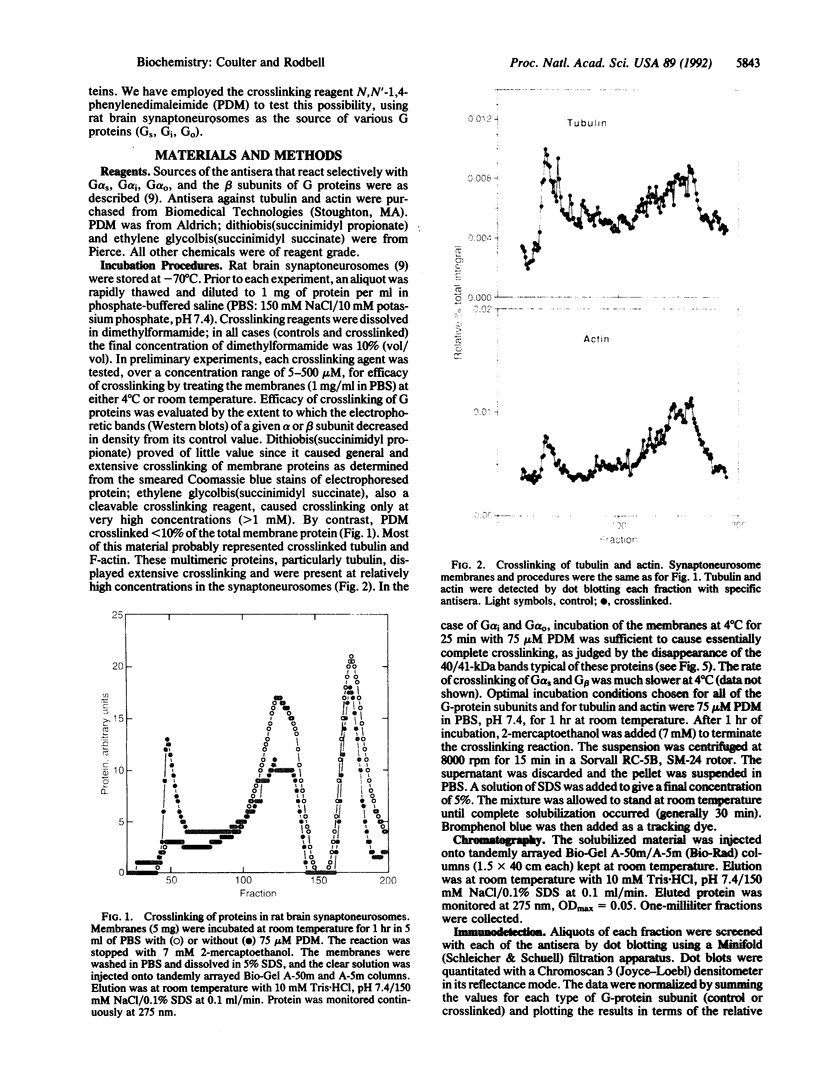
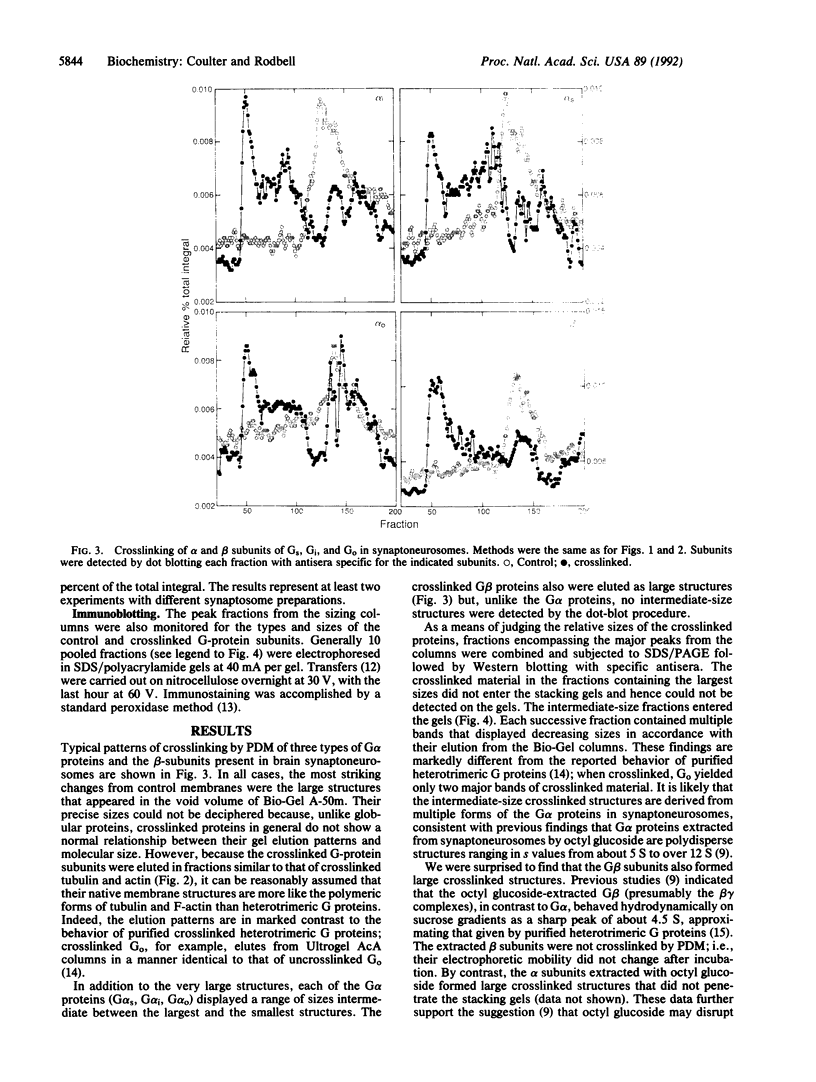
We have treated rat brain synaptoneurosomes with the crosslinking agent N,N'-1,4-phenylenedimaleimide under conditions that cause extensive crosslinking of tubulin, F-actin, and the alpha and beta subunits of three major types of heterotrimeric GTP-binding regulatory proteins (G(o), Gs, Gi) present in brain membranes. The major crosslinked products are coeluted from Bio-Gel sizing columns as very large structures that do not penetrate stacking gels during SDS/PAGE. The alpha subunits but not the beta subunits of Gs, G(o) and Gi also yield crosslinked products of intermediate sizes. None of the products are as small as the heterotrimeric G proteins extracted from brain by cholate or Lubrol. However, the large and intermediate crosslinked structures are strikingly similar to the large, polydisperse structures of the alpha subunits of Gs, Gi, and G(o) extracted from synaptoneurosomes by the detergent octyl glucoside, which have sedimentation properties of multimeric proteins. Several ways in which multimeric forms of G proteins can explain the dynamic and pleiotropic actions of hormones and GTP on signal-transducing systems are discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abou-Samra A. B., Jüppner H., Force T., Freeman M. W., Kong X. F., Schipani E., Urena P., Richards J., Bonventre J. V., Potts J. T., Jr Expression cloning of a common receptor for parathyroid hormone and parathyroid hormone-related peptide from rat osteoblast-like cells: a single receptor stimulates intracellular accumulation of both cAMP and inositol trisphosphates and increases intracellular free calcium. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2732–2736. doi: 10.1073/pnas.89.7.2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlier M. F. Actin polymerization and ATP hydrolysis. Adv Biophys. 1990;26:51–73. doi: 10.1016/0065-227x(90)90007-g. [DOI] [PubMed] [Google Scholar]
- Carlier M. F., Didry D., Simon C., Pantaloni D. Mechanism of GTP hydrolysis in tubulin polymerization: characterization of the kinetic intermediate microtubule-GDP-Pi using phosphate analogues. Biochemistry. 1989 Feb 21;28(4):1783–1791. doi: 10.1021/bi00430a054. [DOI] [PubMed] [Google Scholar]
- Cerione R. A., Kroll S., Rajaram R., Unson C., Goldsmith P., Spiegel A. M. An antibody directed against the carboxyl-terminal decapeptide of the alpha subunit of the retinal GTP-binding protein, transducin. Effects on transducin function. J Biol Chem. 1988 Jul 5;263(19):9345–9352. [PubMed] [Google Scholar]
- Chabre M., Bigay J., Bruckert F., Bornancin F., Deterre P., Pfister C., Vuong T. M. Visual signal transduction: the cycle of transducin shuttling between rhodopsin and cGMP phosphodiesterase. Cold Spring Harb Symp Quant Biol. 1988;53(Pt 1):313–324. doi: 10.1101/sqb.1988.053.01.038. [DOI] [PubMed] [Google Scholar]
- Codina J., Carty D. J., Birnbaumer L., Iyengar R. Purification of G proteins. Methods Enzymol. 1991;195:177–188. doi: 10.1016/0076-6879(91)95164-f. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Gudermann T., Birnbaumer M., Birnbaumer L. Evidence for dual coupling of the murine luteinizing hormone receptor to adenylyl cyclase and phosphoinositide breakdown and Ca2+ mobilization. Studies with the cloned murine luteinizing hormone receptor expressed in L cells. J Biol Chem. 1992 Mar 5;267(7):4479–4488. [PubMed] [Google Scholar]
- Hingorani V. N., Tobias D. T., Henderson J. T., Ho Y. K. Chemical cross-linking of bovine retinal transducin and cGMP phosphodiesterase. J Biol Chem. 1988 May 15;263(14):6916–6926. [PubMed] [Google Scholar]
- Ho Y. K., Hingorani V. N., Navon S. E., Fung B. K. Transducin: a signaling switch regulated by guanine nucleotides. Curr Top Cell Regul. 1989;30:171–202. doi: 10.1016/b978-0-12-152830-0.50008-6. [DOI] [PubMed] [Google Scholar]
- Iwanij V., Vincent A. C. Characterization of the glucagon receptor and its functional domains using monoclonal antibodies. J Biol Chem. 1990 Dec 5;265(34):21302–21308. [PubMed] [Google Scholar]
- Iyengar R., Birnbaumer L. Roles of G proteins and G protein subunits in signal transduction. Lymphokine Res. 1990 Winter;9(4):533–537. [PubMed] [Google Scholar]
- Iyengar R., Rich K. A., Herberg J. T., Premont R. T., Codina J. Glucagon receptor-mediated activation of Gs is accompanied by subunit dissociation. J Biol Chem. 1988 Oct 25;263(30):15348–15353. [PubMed] [Google Scholar]
- Krupinski J., Coussen F., Bakalyar H. A., Tang W. J., Feinstein P. G., Orth K., Slaughter C., Reed R. R., Gilman A. G. Adenylyl cyclase amino acid sequence: possible channel- or transporter-like structure. Science. 1989 Jun 30;244(4912):1558–1564. doi: 10.1126/science.2472670. [DOI] [PubMed] [Google Scholar]
- Kühn H. Light-regulated binding of rhodopsin kinase and other proteins to cattle photoreceptor membranes. Biochemistry. 1978 Oct 17;17(21):4389–4395. doi: 10.1021/bi00614a006. [DOI] [PubMed] [Google Scholar]
- Lad P. M., Welton A. F., Rodbell M. Evidence for distinct guanine nucleotide sites in the regulation of the glucagon receptor and of adenylate cyclase activity. J Biol Chem. 1977 Sep 10;252(17):5942–5946. [PubMed] [Google Scholar]
- Levitzki A., Bar-Sinai A. The regulation of adenylyl cyclase by receptor-operated G proteins. Pharmacol Ther. 1991;50(3):271–283. doi: 10.1016/0163-7258(91)90045-n. [DOI] [PubMed] [Google Scholar]
- Muntz K. H., Sternweis P. C., Gilman A. G., Mumby S. M. Influence of gamma subunit prenylation on association of guanine nucleotide-binding regulatory proteins with membranes. Mol Biol Cell. 1992 Jan;3(1):49–61. doi: 10.1091/mbc.3.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S., Rodbell M. Glucagon induces disaggregation of polymer-like structures of the alpha subunit of the stimulatory G protein in liver membranes. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7150–7154. doi: 10.1073/pnas.88.16.7150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S., Rodbell M. Octyl glucoside extracts GTP-binding regulatory proteins from rat brain "synaptoneurosomes" as large, polydisperse structures devoid of beta gamma complexes and sensitive to disaggregation by guanine nucleotides. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6413–6417. doi: 10.1073/pnas.87.16.6413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendell M. S., Rodbell M., Berman M. Activation of hepatic adenylate cyclase by guanyl nucleotides. Modeling of the transient kinetics suggests an "excited" state of GTPase is a control component of the system. J Biol Chem. 1977 Nov 25;252(22):7909–7912. [PubMed] [Google Scholar]
- Rodbell M. The role of GTP-binding proteins in signal transduction: from the sublimely simple to the conceptually complex. Curr Top Cell Regul. 1992;32:1–47. doi: 10.1016/b978-0-12-152832-4.50003-3. [DOI] [PubMed] [Google Scholar]
- Rodbell M. The role of hormone receptors and GTP-regulatory proteins in membrane transduction. Nature. 1980 Mar 6;284(5751):17–22. doi: 10.1038/284017a0. [DOI] [PubMed] [Google Scholar]
- Roof D. J., Korenbrot J. I., Heuser J. E. Surfaces of rod photoreceptor disk membranes: light-activated enzymes. J Cell Biol. 1982 Nov;95(2 Pt 1):501–509. doi: 10.1083/jcb.95.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegel W., Cooper D. M., Rodbell M. Inhibition and activation of fat cell adenylate cyclase by GTP is mediated by structures of different size. Arch Biochem Biophys. 1980 May;201(2):678–682. doi: 10.1016/0003-9861(80)90559-7. [DOI] [PubMed] [Google Scholar]
- Schlegel W., Kempner E. S., Rodbell M. Activation of adenylate cyclase in hepatic membranes involves interactions of the catalytic unit with multimeric complexes of regulatory proteins. J Biol Chem. 1979 Jun 25;254(12):5168–5176. [PubMed] [Google Scholar]
- Simon M. I., Strathmann M. P., Gautam N. Diversity of G proteins in signal transduction. Science. 1991 May 10;252(5007):802–808. doi: 10.1126/science.1902986. [DOI] [PubMed] [Google Scholar]
- Ting T. D., Ho Y. K. Molecular mechanism of GTP hydrolysis by bovine transducin: pre-steady-state kinetic analyses. Biochemistry. 1991 Sep 17;30(37):8996–9007. doi: 10.1021/bi00101a013. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaillancourt R. R., Dhanasekaran N., Johnson G. L., Ruoho A. E. 2-Azido-[32P]NAD+, a photoactivatable probe for G-protein structure: evidence for holotransducin oligomers in which the ADP-ribosylated carboxyl terminus of alpha interacts with both alpha and gamma subunits. Proc Natl Acad Sci U S A. 1990 May;87(10):3645–3649. doi: 10.1073/pnas.87.10.3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi F., Denker B. M., Neer E. J. Structural and functional studies of cross-linked Go protein subunits. J Biol Chem. 1991 Feb 25;266(6):3900–3906. [PubMed] [Google Scholar]