Abstract
This data article is related to the subject of a publication in Process Biochemistry, entitled “Chloroperoxidase-catalyzed amino alcohol oxidation: Substrate specificity and novel strategy for the synthesis of N-Cbz-3-aminopropanal” (Masdeu et al., 2016) [1]. Here, the products of the chemical reaction involving the amino aldehyde (N-Cbz-3-aminopropanal) and peroxides (tert-butyl hydroperoxide and H2O2) are characterized by NMR. 1H and 13C NMR full characterization of the products was obtained based on 2D NMR, 1D selective NOESY and diffusion spectroscopy (DOSY) experiments.
Keywords: N-Cbz-3-aminopropanal, Aldehyde-peroxide reaction, NMR identification
Specifications Table
| Subject area | Biotechnology, Biochemistry |
| More specific subject area | Biocatalysis |
| Type of data | Figures, scheme, table |
| How data was acquired | NMR data adquired on a Bruker Avance II 600 nuclear magnetic resonance spectrometer (Bruker Biospin) equipped with a 5 mm TBI probe with Z-gradients and a TCU (temperature control unit). |
| Data format | Analyzed |
| Experimental factors | By-products were obtained by 24-h incubation of the amino aldehyde and peroxides. |
| Experimental features | Dried samples were dissolved in CDCl3and/or D2O and analyzed.1H (600.13 MHz) and13C (150.13 MHz) NMR spectra were recorded at 298.0 K of temperature. Spectra of CDCl3samples were calibrated using the residual solvent signal (7.26 ppm for1H and 77.16 for13C) and spectra of aqueous samples using TSP as internal reference. |
| Data source location | Bellaterra, Spain, Universitat Autònoma de Barcelona |
| Data accessibility | Data is with this article. |
Value of the data
-
•
NMR-based concerted analysis is helpful for the identification of complex molecules by NMR correlations.
-
•
A simple 1H NMR experiment and comparison of signal chemical shifts and multiplicities are beneficial for the rapid identification of the products from a chemical reaction aldehyde-peroxide.
-
•
Data in 1H NMR and 13C NMR is valuable for related chemical reactions between peroxides and an aldehyde.
1. Data
This paper deals about the identification of the products from the chemical reaction between N-Cbz-3-aminopropanal (β-CHO) and tert-butyl hydroperoxide (t-BuOOH) or H2O2. It describes the preparation of the samples prior the NMR measurements, and the concerted analysis of the NMR spectra and 2D correlations.
2. Experimental design, materials and methods
Three preparative reactions were carried out in order to obtain enough amounts of by-products (compounds 6–8, Scheme 1) for further analyses. β-CHO (17 mM, maximum solubility) was dissolved in 10 mL water. Selected peroxide was added to the reaction medium and left in incubation for 24 h. For compound 6 preparation, 250 mM t-BuOOH was employed (ca 50% yield); for 7, 600 mM H2O2 (ca 65% yield); for 8, 72 mM H2O2 (ca 99% yield). All reactions were performed at 25 °C, 1000 rpm of MultiTherm™ orbital stirring. Compounds 6–7 were carefully filtered prior the analysis to eliminate impurities. Compound 8 was isolated by filtration and cautiously dried at 35 °C.
Scheme 1.
Reaction scheme extracted from the related research article [1]. The formed amino aldehyde from the chloroperoxidase-catalyzed N-Cbz-3-aminopropanol (β-OH) oxidation reacts with either t-BuOOH or H2O2. Compounds 6–8 have been characterized by NMR data.
For the identification of the product from reaction between β-CHO and t-BuOOH, the reaction medium (containing product 6) was directly analyzed. 200 µL of D2O (99.96% D), containing 0.3% of TSP (trimethylsilyl propanoic acid), were added to a 400 µL aliquot of the aqueous crude and the dissolution was transferred to a 5-mm-diameter NMR tube. To analyze the reaction intermediate in the β-CHO-H2O2 reaction (compound 7), 200 µL of D2O (99.96% D), containing 0.3% of TSP, were added to a 400 µL aliquot of the aqueous sample of the reaction crude. The solution was transferred to a 5-mm-diameter NMR tube. For compound 8 identification, the dried reaction by-product (20.2 mg) of the oxidation reaction between β-CHO and H2O2 was dissolved in 600 µL of CDCl3 (99.96% D).
1H (600.13 MHz) and 13C (150.13 MHz) NMR spectra were recorded at 298.0 K of temperature on a Bruker Avance II 600 nuclear magnetic resonance spectrometer (Bruker Biospin, Rheinstetten, Germany) equipped with a 5 mm TBI probe with Z-gradients and a TCU (temperature control unit). Initially, 1D 1H NMR spectra of all samples were acquired. For that, a standard 90° pulse sequence, with an acquisition time of 1.71 s and a relaxation delay of 2 s was recorded. Data were collected into 32 K computer data points, with a spectral width of 9590 Hz and as the sum of 1024 transients. The resulting free induction decays (FIDs) were Fourier transformed manually phased and baseline corrected. In the case of samples containing H2O, the peak of the protonated water was suppressed by the standard presaturation of the signal.
The structural characterization of compounds was carried out with the aid of 2D NMR experiments, such as COSY (Correlated Spectroscopy), HSQC (Heteronuclear Single Quantum Correlation), HMBC (Heteronuclear Multiple Bond Correlation), NOESY (Nuclear Overhauser and Exchange Spectroscopy), DOSY (Diffusion Spectroscopy) and 1D selective NOESY experiments performed under standard conditions. When required, solvent suppression techniques were applied. Spectra of CDCl3 samples were calibrated using the residual solvent signal (7.26 ppm for 1H and 77.16 for 13C) and spectra of aqueous samples using TSP as internal reference.
The NMR analysis of the reaction media of β-CHO and t-BuOOH oxidation, revealed the formation of compound 6. The results obtained from 1D 1H, COSY and 1D selective NOESY experiments of the aqueous media of reaction, allowed the 1H NMR characterization of the molecule. Fig. 1 shows the structure of molecule 6 and the 1H spectrum of the reaction media with the assignment of proton signals of 6. Fig. 1b and c shows, respectively, the 1D selective NOESY spectra obtained when signals in the t-butyl region (1.15 ppm) and when signal corresponding to H1 (5.13 ppm) was irradiated. NOESY correlation between H1 and H13 confirmed the presence of the t-butyl moiety in the molecule (see Table 1).
Fig. 1.
NMR spectra of the reaction medium of β-CHO and t-BuOOH oxidation with solvent H2O–D2O (67:33). (a) 1H NMR spectrum of the sample with suppression of the H2O signal, (b) 1H 1D selective NOESY spectrum with irradiation of H13 signal at 1.15 ppm, and (c) 1H 1D selective NOESY spectrum with irradiation of H1 signal at 5.13 ppm. Experiments acquired at 298.0 K and at a magnetic field of 600 MHz.
Table 1.
1H and 13C NMR chemical shifts (δ) and multiplicity of β-CHO and compounds 6–8 at 298.0 K of temperature.
|
β-CHOa |
6b |
7b |
8a |
||||
|---|---|---|---|---|---|---|---|
| δ (1H) | δ (13C) | δ (1H) | δ (1H) | δ (13C) | δ (1H) | δ (13C) | |
| Id. | [ppm] | [ppm] | [ppm] | [ppm] | [ppm] | [ppm] | [ppm] |
| 1 | 9.79 (s)c | 201.0 | 5.13 (t) | 5.09 (t) | 99.3 | 5.39 (m) | 99.4 |
| 2 | 2.73 (t) | 43.9 | 1.69 (m)/1.75 (m) | 1.69 (m)/1.74 (m) | 32.4 | 1.79 (m) | 33.6 |
| 3 | 3.48 (t) | 34.2 | 3.17 (m) | 3.17 (m) | 36.5 | 3.36 (m) | 36.1 |
| 4 | – | 156.5 | – | – | 158.1 | – | 157.0 |
| 5 | 5.08 (s, br) | 66.7 | 5.03 (s, br) | 5.04 (s, br) | 66.8 | 5.09 (s, br) | 66.7 |
| 6 | – | 136.7 | – | – | 136.4 | – | 136.2 |
| 7-11 | 7.29–7.39 (m, br) | 126.2–130.3 | 7.29–7.39 (m, br) | 7.29–7.39 (m, br) | 126.2–130.3 | 7.29–7.39 (m, br) | 126.2–130.3 |
| 13 | – | – | 1.15 (s) | – | – | – | – |
In CDCl3.
In H2O–D2O (67:33).
Signal multiplicity: s, singlet; t, triplet; m, multiplet; br, broad.
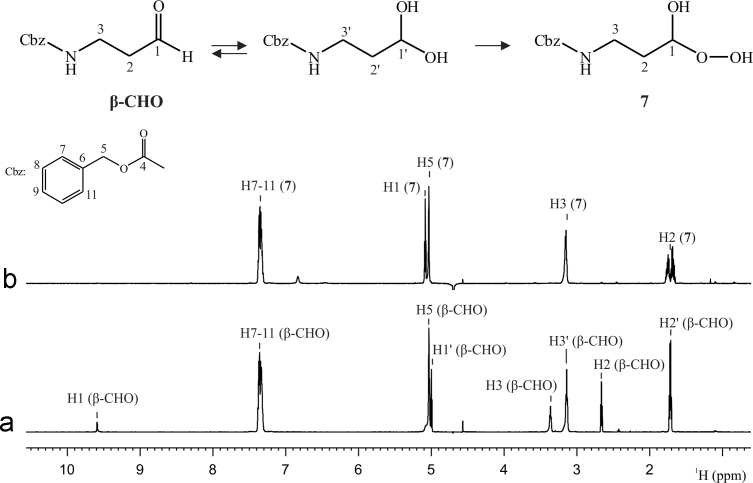
The intermediate of the reaction β-CHO-H2O2 was identified as the hydroxy peroxy derivative of β-CHO, compound 7. The 1H NMR spectra of β-CHO dissolved in D2O and of an aliquot from the mentioned chemical reaction in H2O–D2O (67:33) were compared. In the case of β-CHO, two species were observed in the aqueous solution, which corresponded to the equilibria of the aldehydic and the acetal forms of the molecule (see Fig. 2a). Some important differences were observed between the β-CHO spectra and that of the reaction sample in aqueous media. In the case of the later, just one species was observed, meaning that the aldehydic and/or acetal forms were not present anymore. Besides, the signal corresponding to H1 was slightly down field shifted compared to the acetal form of β-CHO (from 5.00 to 5.09 ppm), which was consistent with the presence of a hydroxy peroxy moiety (see Table 1). Also, no other unidentified signals were observed in the 1H spectrum (see Fig. 2b).
Fig. 2.
1H NMR spectrum of (a) β-CHO in D2O and of (b) an aliquot of the β-CHO-H2O2 reaction media in H2O–D2O (67:33). Experiments acquired at 298.0 K and at a magnetic field of 600 MHz.
NMR spectroscopy allowed the identification of compound 8 yielded by the oxidation of β-CHO with hydrogen peroxide (see Fig. 3). Initially, β-CHO was 1H and 13C fully characterized by the concerted analysis of the 2D NMR correlations COSY, HSQC and HMBC (see Fig. 3 and Table 1) [2], [3] Likewise, a second sample consisting in the product of the oxidation reaction, dissolved in CDCl3, was analyzed. The analysis showed a new molecule, 8, and the presence of reactive β-CHO in a smaller amount. Fig. 3 shows the structure of 8 and the 1H NMR spectra of the analyzed samples with the assignment of the signals. Briefly, comparing with the spectrum of β-CHO, compound 8 showed no aldehydic proton and a new signal appeared at δ(1H) 5.39 ppm and δ(13C) 99.37 ppm. Also, protons H2 and H3 resonated at lower frequencies regarding their analogues of β-CHO (see Fig. 3). The DOSY experiment performed in the second sample (see Fig. 3c and Table 1) showed a significant lower diffusion (i.e. a smaller diffusion coefficient, D) of molecule 8 respect to β-CHO. This result, together with the information yielded by the 2D correlations, confirmed the dimeric structure of compound 8.
Fig. 3.
NMR spectra of β-CHO and 8 in CDCl3. (a) 1H NMR spectrum of β-CHO, (b) 1H NMR spectrum and c) 1H DOSY spectrum of sample containing compound 8 and reactive β-CHO (indicated with *). Experiments acquired at 298.0 K and at a magnetic field of 600 MHz.
Products 6–8 masses were confirmed by mass spectrometry (protocol not detailed, Supplementary material): HPLC-MS-MS (1200RR LC – Agilent Technologies, Santa Clara, CA, USA – and micrOTOF-Q with Apollo II Electrospray ion source – Bruker Technologies, Billerica, MA, USA) or MS (micrOTOF-Q II with Apollo II Electrospray ion source – Bruker Technologies). Those analyses were executed by SAQ (Servei d’Anàlisi Química, UAB, Barcelona, Spain).
2.1. Complete characterization of the three compounds is detailed below
Benzyl (3-(tert-butylperoxy)-3-hydroxypropyl)carbamate (6): Colorless; 1H NMR (600 MHz, H2O–D2O 67:33): δ=7.39–7.29 (br m, 5H), 5.13 (t, J=5.8, 1H), 5.03 (br s, 2H), 3.17 (br m, 2H), 1.75 (m, 1H), 1.69 (m, 1H), 1.15 (s, 9H); MS-ESI+: m/z=320.1469, calcd. for C15H23NO5: 320.1468.
Benzyl (3-hydroperoxy-3-hydroxypropyl)carbamate (7): Colorless; 1H NMR (600 MHz, H2O–D2O 67:33): δ=7.39–7.29 (br m, 5H), 5.09 (t, J=5.8, 1H), 5.04 (br s, 2H), 3.17 (br m, 2H), 1.74 (m, 1H), 1.69 (m, 1H); 13C NMR (150 MHz, CDCl3): δ=158.1, 136.4, 128.9, 128.5, 128.3, 99.3, 66.8, 36.5, 32.4; HPLC-MS-ESI+: m/z=264.0856, calcd. for C11H15NO5: 264.0842.
Dibenzyl (peroxybis(3-hydroxypropane-3,1-diyl))dicarbamate (8): White solid; 1H NMR (600 MHz, CDCl3): δ=7.39–7.29 (br m, 10H), 5.39 (br m, 2H), 5.09 (br m, 4H), 3.36 (br m, 4H), 1.79 (br m, 4H); 13C NMR (150 MHz, CDCl3): δ=157.0, 136.2, 128.9, 128.5, 128.3, 99.4, 66.7, 36.1, 33.6; MS-ESI+: m/z=471.1744, calcd. for C22H28N2O8: 471.1738.
Acknowledgments
We thank Dr. Alba Eustaquio and Dr. Maria Jesús Ibarz at Servei d’Anàlisi Química of the Universitat Autònoma de Barcelona for their assistance on mass spectrometry analyses. This work has been supported by Spanish MINECO (project number CTQ2014-53114R), cofinanced by European Regional Development Fund (ERDF).
The Department of Chemical, Biological and Environmental Engineering of UAB constitutes the Biochemical Engineering Unit of the Reference Network in Biotechnology (XRB) and the research group 2014 SGR 452, Generalitat de Catalunya. Financial support from Spanish MINECO for Ph.D. scholarship of Gerard Masdeu is acknowledged.
Footnotes
Transparency data associated with this article can be found in the online version at doi:10.1016/j.dib.2016.06.028.
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.dib.2016.06.028.
Transparency document. Supplementary material
Supplementary material
Appendix A. Supplementary material
Supplementary material
.
References
- 1.Masdeu G., Pérez-Trujillo M., López-Santín J., Álvaro G. Chloroperoxidase-catalyzed amino alcohol oxidation: substrate specificity and novel strategy for the synthesis of N-Cbz-3-aminopropanal. Process. Biochem. 2016 [Google Scholar]
- 2.Breitmaier E. John Wiley & Sons; Chichester: 2003. Structure Elucidation by NMR in Organic Chemistry: A Practical Guide. [Google Scholar]
- 3.Berger S., Braun S. John Wiley & Sons; Chichester: 2004. 200 and More NMR Experiments. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material