Abstract
Ethylene plays an essential role in many biological processes including fruit ripening via modulation of ethylene signaling pathway. Ethylene Response Factors (ERFs) are key transcription factors (TFs) involved in ethylene perception and are divided into AP2, RAV, ERF, and DREB sub-families. Although a number of studies have implicated the involvement of DREB sub-family genes in stress responses, little is known about their roles in fruit ripening. In this study, we identified a DREB TF with a EAR motif, designated as MaDEAR1, which is a nucleus-localized transcriptional repressor. Expression analysis indicated that MaDEAR1 expression was repressed by ethylene, with reduced levels of histone H3 and H4 acetylation at its regulatory regions during fruit ripening. In addition, MaDEAR1 promoter activity was also suppressed in response to ethylene treatment. More importantly, MaDEAR1 directly binds to the DRE/CRT motifs in promoters of several cell wall-modifying genes including MaEXP1/3, MaPG1, MaXTH10, MaPL3, and MaPME3 associated with fruit softening during ripening and represses their activities. These data suggest that MaDEAR1 acts as a transcriptional repressor of cell wall-modifying genes, and may be negatively involved in ethylene-mediated ripening of banana fruit. Our findings provide new insights into the involvement of DREB TFs in the regulation of fruit ripening.
Keywords: banana (Musa acuminata), cell wall modification, DREB, fruit ripening, transcriptional repression
Introduction
The phytohormone ethylene plays an essential role in many biological processes of plant growth and development, including germination, organ senescence, stress response, and fruit ripening (Salehin and Estelle, 2015). The ethylene signaling pathway is well studied in Arabidopsis, which reveals a linear transduction pathway with the transduction of ethylene signal from receptors to dedicated transcription factors (TFs) (Pirrello et al., 2012). The last components of the ethylene signaling pathway are the Ethylene Response Factor (ERF) TFs which possess a highly conserved DNA-binding domain called the APETALA2/ethylene-responsive element binding (AP2/ERF) domain (Ohme-Takagi and Shinshi, 1995).
The AP2/ERF proteins are divided into four major sub-families, namely the AP2, related to ABI3/VP1 (RAV), ERF and dehydration-responsive element-binding protein (DREB), according to the number and similarity of the AP2/ERF domains (Sakuma et al., 2002). DREB TFs, as a sub-family of the AP2/ERF proteins, were first isolated using an 6-bp conserved sequence (A/GCCGAC), named the dehydration responsive element (DRE), in yeast one-hybrid screening in Arabidopsis cDNA (Stockinger et al., 1997; Liu et al., 1998). Extensive studies have established important regulatory roles for DREB TFs in response to environmental stimuli. For example, in Arabidopsis, DREB1A was induced by cold, while DREB2 like genes (DREB2A and DREB2B) were induced by drought, salt and heat (Nakashima et al., 2000). By contrast, other DREB1-related genes such as DREB1D regulate high osmotic stress-induced gene expression (Haake et al., 2002), whereas DREB1E and DREB1F are responsive to high salinity (Mizoi et al., 2012). Except for these transcriptional activators, several members of DREB TFs with ERF-associated amphiphilic repression (EAR) motif at C-terminus act as transcriptional repressors of stress responses (Ohta et al., 2001; Kagale and Rozwadowski, 2010). These EAR motif-containing DREB repressors negatively modulate the responses of plants to cold and dehydration, as are the cases of DEAR1 (Tsutsui et al., 2009), RAP2.1 (Dong and Liu, 2010), and GhDREB (Gao et al., 2009). Despite these findings, less is known about the functions of these proteins in agricultural crops, especially in relation to natural processes like fruit ripening where ethylene plays a major role.
Banana is one of the most important fruit species in tropical and sub-tropical countries, ranking as the world’s second largest fruit crop and listing among the world’s ten most important food commodities (Sreedharan et al., 2012). Banana is a typical climacteric fruit, characterized by a burst in respiration and a typical increase in ethylene biosynthesis that initiates ripening-associated processes. This, from an economic perspective, limits fruit shelf-life with rapid deterioration of peel color and pulp firmness (Ba et al., 2016). For example, ripened bananas become unmarketable within 1–3 days at ambient temperature (Ahmed and Palta, 2016). Although numerous post-harvest practices such as low temperature storage, thermal processing, chemical, and biological treatments coupled with other preservation techniques are applied on fresh produces to maintain or extend the shelf-life, severe post-harvest losses still occur (Kuan et al., 2015). Therefore, a better understanding of the regulators involved in banana fruit ripening will help develop more effective post-harvest storage technologies. Since bananas are climacteric fruits, considerable effort has been directed to study genes involved in ethylene biosynthesis and signaling pathways including 1-aminocyclopropane-1-carboxylic acid (ACC) synthase (ACS), ACC oxidase (ACO), ethylene receptor, CTR1 ortholog, ethylene insensitive3 (EIN3)/EIN3-like (EIL), EIN3 binding F-box (EBF) and ERF genes (Liu et al., 1999; Mbéguié-A-Mbéguié et al., 2008; Kuang et al., 2013; Xiao et al., 2013; Jourda et al., 2014). Interestingly, opposing functions have been reported for banana ERF genes. For instance, among the fifteen ERF TFs that have been isolated from banana fruit, MaERF11 binds to MaACS1 and MaACO1 promoters to suppress their activities whereas MaERF9 activates MaACO1 promoter activity (Xiao et al., 2013). Whilst DREB and ERF TFs belong to the AP2/ERF families, little is known about DREBs role in fruit ripening, especially those with EAR motif.
In this study, we identified a DREB TF with EAR motif, designated as MaDEAR1, which is a nucleus-localized transcriptional repressor. MaDEAR1 was ethylene- and ripening-inhibited, with reduced levels of histone H3 and H4 acetylation at its regulatory regions during fruit ripening. More importantly, MaDEAR1 binds to and represses promoters of several cell wall-modifying genes associated with fruit softening, including expansins (MaEXP1/3), polygalacturonase (MaPG1), xyloglucan endotransglycosylase/hydrolase (MaXTH10), pectate lyase (MaPL3), and pectin methylesterase (MaPME3). Our results suggest that MaDEAR1 may be acting as a negative regulator of cell wall-modifying genes, unraveling new information on EAR motif-containing DREB TFs in relation to fruit ripening.
Materials and Methods
Plant Materials and Treatments
Pre-climacteric banana (Musa acuminata, AAA group, cv. Cavendish) fruit at the 70–80% plump stage were obtained from a local commercial plantation near Guangzhou, southern China. Harvested fruit were separated into fingers, and fruit of uniform weight, shape, and maturity with no visual defects were selected, rinsed in tap water, and then air-dried before treatments were applied. The post-harvest treatments include a control (natural ripening), ethylene-induced ripening (100 μL L-1 ethylene, 18 h), and 1-methylcyclopropene (1-MCP)-delayed ripening (0.5 μL L-1 1-MCP, 18 h), as described previously by Shan et al. (2012). All assessments were conducted using three biological replicates and fruit pulp of all samples were frozen in liquid nitrogen immediately after sampling, and stored at –80°C for further use.
Tobacco bright yellow 2 (BY-2) suspension cells were cultured and prepared as described by Kumagai-Sano et al. (2006). Tobacco (Nicotiana benthamiana) plants were grown under a 16-h light (25°C) and 8-h dark (22°C) photoperiod. Four- to six-week-old tobacco plants were used for transient assays.
RNA Extraction, Gene Isolation, and Sequence Analysis
Frozen banana fruit pulp were ground in liquid nitrogen using a mortar and pestle. Total RNA was extracted using the hot borate method described by Wan and Wilkins (1994). Total RNA (∼1 μg) from each sample was treated with DNAse I digestion using an RNAse-free kit (Promega Madison, Fitchburg, WI, USA). The above DNA-free total RNA was then used as template for RT-PCR. The first-strand cDNA of the product was subjected to PCR amplification. According to gene annotation, bioinformatics and RNA sequencing analyses (D’Hont et al., 2012), one full-length DREB gene containing an EAR motif, with complete start and stop codons, termed MaDEAR1 (GSMUA_Achr3T13190_001 in Banana Genome Hub, XP_009392127 in NCBI), was identified and selected from banana whole-genome sequence. This segment was cloned and sequenced. Alignments were carried out on ClustalX (version 1.83) and GeneDoc software, and a phylogenetic tree was constructed using the Neighbor–Joining method in the MEGA5 program.
Quantitative Real-Time PCR (qRT-PCR) Analysis
All qRT-PCR analysis and synthesis of first-strand cDNA were performed as described previously (Chen et al., 2011; Shan et al., 2012). The sequences of all primers used for qRT-PCR analysis are listed in Supplementary Table S1. qRT-PCR was carried out on a Bio-Rad CFX96 Real-Time PCR System using the SYBR®Green PCR Supermix Kit (Bio-Rad Laboratories) following the manufacturer’s instructions. MaRPS2 (ribosomal protein 2) was selected as a reference gene according to our previous study on the selection of reliable reference genes under different experimental conditions (Chen et al., 2011). All qRT-PCR reactions were normalized using Ct value corresponding to the reference gene. The relative expression levels of target gene were calculated with the formula 2-ΔΔCT. Three independent biological replicates were used in the analysis.
Sub-cellular Localization of MaDEAR1 Protein
The coding sequences of MaDEAR1 were amplified and cloned into the pEAQ-GFP vectors (kindly gifted by Dr. George P. Lomonossoff), then the fusion construct and positive control GFP vector were electroporated into the Agrobacterium tumefaciens strain GV3101 using Gene PulserXcellTM Electroporation Systems (Bio-Rad, Hercules, CA, USA). The primers for construct development are listed in Supplementary Table S1. The Agrobacterium harboring MaDEAR1-GFP or the positive control was inoculated for 16 h at 28°C. Cells were pelleted, resuspended at OD600 = 0.1 in infiltration buffer [10 mM MgCl2, 10 mM MES (pH 5.6), 100 μM acetosyringone], incubated for 4 h at room temperature, then was infiltrated into the abaxial side of 4- to 6-week-old tobacco leaves using a 1-mL needleless syringe as described previously by Sainsbury et al. (2009). Two days after infiltration, GFP fluorescence signals were observed by a fluorescence microscope (Zeiss Axioskop 2 Plus) with a beam splitter for excitation at 500 nm. All assays were repeated at least three times.
Promoter Isolation and Analysis
Genomic DNA was extracted from banana leaves using the DNeasy Plant Mini Kit (Qiagen). The promoters of MaDEAR1, and genes including MaEXP1/3, MaPG1, MaXTH10, MaPL3, and MaPME3 involved in cell wall loosening of banana fruit associated with softening (Pua et al., 2001; Asif and Nath, 2005; Asha et al., 2007; Mbéguié-A-Mbéguié et al., 2009; Asif et al., 2014), were isolated using a Genome Walker Kit (Clontech) with nested PCR according to the manufacturer’s instructions (specific primers are listed in Supplementary Table S1). After sequencing, conserved cis-element motifs of promoters were predicted using Plant-CARE1 databases.
Promoter Activity Assay
The MaDEAR1 promoter region was amplified by PCR using the specific primers listed in Supplementary Table S1. The PCR product was inserted into the pGreenII 0800-LUC double reporter vector (Hellens et al., 2005) at the KpnI and NcoI sites to fuse it with the Firefly luciferase (LUC) reporter gene (MaDEAR1 pro-LUC). A Renilla luciferase (REN) under the control of the 35S promoter at the same vector was used as an internal control. The construct CaMV35S-REN/MaDEAR1 pro-LUC (∼20 μg) was transformed into tobacco BY-2 protoplasts (∼2 × 104) by polyethylene glycol (PEG) methods as described previously (Abel and Theologis, 1994).
The promoter activity was assayed according to Ba et al. (2014a). The transformed protoplasts were subjected to 0 mM (control) or 0.8 mM of ethrel (ethylene releaser) treatment and then incubated at 23°C for 14 h, and LUC and REN activities were assayed using the dual luciferase assay kits (Promega), and the promoter activity is indicated by the ratio of LUC to REN. The analysis was carried out using the Luminoskan Ascent Microplate Luminometer (Thermo) according to the manufacturer’s instructions, with a 5-s delay and 15-s integrated measurements. At least six assay measurements were included for each.
Chromatin Immunoprecipitation (ChIP) and Quantitative PCR Analysis
Chromatin immunoprecipitation (ChIP) was performed as described earlier (Gendrel et al., 2005; Benhamed et al., 2006). Unripe and ripe banana fruit pulp were collected and crosslinked in 1% formaldehyde for 15 min in a vacuum and then neutralized by 0.125 M glycine. After washing with sterilized water, 5 g of banana fruit pulp were ground in liquid nitrogen, and suspended in a buffer containing 0.25 M sucrose, 10 mM Tris-HCl (pH 8.0), 10 mM MgCl2, 1% Triton X-100, 5 mM β-mercaptoethanol, 0.1 mM PMSF, and protease inhibitors (one minitablet per milliliter; Roche). The suspensions were transferred to microfuge tubes and centrifuged at 12,000 g for 10 min. The pellets were suspended in 1.7 M sucrose, 10 mM Tris-HCl (pH 8.0), 2 mM MgCl2, 0.15% Triton X-100, 5 mM β-mercaptoethanol, 0.1 mM PMSF, and protease inhibitors and centrifuged through a layer of the same buffer in microfuge tubes. The chromatin extracts were lysed in a buffer containing 50 mM Tris-HCl, pH 8, 10 mM EDTA, 1% SDS, and protease inhibitors, and were sheared to an average length of 500 bp by sonication at 4°C using the Sonics VCX800 apparatus followed by centrifugation. The supernatants were diluted 10-fold with 1% Triton X-100, 1.2 mM EDTA, 16.7 mM Tris-HCl (pH 8.0), and 167 mM NaCl. A 1 mL aliquot of the dilution was immunoprecipitated with specific antibodies of anti-acetyl-histone H3 (anti-H3ac) and anti-acetyl-histone H4 (anti-H4ac) (Millipore), while immunoglobulin G (IgG) was used as a negative control. ChIP assays were repeated with three biological replicates. The DNA cross-linked to immunoprecipitated proteins and input DNA were detected by qRT-PCR. MaACT2 (GSMUA_Achr9G03170_001) was used as internal control since its histone acetylation level is stable during ripening (Han et al., 2016). The percentage of IP/Input was calculated by determining 2-ΔCt (=2-[Ct(IP)-Ct(Input)]). The primers used for ChIP-qPCR analysis are listed in Supplementary Table S1.
Protein Expression and Electrophoretic Mobility Shift Assay (EMSA)
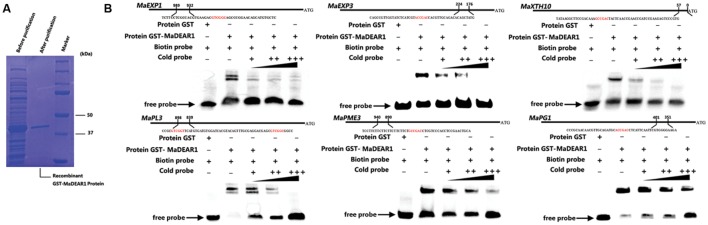
MaDEAR1 was cloned into pGEX-4T-1 (Amersham Biosciences) to fuse in frame with GST and expressed in BM Rosetta (DE3) by induction with 1 mM of isopropyl-β-D-thiogalactopyramoside (IPTG) for 6 h at 30°C. The recombinant protein was purified with Glutathione Sepharose 4B (GE Healthcare). The EMSA was performed using the EMSA kit (Thermo) according to the manufacturer’s instructions. The probes containing the DRE/CRT (core sequence for A/GCCGAC) element (2 × 10-6 μmol) derived from MaEXP1/3, MaPG1, MaXTH10, MaPL3, and MaPME3 promoters were labeled with biotin using DNA 3′ End Biotinylation Kit (Thermo). The same unlabeled DNA fragment with 2 × 10-5 μmol, 2 × 10-4 μmol, or 2 × 10-3 μmol, respectively, was used as a competitor. After cross-linking, the membrane was detected by the chemiluminescence method according to the manufacturer’s protocol on a ChemiDocTM MP Imaging System (Bio-Rad). The primers used in protein expression and EMSA assays are listed in Supplementary Table S1.
Transient Assay in Tobacco Leaves
A dual-luciferase reporter system was used in the transient assay, and all primers used for the following constructs are listed in Supplementary Table S1. For transcriptional activity analysis of MaDEAR1, the coding sequence of MaDEAR1 was inserted into the constructed pBD vector driven by the 35S promoter as the effector, and the double reporter vector includes a native GAL4-LUC, and an internal control REN driven by 35S promoter, which was modified based on pGreenII 0800-LUC reporter vector (Hellens et al., 2005). GAL4-LUC contains five copies of GAL4 binding element and 35S promoter, and these sequences are located upstream of the LUC. For the assay of MaDEAR1 repressing the MaEXP1/3, MaPG1, MaXTH10, MaPL3, and MaPME3 promoters, MaDEAR1 were inserted into the pEAQ vector as effector, while the promoters were cloned into pGreenII 0800-LUC double-reporter vector as reporter.
The constructed effector and reporter plasmids were co-transformed into tobacco leaves by Agrobacterium tumefaciens strain GV3101. LUC and REN luciferase activities were measured as described above. The transcriptional activity of MaDEAR1 and the binding activity of MaDEAR1 to the promoter are indicated by the ratio of LUC to REN. At least six biological replicates were assayed for each combination.
Statistical Analysis
Experiments were conducted using a completely randomized design. Each sample time point for each treatment comprised three independent biological replicates. Data were plotted as means ± standard errors (SE) in figures. Least significant difference (LSD) at the 5% level was estimated using DPS software (version 3.01; Zhejiang University, Hangzhou, China).
Results
MaDEAR1 Is an A-5 Sub-group Member of the DREB Family
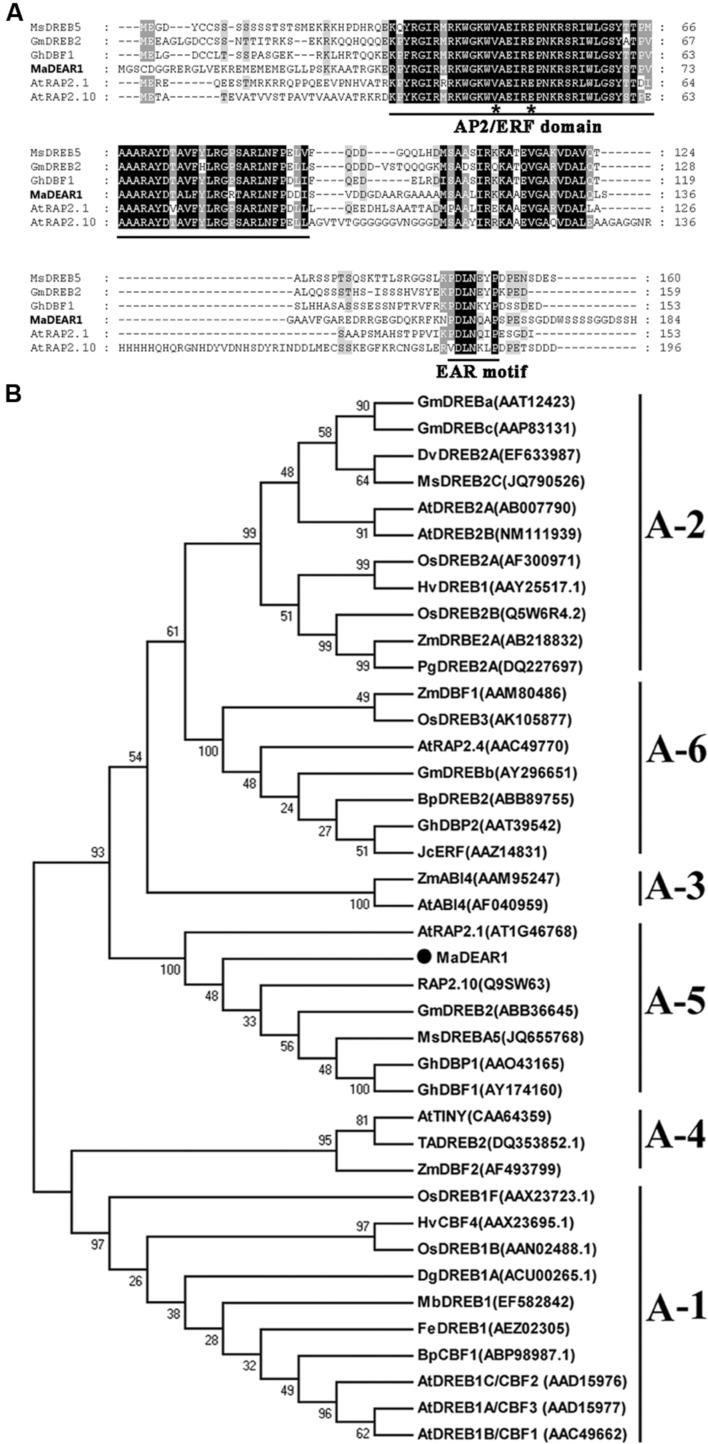
Based on gene annotation, bioinformatics and RNA sequencing analyses (D’Hont et al., 2012), one full-length DREB gene containing an EAR motif, designated as MaDEAR1 (M. acuminata DREB and EAR motif protein 1) (GSMUA_Achr3T13190_001 in Banana Genome Hub, XP_009392127 in NCBI), which was found down-regulated during fruit ripening, did attract our attention. MaDEAR1 encodes a protein of 184 amino acids, with calculated molecular weight of 20.24 kDa and pI value of 10.10. Analysis of deduced amino acid sequence of MaDEAR1 revealed a typical AP2/ERF domain of 58 amino acids with the conserved valine (V) and glutamic acid (E) at the 14th and 19th positions, respectively (Figure 1A), which are considered to be essential sites for the binding of DREBs to the DRE cis-elements (Sakuma et al., 2002). In addition, a repressor domain, the ERF-associated amphiphilic repression (EAR) motif was observed at the C-terminus of the protein (Figure 1A), suggesting that the MaDEAR1 might function as a transcriptional repressor. Phylogenetic analyses showed that DREB proteins can be classified into six sub-groups (A1–A6), in which MaDEAR1 together with AtRAP2.1, GmDREB2, GhDBP1, and MsDREB5 belong to A-5 sub-group (Figure 1B). Collectively, these data suggest that MaDEAR1 is an A-5 sub-group member of the DREB family.
FIGURE 1.
Sequence analysis of MaDEAR1. (A) Alignment of the deduced amino acid sequences of the banana MaDEAR1 and its respective homologs MsDREBA5 (JQ655768), GmDREB2 (DQ208968), AtRAP2.1 (AT1G46768), and AtRAP2.10 (AT4G36900). Identical and similar amino acids were presented by black and gray shading, respectively. The AP2/ERF domain and the EAR-motif (DLNXXP) were underlined. The 14th and 19th amino acids were indicated by asterisks. (B) Phylogenetic analysis of plant DREB family proteins. The multiple alignment was made using ClustalW, and the phylogenetic tree was constructed with MEGA 5.0 using a bootstrap test of phylogeny with minimum evolution test and default parameters. Numbers indicate bootstrap values.
MaDEAR1 Is a Nucleus-Localized Transcriptional Repressor
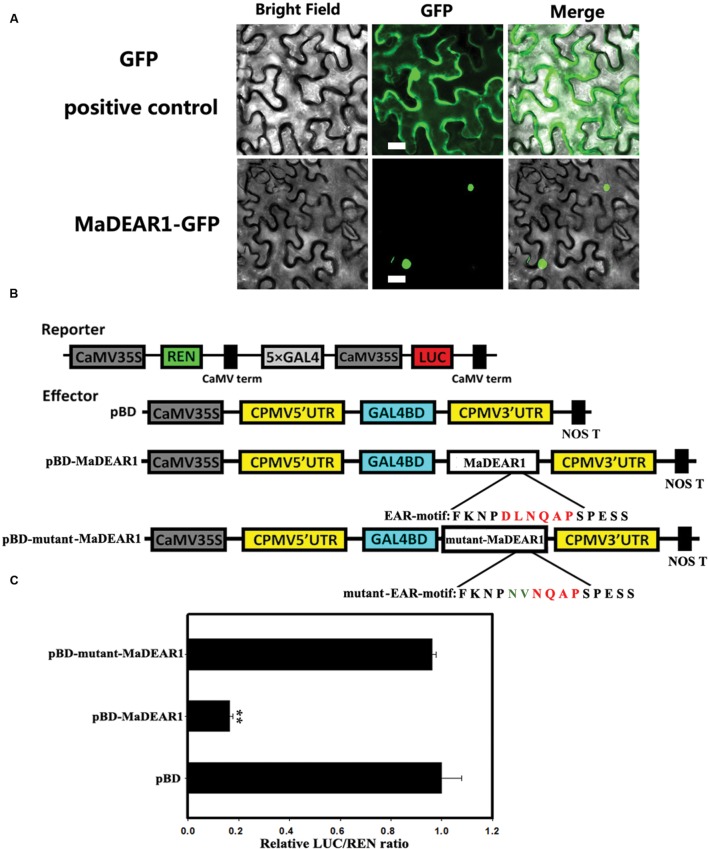
To examine the sub-cellular localization of MaDEAR1 in vivo, we fused the MaDEAR1 coding region in-frame with the N-terminal side of green fluorescent protein (GFP) under the control of CaMV 35S promoter. The MaDEAR1-GFP and GFP control plasmids were transiently expressed in tobacco leaves by Agrobacterium infiltration. While control GFP accumulated in both nucleus and cytoplasm, the MaDEAR1-GFP fusion protein was clearly localized in the nucleus (Figure 2A). This suggests that MaDEAR1, like other reported DREB proteins, is a nuclear protein which is a typical feature of TFs.
FIGURE 2.
Sub-cellular localization and transcriptional repression activity of MaDEAR1. (A) MaDEAR1 is localized in nucleus. Agrobacterium tumefaciens carrying MaDEAR1-GFP or GFP positive control were infiltrated into tobacco leaves. After 48 h, the fluorescence of MaDEAR1 protein was localized exclusively in the nucleus, while the fluorescence of the GFP positive control was distributed in both nucleus and cytoplasm. Bar, 20 μM. (B) Reporter and effector constructs. The dual luciferase reporter construct contained the LUC reporter gene fused with 5 × GAL4 and CaMV35S. The effector plasmid contained the MaDEAR1 gene or with mutant EAR motif fused to GAL4BD driven by the CaMV35S. The two conserved amino acids (DL) of the EAR-motif without or with site-mutation (NV) are also shown. pBD was used as a negative control. (C) Transcriptional repression ability of MaDEAR1 in vivo. Compared with the pBD control, pBD-MaDREB5 significantly repressed the expression of the LUC reporter. The ratio of LUC to REN of the pBD vector was used as a calibrator (set as 1). Each value represents the means of six biological replicates, and vertical bars represent the SE Asterisks indicate a statistically significant difference compared with pBD by Student’s t-test. ∗∗P < 0.01.
To investigate whether MaDEAR1 possesses transcriptional repression activity in vivo, a dual luciferase assay was performed. The dual-luciferase reporter harbors five copies of the GAL4 DNA-binding element and CaMV 35S fused to the firefly luciferase (LUC) reporter, whereas a renilla luciferase (REN) reporter under the control of the 35S promoter was used as an internal control. Full-length MaDEAR1 was fused with GAL4 DNA-binding domain (GAL4-BD) as the effector, and the empty GAL4-BD (pBD-empty) was used as a negative control (Figure 2B). As shown in Figure 2C, compared with the pBD-empty control, pBD-MaDEAR1 significantly repressed the expression of the LUC reporter, with approximately fivefold less LUC/REN value than the control. To further confirm whether the conserved EAR motif (DLNQAP) was important for the MaDEAR1-mediated repression, site-specific mutations were made to convert two conserved amino acids (DL) to NV (Figure 2B). As expected, the transcriptional repression ability of MaDEAR1 was abolished when the EAR-motif was mutated (Figure 2C). These results confirm that MaDEAR1 may act as a transcriptional repressor, and the EAR motif is important for its repression activity.
MaDEAR1 Is Inhibited by Ethylene and Ripening
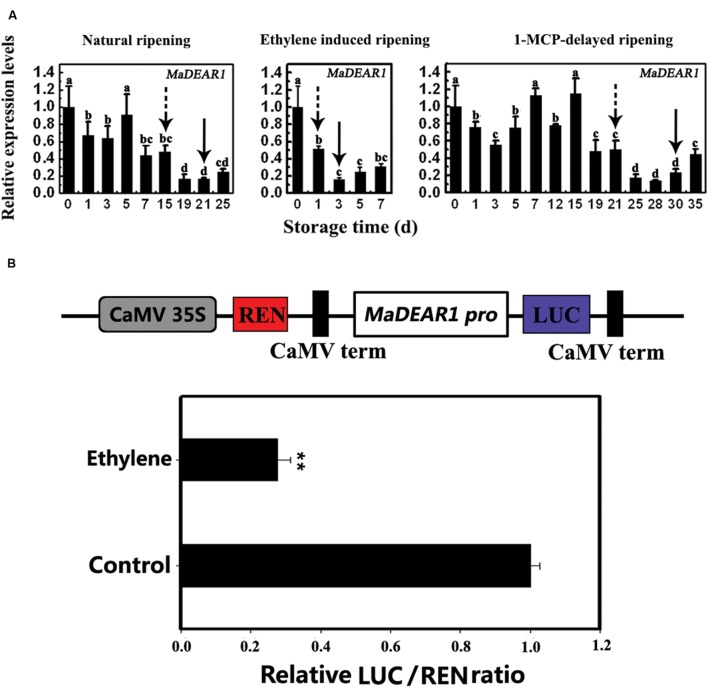
Our previous study showed that fruits in natural ripening group start ethylene production after storage at 22°C and 90% relative humidity for 15 days, which peaks around day-21, and declines thereafter. Ethylene-treated fruit ripened rapidly, with an ethylene peak appearing at day 3 following treatment. In contrast, 1-MCP treatment delayed ripening, with ethylene peaking at day 30 (Shan et al., 2012). To investigate the expression of MaDEAR1 during banana fruit ripening, we performed quantitative RT-PCR analysis using banana fruit in three different ripening behaviors caused by natural, ethylene-induced, and 1-MCP-delayed ripening treatments. As shown in Figure 3A, MaDEAR1 expression was repressed by ethylene, and its transcript level in natural, ethylene-induced or 1-MCP-delayed ripening was decreased following ethylene production appearance, revealing that MaDEAR1 expression was suppressed by ethylene and ripening.
FIGURE 3.
MaDEAR1 is inhibited by ethylene and ripening. (A) Expression of MaDEAR1 in pulp during three ripening conditions, which include natural (control), ethylene-induced, and 1-MCP-delayed ripening. The expression levels of MaDEAR1 are expressed as a ratio relative to the harvest time (0 days of control), which was set at 1. Each value represents the mean ± SE of three biological replicates. Different letters above bars indicate significant difference at the 5% level by Student’s t-test. The broken arrow and full arrow indicate the time point at which ethylene production began to increase and its peak for each treatment, respectively. The physiological data such as changes in fruit firmness and ethylene production during banana fruit ripening and softening have been presented in Shan et al. (2012). (B) MaDEAR1 promoter activity in response to ethylene. The dual luciferase reporter vector containing MaDEAR1 promoter (CaMV35S–REN/MaDEAR1 pro-LUC) was transiently transformed into tobacco BY-2 protoplasts using a modified PEG method, and the transformed protoplasts were subjected to 0 (control) or 0.8 mM ethrel (ethylene releaser) treatment. After incubation for 14 h, LUC and REN luciferase activities were assayed, and the promoter activity is indicated by the ratio of LUC to REN. Each value represents the means of six biological replicates, and vertical bars represent the SE ∗∗P < 0.01 by Student’s t-test.
To better understand the mechanism(s) of MaDEAR1 expression modulation, a 976 bp upstream sequence from the start codon of MaDEAR1 was isolated from the genome of M. acuminata using a genome-walking PCR method. Analysis of the promoter using the PLACE and Plant-CARE databases revealed a site for the ethylene-responsive element (ERE), ATTTCAAA, with one nucleotide change found in the promoter at –507 to –515 bp from the initiation codon (Supplementary Data Sheet S1), indicating that MaDEAR1 promoter might respond to ethylene. We then fused the MaDEAR1 promoter in front of LUC in the dual luciferase reporter vector, while the REN driven by the CaMV 35S promoter at the same vector was used as an internal control (Figure 3B). The resultant vector was transiently expressed in tobacco BY2 protoplasts with or without ethrel treatment, and the luciferases were assayed thereafter. As shown in Figure 3B, after the treatment with ethrel, the promoter activity of MaDEAR1 was dramatically decreased, as evidenced by the much lower relative LUC/REN ratio compared that of the control, indicating that MaDEAR1 promoter activity was suppressed by ethylene.
Decrease Expression of MaDEAR1 Is Correlated with Histone Acetylation Changes during Fruit Ripening
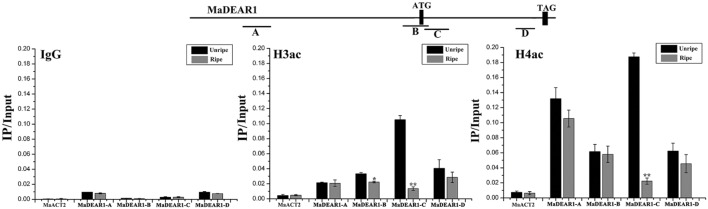
Histone acetylation is a type of chromatin modification facilitating the gene expression, which is closely associated with gene activation. To assess whether the expression of MaDEAR1 during fruit ripening is associated with histone acetylation, we examine the histone acetylation levels of MaDEAR1 in fruit of unripe and ripe stages by ChIP-qPCR assays using antibodies such as anti-acetyl-histone H3 (H3ac) and anti-acetyl-histone H4 (H4ac). As shown in Figure 4, as a negative control, the enrichments of IgG in the promoter and coding region of MaDEAR1 were low. In addition, no significant difference of histone H3 or H4 acetylation levels of MaACT2 between unripe and ripe banana fruit was observed. On the contrary, the histone H3 acetylation (H3ac) levels of MaDEAR1 in region B and C as well as histone H4 acetylation (H4ac) levels of MaDEAR1 in region C were decreased in ripening bananas (Figure 4), which is consistent with its decreased level of expression during ripening (Figure 3A). These findings, together with the observations of its gene expression and promoter activity, suggest that MaDEAR1 is suppressed by ethylene and ripening, and its decreased expression might be associated with reduced levels of histone H3 and H4 acetylation during ripening.
FIGURE 4.
ChIP-qPCR analysis of histone H3 (H3ac) or H4 acetylation (H4ac) levels at the regions of MaDEAR1 in unripe and ripe banana fruit. The four regions of MaDEAR1 (A–D) examined by ChIP-qPCR are shown in the top panel. The amounts of DNA after ChIP were quantified and normalized to MaACT2. IgG and MaACT2 were used as negative controls. Data are shown as the ratio of IP to input (IP/Input). Each experiment was repeated with three biological replicates. Error bars represent SE ∗P < 0.05 and ∗∗P < 0.01 by Student’s t-test, compared with unripe fruit.
Genes Involving in Cell Wall Loosening of Banana Fruit, MaEXP1/3, MaPG1, MaXTH10, MaPL3, and MaPME3, Are Direct Targets of MaDEAR1
To understand the possible roles of MaDEAR1 in banana fruit ripening, we identified the potential targets of MaDEAR1 in relation to fruit ripening. Previous studies indicated that DREB proteins bind to the cis-acting dehydration-responsive element/C-repeat (DRE/CRT) in the promoter of their target genes, including RD29A, RD17, COR15A, ERD10, KIN1, and COR6.6 (Lucas et al., 2011). Softening is an important indicator of fruit ripening, which is related to cell wall modifications, including enzymatic and non-enzymatic degradation of cell wall components (Li et al., 2010). Previously, 23 cell wall-modifying genes, including five EXP, nine XET/XTH, four PG, two PE/PL and three PME have been reported to be related to banana fruit softening (Pua et al., 2001; Asif and Nath, 2005; Asha et al., 2007; Mbéguié-A-Mbéguié et al., 2009; Asif et al., 2014), and we have examined the presence of the DRE/CRT core consensus sequence (A/GCCGAC) in their promoters. It was found that MaEXP1, MaXTH10, MaPME3, MaPG1, and MaPL3 have one or two DRE/CRT elements in their promoters, while MaEXP3 promoter contained four DRE/CRT elements (Supplementary Data Sheet S1), suggesting that these genes might be the targets of MaDEAR1.
To test whether MaDEAR1 could directly bind to these promoters, electrophoretic mobility shift assay (EMSA) was performed. DNA fragments containing the DRE/CRT element in the region of these promoters were used as probe. Recombinant glutathione S-transferase (GST)-MaDEAR1 fusion proteins were expressed in E. coli and purified (Figure 5A). As shown in Figure 4B, MaDEAR1 can directly bind to the DNA probes containing the DRE-motif in MaEXP1/3, MaPG1, MaXTH10, MaPL3, and MaPME3 promoters, and the bindings were abolished by the addition of increasing amounts of unlabeled competitors with the same sequence. In addition, no parallel band shift was detected with only the GST tag (Figure 5B). These results show that MaDEAR1 specifically binds to the DRE motif in the promoters of MaEXP1/3, MaPG1, MaXTH10, MaPL3, and MaPME3, demonstrating that these genes are likely direct targets of MaDEAR1.
FIGURE 5.
MaDEAR1 binds to promoters of cell wall-modifying genes. (A) SDS-PAGE gel stained with Coomassie blue demonstrating affinity purification of the recombinant MaDEAR1 protein used for the electrophoretic mobility shift assay (EMSA). (B) EMSA showing MaDEAR1 binding to the promoter of MaEXP1/3, MaPG1, MaXTH10, MaPL3, and MaPME3 containing DRE/CRT cis-acting element. Biotin-labeled DNA probe from the promoter was incubated with GST-MaDEAR1 protein, and the DNA-protein complexes were separated on 6% native polyacrylamide gels. Triangles indicate increasing amounts of unlabeled probes (2 × 10-5 μmol, 2 × 10-4 μmol, or 2 × 10-3 μmol) for competition.
MaDEAR1 Represses Promoter Activities of MaEXP1/3, MaPG1, MaXTH10, MaPL3, and MaPME3
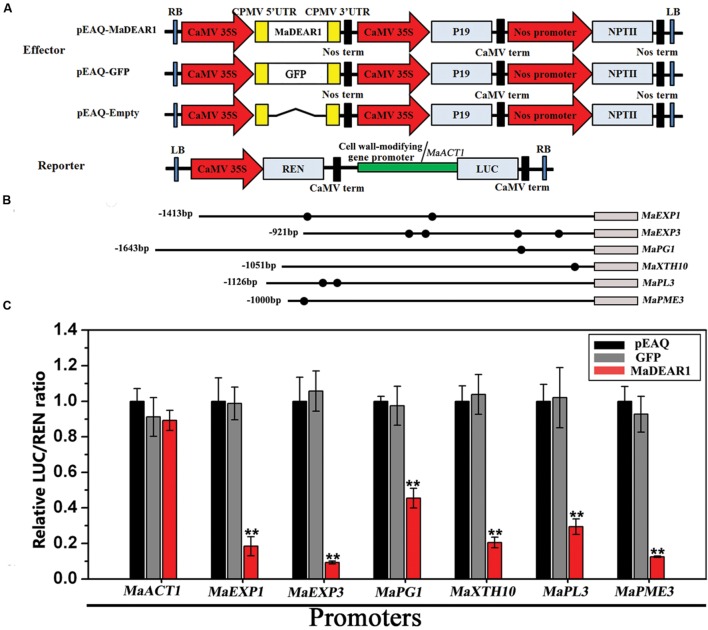
To further understand the regulation of MaEXP1/3, MaPG1, MaXTH10, MaPL3, and MaPME3 by MaDEAR1, we performed transient expression assays using the dual luciferase reporter system. The dual luciferase reporter plasmid harbor MaEXP1/3-, MaPG1-, MaXTH10-, MaPL3-, or MaPME3-promoter fused to LUC, and the REN driven by the CaMV 35S promoter, while an effector plasmid carried MaDEAR1 expressed under the control of the CaMV 35S promoter (Figure 6A). As shown in Figure 6B, compared with the negative controls, tobacco leaves expressing MaDEAR1 showed significantly lower luciferase activity with the constructs harboring the LUC reporter gene driven by the MaEXP1/3-, MaPG1-, MaXTH10-, MaPL3-, or MaPME3-promoter, which is consistent with the finding that MaDEAR1 is a transcriptional repressor (Figure 2B).
FIGURE 6.
Transient dual-luciferase reporter assays showing MaDEAR1’s ability to repress the promoter activity of MaEXP1/3, MaPG1, MaXTH10, MaPL3, or MaPME3. (A) Constructs used in the transient assays. The reporter contained the MaEXP1/3, MaPG1, MaXTH10, MaPL3, or MaPME3 promoters fused to LUC luciferase and REN luciferase driven by CaMV 35S as internal control. The effector contained the MaDEAR1 driven by the CaMV35S. The effector vector also contained the P19 suppressor of gene silencing, and the NPTII kanamycin resistance gene. (B) Schematics of the promoter of MaEXP1/3, MaPG1, MaXTH10, MaPL3, or MaPME3. Promoter length and DRE/CRT cis-acting elements are indicated with lines and black circles, respectively. (C) MaDEAR1 represses the promoter activity of MaEXP1/3, MaPG1, MaXTH10, MaPL3, or MaPME3. Agrobacterium tumefaciens strain GV3101 carrying the LUC reporter plasmid and different combinations of effector plasmids was infiltrated into N. benthamiana leaves, and the luciferase activity at the site of infiltration was measured 2 days after infiltration. The repression ability of MaDEAR1 to the promoter was shown by the ratio of LUC to REN. The ratio of LUC to REN of the empty vector (pEAQ) plus promoter was used as a calibrator (set as 1). The reporter containing the MaACT1 promoter, and effector carrying the GFP gene were used as negative controls. Each value represents the means of six biological replicates, and vertical bars represent the SE ∗∗P < 0.01 by Student’s t-test, compared with pEAQ.
Discussion
Dehydration-responsive element-binding proteins are a class of AP2/ERF family of TFs, which are involved in plant response to drought, high salinity, low-temperature, and other environmental stresses (Agarwal et al., 2006; Lata and Prasad, 2011). The DREB proteins are divided into six small groups (A-1 to A-6) based on the sequence signature of DNA-binding domain and the existence of other motifs, of which A-5 group members share a conserved EAR motif at their C-terminus and act as transcriptional repressors (Ohta et al., 2001; Sakuma et al., 2002). In this study, we identified a banana DREB gene, MaDEAR1, whose predicted protein possesses an APETALA2 (AP2) domain that binds to DREs and an EAR motif that is responsible for transcriptional repression (Figures 1A and 2), and thus belongs to A-5 group (Figure 1B). Sub-cellular localization and transcriptional activation assays indicated that MaDEAR1 is nuclear-localized and possesses transcriptional repression activity (Figure 2), similar to GhDBP1 in cotton (Huang and Liu, 2006) and MsDREBA5 in Malus sieversii Roem (Zhao et al., 2012).
To date, most reports about DREBs focus on A-1 and A-2 sub-groups, while investigation into others, such as the A-5 sub-group, is limited. Generally, the A-5 sub-group DREB proteins are transcriptional repressors of gene expression during stress. For example, transgenic Arabidopsis over-expressing DEAR1 showed a cell death phenotype, resulting in reduced freezing tolerance (Tsutsui et al., 2009). Similarly, mutations in RAP2.1, another A-5 DREB, led to increased expression of DREB1/CBF and DREB2 target genes with enhanced tolerance to drought and freezing (Dong and Liu, 2010). More recently, over-expression of TaRAP2.1L (a homolog of RAP2.1 in wheat) under constitutive and stress-inducible promoters in transgenic wheat and barley caused dwarfism and decreased frost tolerance, supporting the notion that most DREB members in A-5 sub-group are negative regulators of stress tolerance (Amalraj et al., 2016). However, in addition to their role in stress responses, whether A-5 DREBs are involved in other biological processes such as fruit ripening is unknown. Here we showed that the MaDEAR1 expression was down-regulated by ethylene and ripening. The ripening associated down-regulation is, at least in part, likely to be mediated by the repression of its promoter activity by ethylene produced during ripening (Figure 3). It is also important to note that the decline in MaDEAR1 expression showed a concomitant increase in ethylene production during fruit ripening. These findings suggest that MaDEAR1 is a transcriptional repressor associated with fruit ripening, which is consistent with its transcriptional repression activity reported here (Figure 2B). Interestingly, we also found reduced levels of histone H3ac and H4ac in MaDEAR1 promoter in the ripening stage of banana fruit (Figure 4), which closely corresponds with its decreased expression during fruit ripening (Figure 3). Several reports have implicated histone acetylation as an important mechanism for controlling DREB gene expression. For example, treatment of maize with the HDAC inhibitor trichostatin A (TSA) under cold stress conditions selectively inhibited the induction of the cold-responsive gene ZmDREB1 through histone modification in the promoter region (Hu et al., 2011). Reports on enhanced transcript level of rice OsDREB1b and maize ZmDREB2A, as well as histone acetylation in their promoters further attest the regulation of DREB gene expression through histone modification (Roy et al., 2014; Zhao et al., 2014).
Fruit softening is one of the most important features that characterize the ripening process of fleshy climacteric fruits like bananas, and cell wall modification that occurs during the ripening process plays a critical role in the softening process (Li et al., 2010). The process of fruit ripening involves enzymatic and non-enzymatic factors. Expansins are non-enzymatic cell wall proteins that primarily induce cell wall extension (Cosgrove, 2000). Secondary wall-loosening factors such as XTH and PG, which enzymatically modify the structures of the cell wall, render it more responsive to wall-loosening events mediated by expansins (Cosgrove, 2000; Péret et al., 2009). XTH proteins can display two distinct enzymatic activities, including transglycosylase enzymatic (XET) activity leading to xyloglycan chain synthesis, and xyloglucan hydrolase activity (XEH) resulting in their degradation (Saladie et al., 2006). According to the previous reports, 23 cell wall-modifying genes, including five EXP, nine XET/XTH, four PG, two PE/PL and three PME have been isolated from banana fruit (Pua et al., 2001; Asif and Nath, 2005; Asha et al., 2007; Mbéguié-A-Mbéguié et al., 2009; Asif et al., 2014). It was found that transcripts of these genes were differentially expressed during post-harvest ripening (Mbéguié-A-Mbéguié et al., 2009; Asif et al., 2014), supporting their involvement in fruit ripening and softening. It is worth noting that transcriptional regulation of cell wall-modifying genes might be a conserved mechanism by which TFs regulate fruit ripening, as the cases of RIN in tomato (Fujisawa et al., 2013) and AdEILs and AdERFs in kiwifruit (Yin et al., 2010). Similar results were also found in bananas. For instance, MaMADS5 binds to the CArG-box sequence in the promoters of several ripening genes including MaEXPs (Roy Choudhury et al., 2012). Our previous studies also indicated that MaLBDs and MaBSD1 TFs are involved in fruit ripening, via transcriptional regulation of MaEXP1/2 (Ba et al., 2014a,b). Also we found that, a transcriptional repressor MaDof23 physically interacts with a transcriptional activator MaERF9, and they act antagonistically to regulate 10 ripening-related genes including MaEXP1/2/3/5, MaXET7, MaPG1, MaPME3, MaPL2, MaCAT, and MaPDC that are associated with cell wall degradation and aroma formation during banana ripening (Feng et al., 2016). In this work, we show that MaDEAR1 binds and represses the activity of six cell wall-modifying genes, such as MaEXP1/3, MaPG1, MaXTH10, MaPL3, and MaPME3 (Figures 5 and 6), suggesting its regulatory role in cell wall degradation during banana fruit ripening. Nuclear localization and promoter activity of MaDEAR1 in banana protoplasts will further substantiate these results. In addition to these fruit softening-associated genes, those involved in ethylene production and aroma formation such as MaACS1, MaACO1 and MaPDC are also associated with banana ripening (Yang et al., 2011; Xiao et al., 2013), but whether they are direct targets of MaDEAR1 remains unknown. As fruit ripening is controlled by transcriptional regulatory networks involving several TFs, it would be interesting to investigate whether MaDEAR1 interacts with other reported ripening-related TFs of banana fruit, including MaERFs (Xiao et al., 2013), MaMADSs (Roy Choudhury et al., 2012), MaNACs (Shan et al., 2012), MaLBDs and MaBSDs (Ba et al., 2014a,b), and the effects of such interactions on fruit ripening.
Conclusion
The data reported here represent an EAR-motif-containing DREB TF, MaDEAR1, which was found to be a nuclear-localized transcriptional repressor. Expression and promoter activity of MaDEAR1 were repressed by ethylene and ripening, and its expression is likely to be regulated by, at least in part, by histone modification. MaDEAR1 binds to and represses several cell wall-modifying genes, including MaEXP1/3, MaPG1, MaXTH10, MaPL3, and MaPME3. Taken together, our results report new insights into the mechanisms underpinning ethylene-mediated banana fruit ripening, of which MaDEAR1 may be playing a role via transcriptional repression of genes involved in cell wall modification and softening of fruit.
Author Contributions
JC, JK, WL, PL, and XD designed the research. ZF, WS, YH, YX, and YY performed the experiments. ZF, JK, WL, PL, and JC wrote the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Professor Seiichiro Hasezawa (Department of Integrated Biosciences, the University of Tokyo), Professor Shouyi Chen (Institute of Genetics and Developmental Biology, Chinese Academy of Sciences), Professor Junping Gao (Department of Ornamental Horticulture, China Agricultural University), and Professor George P. Lomonossoff (Department of Biological Chemistry, John Innes Centre, Norwich Research Park) for the generous gift of tobacco BY-2 suspension cells, the transient expression vectors, and pEAQ vectors, respectively.
Funding. This work was supported in part by the National Basic Research Program of China (grant No. 2013CB127104) and the China Agriculture Research System (grant no. CARS-32-09).
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fpls.2016.01021
References
- Abel S., Theologis A. (1994). Transient transformation of Arabidopsis leaf protoplasts: a versatile experimental system to study gene expression. Plant J. 5 421–427. 10.1111/j.1365-313X.1994.00421.x [DOI] [PubMed] [Google Scholar]
- Agarwal P. K., Agarwal P., Reddy M., Sopory S. K. (2006). Role of DREB transcription factors in abiotic and biotic stress tolerance in plants. Plant Cell Rep. 25 1263–1274. 10.1007/s00299-006-0204-8 [DOI] [PubMed] [Google Scholar]
- Ahmed Z. F. R., Palta J. P. (2016). Postharvest dip treatment with a natural lysophospholipid plus soy lecithin extended the shelf life of banana fruit. Postharvest Biol. Technol. 113 58–65. 10.1016/j.postharvbio.2015.10.016 [DOI] [Google Scholar]
- Amalraj A., Luang S., Kumar M. Y., Sornaraj P., Eini O., Kovalchuk N., et al. (2016). Change of function of the wheat stress-responsive transcriptional repressor TaRAP2.1L by repressor motif modification. Plant Biotech. J. 14 820–832. 10.1111/pbi.12432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asha S. V. A., Sane A. P., Nath P. (2007). Multiple forms of alpha-expansin genes are expressed during banana fruit ripening and development. Postharvest Biol. Technol. 45 184–192. 10.1016/j.postharvbio.2007.03.003 [DOI] [Google Scholar]
- Asif M., Nath P. (2005). Expression of multiple forms of polygalacturonase gene during ripening in banana fruit. Plant Physiol. Biochem. 43 177–184. 10.1016/j.plaphy.2005.01.011 [DOI] [PubMed] [Google Scholar]
- Asif M. H., Lakhwani D., Pathak S., Gupta P., Bag S. K., Nath P., et al. (2014). Transcriptome analysis of ripe and unripe fruit tissue of banana identifies major metabolic networks involved in fruit ripening process. BMC Plant Biol. 14:316 10.1186/s12870-014-0316-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ba L., Shan W., Kuang J., Feng B., Xiao Y., Lu W., et al. (2014a). The banana MaLBD (LATERAL ORGAN BOUNDARIES DOMAIN) transcription factors regulate EXPANSIN expression and are involved in fruit ripening. Plant Mol. Biol. Rep. 32 1103–1113. 10.1007/s11105-014-0720-6 [DOI] [Google Scholar]
- Ba L., Shan W., Xiao Y., Chen J., Lu W., Kuang J. (2014b). A ripening-induced transcription factor MaBSD1 interacts with promoters of MaEXP1/2 from banana fruit. Plant Cell Rep. 33 1913–1920. 10.1007/s00299-014-1668-6 [DOI] [PubMed] [Google Scholar]
- Ba L. J., Kuang J. F., Chen J. Y., Lu W. J. (2016). MaJAZ1 attenuates the MaLBD5-mediated transcriptional activation of jasmonate biosynthesis gene MaAOC2 in regulating cold tolerance of banana fruit. J. Agric. Food Chem. 64 738–745. 10.1021/acs.jafc.5b05005 [DOI] [PubMed] [Google Scholar]
- Benhamed M., Bertrand C., Servet C., Zhou D. X. (2006). Arabidopsis GCN5, HD1, and TAF1/HAF2 interact to regulate histone acetylation required for light-responsive gene expression. Plant Cell 18 2893–2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Zhong H. Y., Kuang J. F., Li J. G., Chen J. Y., Lu W. J. (2011). Validation of reference genes for RT-qPCR studies of gene expression in banana fruit under different experimental conditions. Planta 234 377–390. 10.1007/s00425-011-1410-3 [DOI] [PubMed] [Google Scholar]
- Cosgrove D. J. (2000). Loosening of plant cell walls by expansins. Nature 407 321–326. 10.1038/35030000 [DOI] [PubMed] [Google Scholar]
- D’Hont A., Denoeud F., Aury J. M., Baurens F. C., Carreel F., Garsmeur O., et al. (2012). The banana (Musa acuminata) genome and the evolution of monocotyledonous plants. Nature 488 213–217. 10.1038/nature11241 [DOI] [PubMed] [Google Scholar]
- Dong C. J., Liu J. Y. (2010). The Arabidopsis EAR-motif-containing protein RAP2.1 functions as an active transcriptional repressor to keep stress responses under tight control. BMC Plant Biol. 16:10–47. 10.1186/1471-2229-10-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng B., Han Y., Xiao Y., Kuang J., Fan Z., Chen J., et al. (2016). The banana fruit Dof transcription factor MaDof23 acts as a repressor and interacts with MaERF9 in regulating ripening-related genes. J. Exp. Bot. 67 2263–2275. 10.1093/jxb/erw032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa M., Nakano T., Shima Y., Ito Y. (2013). A large-scale identification of direct targets of the tomato MADS box transcription factor RIPENING INHIBITOR reveals the regulation of fruit ripening. Plant Cell 25 371–386. 10.1105/tpc.112.108118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao S. Q., Chen M., Xia L. Q., Xiu H. J., Xu Z. S., Li L. C., et al. (2009). A cotton (Gossypium hirsutum) DRE-binding transcription factor gene, GhDREB, confers enhanced tolerance to drought, high salt, and freezing stresses in transgenic wheat. Plant Cell Rep. 28 301–311. 10.1007/s00299-008-0623-9 [DOI] [PubMed] [Google Scholar]
- Gendrel A. V., Lippman Z., Martienssen R., Colot V. (2005). Profiling histone modification patterns in plants using genomic tiling microarrays. Nat. Methods 2 213–218. 10.1038/nmeth0305-213 [DOI] [PubMed] [Google Scholar]
- Haake V., Cook D., Riechmann J., Pineda O., Thomashow M. F., Zhang J. Z. (2002). Transcription factor CBF4 is a regulator of drought adaptation in Arabidopsis. Plant Physiol. 130 639–648. 10.1104/pp.006478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y. C., Kuang J. F., Chen J. Y., Liu X. C., Xiao Y. Y., Fu C. C., et al. (2016). Banana transcription factor MaERF11 recruits histone deacetylase MaHDA1 and represses the expression of MaACO1 and expansins during fruit ripening. Plant Physiol. 171 1070–1084. 10.1104/pp.16.00301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellens R., Allan A., Friel E., Bolitho K., Grafton K., Templeton M., et al. (2005). Transient expression vectors for functional genomics, quantification of promoter activity and RNA silencing in plants. Plant Methods 1 13 10.1186/1746-4811-1-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y., Zhang L., Zhao L., Li J., He S., Zhou K., et al. (2011). Trichostatin A selectively suppresses the cold-induced transcription of the ZmDREB1 gene in maize. PLoS ONE 6:e22132 10.1371/journal.pone.0022132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B., Liu J. Y. (2006). A cotton dehydration responsive element binding protein functions as a transcriptional repressor of DRE element-mediated gene expression. Biochem. Biophys. Res. Commun. 343 1023–1031. 10.1016/j.bbrc.2006.03.016 [DOI] [PubMed] [Google Scholar]
- Jourda C., Cardi C., Mbeguie-A-Mbeguie D., Bocs S., Garsmeur O., D’Hont A., et al. (2014). Expansion of banana (Musa acuminata) gene families involved in ethylene biosynthesis and signalling after lineage-specific whole-genome duplications. New Phytol. 202 986–1000. 10.1111/nph.12710 [DOI] [PubMed] [Google Scholar]
- Kagale S., Rozwadowski K. (2010). Small yet effective: the ethylene responsive element binding factor-associated amphiphilic repression (EAR) motif. Plant Signal. Behav. 5 691–694. 10.4161/psb.5.6.11576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuan C. H., Ahmad S. H., Son R., Yap E. S. P., Zamri M. Z., Shukor N. I. A., et al. (2015). Influence of forced-air precooling time on the changes in quality attributes and consumer acceptance of Musa AAA Berangan. Int. Food Res. J. 22 1864–1869. [Google Scholar]
- Kuang J., Chen L., Shan W., Yang S., Lu W., Chen J. (2013). Molecular characterization of two banana ethylene signaling components MaEBFs during fruit ripening. Postharvest Biol. Technol. 85 94–101. 10.1016/j.postharvbio.2013.05.004 [DOI] [Google Scholar]
- Kumagai-Sano F., Hayashi T., Sano T., Hasezawa S. (2006). Cell cycle synchronization of tobacco BY-2 cells. Nat. Protoc. 1 2621–2627. 10.1038/nprot.2006.381 [DOI] [PubMed] [Google Scholar]
- Lata C., Prasad M. (2011). Role of DREBs in regulation of abiotic stress responses in plants. J. Exp. Bot. 62 4731–4748. 10.1093/jxb/err210 [DOI] [PubMed] [Google Scholar]
- Li X., Xu C., Korban S. S., Chen K. (2010). Regulatory mechanisms of textural changes in ripening fruits. Crit. Rev. Plant Sci. 29 222–243. 10.1080/07352689.2010.487776 [DOI] [Google Scholar]
- Liu Q., Kasuga M., Sakuma Y., Abe H., Miura S., Yamaguchi-Shinozak K., et al. (1998). Two transcription factors, DREB1 and DREB2,with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought- and low-temperature responsive gene expression, respectively, in Arabidopsis. Plant Cell 10 1391–1406. 10.2307/3870648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X. J., Shiomi S., Nakatsuka A., Kubo Y., Nakamura R., Inaba A. (1999). Characterization of ethylene biosynthesis associated with ripening in banana fruit. Plant Physiol. 121 1257–1265. 10.1104/pp.121.4.1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas S., Durmaz E., Akpınar B. A., Budak H. (2011). The drought response displayed by a DRE-binding protein from Triticum dicoccoides. Plant Physiol. Biochem. 49 346–351. 10.1016/j.plaphy.2011.01.016 [DOI] [PubMed] [Google Scholar]
- Mbéguié-A-Mbéguié D., Hubert O., Baurens F. C., Matsumoto T., Chillet M., Fils-Lycaon B., et al. (2009). Expression patterns of cell wall-modifying genes from banana during fruit ripening and in relationship with finger drop. J. Exp. Bot. 60 2021–2034. 10.1093/jxb/erp079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbéguié-A-Mbéguié D., Hubert O., Fils-Lycaon B., Chillet M., Baurens F. C. (2008). EIN3-like gene expression during fruit ripening of Cavendish banana (Musa acuminata cv. Grande Naine). Physiol. Plant. 133 435–448. 10.1111/j.1399-3054.2008.01083.x [DOI] [PubMed] [Google Scholar]
- Mizoi J., Shinozaki K., Yamaguchi-Shinozaki K. (2012). AP2/ERF family transcription factors in plant abiotic stress responses. Biochim. Biophys. Acta 1819 86–96. 10.1016/j.bbagrm.2011.08.004 [DOI] [PubMed] [Google Scholar]
- Nakashima K., Shinwari Z. K., Sakuma Y., Seki M., Miura S., Shinozaki K., et al. (2000). Organization and expression of two Arabidopsis DREB2 genes encoding DRE-binding proteins involved in dehydration- and high-salinity-responsive gene expression. Plant Mol. Biol. 42 657–665. 10.1023/A:1006321900483 [DOI] [PubMed] [Google Scholar]
- Ohme-Takagi M., Shinshi H. (1995). Ethylene-inducible DNA binding proteins that interact with an ethylene-responsive element. Plant Cell 7 173–182. 10.1105/tpc.7.2.173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta M., Matsui K., Hiratsu K., Shinshi H., Ohme-Takagi M. (2001). Repression domains of class II ERF transcriptional repressors share an essential motif for active repression. Plant Cell 13 1959–1968. 10.1105/tpc.13.8.1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Péret B. D., Rybel B., Casimiro I., Benková E., Swarup R., Laplaze L., et al. (2009). Arabidopsis lateral root development: an emerging story. Trends Plant Sci. 14 399–408. 10.1016/j.tplants.2009.05.002 [DOI] [PubMed] [Google Scholar]
- Pirrello J., Prasad N. B. C., Zhang W., Chen K., Isabelle M., Zouine M., et al. (2012). Functional analysis and binding affinity of tomato ethylene response factors provide insight on the molecular bases of plant differential responses to ethylene. BMC Plant Biol. 12:190 10.1186/1471-2229-12-190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pua E. C., Ong C. K., Liu P., Liu J. Z. (2001). Isolation and expression of two pectate lyase genes during fruit ripening of banana (Musa acuminata). Physiol. Plant. 113 92–99. 10.1034/j.1399-3054.2001.1130113.x [DOI] [Google Scholar]
- Roy D., Paul A., Roy A., Ghosh R., Ganguly P., Chaudhuri S. (2014). Differential acetylation of histone H3 at the regulatory region of OsDREB1b promoter facilitates chromatin remodeling and transcription activation during cold stress. PLoS ONE 9:e100343 10.1371/journal.pone.0100343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy Choudhury S., Roy S., Nag A., Singh S. K., Sengupta D. N. (2012). Characterization of an AGAMOUS-like MADS box protein, a probable constituent of flowering and fruit ripening regulatory system in banana. PLoS ONE 7:e44361 10.1371/journal.pone.0044361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainsbury F., Thuenemann E. C., Lomonossoff G. P. (2009). pEAQ: versatile expression vectors for easy and quick transient expression of heterologous proteins in plants. Plant Biotechnol. J. 7 682–693. 10.1111/j.1467-7652.2009.00434.x [DOI] [PubMed] [Google Scholar]
- Sakuma Y., Liu Q., Dubouzet J. G., Abe H., Shinozaki K., Yamaguchi-Shinozaki K. (2002). DNA-binding specificity of the ERF/AP2 domain of Arabidopsis DREBs, transcription factors involved in dehydration- and cold-inducible gene expression. Biochem. Biophys. Res. Commun. 290 998–1009. 10.1006/bbrc.2001.6299 [DOI] [PubMed] [Google Scholar]
- Saladie M., Rose J. R. C., Cosgrove D. J., Catala C. (2006). Characterization of a new xyloglucan endotransglucosylase/hydrolase (XTH) from ripening tomato fruit and implications for the diverse modes of enzymicaction. Plant J. 47 282–295. 10.1111/j.1365-313X.2006.02784.x [DOI] [PubMed] [Google Scholar]
- Salehin M., Estelle M. (2015). Ethylene prunes translation. Cell 163 543–544. 10.1016/j.cell.2015.10.032 [DOI] [PubMed] [Google Scholar]
- Shan W., Kuang J. F., Chen L., Xie H., Peng H. H., Xiao Y. Y., et al. (2012). Molecular characterization of banana NAC transcription factors and their interactions with ethylene signalling component EIL during fruit ripening. J. Exp. Bot. 63 5171–5187. 10.1093/jxb/ers178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreedharan S., Shekhawat U. K. S., Ganapathi T. R. (2012). MusaSAP1, a A20/AN1zinc finger gene from banana functions as a positive regulator in different stress responses. Plant Mol. Biol. 80 503–517. 10.1007/s11103-012-9964-4 [DOI] [PubMed] [Google Scholar]
- Stockinger E. J., Gilmour S. J., Thomashow M. F. (1997). Arabidopsis thaliana CBF1 encodes an AP2 domain-containing transcriptional activator that binds to the C-repeat/DRE, a cis-acting DNA regulatory element that stimulates transcription in response to low temperature and water deficit. Proc. Natl. Acad. Sci. U.S.A. 94 1035–1040. 10.1073/pnas.94.3.1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsui T., Kato W., Asada Y., Sako K., Sato T., Sonoda Y., et al. (2009). DEAR1, a transcriptional repressor of DREB protein that mediates plant defense and freezing stress responses in Arabidopsis. J. Plant Res. 122 633–643. 10.1007/s10265-009-0252-6 [DOI] [PubMed] [Google Scholar]
- Wan C. Y., Wilkins T. A. (1994). A modified hot borate method significantly enhances the yield of high-quality RNA from cotton (Gossypium hirsutum L.). Anal. Biochem. 223 7–12. 10.1006/abio.1994.1538 [DOI] [PubMed] [Google Scholar]
- Xiao Y. Y., Chen J. Y., Kuang J. F., Shan W., Xie H., Jiang Y. M., et al. (2013). Banana ethylene response factors are involved in fruitripening through their interactions with ethylene biosynthesis genes. J. Exp. Bot. 64 2499–2510. 10.1093/jxb/ert108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X. T., Song J., Fillmore S., Pang X. Q., Zhang Z. Q. (2011). Effect of high temperature on color, chlorophyll fluorescence and volatile biosynthesis in green-ripe banana fruit. Postharvest Biol. Technol. 62 246–257. 10.1016/j.postharvbio.2011.06.011 [DOI] [Google Scholar]
- Yin X. R., Allan A. C., Chen K. S., Ferguson I. B. (2010). Kiwifruit EIL and ERF genes involved in regulating fruit ripening. Plant Physiol. 153 1280–1292. 10.1104/pp.110.157081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L., Wang P., Yan S., Gao F., Li H., Hou H., et al. (2014). Promoter-associated histone acetylation is involved in the osmotic stress-induced transcriptional regulation of the maize ZmDREB2A gene. Physiol. Plant. 151 459–467. 10.1111/ppl.12136 [DOI] [PubMed] [Google Scholar]
- Zhao X. J., Lei H. J., Zhao K., Yuan H. Z., Li T. H. (2012). Isolation and characterization of a dehydration responsive element binding factor MsDREBA5 in Malus sieversii Roem. Sci. Hort. 142 212–220. 10.1016/j.scienta.2012.05.020 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.