Abstract
The aim of this study is to examine the amyloid β (Aβ) inhibition mechanism of plant sprouts' aqueous extracts (PSAE). In this study, we screened the effects of five plant sprouts' extracts on Aβ (1–42) structure modification using gel electrophoresis. In PSAE, no band of Aβ monomer was recognized in Japanese butterbur. Similarly, the Aβ monomer band became light in buckwheat, red cabbage, broccoli, and brussels. The neuroprotective effects of PSAE were evaluated by measuring levels of Aβ in mixtures (Aβ and PSAE) with Aβ ELISA assay. The treatment with PSAE decreased Aβ levels. The results indicated that the levels of red cabbage, Japanese butterbur, and broccoli were 9.6, 28.0, and 44.0%, respectively. The lowest value was observed with buckwheat. Furthermore, we carried out a Congo Red (CR) and Aβ binding experiment of PSAE to confirm the modification mechanism of PSAE. The correlation coefficient for the absorption spectrum peak of CR was found to be bigger than 0.8 (r = 0.882) which proved that the Aβ levels could be attributed to the peak of CR. In conclusion, we demonstrated that treatment with PSAE effectively decreases Aβ concentration. Thus, the mechanism that decreased the Aβ levels may be modification by PSAE.
1. Introduction
Alzheimer's disease (AD) [1] is currently the most lethal neurodegenerative disorder known. It is characterized by progressive neuronal loss and neuroinflammation in the brain. Neuropathology detects neuronal loss in association with the deposition of amyloid plaques. The aggregation of amyloid β (Aβ) peptides starts with changes in their secondary structure leading to β-sheet formation, progresses with aggregation of the misfolded peptides into oligomers, and culminates in the production of amyloid fibres that precipitate into the brain forming amyloid plaques. Aβ can be neurotoxic by a mechanism linked to peptide fibril formation. Therefore, Aβ peptides are very important in the research of AD. However, the mechanism by which Aβ produces brain dysfunction in patients with AD is largely unknown.
Sprouts are a source of various biologically active phytomolecules, including phenolic compounds, flavonoids, and vitamins [2, 3]. The health properties of sprouts depend on their phenolic compounds [4], which have interesting pharmacological properties. Much research has assessed the dietary role of polyphenolic substances and their characteristics, metabolic pathways, and biological effects [5]. Sprouts have been widely used to scavenge reactive oxygen species (ROS) and treat a variety of diseases [6]. We propose that extracts of sprouts have significant antioxidant activity and suspect the extract might be useful in preventing AD.
Złotek et al. proposed that the glycation process might contribute to both extensive protein cross-linking and oxidative stress in AD [7]. Nonenzymatic protein glycation is an endogenous process in which reducing the sugars that react with amino groups in proteins through a series of Maillard reactions forming reversible Schiff-base and Amadori compounds produces a heterogeneous class of molecules, collectively termed advanced glycation end products (AGEs) [8, 9]. A previous study discussed the globular amyloid-like deposits of D-ribose glycation of bovine serum albumin (BSA) aggregates. The amyloid-like aggregation of glycated BSA induces apoptosis in the neuronal cell. D-ribose saccharifies BSA which then misfolds rapidly and forms globular amyloid-like aggregations, which play an important role in cytotoxicity of neuronal cells [10]. In addition, glycation of Aβ markedly enhances its aggregation in vitro. Therefore, in the present study, we have investigated the effect of glycation on the aggregation pathways of BSA and lactalbumin (LAB). Although this reaction may not be directly related to AD, it is thought to be a good representative model of proteins that intrinsically evolve toward the formation of amyloid aggregates.
Although there are many reports on beneficial effects of plant food extracts to Aβ-induced neuronal cell death, their inhibition mechanisms are yet to be well understood. Therefore, study on the mechanisms is very important in the research of AD.
As a result of having examined modification to Aβ (1–42) by SDS-polyacrylamide gel electrophoresis of about 15 samples (5 sprouts × 3 extract methods) of the plant sprouts, we decided to focus on plant sprouts' aqueous extracts (PSAE) which showed the remarkable result. In addition, we experimented with Aβ alone and with a mixed sample (Aβ+PSAE). Using an amyloid β ELISA kit, we found that PSAE showed the inhibition effect on Aβ. Considering these findings, we examined the Aβ-inhibition mechanism of PSAE.
2. Materials and Methods
2.1. Preparation of Extracts from Plant Sprouts (PSE)
We selected five plant sprouts (buckwheat (Fagopyrum esculentum) sprout (BWS), red cabbage (Brassica oleracea var. capitata) sprout (RCS), broccoli (Brassica oleracea var. italica) sprout (BS), brussels (Brassica oleracea var. gemmifera) sprout (BRS), and Japanese butterbur sprout flower buds (Petasites japonicus) (JBB)). Plant sprouts were purchased from the market in Japan. Plant sprouts (100 g) were minced into 5 mm fragments and extracts were obtained with cold water (CW, 20°C), hot water (HW, 100°C, 10 min), and methanol (MH) overnight at room temperature (Table 1). The extracts were filtered. The MH filtrates were evaporated in a vacuum slightly below 40°C in a rotary evaporator. The MH extracts were prepared as a solution in dimethyl sulfoxide (DMSO). The collected filtrate was then stored at under −20°C until use. When used in assays, each sample was returned to ambient temperature, followed by filtration through a membrane filter (pore size 0.2 μm).
Table 1.
Extract concentrations from plant sprouts. We selected five plant sprouts (buckwheat sprout (BWS), red cabbage sprout (RCS), broccoli sprout (BS), brussels sprout (BRS), and Japanese butterbur sprout flower buds (JBB)). Plant sprouts (100 g) were minced into 5 mm fragments and extracts were obtained with cold water (CW, 20°C), hot water (HW, 100°C, 10 min), and methanol (MH) overnight at room temperature. Extract concentrations were expressed as gram of dry weight/mL of extract volume. The concentration of plant sprouts' extracts (PSE) was calculated as catechin equivalents per gram of plant sprouts. ∗Means ± standard deviation (SD) of three independent experiments.
| Extract | Initial dry weight (g)∗ | Extract volume (mL)∗ | Extract concentration (g of dry weight/mL of extract volume)∗ | Total phenolic content (mg of catechin equiv/g)∗ |
|---|---|---|---|---|
| CW-BWS | 8.40 ± 0.53 | 2.90 ± 0.45 | 2.90 ± 0.53 | 15.9 ± 0.37 |
| CW-RCS | 4.87 ± 0.45 | 2.50 ± 0.38 | 1.95 ± 0.34 | 10.8 ± 0.41 |
| CW-BS | 5.43 ± 0.52 | 2.50 ± 0.31 | 2.17 ± 0.32 | 6.5 ± 0.21 |
| CW-BRS | 10.4 ± 0.65 | 9.20 ± 0.63 | 1.13 ± 0.17 | 9.2 ± 0.71 |
| CW-JBB | 10.0 ± 0.65 | 11.5 ± 0.55 | 0.87 ± 0.08 | 223.3 ± 7.37 |
| HW-BWS | 8.40 ± 0.45 | 4.30 ± 0.35 | 1.95 ± 0.13 | 339.1 ± 8.37 |
| HW-RCS | 4.87 ± 0.25 | 3.20 ± 0.28 | 1.52 ± 0.22 | 36.1 ± 1.41 |
| HW-BS | 5.43 ± 0.35 | 5.50 ± 0.31 | 0.99 ± 0.22 | 242.1 ± 6.21 |
| HW-BRS | 10.0 ± 0.55 | 7.10 ± 0.46 | 1.41 ± 0.27 | 76.5 ± 3.71 |
| HW-JBB | 8.48 ± 0.45 | 7.50 ± 0.45 | 1.13 ± 0.36 | 397.8 ± 9.37 |
| MH-BWS | 10.0 ± 0.55 | 3.95 ± 0.25 | 2.53 ± 0.39 | 433.1 ± 9.37 |
| MH-RCS | 10.0 ± 0.47 | 3.30 ± 0.28 | 3.03 ± 0.34 | 235.2 ± 5.41 |
| MH-BS | 10.0 ± 0.45 | 3.30 ± 0.11 | 3.03 ± 0.22 | 241.0 ± 5.21 |
| MH-BRS | 10.0 ± 0.48 | 3.20 ± 0.16 | 3.13 ± 0.27 | 317.3 ± 7.71 |
| MH-JBB | 37.2 ± 1.05 | 12.2 ± 0.56 | 3.05 ± 0.38 | 371.0 ± 8.37 |
2.2. Measurement of SDS-Polyacrylamide Gel Electrophoresis
As described previously [20], the Aβ modification by PSE was used with measurements of SDS-polyacrylamide gel electrophoresis (PAGE). Briefly, 75 μL of PSE or H2O was combined with 75 μL of Aβ (1–42) (10 μM, Wako Pure Chemical Industries, Ltd.) solution. We allowed the mixture to sit for 20 h at 37°C before measuring SDS-PAGE. Ten μL of incubated samples was mixed with 10 μL of SDS-PAGE sample buffer and loaded on 15% SDS-polyacrylamide gel. The samples were electrophoresed at 40 A for 1 h. The gels were stained for protein with Quick CBB PLUS (Wako Co. Ltd). Molecular masses of the bands obtained were calculated with the help of the standard molecular weight markers (Precision Plus Protein™ Prestained Standards, Bio-Rad Laboratories).
2.3. Determination of Total Phenolic Content (TPC)
We measured total phenolic content (TPC) with a modified version of the Folin-Ciocalteu method [21] using 0–0.1 mg/mL catechin as a standard. Briefly, 100 μL of sample or standard was combined with 100 μL of Folin-Ciocalteu reagent and 100 μL of 2% Na2CO3 solution. We allowed the mixture to sit for 60 min before reading absorbance at 750 nm using an Ultrospec Visible Plate Reader II 96 (GE Healthcare Ltd., England) and calculating the concentration of plant sprouts' aqueous extracts (PSAE) as catechin equivalents per gram of plant sprouts.
2.4. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Radical Scavenging Activity
Scavenging of DPPH free radical is the basis of a common antioxidant assay. Therefore, we used the DPPH method. Employing the procedure described by Negro et al. [22], we measured the free radical scavenging activity of PSAE. After mixing a sample solution (100 μL) with 100 μL of 800 μM DPPH-methanol solution and waiting for 30 min, we measured the absorbance of the sample solution at 520 nm against a blank. DPPH radical scavenging activity was exhibited as follows. The control ratio (%) was expressed as a percentage of the untreated control as follows: % control ratio = (A520 nm of PSAE treated DPPH-methanol solution/A520 nm of untreated control) × 100. All tests and analyses were run in triplicate and averaged.
2.5. Assessment of Aβ (1–42) Concentration
Levels of Aβ (1–42) in mixtures (10 μM Aβ (1–42) 55 μL and PSAE 55 μL) were determined with human specific enzyme-linked immunosorbent assay (ELISA) (Wako, Osaka), according to the manufacturer's instructions. The mixtures (110 μL) were added to microplate wells. The mixtures were then incubated at room temperature for 24 h. After 24 h incubation, we obtained 100 μL of sample and distributed some of the sample into each well coated with a monoclonal antibody specific to the NH2-terminus region of human Aβ. Detected Aβ (1–42) was analyzed with a commercial kit according to instructions provided by the manufacturer (Wako Pure Chemical Industries). The detection limit of the assay was 0.1 pmol/L for Aβ (1–42). Epigallocatechin gallate (EGCG) was used as the positive control. The Aβ (1–42) level was measured as A450 nm. The control ratio (%) was expressed as a percentage of the untreated control as follows: % control ratio = (A450 nm of treated cells/A450 nm of untreated cells) × 100.
2.6. In Vitro Glycation of Bovine Serum Albumin (BSA) and Lactalbumin (LAB) Induced by D-Ribose
Inhibition of glycation was measured with a modified version of the Wei method [10]. After sterilization, using a Millex GV filter (Millipore, Cork, Ireland) to prevent bacterial growth, BSA and LAB were dissolved in 20 mM Tris-HCl (pH 7.4) to yield a stock solution of 20 mg/mL. D-ribose (1 M, a final concentration) was then prepared in Tris-HCl to final concentrations of 10 mg/mL BSA or LAB. PSAE were added to 20 mM Tris-HCl containing 1 M D-ribose and either BSA or LAB to acquire final concentrations of 10 mg/mL. EGCG was used as the positive control. Then, the solutions were incubated at 37°C for up to 24 h. After incubation, the fluorescent reaction products were assayed on a fluorophotometer (λ ex 360 nm/λ em 465 nm, multimode microplate reader Infinite F200, Tecan Trading AG, Switzerland). BSA or LAB, in the presence of D-ribose, was used as a control. The control ratio (%) was expressed as a percentage of the untreated control as follows: % control ratio = (fluorescence intensity of treated cells/fluorescence intensity of untreated cells) × 100. Each experimental condition was performed in triplicate.
2.7. In Vitro Aggregation of BSA and LAB Induced by D-Ribose
In vitro aggregation of BSA and LAB was measured by methods previously described [20]. Briefly, PSAE was added to 20 mM Tris-HCl containing 1 M (a final concentration) D-ribose and either BSA or LAB to acquire final concentrations of 10 mg/mL. The solutions were then incubated at 37°C for up to 24 h. After incubation, Thioflavin T (ThT, 30 μM), commonly used to detect protein aggregations, was added to the solution to investigate whether any amyloid-like deposits formed at 37°C. After incubation for 10 min, the fluorescent reaction products were assayed on a fluorophotometer (λ ex 430 nm/λ em 465 nm). BSA or LAB, in the presence of D-ribose, was used as a control. In addition, EGCG was used as the positive control. The control ratio (%) was expressed as a percentage of the untreated control as follows: % control ratio = (fluorescence intensity of treated cells/fluorescence intensity of untreated cells) × 100.
2.8. Congo Red (CR) Binding Assay
The binding of CR was monitored using absorption spectroscopy. A 5.0 mM stock solution of CR was prepared in PBS. PSAE was added to PBS containing 10 mM (a final concentration) Aβ (25–35) and Congo Red (CR) solution to acquire final concentrations of 5.0 × 10−6 M. The solutions were then incubated at 25°C for up to 168 h. And then, the mixture was assayed on a UV-VIS spectrophotometer (Multiskan™ GO Microplate Spectrophotometer, Thermo Fisher Scientific, MA, USA).
2.9. Statistical Analysis
We present all data as mean ± standard deviation of the three measurements. A statistical comparison between the groups was carried out using either ANOVA or Student's t-test. P < 0.05 were considered as statistically significant.
3. Results
3.1. Amyloid β Electrophoretic Analysis
In the present study, we investigated the effects of PSE on Aβ (1–42) structure modification. The mixture samples were electrophoresed at 40 A for 1 h (Figure 1). No band of Aβ monomer (4.5 kDa) was recognized in CW-JBB. Similarly, the Aβ monomer (4.5 kDa) band became light in CW-RCS, CW-BS, CW-BRS, and CW-BWS. These suggest that some special conformation, presumably in protein, was present in a major constituent of amyloid. Therefore, we carried out the following experiments involving PSAE that showed that its effect was remarkable in this electrophoretic experiment.
Figure 1.
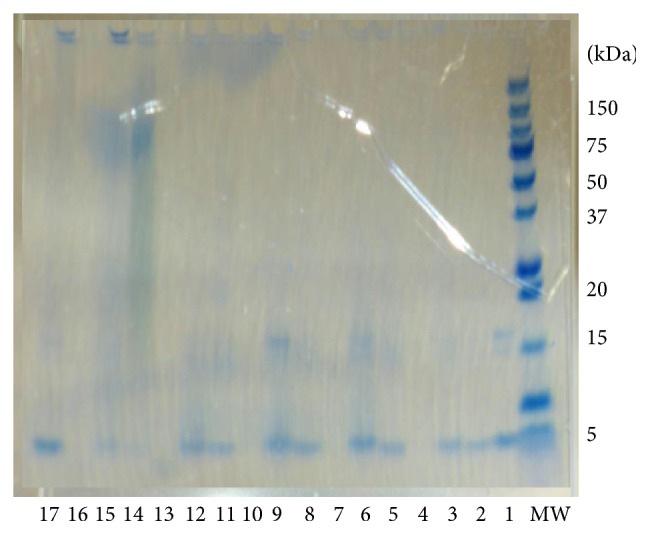
15% SDS-PAGE of the products of amyloid β (Aβ) (1–42) incubation with extracts from plant sprouts (PSE). SDS-PAGE illustrating the inhibition effect of PSE on Aβ (1–42). Lanes (from right to left) indicating the electrophoretic migration of Aβ (1–42) in the absence of PSE and in the presence of PSE. 1: Aβ (1–42); 2: cold water- (CW-) buckwheat sprout (BWS); 3: hot water- (HW-) buckwheat sprout (BWS); 4: methanol- (MH-) buckwheat sprout (BWS); 5: CW-red cabbage sprout (RCS); 6: HW-red cabbage sprout (RCS); 7: MH-red cabbage sprout (RCS); 8: CW-broccoli sprout (BS); 9: HW-broccoli sprout (BS); 10: MH-broccoli sprout (BS); 11: CW-brussels sprout (BRS); 12: HW-brussels sprout (BRS); 13: MH-brussels sprout (BRS); 14: CW-Japanese butterbur (JBB); 15: HW-Japanese butterbur (JBB); 16: MH-Japanese butterbur (JBB); 17: epigallocatechin gallate (EGCG). MW shows a molecular weight marker. All tested samples were analyzed by 15% Tris-HCl-SDS-PAGE, followed by Quick-CBB PLUS staining, as described in the experimental part.
3.2. PSAE Reduces Aβ (1–42) Concentration
To examine the effects of PSAE on the Aβ (1–42) concentration, PSAE mixture samples were analyzed by Aβ (1–42) ELISA. Figure 2 illustrates that PSAE were associated with differential reduction in the levels of Aβ (1–42) (CW-RCS, 9.6%; CW-JBB, 28.0%; CW-BS, 44.0%; CW-BRS, 86.7%; CW-BWS, 89.6%), demonstrating that treatment with PSAE effectively decreases Aβ (1–42) concentration. CW-RCS treatment demonstrated the strongest Aβ-inhibition potential (Figure 2). These showed low values from 10 μM EGCG (95.0%).
Figure 2.
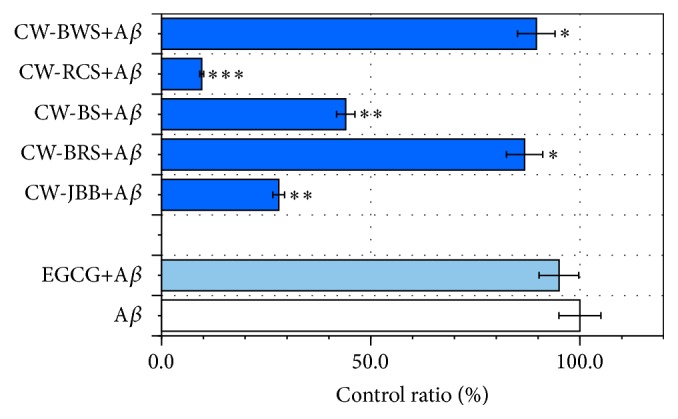
Inhibitory effects of plant sprouts' aqueous extracts (PSAE) on Aβ (1–42) concentration. Levels of Aβ (1–42) in mixtures (10 μM Aβ (1–42) 55 μL and PSAE 55 μL) were determined with human specific enzyme-linked immunosorbent assay. The mixtures (110 μL) were added to the microplate wells and then incubated at room temperature for 20 h. After 20 h incubation, Aβ (1–42) was analyzed with a commercial kit. Values are mean ± SD of the three measurements. ∗∗∗ P < 0.001, ∗∗ P < 0.01, and ∗ P < 0.05 compared with the controls.
3.3. Relationships between Total Phenolic Content (TPC), DPPH Radical Scavenging Activity, and Aβ (1–42) Levels of PSAE
TPC was expressed as mg of catechin equiv/g of matter. Significant differences were observed for TPC among the 5 plant sprout varieties. The total phenolic content varied among species from 6.5 to 433.1 mg (Table 1). The aqueous extracts from broccoli sprouts (CW-BS) contained the lowest amounts. The aqueous extracts from buckwheat, red cabbage, and brussels sprouts contained roughly 1.5–2.5 times the total phenolic content of broccoli sprouts. The aqueous extracts from Japanese butterbur sprouts contained the highest amount of total phenolics (223.3 mg (+)-catechin equivalents/g). Broccoli sprouts contained 1/34 of the phenolics observed in Japanese butterbur sprouts.
The radical scavenging effect is proportional to the disappearance of DPPH in test samples. Based on this principle, the radical scavenging effect of each plant sprouts extract was measured. The extracts of 5 plant sprouts were compared for their radical scavenging activities against DPPH radical. With regard to the value, the highest radical scavenging activity was found in the extract of CW-BWS (25.0%) and the lowest activity was found in CW-RCS (100.6%). The order of the scavenging activity was 25.0, 55.6, 90.8, 91.0, and 100.6% for CW-BWS, CW-JBB, CW-BS, CW-BRS, and CW-RCS, respectively. However, the scavenging activity of all extracts except for CW-BWS was less than that of ±catechin (50.7%). The activity of CW-BWS was approximately 4 times higher than that of CW-RCS.
On the other hand, the correlations between Aβ (1–42) levels and TPC and DPPH radical scavenging activity were analyzed (Figures 3 and 4). As shown in Figure 3, the Aβ (1–42) levels were not observed to increase depending on TPC levels in PSAE. Specifically, no significant correlation (r = 0.358) was observed between Aβ (1–42) levels and TPC among our experimental samples. As shown in Figure 4, the DPPH radical scavenging activity of the 5 PSAE did not significantly correlate with Aβ (1–42) levels. The correlation coefficient for Aβ (1–42) levels was found to be smaller than 0.5 (r = 0.462) which proved that the DPPH radical scavenging activity could not be attributed to the Aβ (1–42) levels of the 5 PSAE. These results proved that the TPC and DPPH radical scavenging activity of these plant sprouts could not be clearly attributed to their Aβ-inhibition potential.
Figure 3.
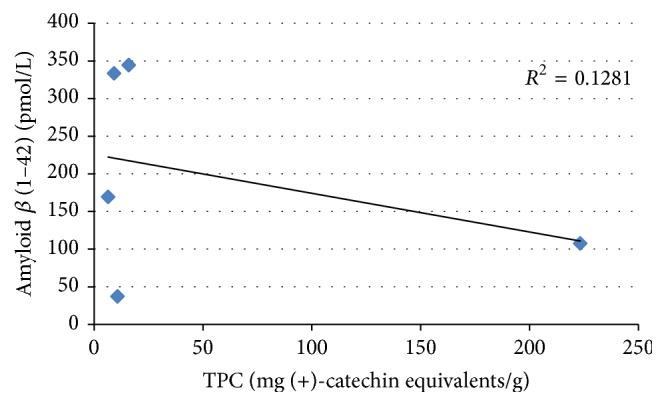
The correlation between levels of Aβ (1–42) and total phenolic contents of aqueous extracts from the sprouts of five plants.
Figure 4.
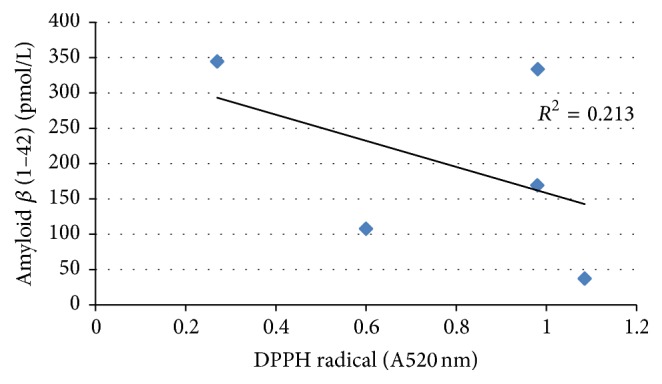
The correlation between levels of Aβ (1–42) and DPPH radical scavenging activities of aqueous extracts from the sprouts of five plants.
3.4. Effects of PSAE on D-Ribose Induced Glycation of BSA or LAB
In the present study, we investigated the effects of PSAE on glycation of BSA or LAB induced by D-ribose. The mixture samples were incubated at 37°C for up to 24 h (Figures 5 and 6). Inhibition of glycation was recognized in CW-JBB in BSA and LAB. Among the 5 PSAE, CW-JBB treatment demonstrated the strongest antiglycation potential. EGCG (100 μM) treatment exhibited the greatest potential for antiglycation. In Figure 5, fluorescence assay results showed that BSA glycation levels did not decrease in the 24 h PSAE-loaded treatments relative to the control. However, BSA glycation by CW-JBB and EGCG inhibited 67.2 ± 3.58% and 85.0 ± 4.55%, respectively (P < 0.01). Among the 5 PSAE, CW-JBB caused a maximum decrease in BSA glycation (Figure 5).
Figure 5.
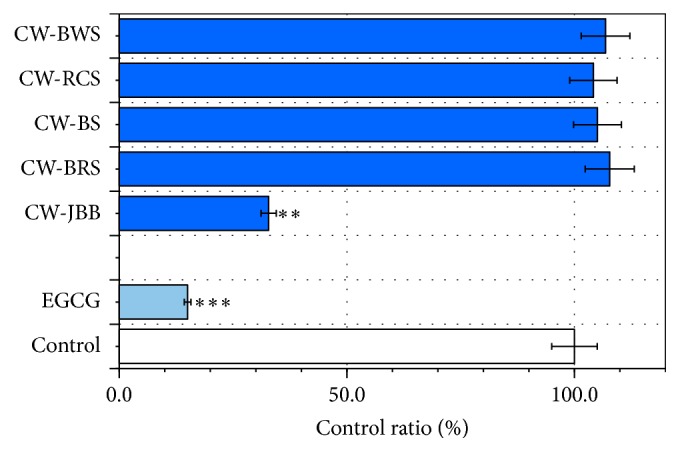
Changes in the fluorescence of BSA+D-ribose treated with plant sprouts' aqueous extracts (PSAE). BSA (final concentration 10 mg/mL) in the presence of D-ribose (final concentration 1 M) was kept at 37°C in Tris-HCl buffer (pH 7.4). PSAE was mixed with samples of BSA+D-ribose for up to 24 h. The fluorescence intensity of glycation was recorded (λ ex 360 nm; λ em 465 nm). BSA (or LAB) and D-ribose were used as a control. Aliquots were taken for measurements of fluorescence spectra (λ ex = 360 nm). Values are mean ± SD of the three measurements. ∗∗∗ P < 0.001 and ∗∗ P < 0.01 compared with the controls.
Figure 6.
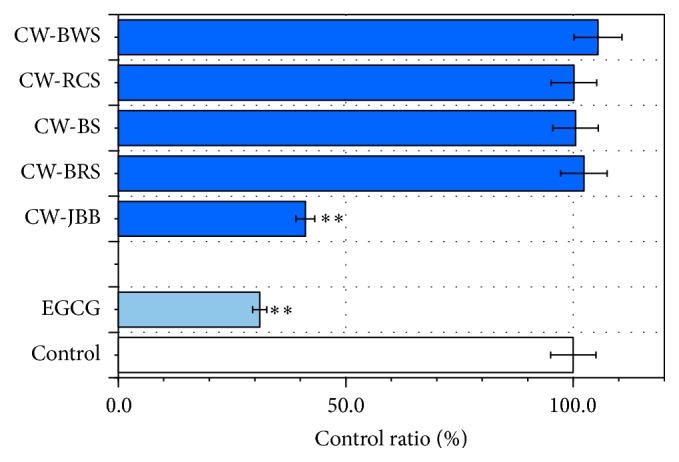
Changes in the fluorescence of LAB+D-ribose treated with plant sprouts' aqueous extracts (PSAE). LAB (final concentration 10 mg/mL) in the presence of D-ribose (final concentration 1 M) was kept at 37°C in Tris-HCl buffer (pH 7.4). PSAE was mixed with samples of LAB+D-ribose for up to 24 h. The fluorescence intensity of glycation was recorded (λ ex 360 nm; λ em 465 nm). LAB and D-ribose were used as a control. Aliquots were taken for measurements of fluorescence spectra (λ ex = 360 nm). Values are mean ± SD of the three measurements. ∗∗ P < 0.01 compared with the controls.
The correlations between BSA glycation inhibition and Aβ (1–42) levels were analyzed. The Aβ (1–42) levels of the 5 PSAE did not significantly correlate with BSA glycation inhibition. The correlation coefficient for BSA glycation inhibition was found to be smaller than 0.5 (r = 0.409) which proved that the Aβ (1–42) levels could not be attributed to BSA glycation inhibition.
Fluorescence assay results (Figure 6) showed that LAB glycation level did not decrease in the 24 h PSAE-loaded treatment relative to the control. However, LAB glycation by CW-JBB inhibited 58.9 ± 2.85% (P < 0.01). EGCG (100 μM) treatment exhibited the greatest potential for antiglycation (68.9 ± 3.59%). The correlations between LAB glycation inhibition and Aβ (1–42) levels were analyzed. The Aβ (1–42) levels of the 5 PSAE did not significantly correlate with LAB glycation inhibition. The correlation coefficient for LAB glycation inhibition was found to be smaller than 0.5 (r = 0.429) which proved that the Aβ (1–42) levels could not be attributed to the LAB glycation inhibition of the 5 PSAE. These results proved that the Aβ (1–42) levels of these plants could not be clearly attributed to their LAB glycation inhibition.
3.5. Effects of PSAE on Aggregates of D-Ribose-Glycated BSA or LAB
We added ThT (a fluorescent reagent) to test whether PSAE is an inhibitor of amyloid-like aggregates (Figure 7). Fluorescence of ThT at 465 nm significantly increased in the presence of BSA incubated with D-ribose for 24 h. Fluorescence intensity showed about 36548 ± 1288 counts in BSA+D-ribose (Figure 7). Inhibition of aggregates was recognized in CW-BWS in BSA and CW-JBB in BSA and LAB. Among the 5 PSAE, CW-JBB treatment demonstrated the strongest antiaggregation potential. EGCG (100 μM) treatment exhibited the second greatest potential for antiaggregation. In Figure 7, fluorescence assay results showed that BSA aggregation levels did not decrease in the 24 h PSAE-loaded treatments relative to the control. However, BSA aggregation by CW-JBB and CW-BWS inhibited 13.1 ± 1.15% and 11.5 ± 0.85%, respectively (P < 0.05). EGCG (100 μM) treatment exhibited the second greatest potential for antiaggregation (12.5 ± 1.38%). BSA+D-ribose incubated with CW-JBB and CW-BWS showed decreases in ThT fluorescence under our experimental conditions.
Figure 7.
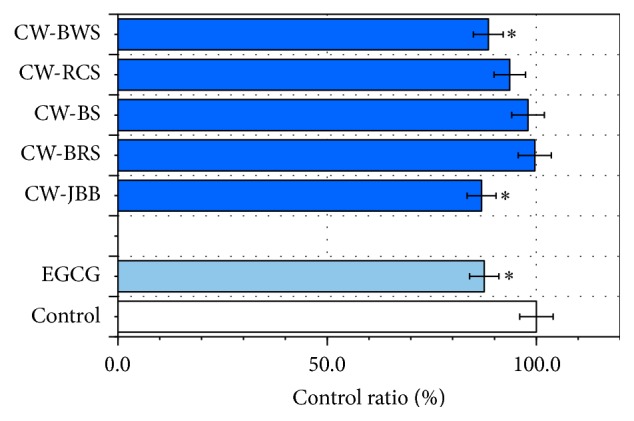
Changes in the Thioflavin T fluorescence of BSA+D-ribose treated with plant sprouts' aqueous extracts (PSAE). BSA (final concentration 10 mg/mL) in the presence of D-ribose (final concentration 1 M) was kept at 37°C in Tris-HCl buffer (pH 7.4). Thioflavin T (final concentration 30 μM) was mixed with samples of BSA+D-ribose+PSAE, as described in Section 2.7. The fluorescence intensity of Thioflavin T was recorded (λ ex 430 nm; λ em 465 nm). BSA and D-ribose were used as a control. Aliquots were taken for measurements of fluorescence spectra (λ ex = 430 nm). Values are mean ± SD of the three measurements. ∗ P < 0.05 compared with the controls.
The correlations between BSA aggregation inhibition and Aβ (1–42) levels were analyzed. The Aβ (1–42) levels of the 5 PSAE did not significantly correlate with BSA aggregation inhibition. The correlation coefficient for BSA aggregation inhibition was found to be smaller than 0.2 (r = 0.176) which proved that the Aβ (1–42) levels could not be attributed to BSA aggregation inhibition.
Again, we added ThT to test whether PSAE is an inhibitor of amyloid-like aggregates (Figure 8). This time, however, fluorescence of ThT at 465 nm did not significantly decrease in the presence of LAB incubated with D-ribose for 24 h except for CW-JBB. Fluorescence intensity showed about 35751 ± 1276 counts in LAB+D-ribose. LAB+D-ribose incubated with PSAE except for CW-JBB showed no significant changes in ThT fluorescence under our experimental conditions. However, LAB+D-ribose incubated with CW-JBB showed significant decrease in ThT fluorescence (10.0 ± 0.43%). EGCG (100 μM) treatment exhibited the second greatest potential for antiaggregation (9.6 ± 0.45%). The correlations between LAB aggregation inhibition and Aβ (1–42) levels were analyzed. The Aβ (1–42) levels of the 5 PSAE did not significantly correlate with LAB aggregation inhibition. The correlation coefficient for LAB aggregation inhibition was found to be smaller than 0.5 (r = 0.452) which proved that the Aβ (1–42) levels could not be attributed to LAB aggregation inhibition.
Figure 8.
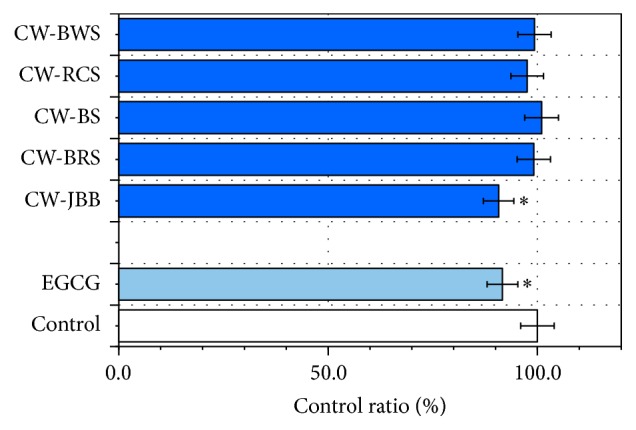
Changes in the Thioflavin T fluorescence of LAB+D-ribose treated with plant sprouts' aqueous extracts (PSAE). LAB (final concentration 10 mg/mL) in the presence of D-ribose (final concentration 1 M) was kept at 37°C in Tris-HCl buffer (pH 7.4). Thioflavin T (final concentration 30 μM) was mixed with samples of LAB+D-ribose+PSAE, as described in Section 2.7. The fluorescence intensity of Thioflavin T was recorded (λ ex 430 nm; λ em 465 nm). LAB and D-ribose were used as a control. Aliquots were taken for measurements of fluorescence spectra (λ ex = 430 nm). Values are mean ± SD of the three measurements. ∗ P < 0.05 compared with the controls.
3.6. Congo Red (CR) Assay
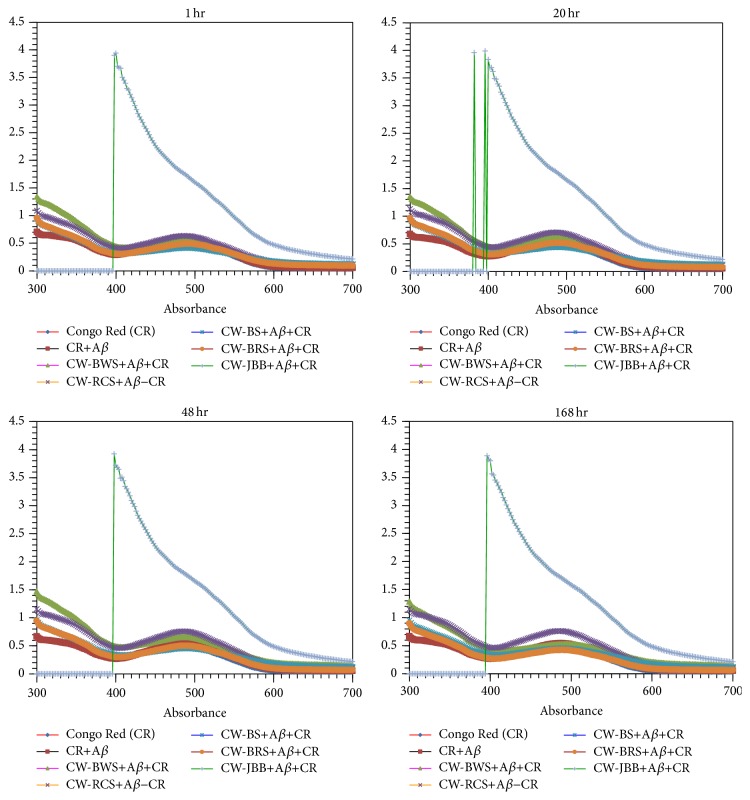
CR is a diazo dye that is widely used to identify amyloid deposits because of its ability to bind preferentially to the aggregated amyloid peptides [23]. We performed CR and Aβ binding experiments to determine whether PSAE are the modification of amyloid structures. CR and Aβ binding is detected as a red shift in the absorbance spectrum (shown by Table 3 and Figure 9). Figure 9 (1, 20, 48, and 168 hr) shows the absorption spectrum of CR and Aβ in the presence of PSAE. A significant red shift from 482 nm to 494 nm occurred upon binding of the dye to Aβ in 168 hr (Table 3 and Figure 9, 168 hr). Similarly, a significant red shift from 482 to 494 nm occurred upon binding of the dye to Aβ in the presence of CW-BWS. On the other hand, a red shift from 482 to 490 nm occurred upon binding of the dye to Aβ in the presence of CW-BS or CW-BRS. However, CW-RCS and Aβ mixture (484 nm) hardly influenced the absorption spectrum peak of CR. Namely, CR and Aβ binding in the presence of CW-RCS was not detected as a red shift in its absorbance spectrum. CW-JBB did not show a peak of the absorption spectrum.
Table 3.
Absorption spectrum peak determined using Congo Red (CR) assay by spectrophotometry in plant sprouts' aqueous extracts (PSAE). We selected five plant sprouts (buckwheat sprout (BWS), red cabbage sprout (RCS), broccoli sprout (BS), brussels sprout (BRS), and Japanese butterbur sprout flower buds (JBB)). This table shows the absorption spectrum peak of CR and Aβ (1–42). The binding of CR and Aβ was monitored using absorption spectroscopy. Values are the means, n = 3.
| Samples | Absorbance peak |
|---|---|
| CW-BWS+Aβ+CR | 494 |
| CW-RCS+Aβ-CR | 484 |
| CW-BS+Aβ+CR | 490 |
| CW-BRS+Aβ+CR | 490 |
| CW-JBB+Aβ+CR | None |
|
| |
| CR+Aβ | 494 |
| Congo Red (CR) | 482 |
Figure 9.
Absorption spectra of 5.0 × 10−6 M Congo red with and without plant sprouts' aqueous extracts (PSAE). The colour lines indicate the difference spectrum. The binding of CR was monitored using absorption spectroscopy for up to 168 h.
The correlations between the absorption spectrum peaks of CR and Aβ (1–42) levels were analyzed. The Aβ (1–42) levels of the 4 (except for JBB) PSAE significantly correlate with the absorption spectrum peak of CR (Table 3). The correlation coefficient for the absorption spectrum peak of CR was found to be bigger than 0.8 (r = 0.882) which proved that the Aβ (1–42) levels could be attributed to the absorption spectrum peak of CR.
4. Discussion
In the present study, we investigated the effects of PSAE on Aβ (1–42) structure modification and demonstrated that treatment with PSAE effectively decreases Aβ (1–42) concentration (Figure 2).
Alzheimer's disease (AD) is receiving attention as “type 3 diabetes” and it is evident that this neurodegenerative disease has multiple shared pathologies with diabetes mellitus. Recently, we published two papers describing the utilization of plant extracts for improved treatment of diabetic complications [24, 25]. We investigated the impact of methanolic extracts from edible plants on the receptor for advanced glycation end products (RAGE) and endogenous secretory receptor for advanced glycation end products (esRAGE) production in human umbilical vein endothelial cells cultured in high glucose (4.5 g/L). The results showed that several extracts reduced RAGE production, and several extracts showed an increase in esRAGE. RAGE-mediated signaling pathway is related to Aβ-induced pathogenic responses [26]. Also, Yamagishi and Matsui indicated that V1- and C1-type domains of RAGE have a net positive charge that might act as an electrostatic trap for negatively charged macromolecules such as AGEs, high-mobility group protein box-1, S-100 calcium-binding protein, and amyloid β protein [27]. Li et al. described that the glycated amyloid β is more toxic [28]. Therefore, inhibition of glycation could be a molecular target for life-threatening disorders such as AD.
On the other hand, the protective effects of sprouts extract and its constituents against type 2 diabetes were reported. For instance, sunflower sprouts possess antiglycative and antioxidant activity [29], and broccoli sprouts powder could improve serum triglycerides and oxidized LDL/LDL-cholesterol ratio in type 2 diabetes [30]. Mung bean sprout extracts exerted an antidiabetic effect in diabetic KK-Ay mice [31], chickpea sprout was found to ameliorate some hyperglycemic symptoms of the diabetic rats, that is, reducing impairment of diabetic related spatial learning and memory [32], and Japanese radish sprout had a hypoglycemic activity in both normal and diabetic rats [33]. However, there is no article related to Aβ protein and glycation by plant sprouts. Therefore, we furthered this research by examining sprouts of plants, to determine whether or not they also exhibit the anti-Aβ activity and antiglycation.
A full understanding of the pathogenesis of AD has remained elusive, and an increasing amount of evidence is confirming that AD is a disease with numerous contributing factors, both genetic and environmental. It has been proposed that a chemical process known as glycation may contribute to both extensive protein cross-linking and oxidative stress in AD [34]. Glycation is the reaction of reducing sugars to proteins and lipids, resulting in protein modification. Glycation reactions are also elevated during metabolic dysfunction. Nonenzymatic protein glycation is an endogenous process in which reducing sugars react with amino groups in proteins through a series of Maillard reactions forming reversible Schiff-base and Amadori compounds. Wu et al. [35] show that the Schiff base is oxidized in the first stage of glycation and easily produces free radicals. These reactions increase the misfolding of proteins such as Aβ in AD. Thus, glycation is linked to metabolic dysfunction and may have a causal role in AD.
Taking the statement above into account, we examined the glycation inhibition ability of PSAE. In this study, we used the modification model of BSA and D-ribose by Wei et al. [10]. We considered whether PSAE might be inhibiting BSA or LAB glycation. Based on its fluorescence properties, we studied the influence of PSAE on the glycation of D-ribose with BSA or LAB. Our results demonstrated that CW-JBB in BSA and LAB inhibited glycation of BSA or LAB.
As a result, we found that PSAE except in CW-JBB did not inhibit glycation of BSA (Figure 5). A similar result was provided for the different protein, LAB (Figure 6). Significant results were evident in BSA or LAB aggregation except in CW-BWS in BSA and CW-JBB in BSA and LAB (Figures 7 and 8). CW-JBB had the ability to inhibit glycation and also the ability to inhibit aggregation of the protein. In other words, reactions to protein and sugar were inhibited by CW-JBB, and CW-JBB inhibited aggregation of the cross-linked, structure producing protein. Our results agreed with our prediction. It is thought likely that, in the stage before protein is modified by sugar, CW-JBB binds to the protein and disturbs protein modification by sugar. Namely, we propose that the sugar-protein binding site may also be the binding site for the CW-JBB protein and that CW-JBB may compete with D-ribose.
The process of BSA glycation is triggered by the production of an irreversible heterologous byproduct. Bhattacherjee and Chakraborti [36] reported that the extract of Piper betle Linn. leaf may have beneficial effect in preventing protein glycation, and considering its relative amounts present in the extract, rutin appeared to be the most active antiglycating agent. On the other hand, rutin was isolated from flower buds of Japanese butterbur (Petasites japonicus subsp. gigantea Kitam.) [16]. Therefore, the likelihood that rutin occurs in CW-JBB is quite high. As for the result of 24 h incubation under the coexistence of BSA and D-ribose (Figure 5), CW-JBB is thought to have produced the result that contributed to the carbonyl formation of protein, as the extract of Piper betle Linn. leaf controlled the reaction. Therefore, because we have shown it is possible that CW-JBB competes with sugar in protein modification, CW-JBB may contribute to a meaningful delay in the pathological progress of diseases such as AD. To examine the effects of PSAE on the Aβ (1–42) levels, PSAE+Aβ samples were analyzed by Aβ ELISA. We demonstrated that treatment with PSAE effectively decreases Aβ levels (Figure 2). The above data (Figures 2, 5, and 6) show that CW-JBB induced the inhibition of both Aβ (1–42) levels and protein glycation. Notably, CW-JBB may reduce Aβ (1–42) levels by affecting protein conformation. Whereas Fu et al. [37] reported that curcumin and resveratrol bind to the N-terminus of Aβ42 monomers and cap the height of the oligomers, Feng et al. also described that resveratrol may directly bind to Aβ42, interfere in Aβ42 aggregation, and change the Aβ42 oligomer conformation [38]. However, in this case, resveratrol could not prevent Aβ42 oligomer formation.
Our data indicated that the Aβ (1–42) levels of the 4 (except for JBB) PSAE significantly correlate with the absorption spectrum peak of CR. We showed here that the correlation coefficient for the absorption spectrum peak of CR was found to be bigger than 0.8 (r = 0.882) which proved that the Aβ (1–42) levels could be attributed to the absorption spectrum peak of CR. And, no band of Aβ monomer (4.5 kDa) was recognized in CW-JBB. Similarly, the Aβ monomer (4.5 kDa) band became light in CW-RCS, CW-BS, CW-BRS, and CW-BWS. Thus, the mechanism that decreased a level of amyloid β may be modification by PSAE. It might act in similar ways to curcumin, resveratrol, and Ginkgo biloba extract.
On the other hand, Yao et al. showed that the Ginkgo biloba extract EGb 761 rescues PC12 neuronal cells from Aβ-induced cell death by inhibiting the formation of Aβ-derived diffusible neurotoxic ligands [39]. Addition of the extract of Ginkgo biloba leaves, EGb 761, in combination with the Aβ protein prevented, in a dose-dependent manner, Aβ-induced free radical production and cell death. These results indicate that the terpenoid and flavonoid constituents of EGb 761 are responsible for rescuing the neuronal cells from Aβ-induced cell death, their mechanism of action being distinct from their antioxidant properties. Because pre- and posttreatment with EGb 761 did not protect the cells from Aβ-induced neurotoxicity, they examined whether EGb 761 interacts directly with Aβ. In vitro reconstitution studies demonstrated that EGb 761 inhibits the formation of Aβ-derived diffusible neurotoxic soluble ligands, suggested to be involved in the pathogenesis of AD. We also indicated that the DPPH radical scavenging activity of the 5 PSAE did not significantly correlate with Aβ (1–42) levels. Based upon the foregoing, the PSAE mechanism may be the same as that of EGb 761. PSAE may interact with Aβ. In addition, there were reports [12, 13, 16] revealing the presence of terpenoids or flavonoids in water soluble extract of sprout (Table 2). For example, it is known that buckwheat sprouts contain several flavonoids, including orientin, isoorientin, vitexin, isovitexin, rutin, and quercetrin [12]. Judging from the mechanism of Ginkgo biloba extract, it is likely that PSAE also contains a terpenoid and a flavonoid and might act in similar ways to Ginkgo biloba extract.
Table 2.
Bioactive compounds of sprouts or matured plants. We selected five plant sprouts (buckwheat (BW) sprout (BWS), red cabbage (RC) sprout (RCS), broccoli (B) sprout (BS), brussels (BR) sprout (BRS), and Japanese butterbur sprout flower buds (JBB)). This table shows the reported bioactive compounds of sprouts or matured plants.
| Samples name | Compounds | References |
|---|---|---|
| BWS or BW | Quercetin glucoside | [11] |
|
| ||
| RCS or RC | Orientin, isoorientin, vitexin, isovitexin, rutin, and quercetin | [12] |
| Kaempferol, quercetin derivatives, hydroxybenzoic acid, and cyanidin | [13] | |
|
| ||
| BS or B | Quercetin and sulforaphane | [14] |
| Sinigrin | [15] | |
|
| ||
| BRS or BR | Kaempferol and quercetin derivatives | [13] |
| Hydroxybenzoic acid and sinigrin | [15] | |
|
| ||
| JBB (flower buds) or JBB | Quercetin glucoside | [16] |
| Petasiphenol | [17] | |
| Fukinolic acid | [18] | |
| Triterpene glycosides | [19] | |
This study found that treatment with PSAE effectively decreases Aβ concentration, showing promise in the development of a novel and functional food for suppressing AD.
5. Conclusion
The neuroprotective effects of PSAE were evaluated by measuring levels of Aβ in mixtures (Aβ and PSAE) with Aβ ELISA assay. The results demonstrate that the treatment with PSAE decreased Aβ levels. The highest value was observed with CW-RCS and Aβ mixture. Furthermore, we performed CR and Aβ binding experiments to determine whether PSAE are the modification of amyloid structures. CW-RCS and Aβ mixture did not affect the CR spectrum. Namely, CR binding in CW-RCS and Aβ mixture was not detected as a red shift in its absorbance spectrum. And, the Aβ (1–42) levels of the 4 (except for JBB) PSAE significantly correlate with the absorption spectrum peak of CR. The mechanism that decreased a level of Aβ may be modification by PSAE. It might act in similar ways to curcumin, resveratrol, and Ginkgo biloba extract.
Competing Interests
The authors declare that they have no conflict of interests.
References
- 1.Angeloni C., Zambonin L., Hrelia S. Role of methylglyoxal in alzheimer's disease. BioMed Research International. 2014;2014:12. doi: 10.1155/2014/238485.238485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guo L., Yang R., Wang Z., Guo Q., Gu Z. Effect of NaCl stress on health-promoting compounds and antioxidant activity in the sprouts of three broccoli cultivars. International Journal of Food Sciences and Nutrition. 2014;65(4):476–481. doi: 10.3109/09637486.2013.860583. [DOI] [PubMed] [Google Scholar]
- 3.Chiavaro E., Mazzeo T., Visconti A., Manzi C., Fogliano V., Pellegrini N. Nutritional quality of sous vide cooked carrots and brussels sprouts. Journal of Agricultural and Food Chemistry. 2012;60(23):6019–6025. doi: 10.1021/jf300692a. [DOI] [PubMed] [Google Scholar]
- 4.Jaiswal A. K., Rajauria G., Abu-Ghannam N., Gupta S. Phenolic composition, antioxidant capacity and antibacterial activity of selected Irish Brassica vegetables. Natural Product Communications. 2011;6(9):1299–1304. [PubMed] [Google Scholar]
- 5.Shetty K., Wahlqvist M. L. A model for the role of the proline-linked pentose-phosphate pathway in phenolic phytochemical bio-synthesis and mechanism of action for human health and environmental applications. Asia Pacific Journal of Clinical Nutrition. 2004;13(1):1–24. [PubMed] [Google Scholar]
- 6.Hoelzl C., Bichler J., Ferk F., et al. Methods for the detection of antioxidants which prevent age related diseases: a critical review with particular emphasis on human intervention studies. Journal of Physiology and Pharmacology. 2005;56(2):49–64. [PubMed] [Google Scholar]
- 7.Złotek U., Świeca M., Jakubczyk A. Effect of abiotic elicitation on main health-promoting compounds, antioxidant activity and commercial quality of butter lettuce (Lactuca sativa L.) Food Chemistry. 2014;148:253–260. doi: 10.1016/j.foodchem.2013.10.031. [DOI] [PubMed] [Google Scholar]
- 8.Schalkwijk C. G., Stehouwer C. D. A., van Hinsbergh V. W. M. Fructose-mediated non-enzymatic glycation: sweet coupling or bad modification. Diabetes/Metabolism Research and Reviews. 2004;20(5):369–382. doi: 10.1002/dmrr.488. [DOI] [PubMed] [Google Scholar]
- 9.Yamagishi S.-I. Potential clinical utility of advanced glycation end product cross-link breakers in age- and diabetes-associated disorders. Rejuvenation Research. 2012;15(6):564–572. doi: 10.1089/rej.2012.1335. [DOI] [PubMed] [Google Scholar]
- 10.Wei Y., Chen L., Chen J., Ge L., He R. Q. Rapid glycation with D-ribose induces globular amyloid-like aggregations of BSA with high cytotoxicity to SH-SY5Y cells. BMC Cell Biology. 2009;10, article 10 doi: 10.1186/1471-2121-10-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muhammad S., Fatima N. In silico analysis and molecular docking studies of potential angiotensin-converting enzyme inhibitor using quercetin glycosides. Pharmacognosy Magazine. 2015;11(42):S123–S126. doi: 10.4103/0973-1296.157712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nam T. G., Lee S. M., Park J. H., Kim D. O., Baek N. I., Eom S. H. Flavonoid analysis of buckwheat sprouts. Food Chemistry. 2015;170:97–101. doi: 10.1016/j.foodchem.2014.08.067. [DOI] [PubMed] [Google Scholar]
- 13.Duchnowicz P., Bors M., Podsedek A., Koter-Michalak M., Broncel M. Effect of polyphenols extracts from Brassica vegetables on erythrocyte membranes (in vitro study) Environmental Toxicology and Pharmacology. 2012;34(3):783–790. doi: 10.1016/j.etap.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 14.Lozanovski V. J., Houben P., Hinz U., Hackert T., Herr I., Schemmer P. Pilot study evaluating broccoli sprouts in advanced pancreatic cancer (POUDER trial)—study protocol for a randomized controlled trial. Trials. 2014;15, article 204 doi: 10.1186/1745-6215-15-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Awasthi S., Saraswathi N. Sinigrin, a major glucosinolate from cruciferous vegetables restrains non-enzymatic glycation of albumin. International Journal of Biological Macromolecules. 2016;83:410–415. doi: 10.1016/j.ijbiomac.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 16.Matsuura H., Amano M., Kawabata J., Mizutani J. Isolation and measurement of quercetin glucosides in flower buds of Japanese butterbur (Petasites japonicus subsp. gigantea Kitam.) Bioscience, Biotechnology and Biochemistry. 2002;66(7):1571–1575. doi: 10.1271/bbb.66.1571. [DOI] [PubMed] [Google Scholar]
- 17.Mizushina Y., Kamisuki S., Kasai N., et al. Petasiphenol: a DNA polymerase λ inhibitor. Biochemistry. 2002;41(49):14463–14471. doi: 10.1021/bi020476q. [DOI] [PubMed] [Google Scholar]
- 18.Hasa Y., Tazaki H. Biosynthesis of fukinolic acid isolated from Petasites japonicus . Bioscience, Biotechnology and Biochemistry. 2004;68(10):2212–2214. doi: 10.1271/bbb.68.2212. [DOI] [PubMed] [Google Scholar]
- 19.Shimoda H., Tanaka J., Yamada E., Morikawa T., Kasajima N., Yoshikawa M. Anti type I allergic property of Japanese butterbur extract and its mast cell degranulation inhibitory ingredients. Journal of Agricultural and Food Chemistry. 2006;54(8):2915–2920. doi: 10.1021/jf052994o. [DOI] [PubMed] [Google Scholar]
- 20.Okada Y., Okada M. Komatsuna seed extracts protection against amyloid β(1-42)-induced neuronal cell death. Journal of Diabetes & Metabolism. 2014;5(5, article 368) doi: 10.4172/2155-6156.1000368. [DOI] [Google Scholar]
- 21.Gao X., Ohlander M., Jeppsson N., Björk L., Trajkovski V. Changes in antioxidant effects and their relationship to phytonutrients in fruits of sea buckthorn (Hippophae rhamnoides L.) during maturation. Journal of Agricultural and Food Chemistry. 2000;48(5):1485–1490. doi: 10.1021/jf991072g. [DOI] [PubMed] [Google Scholar]
- 22.Negro C., Tommasi L., Miceli A. Phenolic compounds and antioxidant activity from red grape marc extracts. Bioresource Technology. 2003;87(1):41–44. doi: 10.1016/S0960-8524(02)00202-X. [DOI] [PubMed] [Google Scholar]
- 23.Chen J., Armstrong A. H., Koehler A. N., Hecht M. H. Small molecule microarrays enable the discovery of compounds that bind the Alzheimer's Aβ peptide and reduce its cytotoxicity. Journal of the American Chemical Society. 2010;132(47):17015–17022. doi: 10.1021/ja107552s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okada M., Okada Y. Effects of methanolic extracts of edible plants on RAGE in high-glucose-induced human endothelial cells. Bio-Medical Materials and Engineering. 2015;25(3):257–266. doi: 10.3233/BME-151280. [DOI] [PubMed] [Google Scholar]
- 25.Okada Y., Okada M. Effects of methanolic extracts from edible plants on endogenous secretory receptor for advanced glycation end products induced by the high glucose incubation in human endothelial cells. Journal of Pharmacy and Bioallied Sciences. 2015;7(2):145–150. doi: 10.4103/0975-7406.148783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lv C., Wang L., Liu X., et al. Multi-faced neuroprotective effects of geniposide depending on the RAGE-mediated signaling in an Alzheimer mouse model. Neuropharmacology. 2015;89:175–184. doi: 10.1016/j.neuropharm.2014.09.019. [DOI] [PubMed] [Google Scholar]
- 27.Yamagishi S.-I., Matsui T. Role of receptor for advanced glycation end products (RAGE) in liver disease. European Journal of Medical Research. 2015;20, article 15 doi: 10.1186/s40001-015-0090-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li X.-H., Du L.-L., Cheng X.-S., et al. Glycation exacerbates the neuronal toxicity of β-amyloid. Cell Death and Disease. 2013;4(6, article e673) doi: 10.1038/cddis.2013.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun Z., Chen J., Ma J., et al. Cynarin-rich sunflower (Helianthus annuus) sprouts possess both antiglycative and antioxidant activities. Journal of Agricultural and Food Chemistry. 2012;60(12):3260–3265. doi: 10.1021/jf300737y. [DOI] [PubMed] [Google Scholar]
- 30.Bahadoran Z., Mirmiran P., Hosseinpanah F., Rajab A., Asghari G., Azizi F. Broccoli sprouts powder could improve serum triglyceride and oxidized LDL/LDL-cholesterol ratio in type 2 diabetic patients: a randomized double-blind placebo-controlled clinical trial. Diabetes Research and Clinical Practice. 2012;96(3):348–354. doi: 10.1016/j.diabres.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 31.Yao Y., Chen F., Wang M., Wang J., Ren G. Antidiabetic activity of Mung bean extracts in diabetic KK-Ay mice. Journal of Agricultural and Food Chemistry. 2008;56(19):8869–8873. doi: 10.1021/jf8009238. [DOI] [PubMed] [Google Scholar]
- 32.Mao X., Zhang L., Xia Q., et al. Vanadium-enriched chickpea sprout ameliorated hyperglycemia and impaired memory in streptozotocin-induced diabetes rats. BioMetals. 2008;21(5):563–570. doi: 10.1007/s10534-008-9142-y. [DOI] [PubMed] [Google Scholar]
- 33.Taniguchi H., Kobayashi-Hattori K., Tenmyo C., et al. Effect of Japanese radish (Raphanus sativus) sprout (Kaiware-daikon) on carbohydrate and lipid metabolisms in normal and streptozotocin-induced diabetic rats. Phytotherapy Research. 2006;20(4):274–278. doi: 10.1002/ptr.1851. [DOI] [PubMed] [Google Scholar]
- 34.Fawver J. N., Schall H. E., Petrofes Chapa R. D., Zhu X., Murray I. V. J. Amyloid-β Metabolite sensing: biochemical linking of glycation modification and misfolding. Journal of Alzheimer's Disease. 2012;30(1):63–73. doi: 10.3233/jad-2012-112114. [DOI] [PubMed] [Google Scholar]
- 35.Wu C.-H., Huang S.-M., Lin J.-A., Yen G.-C. Inhibition of advanced glycation endproduct formation by foodstuffs. Food and Function. 2011;2(5):224–234. doi: 10.1039/c1fo10026b. [DOI] [PubMed] [Google Scholar]
- 36.Bhattacherjee A., Chakraborti A. S. Inhibitory effect of Piper betle Linn. leaf extract on protein glycation—quantification and characterization of the antiglycation components. Indian Journal of Biochemistry and Biophysics. 2013;50(6):529–536. [PubMed] [Google Scholar]
- 37.Fu Z., Aucoin D., Ahmed M., Ziliox M., Van Nostrand W. E., Smith S. O. Capping of Aβ42 oligomers by small molecule inhibitors. Biochemistry. 2014;53(50):7893–7903. doi: 10.1021/bi500910b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feng Y., Wang X.-P., Yang S.-G., et al. Resveratrol inhibits beta-amyloid oligomeric cytotoxicity but does not prevent oligomer formation. NeuroToxicology. 2009;30(6):986–995. doi: 10.1016/j.neuro.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 39.Yao Z.-X., Drieu K., Papadopoulos V. The Ginkgo biloba extract EGb 761 rescues the PC12 neuronal cells from β-amyloid-induced cell death by inhibiting the formation of β-amyloid-derived diffusible neurotoxic ligands. Brain Research. 2001;889(1-2):181–190. doi: 10.1016/s0006-8993(00)03131-0. [DOI] [PubMed] [Google Scholar]