Abstract
Purpose
Computed tomography (CT) scans are an important source of ionizing irradiation (IR) in medicine that can induce a variety of DNA damage in human tissues. With technological improvements CT scans at reduced absorbed doses became feasible presumably lowering genotoxic side effects.
Materials and methods
For measuring DNA damage we performed γH2AX foci microscopy in peripheral blood mononuclear cells (PBMC) after exposure to reduced and conventional absorbed radiation doses using 3rd generation dual-source CT (DSCT) technology.
Results
CT scans performed at reduced absorbed doses of 3 mGy induced significant lower levels (p < 0.0001) of DNA damage (0.05 focus per cell ± 0.01 [mean ± standard error of mean]) at 5 min after IR compared to conventional absorbed doses of 15 mGy (0.30 focus per cell ± 0.03). With ongoing DNA repair background γH2AX foci levels (0.05 focus per cell) were approached at 24 h after CT with both protocols.
Conclusion
Our results provide evidence that reduced absorbed doses mediated by adjusted tube current in 3rd generation DSCT induce lower levels of DNA damage in PBMC compared to conventional absorbed doses suggesting a lower genotoxic risk for state-of-the-art tube current reduced CT protocols.
Keywords: γH2AX foci, DNA double-strand breaks, 3rd generation dual-source CT, X-rays
1. Introduction
With increased availability of multi-detector row computed tomography (CT), the use of CT examinations increased rapidly in all age groups in the last decade [1], [2]. In parallel, radiation exposure and the resulting biologic effects became topics of ongoing discussion [3]. Although the risk for induction of cancer by a single CT scan is low (about 1:2000 assuming an effective dose of 10 mSv per scan) [4], it may translate into a considerable number of cancer cases in an epidemiologic scale [5]. In addition to theoretical risk calculations two large prospective cohort studies from the UK and Australia provided evidence for a significant increased cancer risk in individuals exposed to CT scans in childhood and adolescence [6], [7]. Furthermore, the risk is also increased in individuals undergoing repeated CT scans, which is often required in cancer patients for adequate treatment planning and follow-up, so that individual cumulative radiation doses may exceed >300 mSv [8]. Thus, radiologists, physicists and CT scanner manufacturers seek to keep radiation exposure by CT scans “as low as reasonably achievable” (ALARA principle). Importantly, advances in CT hard- and software including automatic tube voltage selection, automatic tube current selection and iterative image reconstruction (e.g., provided by 3rd generation dual-source CT (DSCT)) allow now for reduction of the applied irradiation (IR) dose and thereby potentially lower genotoxic side effects.
Generally, for dose measurements conventional dosimeters such as ionization chambers are widely-used as they deliver fast and exact dose readings. However, for monitoring biological effects of IR, methods assaying specific molecular changes might be more appropriate. Since many years cytogenetic analysis of dicentric chromosomes has been the gold standard for biological dosimetry, however, sensitivity of this assay is limited to doses >100 mGy [9]. For monitoring DNA damage at lower doses >1 mGy, γH2AX immunofluorescence microscopy has evolved as a more sensitive and appropriate method in recent years. This assay relies on detection of the Ser139-phosphorylated form of histone H2AX (also named γH2AX), that accumulates in a region of several megabase pairs around each DNA double-strand break (DSB) [10]. There, multiple γH2AX histones form a platform for other proteins involved in DNA repair, chromatin remodeling and signal transduction [11]. γH2AX foci emerge rapidly after exposure to IR in the nucleus and usability of this assay for monitoring formation and repair of DSB after CT has been demonstrated previously [12], [13], [14], [15].
The hypothesis of our study was that lower radiation dose levels, that became clinically feasible with state-of-the-art CT technologies, directly translate into less measurable DNA damage. Therefore, we prospectively compared γH2AX immunofluorescence microscopy based DNA damage measurements in peripheral blood mononuclear cells (PBMC) between a conventional radiation dose as well as a radiation dose reduced CT protocol.
2. Materials and methods
2.1. Peripheral blood samples
After institutional review board approval (Ethics Committee II, Medical Faculty Mannheim, University of Heidelberg) and written informed consent were obtained, peripheral blood samples were withdrawn from 12 healthy volunteers (5 male, 7 female, mean age: 39.3 years, range 25 to 55). PBMC were isolated by Ficoll density gradient centrifugation and cultivated in RPMI-1640 medium supplemented with 10% fetal calf serum, 4 mM glutamine and 1% penicillin/streptomycin. Cells were incubated in a humidified 5% CO2 atmosphere at 37 °C until exposure to IR by CT.
2.2. In vitro x-ray irradiation
All PBMC samples were scanned in flasks using a 3rd generation DSCT (SOMATOM Force, Siemens Healthcare Sector, Forchheim, Germany). The following imaging parameters were used: 120 kV, effective tube time-current 45 mAs (absorbed doses of 3 mGy), 224 mAs (absorbed doses of 15 mGy) and 746 mAs (absorbed doses of 50 mGy). A rotation time of 0.5 s, a pitch of 35, a collimation of 2 × 198 × 0.6 mm and a scan length of 12 cm were chosen and kept constant for each scan. All samples were positioned in the isocenter of the CT scanner in order to guarantee same IR for each sample. After IR samples were incubated again in a humidified 5% CO2 atmosphere at 37 °C until analysis of γH2AX foci at indicated time points. All 12 PBMC samples from 12 healthy volunteers were analyzed for γH2AX foci yields at 5 min and 24 h after CT with doses of 3 mGy and 15 mGy, respectively. In 12 PBMC samples γH2AX foci yields were also analyzed at 5 min after CT with a dose of 50 mGy for determination of dose dependence of γH2AX foci formation. In three of 12 PBMC samples γH2AX foci yields were additionally analyzed at 30 min and 5 h after CT with doses of 3 mGy, 15 mGy and 50 mGy, respectively, for determination of DNA repair kinetics.
2.3. Sample processing and γH2AX immunofluorescence staining
PBMC were cytospun (1000 rpm) on glass slides, fixed in paraformaldehyde 4% (10 min) and permeabilized by Triton-X-100 0.1% (10 min). Samples were incubated with a primary mouse monoclonal anti-γH2AX antibody (1:500) (clone JBW301; Merck Millipore, Darmstadt, Germany) overnight at 4 °C. After washing in PBS and blocking in Chemiblocker (Merck Millipore) PBMC were incubated with an Alexa Fluor 488-conjugated goat anti-mouse secondary antibody (1:500) (Invitrogen, Karlsruhe, Germany) for 1 h at room temperature. PBMC were washed again and mounted with Vectashield mounting medium containing 4,6-diamidino-2-phenylindole (Vector Laboratories, Burlingame, US). Images were obtained by an Axioscope A1 fluorescence microscope (Zeiss, Jena, Germany) equipped with a Cool Cube 1 CCD camera (Metasystems, Altlussheim, Germany) and processed by Isis software (Metasystems). Foci analysis was performed by eye during imaging at a 100× objective magnification. At least 100 typical lymphocytic cells were analyzed for each measurement.
2.4. Statistical analysis
Statistical analysis was performed using SAS 9.3 (SAS Institute Inc., Cary, US). The paired sample t-test was used to test the hypotheses whether there is a significant difference in γH2AX foci yields after absorbed doses of 15 mGy and 3 mGy at 5 min after CT and whether there is no significant difference at 24 h after CT. P-values <0.05 were considered as statistically significant.
3. Results
3.1. Validation of γH2AX foci formation in irradiated PBMC in vitro
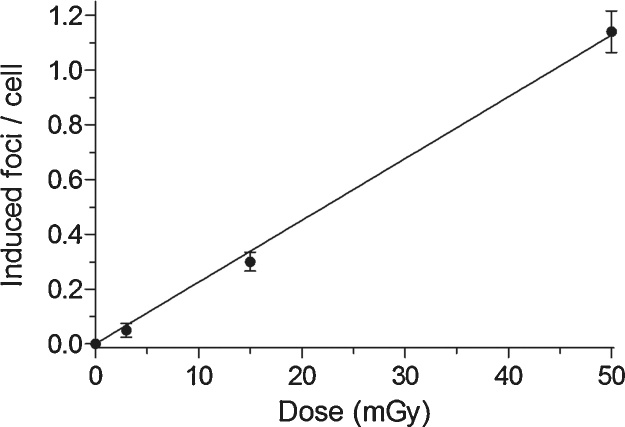
γH2AX foci yields were analyzed in PBMC at a dose range from 0 to 50 mGy. γH2AX foci yields increased linearly with dose and the mean increment of γH2AX foci was 1.14 foci per cell at 50 mGy (Fig. 1). A mean background level of 0.05 γH2AX focus per cell was detected in non-irradiated PBMC.
Fig. 1.
Linear dose dependency of γH2AX foci formation in peripheral blood mononuclear cells (PBMC) after irradiation. PBMC samples were irradiated each with doses of 3 mGy, 15 mGy and 50 mGy by a 3rd generation dual-source CT. Each data point represents the mean γH2AX foci yield ± standard error of the mean of 12 different PBMC samples at 5 min after irradiation exposure. Mean γH2AX foci yields are corrected by subtracting the mean γH2AX foci yield of the background (0.05 focus per cell).
3.2. DNA repair kinetics in PBMC in vitro after IR by CT
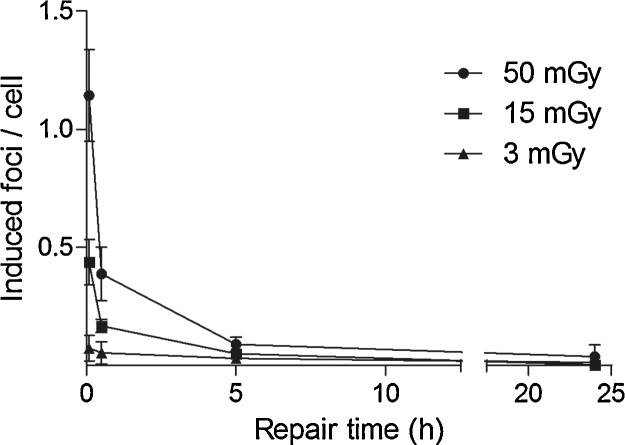
DNA repair kinetics were analyzed in three samples of PBMC in a time interval from 0 to 24 h after exposure to IR by CT at absorbed doses of 3 mGy, 15 mGy and 50 mGy, respectively. After induction of DSB by IR, γH2AX foci reached maximum foci yields at 5 min followed by a non-linear biphasic decline with a fast repair component until 5 h and a slow repair component until 24 h after IR (Fig. 2).
Fig. 2.
Repair kinetics of DNA double-strand breaks in peripheral blood mononuclear cells (PBMC) after irradiation. PBMC samples were irradiated each with doses of 3 mGy, 15 mGy and 50 mGy by a 3rd generation dual-source CT and analyzed for γH2AX foci yields at 5 min, 30 min, 5 h and 24 h after irradiation. All data points represent the mean γH2AX foci yields ± standard errors of the mean of three different PBMC samples. Mean γH2AX foci yields are corrected by subtracting the mean γH2AX foci yield of the background (0.05 focus per cell).
3.3. Formation of γH2AX foci in PBMC in vitro after protocols with low absorbed and conventional absorbed doses
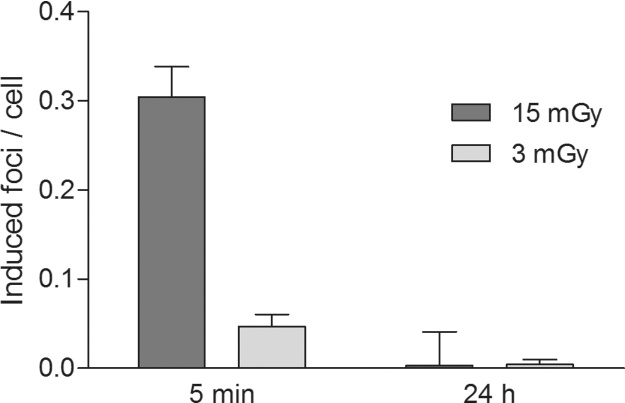
12 samples of PBMC from 12 healthy volunteers were scanned separately using protocols with absorbed doses of 3 mGy and 15 mGy. DNA damage was analyzed by γH2AX foci microscopy (Fig. 3) at 5 min and 24 h after IR. The mean increment of γH2AX foci yields in PBMC was significantly lower (p < 0.0001; 95% confidence interval: 0.1988, 0.3162) for absorbed doses of 3 mGy (0.05 focus per cell ± 0.01 [mean ± standard error of mean]) compared to conventional absorbed doses of 15 mGy (0.30 focus per cell ± 0.03) at 5 min after IR. No significant difference (p = 0.8837; 95% confidence interval: – 0.0131, 0.0114) in γH2AX foci yields was detected at 24 h after IR (Fig. 4).
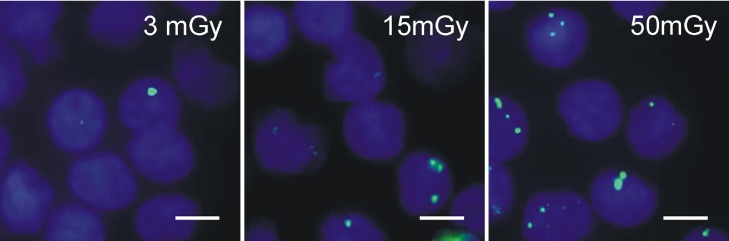
Fig. 3.
Fluorescence microscopic images of γH2AX foci in peripheral blood mononuclear cells (PBMC) after irradiation. Images show γH2AX foci (green, Alexa 488) and nuclei (blue, DAPI) of PBMC at 5 min after irradiation by a 3rd generation dual-source CT using protocols with absorbed doses of 3 mGy, 15 mGy and 50 mGy, respectively (scale bars 5 μm).
Fig. 4.
γH2AX foci yields in peripheral blood mononuclear cells (PBMC) after applying reduced and conventional absorbed doses. Bars represent mean γH2AX foci yields ± standard errors of the mean of 12 different PBMC samples at 5 min and 24 h after application of reduced (3 mGy) and conventional absorbed doses (15 mGy) using a 3rd generation dual-source CT. Mean γH2AX foci yields are corrected by subtracting the mean γH2AX foci yield of the background (0.05 focus per cell).
4. Discussion
The clinical benefit of CT examinations certainly outweighs potential radiation-associated risks of CT by far. However, with continuous increase of millions of CT scans performed annually, the ALARA principle should be kept in mind as small risks may translate into a considerable number of cancer cases in the epidemiologic scale. Moreover, the risk is also increased in patients undergoing several CT examinations. In order to minimize IR-related risks, several dose saving techniques have been introduced throughout the last years. For example, modern CT scanners (e.g., 3rd generation DSCT) automatically select tube voltage and modulate tube current according to the patientś topogram resulting in radiation dose savings of up to 52% [16], [17]. Further, high-pitch acquisitions have become an implement into routine CT protocols leading to higher temporal resolution and to reduction of the applied radiation dose down to a volume computed tomography dose index (CTDIvol) of 1.8 mGy [17], [18]. In addition, recent advances in iterative image reconstruction, especially with the new model-based iterative algorithms, allow for reduction of image noise, when compared to traditional filtered back projection, thus allowing for significantly lower IR doses [19], [20]. When combining these new techniques CTDIvol ranges between 1.7–12.4 mGy can be achieved depending on the tube voltage chosen and the scanned region [16], [19], [20]. Hence, with technical advances (e.g., provided by 3rd generation DSCT technology) it is now possible to significantly reduce absorbed doses and probably DNA-damage in affected tissues. However, one has to be aware, that biological effects of IR can be influenced by unapparent factors, as has been shown for application of iodinated contrast agents that amplify CT-induced DNA damage [21], [22].
For quantification of IR-induced DNA damage in PBMC after 3rd generation DSCT, we performed γH2AX immunofluorescence microscopy. DNA damage in PBMC was significantly reduced at 5 min after IR when using minimum absorbed doses (3 mGy) compared to conventional absorbed doses (15 mGy). Excess of γH2AX foci yields was linearly dose-dependent as published in previous studies determining γH2AX foci yields in cell cultures and lymphocytes after IR by IR devices in vitro or in lymphocytes after conventional CT and angiography in vivo [13], [15], [23], [24], [25], [26]. Furthermore, kinetics of γH2AX foci formation and loss in PBMC from 0 to 24 h after IR in our experiments were comparable to results of previous studies [12], [24]. In addition, we observed approximation of γH2AX foci yields to background levels in PBMC at 24 h after IR in vitro. Noteworthy, no significant difference in γH2AX foci yields was observed in our experiments in PBMC at 24 h after reduced absorbed doses of 3 mGy when compared to conventional absorbed doses of 15 mGy. However, a higher rate of acquired mutations in PBMC is still probable after conventional absorbed doses owing to a higher burden of induced DNA damage, which is repaired by potentially error-prone DNA repair mechanisms (e.g., repair of DSB by classical and alternative non-homologous end joining mechanisms) [27], [28]. Conversely, reduced absorbed doses induce significantly less DNA damage and are therefore probably associated with lower rates of acquired mutations.
Models for risk estimation of CT are based on the so-called linear no-threshold theory (LNT), which holds, that even exposure to low doses of IR is associated with an excess of cancer risk proportional to dose. The LNT is of considerable controversy because of extrapolating risks at intermediate doses (>100 mGy) down to lower doses (<100 mGy). In addition, the model of a linear dose response is challenged by potentially disturbing mechanisms (e.g., adaptive response to ionizing radiation, apoptosis, bystander effect). However, no deviations from a linear dose effect should currently be considered in the absence of convincing data [29]. Thus, even very low radiation doses as feasible by novel CT technologies are presumably associated with a low theoretical risk.
Our study has limitations that need to be addressed e.g., experiments were performed with PBMC in vitro and warrant validation by investigations in vivo. Furthermore, variations in γH2AX foci yields and DNA repair kinetics may occur when DNA damage is analyzed in different tissues of patients exposed to 3rd generation DSCT in the clinical setting.
In conclusion, imaging at reduced absorbed doses is now feasible with the latest technical innovations and holds broad implications for human health care. Importantly, protocols using reduced absorbed doses induce less DNA damage in comparison to protocols using conventional absorbed doses suggesting a lower genotoxic risk for individuals undergoing CT examinations.
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
This study was supported by the Gutermuth Foundation (Frankfurt/Main, Germany).
Acknowledgement
The authors would like thank Dr. Julia Bucher, Live Cell imaging Mannheim, University of Heidelberg, Germany, for advice in immunostaining of γH2AX.
References
- 1.Brenner D.J., Hall E.J. Computed tomography – an increasing source of radiation exposure. N. Engl. J. Med. 2007;357(22):2277–2284. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- 2.Mettler, F.A., Jr., Bhargavan M., Faulkner K., Gilley D.B., Gray J.E., Ibbott G.S., Lipoti J.A., Mahesh M., McCrohan J.L., Stabin M.G., Thomadsen B.R., Yoshizumi T.T. Radiologic and nuclear medicine studies in the United States and worldwide: frequency, radiation dose, and comparison with other radiation sources—1950–2007. Radiology. 2009;253(2):520–531. doi: 10.1148/radiol.2532082010. [DOI] [PubMed] [Google Scholar]
- 3.Fazel R., Krumholz H.M., Wang Y., Ross J.S., Chen J., Ting H.H., Shah N.D., Nasir K., Einstein A.J., Nallamothu B.K. Exposure to low-dose ionizing radiation from medical imaging procedures. N. Engl. J. Med. 2009;361(9):849–857. doi: 10.1056/NEJMoa0901249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.2016. U.S. Food and Drug administration, What are the radiation risks from CT? http://www.fda.gov/Radiation-EmittingProducts/RadiationEmittingProductsandProcedures/MedicalImaging/MedicalX-Rays/ucm115329.htm (accessed 10.06.16) [Google Scholar]
- 5.Berrington de Gonzalez A., Mahesh M., Kim K.P., Bhargavan M., Lewis R., Mettler F., Land C. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch. Intern. Med. 2009;169(22):2071–2077. doi: 10.1001/archinternmed.2009.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mathews J.D., Forsythe A.V., Brady Z., Butler M.W., Goergen S.K., Byrnes G.B., Giles G.G., Wallace A.B., Anderson P.R., Guiver T.A., McGale P., Cain T.M., Dowty J.G., Bickerstaffe A.C., Darby S.C. Cancer risk in 680,000 people exposed to computed tomography scans in childhood or adolescence: data linkage study of 11 million Australians. BMJ. 2013;346:f2360. doi: 10.1136/bmj.f2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pearce M.S., Salotti J.A., Little M.P., McHugh K., Lee C., Kim K.P., Howe N.L., Ronckers C.M., Rajaraman P., Sir Craft A.W., Parker L., Berrington de Gonzalez A. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet. 2012;380(9840):499–505. doi: 10.1016/S0140-6736(12)60815-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pierobon J., Webber C.E., Nayiager T., Barr R.D., Moran G.R., Gulenchyn K.Y. Radiation doses originating from diagnostic procedures during the treatment and follow-up of children and adolescents with malignant lymphoma. J. Radiol. Prot. 2011;31(1):83–93. doi: 10.1088/0952-4746/31/1/005. [DOI] [PubMed] [Google Scholar]
- 9.International Atomic Energy Agency . IAEA; Vienna: 2011. Cytogenetic Dosimetry: Applications in Preparedness for and Response to Radiation Emergencies. [Google Scholar]
- 10.Rogakou E.P., Boon C., Redon C., Bonner W.M. Megabase chromatin domains involved in DNA double-strand breaks in vivo. J. Cell Biol. 1999;146(5):905–916. doi: 10.1083/jcb.146.5.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scully R., Xie A. Double strand break repair functions of histone H2AX. Mutat. Res. 2013;750(1–2):5–14. doi: 10.1016/j.mrfmmm.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lobrich M., Rief N., Kuhne M., Heckmann M., Fleckenstein J., Rube C., Uder M. In vivo formation and repair of DNA double-strand breaks after computed tomography examinations. Proc. Natl. Acad. Sci. U. S. A. 2005;102(25):8984–8989. doi: 10.1073/pnas.0501895102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rothkamm K., Balroop S., Shekhdar J., Fernie P., Goh V. Leukocyte DNA damage after multi-detector row CT: a quantitative biomarker of low-level radiation exposure. Radiology. 2007;242(1):244–251. doi: 10.1148/radiol.2421060171. [DOI] [PubMed] [Google Scholar]
- 14.Beels L., Bacher K., Smeets P., Verstraete K., Vral A., Thierens H. Dose-length product of scanners correlates with DNA damage in patients undergoing contrast CT. Eur. J. Radiol. 2012;81(7):1495–1499. doi: 10.1016/j.ejrad.2011.04.063. [DOI] [PubMed] [Google Scholar]
- 15.Kuefner M.A., Grudzenski S., Hamann J., Achenbach S., Lell M., Anders K., Schwab S.A., Haberle L., Lobrich M., Uder M. Effect of CT scan protocols on x-ray-induced DNA double-strand breaks in blood lymphocytes of patients undergoing coronary CT angiography. Eur. Radiol. 2010;20(12):2917–2924. doi: 10.1007/s00330-010-1873-9. [DOI] [PubMed] [Google Scholar]
- 16.Lurz M., Lell M.M., Wuest W., Eller A., Scharf M., Uder M., May M.S. Automated tube voltage selection in thoracoabdominal computed tomography at high pitch using a third-generation dual-source scanner: image quality and radiation dose performance. Invest. Radiol. 2015;50(5):352–360. doi: 10.1097/RLI.0000000000000133. [DOI] [PubMed] [Google Scholar]
- 17.Meyer M., Haubenreisser H., Schoepf U.J., Vliegenthart R., Leidecker C., Allmendinger T., Lehmann R., Sudarski S., Borggrefe M., Schoenberg S.O., Henzler T. Closing in on the K edge: coronary CT angiography at 100, 80, and 70 kV-initial comparison of a second-versus a third-generation dual-source CT system. Radiology. 2014;273(2):373–382. doi: 10.1148/radiol.14140244. [DOI] [PubMed] [Google Scholar]
- 18.Eloot L., Devos D., Van Meerbeeck S., Achten E., Verstraete K., Thierens H., Bacher K. Organ doses and radiation risk of computed tomographic coronary angiography in a clinical patient population: how do low-dose acquisition modes compare? J. Comput. Assist. Tomogr. 2015;39(4):591–597. doi: 10.1097/RCT.0000000000000253. [DOI] [PubMed] [Google Scholar]
- 19.Meyer M., Klein S.A., Brix G., Fink C., Pilz L., Jafarov H., Hofmann W.K., Schoenberg S.O., Henzler T. Whole-body CT for lymphoma staging: feasibility of halving radiation dose and risk by iterative image reconstruction. Eur. J. Radiol. 2014;83(2):315–321. doi: 10.1016/j.ejrad.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 20.Solomon J., Mileto A., Ramirez-Giraldo J.C., Samei E. Diagnostic performance of an advanced modeled iterative reconstruction algorithm for low-contrast detectability with a third-generation dual-source multidetector CT scanner: potential for radiation dose reduction in a multireader study. Radiology. 2015;275(3):735–745. doi: 10.1148/radiol.15142005. [DOI] [PubMed] [Google Scholar]
- 21.Piechowiak E.I., Peter J.F., Kleb B., Klose K.J., Heverhagen J.T. Intravenous iodinated contrast agents amplify DNA radiation damage at CT. Radiology. 2015;275(3):692–697. doi: 10.1148/radiol.14132478. [DOI] [PubMed] [Google Scholar]
- 22.Grudzenski S., Kuefner M.A., Heckmann M.B., Uder M., Lobrich M. Contrast medium-enhanced radiation damage caused by CT examinations. Radiology. 2009;253(3):706–714. doi: 10.1148/radiol.2533090468. [DOI] [PubMed] [Google Scholar]
- 23.Rothkamm K., Lobrich M. Evidence for a lack of DNA double-strand break repair in human cells exposed to very low x-ray doses. Proc. Natl. Acad. Sci. U. S. A. 2003;100(9):5057–5062. doi: 10.1073/pnas.0830918100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuefner M.A., Grudzenski S., Schwab S.A., Wiederseiner M., Heckmann M., Bautz W., Lobrich M., Uder M. DNA double-strand breaks and their repair in blood lymphocytes of patients undergoing angiographic procedures. Invest. Radiol. 2009;44(8):440–446. doi: 10.1097/RLI.0b013e3181a654a5. [DOI] [PubMed] [Google Scholar]
- 25.Kuefner M.A., Brand M., Ehrlich J., Braga L., Uder M., Semelka R.C. Effect of antioxidants on X-ray-induced gamma-H2AX foci in human blood lymphocytes: preliminary observations. Radiology. 2012;264(1):59–67. doi: 10.1148/radiol.12111730. [DOI] [PubMed] [Google Scholar]
- 26.Redon C.E., Nakamura A.J., Gouliaeva K., Rahman A., Blakely W.F., Bonner W.M. The use of gamma-H2AX as a biodosimeter for total-body radiation exposure in non-human primates. PLoS One. 2010;5(11):e15544. doi: 10.1371/journal.pone.0015544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mladenov E., Iliakis G. Induction and repair of DNA double strand breaks: the increasing spectrum of non-homologous end joining pathways. Mutat. Res. 2011;711(1–2):61–72. doi: 10.1016/j.mrfmmm.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 28.Iliakis G., Murmann T., Soni A. Alternative end-joining repair pathways are the ultimate backup for abrogated classical non-homologous end-joining and homologous recombination repair: implications for the formation of chromosome translocations. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2015;793:166–175. doi: 10.1016/j.mrgentox.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 29.Shah D.J., Sachs R.K., Wilson D.J. Radiation-induced cancer: a modern view. Br. J. Radiol. 2012;85(1020):e1166–73. doi: 10.1259/bjr/25026140. [DOI] [PMC free article] [PubMed] [Google Scholar]