Abstract
Functional MRI (fMRI) is well-established for the study of brain function in healthy populations, although its clinical application has proven more challenging. Specifically, cerebrovascular reactivity (CVR), which allows the assessment of the vascular response that serves as the basis for fMRI, has been shown to be reduced in healthy aging as well as in a range of diseases, including chronic stroke. However, the timing of when this occurs relative to the stroke event is unclear. We used a breath-hold fMRI task to evaluate CVR across gray matter in a group of acute stroke patients (< 10 days from stroke; N = 22) to address this question. These estimates were compared with those from both age-matched (N = 22) and younger (N = 22) healthy controls. As expected, young controls had the greatest mean CVR, as indicated by magnitude and extent of fMRI activation; however, stroke patients did not differ from age-matched controls. Moreover, the ipsilesional and contralesional hemispheres of stroke patients did not differ with respect to any of these measures. These findings suggest that fMRI remains a valid tool within the first few days of a stroke, particularly for group fMRI studies in which findings are compared with healthy subjects of similar age. However, given the relatively high variability in CVR observed in our stroke sample, caution is warranted when interpreting fMRI data from individual patients or a small cohort. We conclude that a breath-hold task can be a useful addition to functional imaging protocols for stroke patients.
Keywords: fMRI, Vascular reactivity, Stroke, Breath-hold, Neurovascular uncoupling
Highlights
-
•
Breath-holding can be used to assess the validity of fMRI in stroke patients.
-
•
Vascular reactivity, estimated by breath-hold fMRI, was greatest in young controls.
-
•
Acute stroke patients and age-matched controls had similar vascular reactivity.
-
•
Modeling the breath-hold response on an individual basis can improve results.
1. Introduction
Functional magnetic resonance imaging (fMRI) has made important contributions to our understanding of post-stroke brain changes. fMRI can provide valuable insight in terms of characterizing brain plasticity changes as well as the prediction of outcomes and assessment of recovery after stroke (Calautti and Baron, 2003, Heiss and Kidwell, 2014, Ward et al., 2003). However, there is growing concern over the integrity of the blood oxygenation level-dependent (BOLD) signal, which is sensitive to changes in cerebral blood flow and volume in addition to the rate of oxygen metabolism, in stroke patients and other groups who may exhibit impairments in cerebrovascular reactivity (CVR)—the ability of cerebral microvasculature to modulate blood flow in response to vasodilatory stimuli, such as neural activity (D'Esposito et al., 2003, Girouard and Iadecola, 2006, Lindauer et al., 2010). Such impairments may manifest as an uncoupling of neural and vascular activity that can preclude the hemodynamic response from reaching a designated threshold during a typical task-fMRI paradigm, especially when assumed to match that of young, healthy subjects (Amemiya et al., 2012, Mazzetto-Betti et al., 2010). The resulting false negatives in the BOLD signal can be misleading to both researchers and clinicians attempting to map changes in brain function after stroke. It is therefore crucial to assess the risk of neurovascular uncoupling (NVU) in stroke patients, and in turn, the validity of BOLD fMRI in this group.
Many studies have sought to assess the validity of fMRI in cerebrovascular patients, often observing a delayed, attenuated, or absent hemodynamic response to behavioral tasks with fMRI (Bonakdarpour et al., 2007, Krainik et al., 2005, Murata et al., 2006, Pineiro et al., 2002), even when a neural response is measured by other means (Amemiya et al., 2012, Binkofski and Seitz, 2004, Bonakdarpour et al., 2015, Mazzetto-Betti et al., 2010, Rossini et al., 2004). However, a majority have focused almost exclusively on the chronic stage (Bonakdarpour et al., 2015, Bonakdarpour et al., 2007, Krainik et al., 2005, Mazzetto-Betti et al., 2010, Rossini et al., 2004), several weeks to months from the stroke. Because functional imaging is often performed just days after a stroke (i.e., at the acute stage), it is critical to determine if NVU is already a significant concern at this point. Since CVR may continue to change throughout the course of stroke recovery (Beaulieu et al., 1999, Siegel et al., 2015, Widder et al., 1994), it is plausible that NVU may be only transient in stroke patients, increasing confidence in fMRI data obtained either before or after a certain risk period. Two longitudinal evaluations of the BOLD signal after stroke have supported this notion, suggesting that NVU risk after stroke is time-sensitive and may be minimal within the first few days of stroke (Altamura et al., 2009, Binkofski and Seitz, 2004). However, the neural variability resulting from conventional fMRI tasks presents a challenge for identifying NVU in this way, and such an approach is inherently limited to the functional region expected to be activated by the task.
In recent years, more robust means for detecting NVU risk have emerged. Specifically, breath-hold CVR mapping, which emerged as a more convenient alternative to other hypercapnic methods such as exogenous CO2 administration, is continuing to garner attention for clinical research and application—particularly to augment functional maps obtained from presurgical tumor patients (Pillai and Mikulis, 2015, Pillai and Zaca, 2011). This non-invasive technique simply requires patients to hold their breath for short intervals inside the MR scanner. Regions of impaired CVR are not as effective in modulating blood flow in response to the induced hypercapnia and can thus be visualized as areas void of activity in the resulting fMRI maps (Pillai and Mikulis, 2015). This method has previously been used at the group level to confirm the deleterious effects of aging on CVR (Handwerker et al., 2007, Kannurpatti et al., 2010), although to our knowledge it has been implemented just twice previously for the same purpose in stroke patients (Geranmayeh et al., 2015, van Oers et al., 2010), the focus being on the subacute and chronic stages.
Thus, the goal of the present study was to quantitatively assess CVR and determine the validity of fMRI in stroke patients at the acute stage, defined in our study as less than ten days after stroke. BOLD parameters computed from a breath-hold task were compared between stroke patients and healthy controls at the group level to identify any stroke-related deficits in CVR that could give rise to NVU. These results are obtained through two separate analyses in order to compare two common approaches to accounting for the extended delay of the vascular response to hypercapnia relative to neural activity—a standard adjustment of the breath-hold model response for all subjects (standard delay) (as in (Di et al., 2013)) and an adjustment optimized on an individual basis (subject-wise delay) (as in (Bright and Murphy, 2013)).
Based on the findings of Binkofski and Seitz (Binkofski and Seitz, 2004) and Altamura et al. (Altamura et al., 2009), we hypothesized that CVR is not yet impaired at a level detectable by fMRI at the acute stage of stroke. Thus, since CVR deficits have been regularly implicated in healthy aging (D'Esposito et al., 2003, Riecker et al., 2003), we predicted that global CVR would be reduced in stroke patients compared to young controls but would not differ from older, age-matched controls. Additionally, although the degree of CVR impairment has been found to be especially pronounced in the lesioned hemisphere (Altamura et al., 2009, Binkofski and Seitz, 2004, Krainik et al., 2005, Mazzetto-Betti et al., 2010), we expected to observe no differences in CVR between the ipsilesional and contralesional hemispheres of stroke patients at this early stage.
2. Methods
2.1. Subjects
Twenty-two acute stroke patients (ages 44–75 years, mean = 59 years, 14 male) (Table 1) were included in the study, originally recruited for an ongoing longitudinal project. Inclusion criteria were at least 18 years of age, ability to provide written informed consent, and initial scan within 10 days of stroke. Exclusion criteria were history of psychiatric illness, confounding neurological disorders, drug abuse, and contraindications to MRI. Twenty-two age-matched healthy controls were also included in the study (ages 50–74 years, mean = 59, 10 male), as well as a group of 22 younger healthy controls (ages 18–27 years, mean = 22, 11 male). The study was conducted in accordance with a protocol approved by the local Health Sciences Institutional Review Board. All subjects provided written informed consent.
Table 1.
Patient characteristics.
| Patient | Sex | Age (years) | Time since stroke (days) | Lesion location | NIH Stroke Scale score | Treatment |
|---|---|---|---|---|---|---|
| 1 | M | 75 | 6 | C; L occipital | 1 | tPA + ST |
| 2 | M | 69 | 4 | L cerebellum, occipital | 0 | ST |
| 3 | M | 74 | 7 | C; R temporal, occipital | 1 | ST |
| 4 | F | 44 | 5 | C; L insula, frontal | 7 | ST |
| 5 | M | 45 | 3 | L cerebellum | 2 | ST |
| 6 | M | 55 | 3 | C; L MCA and MCA/PCA border | 0 | ST |
| 7 | M | 62 | 9 | C; L parietal | 0 | ST |
| 8 | M | 58 | 5 | C; L corticospinal tract, cerebellum | 0 | ST |
| 9 | F | 59 | 7 | C; R MCA | 2 | tPA + ST |
| 10 | M | 59 | 4 | C; R MCA | 2 | tPA + ST |
| 11 | M | 57 | 3 | SC; R pontine | 2 | ST |
| 12 | M | 63 | 5 | SC; R pontine | 0 | ST |
| 13 | F | 47 | 9 | C; R frontal | 0 | ST |
| 14 | F | 58 | 7 | C; L frontal | 1 | ST |
| 15 | F | 59 | 2 | C; L posterior insular, parietal | 2 | ST |
| 16 | F | 46 | 0 | R cerebellum | 2 | ST |
| 17 | M | 67 | 5 | SC; L lateral medulla | 0 | ST |
| 18 | M | 63 | 3 | C; R MCA | 0 | ST |
| 19 | F | 57 | 6 | L cerebellum | 1 | ST |
| 20 | M | 63 | 2 | SC; L posterior putamen | 2 | ST |
| 21 | M | 46 | 3 | C; R occipital | 0 | tPA + ST |
| 22 | F | 67 | 5 | C; R MCA | 4 | tPA + ST |
C, cortical; SC, subcortical; L, left; R, right; MCA, middle cerebral artery; PCA, posterior cerebral artery; tPA, tissue plasminogen activator; ST, standard of care stroke treatment (in most cases consisted of antiplatelet agent (e.g., aspirin, clopidogrel), anticoagulant (e.g. heparin, warfarin), anti-hypertensive (e.g., beta blocker, angiotensin-converting-enzyme inhibitor, and/or statin (e.g., simvastatin, pravastatin)).
2.2. Data collection
All imaging data were obtained on GE 750 3T MRI scanners (GE Healthcare, Waukesha, WI) equipped with an eight-channel head coil. T1-weighted axial anatomical slices were acquired at the beginning of each session following an FSPGR BRAVO sequence (TR = 8.132 ms, TE = 3.18 ms, TI = 450 ms, 256 × 256 matrix, 156 slices, flip angle = 12°, FOV = 25.6 cm, slice thickness = 1 mm). Functional data were acquired via echo-planar T2*-weighted imaging (TR = 2.0 s, 40 slices, 90 volumes, TE = 22 ms, FOV = 22.4 cm, flip angle = 60°, voxel dimensions 3.75 × 3.75 × 4.0 mm3).
Each breath-hold scan followed a block design consisting of four 20-s, end-expiration blocks alternating with four 20-s blocks of rest, for a total scan length of 3 min. Subjects were instructed to begin each task block with a moderate breath.
2.3. Data analysis
All preprocessing of imaging data was performed within the Analysis of Functional Neuroimages (AFNI) suite (Cox, 1996), except where noted. Subject-specific gray matter masks were created via automated segmentation of the skull-stripped anatomical volumes using FSL's FAST (FMRIB Software Library, Oxford, UK) (Zhang et al., 2001). Functional data were first aligned to the anatomical and normalized to standard Montreal Neurological Institute (MNI) space. The first four volumes were discarded to allow for steady-state imaging. Images were then resampled to 3.0 mm isotropic, despiked, volume-registered, and spatially smoothed using a 4-mm full-width at half-maximum Gaussian kernel. The time series within each voxel was scaled to percent signal and estimated in a general linear model with a canonical gamma variate hemodynamic response function (HRF) convolved with a boxcar reference waveform and six rigid-body motion parameters and their derivatives regressed. A composite estimate of head motion was calculated for each subject as the square root of the sum of squares (Euclidean norm) of the twelve motion parameters' (three translational and three rotational) derivatives (Jones et al., 2010). High-motion time points (Euclidean norm exceeding 0.3) were censored in the GLM.
2.4. Breath-hold analyses: Standard delay and subject-wise delay
In an initial analysis, the model HRF was delayed by a standard amount for all subjects (standard delay). A wide range of values have previously been used for this purpose, from as low as 7 s (Di et al., 2013) up to 12 s (Kannurpatti et al., 2011). Given the advanced age of many of the subjects included in the study, we tested a relatively lengthy delay of 11 s for all subjects.
In a separate analysis, the Hilbert transform (AFNI plugin 3ddelay) was used to approximate, at each voxel, the temporal shift of the model HRF needed to best fit the voxel's time course. These delays were averaged over gray matter and incorporated into the model for each subject (subject-wise delay) in order to account for the inter-subject variability in the breath-hold vascular response and potential delays due to slower-responding vasculature in older controls or stroke patients.
2.5. Breath-hold measures and statistical tests
For both the standard and subject-wise analyses, magnitude of activation from the breath-hold task was derived from the beta-weights for the HRF, which approximate BOLD percent signal change during the task, while activation volume was determined as the number of voxels surpassing a liberal threshold of p < 0.01 (uncorrected) (Geranmayeh et al., 2015). The mean delay of the HRF obtained from 3ddelay was also averaged over gray matter and compared between groups. One-way ANOVAs were performed separately for the magnitude, extent, and delay of BOLD activation, as well as gray matter volume, among the three groups. To ensure that ipsilesional impairments in CVR were not overlooked due to averaging with intact contralesional CVR, hemispheric comparisons in stroke patients were performed. Two-tailed paired t-tests were used to compare the above measures between the ipsilesional and contralesional hemispheres of patients. Paired t-tests were also used to compare measures for each group between the two analyses (standard delay versus subject-wise delay). A p value of ≤ 0.05 was considered statistically significant for all tests. For ANOVAs, this was determined by Fisher's least significant difference (LSD) post-hoc test. Statistics are represented as mean ± standard deviation.
3. Results
3.1. Motion during breath-hold task
As described above, a composite measure of head motion, the Euclidean norm, was calculated for each subject. An ANOVA revealed significant between-group differences in the mean of this motion estimate (young controls = 0.09 ± 0.03 mm, old controls = 0.11 ± 0.05 mm, stroke = 0.16 ± 0.07 mm; F(2, 63) = 9.94, p < 0.001). As detailed in the previous section, these differences were minimized through an aggressive motion scrubbing approach in which high-motion time points were censored before HRF fitting, in addition to the regression of six motion parameters and their derivatives.
3.2. Gray matter volume
The volume of the gray matter mask created for each subject was, on average, 85.4 ± 3.7 cm3 for young controls, 78.4 ± 5.9 cm3 for old controls, and 76.4 ± 4.9 cm3 for patients (F(2, 63) = 20.1, p < 0.001). Due to these being significantly different, group activation volumes are reported as percentages of the average size of the gray matter mask for each group (Thomason et al., 2005).
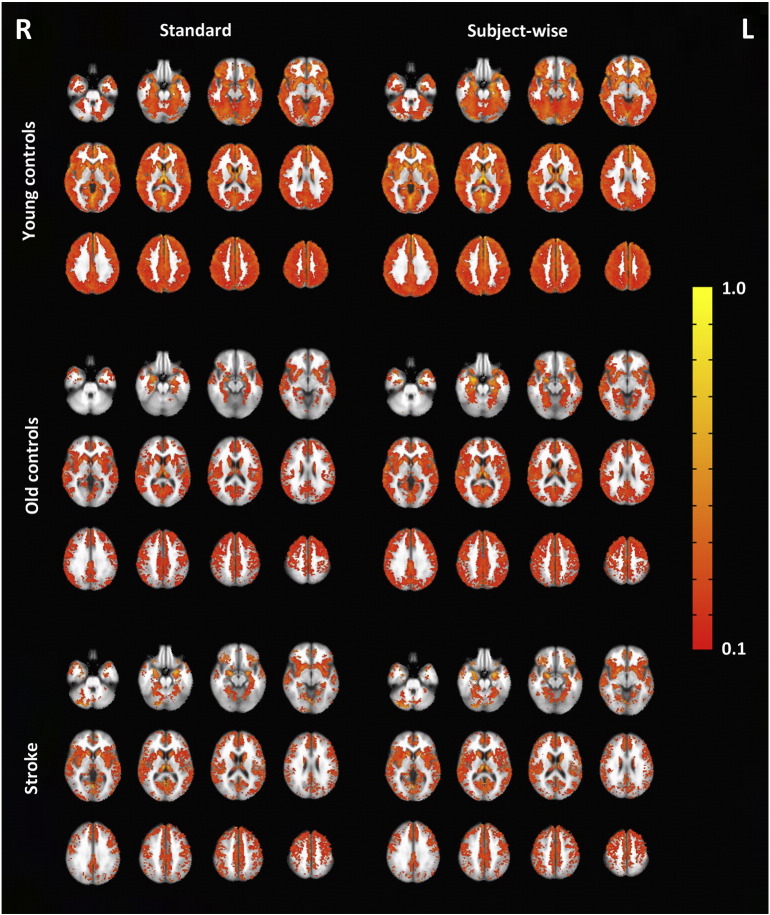
Fig. 1 displays group activation maps for both analyses (standard delay and subject-wise delay). The figure shows large differences in activation volume among the three groups when projected onto a standard MNI mask for both standard and subject delayed approaches. However, for quantitative analysis below, much of this was accounted for by using gray matter masks specific to these different groups.
Fig. 1.
Average breath-hold fMRI group maps from the standard delay (left) and subject-wise delay (right) analyses for young controls (top), old controls (middle), and patients (bottom) overlaid on MNI 152 template. Functional data shown at p < 0.05 with a minimum cluster size of 60 voxels. 11 of the 22 stroke maps were flipped so that all lesioned hemispheres were on the left side (displayed to the right in this figure).
3.3. Standard delay
There were significant differences in magnitude of BOLD activation, calculated as the average percent signal change across gray matter (F(2, 62) = 3.52, p = 0.036). Whereas young controls (1.06 ± 0.27) did not differ from old controls (0.92 ± 0.27; p = 0.090) or patients (1.14 ± 0.30; p = 0.369), the BOLD magnitude of patients was significantly greater than that of old controls (p = 0.011) (Fig. 2). Differences in activation volume also reached significance (F(2, 63) = 3.18, p = 0.048) as, on average, a significantly greater percentage of gray matter voxels were activated in young controls (44.8 ± 19.8%) compared with old controls (25.9 ± 25.2%; p = 0.015) (Fig. 3). Differences between young controls and patients (37.4 ± 29.4) were not significant (p = 0.329), nor were those between old controls and patients (p = 0.133). Magnitude and activation volume in the ipsilesional hemisphere of stroke patients (1.18 ± 0.39 and 37.0 ± 29.0%, respectively) were not significantly different from the contralesional hemisphere (1.18 ± 0.40 and 38.0 ± 30.3%) (magnitude: t(21) = 0.08, p = 0.939; activation volume: t(21) = 0.73, p = 0.476).
Fig. 2.
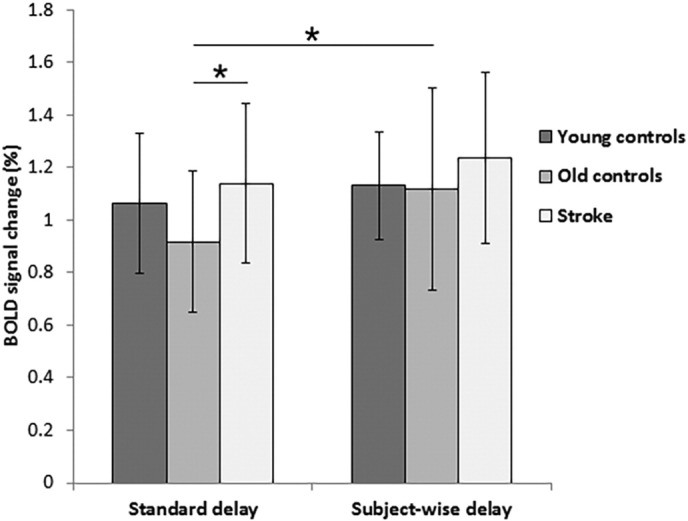
Mean percent signal change in gray matter observed in the BOLD signal during the breath-hold task. Group averages are shown for both standard delay (left) and subject-wise delay (right) analyses. One patient data point from the standard delay was treated as an outlier as it was greater than three standard deviations from the group mean and is not represented here. ⁎p < 0.05.
Fig. 3.
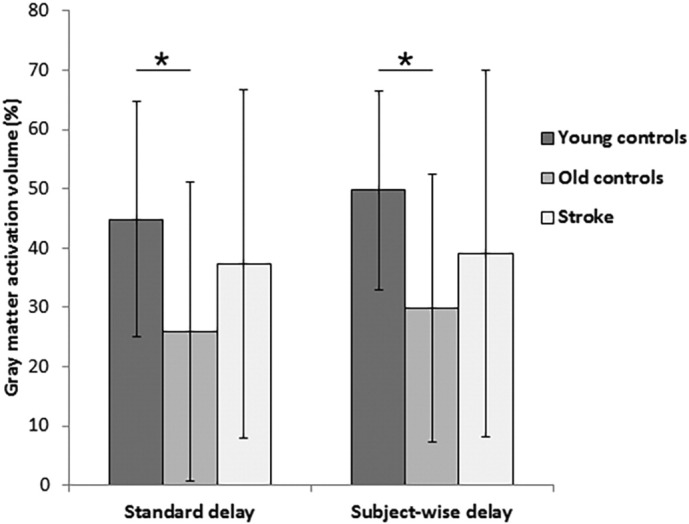
Mean activation volume (as a percentage of gray matter) resulting from the breath-hold task, as calculated from the standard delay (left) and subject-wise delay (right) analyses. * p < 0.05.
3.4. Subject-wise delay
Young controls showed the shortest delay in hemodynamic response (11.2 ± 2.4 s), followed by stroke patients (11.3 ± 2.1 s), while the longest delay was observed in old controls (12.6 ± 2.0 s); however, these differences yielded only a non-significant trend (F(2, 63) = 2.69, p = 0.076) (Fig. 4). Magnitude of BOLD activation did not significantly differ between the three groups (young controls = 1.13 ± 0.20, old controls = 1.12 ± 0.39, stroke patients = 1.25 ± 0.32; F(2, 63) = 1.13, p = 0.329) (Fig. 2). However, there were significant between-group differences in activation volume in the subject-wise delay analysis (F(2, 63) = 3.75, p = 0.029), with young controls (49.7 ± 16.7%) having a significantly greater extent of activation than old controls (29.8 ± 22.6%) (p = 0.008), although not stroke patients (39.1 ± 31.0%) (p = 0.149) (Fig. 3). The difference between old controls and patients did not reach significance (p = 0.207). The average hemodynamic delay did not differ between the ipsilesional (11.2 ± 2.1 s) and contralesional (11.3 ± 2.1 s) hemispheres of stroke patients (t(21) = 0.23, p = 0.822). Further, as in the standard delay analysis, there were no significant differences in either magnitude or activation volume between the ipsilesional (magnitude = 1.24 ± 0.32; activation volume = 38.8 ± 30.5%) and contralesional (magnitude = 1.23 ± 0.34; activation volume = 39.4 ± 31.9%) hemispheres of stroke patients (magnitude: t(21) = 0.15, p = 0.884; activation volume: t = 0.55, p = 0.587).
Fig. 4.
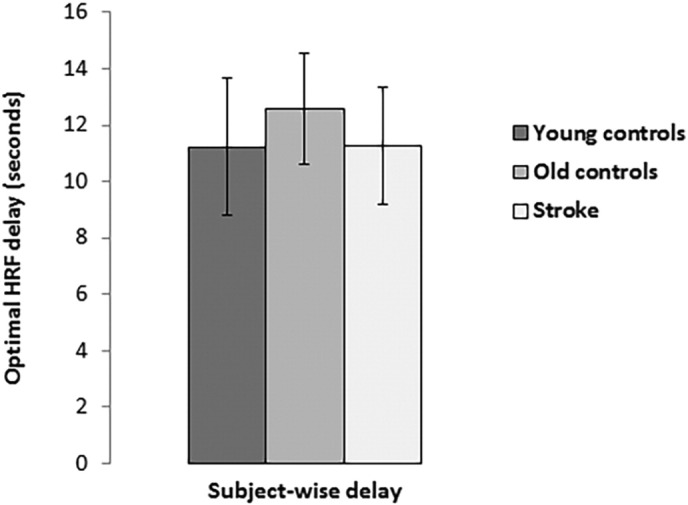
Delay (in seconds) needed for the canonical HRF to optimally fit the time course from each voxel, averaged over each subject's gray matter.
3.5. Comparison of analyses: Standard delay vs. subject-wise delay
The differences in magnitude of activation yielded by the standard delay and subject-wise delay analyses were not significant for young controls (t(21) = 1.18, p = 0.250) or stroke patients (t(21) = 1.03, p = 0.317), although using an individualized value for the delay gave a significantly greater magnitude for old controls (t(21) = 3.35, p = 0.003). Activation volume did not significantly change between the analyses for any of the groups; however, unlike for young controls (t(21) = 1.35, p = 0.191) and patients (t(21) = 1.28, p = 0.215), there was a non-significant trend towards increased activation volume in the subject-wise delay analysis for old controls (t(21) = 1.86, p = 0.077).
4. Discussion
fMRI is a powerful tool that is commonly used to non-invasively localize neural activity in the human brain, although its clinical application has been limited for a variety of reasons (Jezzard and Buxton, 2006, Pillai, 2010). One limitation, NVU, may compromise the validity of the BOLD signal in several patient populations, including brain tumor (Hou et al., 2006, Ulmer et al., 2003), Alzheimer's disease (Iadecola, 2004), cerebrovascular disease (del Zoppo, 2010, Pineiro et al., 2002, Roc et al., 2006), and healthy aging (D'Esposito et al., 2003, Riecker et al., 2003). There is currently a limited understanding of the sequence of events that link neural activity to a hemodynamic response, but in theory, a disruption at any stage in this sequence could result in NVU (Hillman, 2014, Pillai and Mikulis, 2015).
Many studies have indicated the presence of NVU in stroke patients, although it remains unclear exactly how soon after a stroke NVU becomes a significant concern. fMRI has been used to characterize brain changes from just days after a stroke to several months afterward, as results from functional imaging studies have informed our understanding of the prolonged time course of stroke recovery (e.g., (Saur et al., 2006, Ward et al., 2003)). In the present study, we focused on acute stroke patients less than 10 days from stroke. We used a breath-hold task to quantify any disparities in CVR in these patients at the group level relative to healthy controls. Moreover, we analyzed the resulting BOLD response by fitting it with an HRF that was either delayed by a standard amount for all subjects, as is often done to account for the slower vascular response to hypercapnia versus neural activity, or by an amount that was optimized for each subject, accounting for differences in this hemodynamic delay.
Our standard delay analysis, interestingly, revealed a significantly greater magnitude of the breath-hold response in patients compared to old controls. The reason for this is unclear, but this could be due to a combination of the poor fit of the standard delay model for old controls as well as increased motion in the patient group, although a stringent motion censoring approach was undertaken. The subject-wise delay did not reveal any such differences in magnitude. In both analyses, the activation volume of young controls was greater than old controls but not patients. Importantly, neither analysis indicated impaired CVR in the patient group beyond that of age-matched controls. Furthermore, we observed no differences in the breath-hold response between the ipsilesional and contralesional hemispheres of patients. The findings from our study suggest that fMRI may still be valid at the acute stage of stroke, particularly for group comparisons with healthy age-matched subjects.
When considered in the context of existing stroke literature, it may be that NVU first appears at some point beyond the acute stage. This idea is supported by two longitudinal evaluations of the BOLD signal after stroke. Binkofski and Seitz (Binkofski and Seitz, 2004) found task-induced activation volume to be transiently decreased 2–4 weeks after a stroke, relative to both 1 week and 1 month after the stroke, while transcranial magnetic stimulation was used to verify neural responses at each time point. Similarly, dynamic BOLD activation was described by Altamura et al., who observed hemodynamic impairment at the subacute, but not the acute, stage. Although the subacute range in their study, 5–12 days after stroke, had considerable overlap with the present study, the average time since stroke in the present study falls just below this range. It is possible that cerebrovascular changes begin to take place during this range, becoming more apparent in the latter part. More longitudinal studies are necessitated to address such questions.
In addition to our observations in stroke patients, our results from both analyses also support the notion that increasing age adversely impacts the BOLD signal (D'Esposito et al., 2003, Riecker et al., 2003, Tsvetanov et al., 2015). While fMRI still receives widespread use for elderly subjects, comparisons with younger subjects should acknowledge differences in vasculature. The purpose of our standard versus subject-wise delay analysis was to draw attention to the practice of shifting the breath-hold HRF model by an equivalent amount for a group of subjects with variable CVR (e.g., Di et al., 2013, Kannurpatti et al., 2011), rather than modeling the response on an individual basis. Although both approaches yielded the same pattern of results, the more individualized, subject-wise delay analysis showed significantly increased activation volume in old controls—the group with the greatest hemodynamic delay. This finding highlights the importance of the delay chosen for analysis of breath-hold data, as a standard HRF delay used for subjects of varying ages may inadvertently favor younger subjects when determining active voxels. This may represent a larger problem in cognitive neuroscience research, in which a standard model based on young healthy subjects is regularly used for all subjects (Aguirre et al., 1998, D'Esposito et al., 2003, Handwerker et al., 2004). Such modeling may overlook neural activity in non-typical subjects that induces a measureable but less predictable vascular response. The use of an end-tidal CO2 trace as a GLM regressor has been promising for improving the accuracy of breath-hold response modeling (Bright and Murphy, 2013, Geranmayeh et al., 2015, Lipp et al., 2015), although this does not apply to the modeling of traditional fMRI task paradigms.
4.1. Breath-hold CVR mapping in stroke
The present study utilized breath-hold CVR mapping, which is only starting to become utilized in the stroke population. The primary use of this technique has been for evaluating hemodynamic responsiveness in patients – particularly neurosurgical patients – in order to identify regions in which the BOLD signal from a task can be expected to be attenuated (Pillai and Mikulis, 2015). Its correspondence with other validated methods for evaluating CVR has been demonstrated (Biswal et al., 2007, Kastrup et al., 2001). In stroke patients, the importance of such a procedure becomes especially apparent when attempting to compare neuroimaging data with healthy controls or evaluate changes to functional networks over time. To our knowledge, just two published studies have utilized a breath-hold task in stroke patients; interestingly, like the present study, both failed to observe perturbed CVR. In an fMRI study of recovery from aphasia, van Oers et al. (van Oers et al., 2010) implemented a breath-hold task to validate their comparisons from functional imaging by showing that subacute and chronic stroke patients did not differ from controls in CVR. Recently, Geranmayeh et al. (Geranmayeh et al., 2015) also found no differences in CVR in the healthy tissue of subacute and chronic stroke patients and controls. However, the negative findings of these studies contradict much of the literature in chronic stroke. Perhaps more importantly, though, these studies demonstrate the ease and efficacy of a breath-hold task to increase confidence in group functional imaging studies involving chronic stroke patients. Similarly, our study serves this purpose for acute patients.
Given that all three breath-hold studies have indicated CVR to be unaffected in stroke, it is important to note that intact CVR is not equivalent to intact neurovascular coupling. It is plausible in some cases that the ability of vascular smooth muscle cells to modulate blood flow in response to hypercapnia is preserved at the same time that the response to extravascular (e.g., neural) stimulation is compromised. While CVR-based methods of predicting NVU assume a common mechanism to underlie deficits in both, such as an overall reduced capacity for vasodilation, NVU may actually be the result of dysfunction at an earlier stage of the functional hyperemic process—anywhere from the release or synthesis of vasoactive agents in response to neural activity to the binding of these agents, to their corresponding pathways that would otherwise result in vasodilation (Girouard and Iadecola, 2006). BOLD CVR mapping does not exactly test neurovascular coupling, per se, and thus may be of little use in such cases of upstream neurovascular dysfunction (DeYoe and Raut, 2014, Pillai and Mikulis, 2015). This possibility deserves particular consideration in ischemic stroke due to the selective changes in the neurovascular unit that may occur. For example, astrocytes, which link synaptic activity to capillaries and arterioles and thus play a critical role in neurovascular coupling, are often damaged in the stroke event or functionally altered afterward (Pekny and Nilsson, 2005). To the best of our knowledge, there has not yet been a demonstration of impairment at an earlier stage of the coupling process with a preserved vasodilatory response to hypercapnia, but this remains a theoretical possibility.
4.2. Limitations and future directions
Our study had multiple experimental limitations that should be taken into consideration. For one, it is known that differences in breath-hold task performance can confound CVR comparisons (Kannurpatti et al., 2014). Thus, it may be important in future studies to collect physiological data and CO2 recordings in order to verify proper performance of all study subjects on the breath-hold task and improve estimates of CVR (Lipp et al., 2015). In the present study, because no differences in CVR were observed between patients and age-matched controls, it is unlikely that performance was a major issue in the patient group.
Head motion can also become an important concern when working with patient imaging data. The patient group in the present study exhibited more head motion than both groups of controls, and old controls exhibited more head motion than young controls. Although we took an aggressive approach to minimize the risk of head motion obscuring differences in CVR, task-related motion may still have impacted the data, potentially increasing the number of voxels exhibiting task-associated activity as well as the estimated magnitude of BOLD activation. This possibility is acknowledged and highlights one of the most persistent challenges in fMRI research and, in particular, its clinical application.
Finally, the heterogeneity in our patient sample with respect to factors such as type of stroke, location, lesion volume, treatment, and time after stroke onset may have contributed to the high variability observed, making an effect more difficult to identify. For example, differences may exist between cortical and subcortical or supratentorial and infratentorial strokes, or strokes in regions supplied by different major arteries, all of which are included in our sample; however, the extent to which these would differentially affect CVR is unknown. Moreover, changes in the brain can occur quickly in the days following stroke onset, and so the variability in time after onset in our sample could potentially represent different stages in the recovery process. More generally, the course of CVR may be radically different from patient to patient for a number of reasons, and it is possible that any impairments in CVR were averaged out at the group level. Reduced CVR may also be highly focal to the lesion and could have been overlooked when averaging over whole-brain gray matter. To account for this, we compared ipsilesional and contralesional CVR at a group level and confirmed our negative findings, although it is possible that even averaging over a hemisphere is enough to mask reduced CVR inside and proximal to the lesioned area. In short, the highly variable results among patients in our study still warrant precaution when interpreting data from individual patients. In the future, to overcome some limitations of the present study, it would be useful to perform similar analyses in a more homogenous and larger stroke sample.
5. Conclusion
Here we used a breath-hold task to evaluate CVR shortly after stroke. NVU has received increased attention in recent years, particularly in patient populations such as stroke. However, our findings suggest that NVU due to compromised CVR is likely not a serious concern for group comparisons of fMRI data between acute stroke patients and age-matched controls. Thus, group differences in these studies can likely be attributed to brain activity differences. Nonetheless, given the effect of stroke on vasculature and the high variability in CVR observed here, researchers and clinicians should remain cognizant of the limitations of fMRI when dealing with patients at any stage of stroke. In future studies, it will be useful to include more homogenous patient samples and to obtain CO2 recordings to improve the accuracy of breath-hold data.
Acknowledgments
The authors would like to thank Dr. Christian La, Dr. Rasmus Birn, Dr. Wolfgang Gaggl, and Jed Mathis for helpful comments. This work was supported by the Clinical and Translational Science Awards program of the National Center for Research Resources, NIH/NCATS grant 1UL1RR025011, UW ICTR Pilot Grant, KL2 Scholar Award, NIH/NINDS Beeson K23NS086852, Foundation of ASNR, and the AHA Undergraduate Research Program.
References
- Aguirre G.K., Zarahn E., D'esposito M. The variability of human, BOLD hemodynamic responses. NeuroImage. 1998;8:360–369. doi: 10.1006/nimg.1998.0369. [DOI] [PubMed] [Google Scholar]
- Altamura C., Reinhard M., Vry M.S., Kaller C.P., Hamzei F., Vernieri F., Rossini P.M., Hetzel A., Weiller C., Saur D. The longitudinal changes of BOLD response and cerebral hemodynamics from acute to subacute stroke. A fMRI and TCD study. BMC Neurosci. 2009;10:151. doi: 10.1186/1471-2202-10-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amemiya S., Kunimatsu A., Saito N., Ohtomo K. Impaired hemodynamic response in the ischemic brain assessed with BOLD fMRI. NeuroImage. 2012;61:579–590. doi: 10.1016/j.neuroimage.2012.04.001. [DOI] [PubMed] [Google Scholar]
- Beaulieu C., de Crespigny A., Tong D.C., Moseley M.E., Albers G.W., Marks M.P. Longitudinal magnetic resonance imaging study of perfusion and diffusion in stroke: evolution of lesion volume and correlation with clinical outcome. Ann. Neurol. 1999;46:568–578. doi: 10.1002/1531-8249(199910)46:4<568::aid-ana4>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Binkofski F., Seitz R.J. Modulation of the BOLD-response in early recovery from sensorimotor stroke. Neurology. 2004;63:1223–1229. doi: 10.1212/01.wnl.0000140468.92212.be. [DOI] [PubMed] [Google Scholar]
- Biswal B.B., Kannurpatti S.S., Rypma B. Hemodynamic scaling of fMRI-BOLD signal: validation of low-frequency spectral amplitude as a scalability factor. Magn. Reson. Imaging. 2007;25:1358–1369. doi: 10.1016/j.mri.2007.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonakdarpour B., Parrish T.B., Thompson C.K. Hemodynamic response function in patients with stroke-induced aphasia: implications for fMRI data analysis. NeuroImage. 2007;36:322–331. doi: 10.1016/j.neuroimage.2007.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonakdarpour B., Beeson P.M., DeMarco A.T., Rapcsak S.Z. Variability in blood oxygen level dependent (BOLD) signal in patients with stroke-induced and primary progressive aphasia. Neuroimage Clin. 2015;8:87–94. doi: 10.1016/j.nicl.2015.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright M.G., Murphy K. Reliable quantification of BOLD fMRI cerebrovascular reactivity despite poor breath-hold performance. NeuroImage. 2013;83:559–568. doi: 10.1016/j.neuroimage.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calautti C., Baron J.C. Functional neuroimaging studies of motor recovery after stroke in adults: a review. Stroke. 2003;34:1553–1566. doi: 10.1161/01.STR.0000071761.36075.A6. [DOI] [PubMed] [Google Scholar]
- Cox R.W. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- del Zoppo G.J. The neurovascular unit in the setting of stroke. J. Intern. Med. 2010;267:156–171. doi: 10.1111/j.1365-2796.2009.02199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Esposito M., Deouell L.Y., Gazzaley A. Alterations in the BOLD fMRI signal with ageing and disease: a challenge for neuroimaging. Nat. Rev. Neurosci. 2003;4:863–872. doi: 10.1038/nrn1246. [DOI] [PubMed] [Google Scholar]
- DeYoe E.A., Raut R.V. Visual mapping using blood oxygen level dependent functional magnetic resonance imaging. Neuroimaging Clin. N. Am. 2014;24:573–584. doi: 10.1016/j.nic.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di X., Kannurpatti S.S., Rypma B., Biswal B.B. Calibrating BOLD fMRI activations with neurovascular and anatomical constraints. Cereb. Cortex. 2013;23:255–263. doi: 10.1093/cercor/bhs001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geranmayeh F., Wise R.J., Leech R., Murphy K. Measuring vascular reactivity with breath-holds after stroke: a method to aid interpretation of group-level BOLD signal changes in longitudinal fMRI studies. Hum. Brain Mapp. 2015;36:1755–1771. doi: 10.1002/hbm.22735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girouard H., Iadecola C. Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J. Appl. Physiol. 2006;100:328–335. doi: 10.1152/japplphysiol.00966.2005. [DOI] [PubMed] [Google Scholar]
- Handwerker D.A., Ollinger J.M., D'Esposito M. Variation of BOLD hemodynamic responses across subjects and brain regions and their effects on statistical analyses. NeuroImage. 2004;21:1639–1651. doi: 10.1016/j.neuroimage.2003.11.029. [DOI] [PubMed] [Google Scholar]
- Handwerker D.A., Gazzaley A., Inglis B.A., D'Esposito M. Reducing vascular variability of fMRI data across aging populations using a breathholding task. Hum. Brain Mapp. 2007;28:846–859. doi: 10.1002/hbm.20307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiss W.D., Kidwell C.S. Imaging for prediction of functional outcome and assessment of recovery in ischemic stroke. Stroke. 2014;45:1195–1201. doi: 10.1161/STROKEAHA.113.003611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman E.M. Coupling mechanism and significance of the BOLD signal: a status report. Annu. Rev. Neurosci. 2014;37:161–181. doi: 10.1146/annurev-neuro-071013-014111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou B.L., Bradbury M., Peck K.K., Petrovich N.M., Gutin P.H., Holodny A.I. Effect of brain tumor neovasculature defined by rCBV on BOLD fMRI activation volume in the primary motor cortex. NeuroImage. 2006;32:489–497. doi: 10.1016/j.neuroimage.2006.04.188. [DOI] [PubMed] [Google Scholar]
- Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer's disease. Nat. Rev. Neurosci. 2004;5:347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- Jezzard P., Buxton R.B. The clinical potential of functional magnetic resonance imaging. J. Magn. Reson. Imaging. 2006;23:787–793. doi: 10.1002/jmri.20581. [DOI] [PubMed] [Google Scholar]
- Jones T.B., Bandettini P.A., Kenworthy L., Case L.K., Milleville S.C., Martin A., Birn R.M. Sources of group differences in functional connectivity: an investigation applied to autism spectrum disorder. NeuroImage. 2010;49:401–414. doi: 10.1016/j.neuroimage.2009.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannurpatti S.S., Motes M.A., Rypma B., Biswal B.B. Neural and vascular variability and the fMRI-BOLD response in normal aging. Magn. Reson. Imaging. 2010;28:466–476. doi: 10.1016/j.mri.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannurpatti S.S., Motes M.A., Rypma B., Biswal B.B. Increasing measurement accuracy of age-related BOLD signal change: minimizing vascular contributions by resting-state-fluctuation-of-amplitude scaling. Hum. Brain Mapp. 2011;32:1125–1140. doi: 10.1002/hbm.21097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannurpatti S.S., Motes M.A., Biswal B.B., Rypma B. Assessment of unconstrained cerebrovascular reactivity marker for large age-range FMRI studies. PLoS One. 2014;9 doi: 10.1371/journal.pone.0088751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastrup A., Krüger G., Neumann-Haefelin T., Moseley M.E. Assessment of cerebrovascular reactivity with functional magnetic resonance imaging: comparison of CO(2) and breath holding. Magn. Reson. Imaging. 2001;19:13–20. doi: 10.1016/s0730-725x(01)00227-2. [DOI] [PubMed] [Google Scholar]
- Krainik A., Hund-Georgiadis M., Zysset S., von Cramon D.Y. Regional impairment of cerebrovascular reactivity and BOLD signal in adults after stroke. Stroke. 2005;36:1146–1152. doi: 10.1161/01.STR.0000166178.40973.a7. [DOI] [PubMed] [Google Scholar]
- Lindauer U., Dirnagl U., Füchtemeier M., Böttiger C., Offenhauser N., Leithner C., Royl G. Pathophysiological interference with neurovascular coupling – when imaging based on hemoglobin might go blind. Front. Neuroenerg. 2010:2. doi: 10.3389/fnene.2010.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipp I., Murphy K., Caseras X., Wise R.G. Agreement and repeatability of vascular reactivity estimates based on a breath-hold task and a resting state scan. NeuroImage. 2015;113:387–396. doi: 10.1016/j.neuroimage.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzetto-Betti K.C., Leoni R.F., Pontes-Neto O.M., Santos A.C., Leite J.P., Silva A.C., de Araujo D.B. The stability of the blood oxygenation level-dependent functional MRI response to motor tasks is altered in patients with chronic ischemic stroke. Stroke. 2010;41:1921–1926. doi: 10.1161/STROKEAHA.110.590471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata Y., Sakatani K., Hoshino T., Fujiwara N., Kano T., Nakamura S., Katayama Y. Effects of cerebral ischemia on evoked cerebral blood oxygenation responses and BOLD contrast functional MRI in stroke patients. Stroke. 2006;37:2514–2520. doi: 10.1161/01.STR.0000239698.50656.3b. [DOI] [PubMed] [Google Scholar]
- Pekny M., Nilsson M. Astrocyte activation and reactive gliosis. Glia. 2005;50:427–434. doi: 10.1002/glia.20207. [DOI] [PubMed] [Google Scholar]
- Pillai J.J. The evolution of clinical functional imaging during the past 2 decades and its current impact on neurosurgical planning. AJNR Am. J. Neuroradiol. 2010;31:219–225. doi: 10.3174/ajnr.A1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai J.J., Mikulis D.J. Cerebrovascular reactivity mapping: an evolving standard for clinical functional imaging. AJNR Am. J. Neuroradiol. 2015;36:7–13. doi: 10.3174/ajnr.A3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai J.J., Zaca D. Clinical utility of cerebrovascular reactivity mapping in patients with low grade gliomas. World J. Clin. Oncol. 2011;2:397–403. doi: 10.5306/wjco.v2.i12.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineiro R., Pendlebury S., Johansen-Berg H., Matthews P.M. Altered hemodynamic responses in patients after subcortical stroke measured by functional MRI. Stroke. 2002;33:103–109. doi: 10.1161/hs0102.100482. [DOI] [PubMed] [Google Scholar]
- Riecker A., Grodd W., Klose U., Schulz J.B., Gröschel K., Erb M., Ackermann H., Kastrup A. Relation between regional functional MRI activation and vascular reactivity to carbon dioxide during normal aging. J. Cereb. Blood Flow Metab. 2003;23:565–573. doi: 10.1097/01.WCB.0000056063.25434.04. [DOI] [PubMed] [Google Scholar]
- Roc A.C., Wang J., Ances B.M., Liebeskind D.S., Kasner S.E., Detre J.A. Altered hemodynamics and regional cerebral blood flow in patients with hemodynamically significant stenoses. Stroke. 2006;37:382–387. doi: 10.1161/01.STR.0000198807.31299.43. [DOI] [PubMed] [Google Scholar]
- Rossini P.M., Altamura C., Ferretti A., Vernieri F., Zappasodi F., Caulo M., Pizzella V., Del Gratta C., Romani G.L., Tecchio F. Does cerebrovascular disease affect the coupling between neuronal activity and local haemodynamics? Brain. 2004;127:99–110. doi: 10.1093/brain/awh012. [DOI] [PubMed] [Google Scholar]
- Saur D., Lange R., Baumgaertner A., Schraknepper V., Willmes K., Rijntjes M., Weiller C. Dynamics of language reorganization after stroke. Brain. 2006;129:1371–1384. doi: 10.1093/brain/awl090. [DOI] [PubMed] [Google Scholar]
- Siegel J.S., Snyder A.Z., Ramsey L., Shulman G.L., Corbetta M. The effects of hemodynamic lag on functional connectivity and behavior after stroke. J. Cereb. Blood Flow Metab. 2015 doi: 10.1177/0271678X15614846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason M.E., Burrows B.E., Gabrieli J.D., Glover G.H. Breath holding reveals differences in fMRI BOLD signal in children and adults. NeuroImage. 2005;25:824–837. doi: 10.1016/j.neuroimage.2004.12.026. [DOI] [PubMed] [Google Scholar]
- Tsvetanov K.A., Henson R.N., Tyler L.K., Davis S.W., Shafto M.A., Taylor J.R., Williams N., Cam-Can, Rowe J.B. The effect of ageing on fMRI: correction for the confounding effects of vascular reactivity evaluated by joint fMRI and MEG in 335 adults. Hum. Brain Mapp. 2015;36:2248–2269. doi: 10.1002/hbm.22768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmer J.L., Krouwer H.G., Mueller W.M., Ugurel M.S., Kocak M., Mark L.P. Pseudo-reorganization of language cortical function at fMR imaging: a consequence of tumor-induced neurovascular uncoupling. AJNR Am. J. Neuroradiol. 2003;24:213–217. [PMC free article] [PubMed] [Google Scholar]
- van Oers C.A., Vink M., van Zandvoort M.J., van der Worp H.B., de Haan E.H., Kappelle L.J., Ramsey N.F., Dijkhuizen R.M. Contribution of the left and right inferior frontal gyrus in recovery from aphasia. A functional MRI study in stroke patients with preserved hemodynamic responsiveness. Neuroimage. 2010;49:885–893. doi: 10.1016/j.neuroimage.2009.08.057. [DOI] [PubMed] [Google Scholar]
- Ward N.S., Brown M.M., Thompson A.J., Frackowiak R.S. Neural correlates of motor recovery after stroke: a longitudinal fMRI study. Brain. 2003;126:2476–2496. doi: 10.1093/brain/awg245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widder B., Kleiser B., Krapf H. Course of cerebrovascular reactivity in patients with carotid artery occlusions. Stroke. 1994;25:1963–1967. doi: 10.1161/01.str.25.10.1963. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Brady M., Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans. Med. Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]