Abstract
Myotonic dystrophy type 1 (DM1) has a wide phenotypic spectrum and potentially may affect central nervous system with mild to severe involvement. Our aim was to investigate grey matter (GM) and white matter (WM) structural alterations in a sample of adult-onset DM1 patients and to evaluate relationship with clinical and cognitive variables.
Thirty DM1 patients underwent neuropsychological investigation and 3T-MRI protocol. GM and WM changes were evaluated calculating brain parenchymal fraction (BPF), voxel-based morphometry (VBM), white matter lesion load (LL% and Fazekas scale) and tract based spatial statistical (TBSS).
Patients showed main impairment in tests exploring executive and mnesic domains with visuo-spatial involvement, significantly related to BPF. VBM revealed clusters of widespread GM reduction and TBSS revealed areas of decreased fractional anisotropy (FA) and increased radial diffusivity (RD), mean diffusivity (MD) and axial diffusivity (AD) in patients compared to a group of matched healthy controls. Multiple regression analyses showed areas of significant negative relationship between left temporal atrophy and verbal memory, between RD and mnesic and visuo-spatial cognitive domains, and between AD and verbal memory.
TBSS results indicate that the involvement of normal appearance WM, beyond the signal changes detected with conventional MR imaging (Fazekas scale and LL%), was associated with neuropsychological deficit. These data suggest that disrupted complex neuronal networks can underlie cognitive-behavioural dysfunctions in DM1.
Keywords: MR imaging, VBM, TBSS, DM1, Neuropsychology
Highlights
-
•
We performed VBM and TBSS analyses in a sample of adult-onset DM1 patients.
-
•
The relationship between neuroimaging variables and cognitive profile was studied.
-
•
Global atrophy correlated with executive and visuo-spatial abilities.
-
•
TBSS revealed associations between DTI indexes and cognitive performances.
-
•
Disrupted complex neuronal networks can underlie cognitive dysfunction in DM1.
1. Introduction
Myotonic dystrophy type 1 (DM1) is the most common form of muscular dystrophy in adults, with a prevalence of about 1 in 8000 people worldwide. It is an autosomal dominant disorder due to an unstable cytosine-thymine-guanine triplet repeat ([CTG]n) expansions on chromosome 19 (Huang and Kuo, 2005), showing no definitive correlation with phenotypic expression (Udd and Krahe, 2012). DM1 can be classified into four clinical forms: congenital, childhood and classical or late-onset (Ekström et al., 2009, Harper, 2001). Classical DM1 form is a multisystem disorder ranging from mild to more severe phenotypes and affecting many organs and tissues, including central nervous system (CNS), this finally responsible of cognitive and behavioural dysfunctions.
Several studies have demonstrated that DM1 patients show a selective impairment in cognitive functioning, particularly in attentional, visuo-spatial, and executive domains (Meola et al., 2003, Winblad et al., 2010); the existence of a “DM1-related-dysexecutive-syndrome” has been already proposed (Meola and Sansone, 2007). Intelligence assessment documented an IQ below average in the DM1 population as compared to healthy subjects, with no clear evidence of a progressive decline (Meola and Sansone, 2007, Jean et al., 2014).
Beside cognitive impairments, in DM1 patients neuropsychiatric comorbidities are frequently reported with variable pathologic behavioural patterns: lack of interest (apathetic behaviour), a decreased emotional participation and an increased irritability are the main clinical features, defined by some authors as “an emotional imbalance” (Meola and Sansone, 2007, Laberge et al., 2013); moreover, a high prevalence of dysfunctional personality has been described (Meola et al., 2003, Winblad et al., 2010, Sistiaga et al., 2010).
Previous studies have documented brain abnormalities in DM1 through different imaging techniques and investigated their relationship with cognitive impairment without reaching univocal conclusions. Voxel-based morphometry (VBM) technique showed grey matter (GM) atrophy in several regions of temporal and frontal lobes, hippocampi and thalami (Weber et al., 2010, Minnerop et al., 2011, Schneider-Gold et al., 2015); some studies revealed non-specific pathological findings such as ventricular enlargement and white matter (WM) hyperintensities in different cerebral lobes (Minnerop et al., 2011, Romeo et al., 2010); single photon emission tomography and PET studies demonstrated hypoperfusion and glucose hypometabolism of frontal and temporal lobes (Weber et al., 2010, Meola et al., 1999). Recent studies adopting tractography evaluation (Minnerop et al., 2011, Wozniak et al., 2014) provide data showing microstructural WM damage in interhemispheric, corticospinal and limbic pathways and in frontal, temporal, parietal and occipital lobes. To date, only one study demonstrates that brain atrophy and white matter involvement are progressive over time in DM1 (Conforti et al., 2016).
Although considerable structural CNS involvement on one hand and cognitive deficits on the other hand have been detected in DM1, only few studies investigate their relationship. Some studies have found significant correlations between MR imaging features and neuropsychological profiles (Weber et al., 2010, Wozniak et al., 2014, Caso et al., 2014), while others did not (Minnerop et al., 2011, Romeo et al., 2010).
To this purpose we investigated the structural alterations of GM and WM in a sample of adult-onset DM1 patients and evaluated their relationship with clinical and cognitive variables.
2. Material and methods
Thirty patients (24 males, 6 females; mean age 44.6 ± 12.4 years; age range 24–67 years) with clinical and genetic diagnosis of adult form of DM1, according the International Consortium for myotonic dystrophies guidelines (IDMC, 2000), were consecutively recruited at Neurological clinic of University of Pisa. Exclusion criteria were mental retardation (IQ < 70), severe visual impairment, psychiatric illness and a history of substances abuse. None of the patients presented motor or coordination disability sufficient to account for possible delay in any of the neuropsychological tests administered. Patients were grouped on the basis of the number of [CTG]n expansions: 12 patients (40%) were classified as E1 (< 150 [CTG]n) and 18 patients (60%) as E2 (150–1000 [CTG]n). The mean disease duration from symptoms onset to the MRI examination was 16.5 ± 11.8 years, while age at onset was 29.0 ± 12.0 years. Patients' mean educational level was 11.3 ± 3.4 years.
Control group was retrospectively selected from our database and included 30 healthy subjects (22 males, 8 females; mean age 44.8 ± 12.6 years; age range 27–68 years) for VBM analyses and 21 subjects (14 males, 7 females; mean age 44.6 ± 12.7 years; age range 27–61 years) for diffusion tensor imaging (DTI) analysis. Age and gender did not significantly differ between patients and controls either for VBM (Mann–Whitney U test p = 0.95 for age; Fisher's exact test, two tailed p = 0.54 for sex), and for DTI (Mann–Whitney U test p = 0.33 for age; Fisher's exact test, two tailed p = 0.92 for sex). All healthy controls beside not to be affected by neurological or psychiatric disorders, had negative neurological examination and no family history for neuropsychiatric illness.
2.1. Neuropsychological evaluation
An experienced neuropsychologist, who was unaware of the clinical and MRI data, performed the neuropsychological evaluation. For assessment of immediate memory Immediate and Delayed Recall (IR, DR) of Rey Auditory Verbal Learning Test (RAVLT), Immediate and Delayed Recall (IR, DR) of Rey Osterrieth Complex Figure (ROCF), digit span and Corsi Block-tapping Test (CBT) were administered. Trail Making Tests (TMT-A and TMT-B) were used to assess selective attention and cognitive flexibility and Stroop Test was used to assess automatic response inhibition. Frontal and executive functions were examined by phonemic verbal fluency test (FAS), Frontal Assessment Battery (FAB) and Modified Wisconsin Card Sorting Test (WCST). Rey-Osterrieth Complex Figure was used to assess spatial organization and visuo-constructional skills (ROCF-copy). Patients' raw scores were corrected according to Italian normative values (Spinnler and Tognoni, 1987, Lezak et al., 2012). Percentages of impairment of DM1 patients who showed significant neuropsychological dysfunctions across different cognitive domains were established using Italian normative data for both, score adjustment (sex, age, education) and definition of cut-off thresholds; the latter have been determined as the lower limit of the 95% tolerance interval for a confidence level of 95%.
2.2. Data acquisition
MRI imaging was performed with a 3T scanner (Discovery MR750 3.0 T, GE Healthcare, Milwaukee) equipped with an 8-channel head coil with ASSET-technology. The examination protocol included a sagittal CUBE T2 FLAIR sequence (TR 8000 ms; FOV 256 mm; matrix 256 × 256; thickness 1.0 mm; spacing 0 mm; NEX 1.0), a sagittal high resolution 3D T1weighted images with isotropic voxels (TR 8.1 ms; TE 3.2 ms; TI 450 ms; flip angle 12°; FOV 256 mm; matrix 256 × 256; thickness 1.0 mm; spacing 0 mm; NEX 1.0) and DTI performed by using a multiacquisition echoplanar sequence (TR 7000 ms; TE minimum; FOV 240 mm; matrix 128 × 128; thickness 2.9 mm; spacing 0 mm; diffusion gradients applied in 25 directions with b factor = 1000 s/mm2 and 1 b0 volume).
2.3. GM evaluation
The evaluation of GM atrophy was performed with a Region of Interest (ROI) based method and using VBM analysis.
In ROI based method FLAIR images, reformatted in axial slices (thickness 4 mm without spacing), were manually segmented using software AW VolumeShare 4 (ADVANTAGE WORKSTATION 4.3, GE Healthcare, Milwaukee) to measure the parenchymal volume and total intracranial volume. Brain parenchymal fraction (BPF) was calculated through the ratio of brain parenchymal to intracranial volume and was considered as an expression of the degree of atrophy, as done in previous studies of volumetric analysis of DM1 patients (Kassubek et al., 2003). Correlations between BPF values and clinical (age, disease duration) and neuropsychological scores were evaluated by using Pearson correlation coefficient, considering p-value statistically significant at p < 0.05.
2.3.1. VBM
The automated analysis of T1 structural data was carried out by FSL-VBM (Douaud et al., 2007), an optimised VBM protocol (Good et al., 2001) carried out with FMRIB software library package (FSL) (Smith et al., 2004). As preprocessing step T1 images were corrected for WM lesions using the lesion filling toolbox available in FSL (Battaglini et al., 2012). Structural images were brain-extracted using BET (Brain Extraction Tool) (Smith, 2002), and then they were automatically segmented into GM, WM and cerebrospinal fluid (CSF) tissue-type by FAST4 tool (Zhang et al., 2001). The GM volume images were aligned to the Montreal Neurological Institute (MNI) 152 standard space (Mazziotta et al., 1995) by the affine registration tool FLIRT (Jenkinson et al., 2002), followed by non-linear registration using FNIRT (Andersson et al., 2007). The registered GM images of an equal number of healthy controls and DM1 patients were averaged and flipped along the x-axis to create a left-right symmetric, study-specific grey matter template. After that all native GM images were non-linearly registered to this study-specific template, modulated and smoothed with an isotropic Gaussian kernel with a sigma of 3 mm.
A voxel-wise General Linear Model (GLM) was applied using permutation-based non-parametric testing (5000 permutations), correcting for multiple comparisons across space (p < 0.001) by threshold-free cluster enhancement (TFCE) option (Smith and Nichols, 2009). Since voxel based quantification of atrophy is influenced by aging (Draganski et al., 2011) we inserted age and gender of patients and controls as covariate variables within the GLM matrix.
In order to establish a possible relationship with VBM changes multiple regression analyses were also performed inserting neuropsychological scores in different GLM design matrix, using age and gender as covariates of no interest (TFCE p < 0.05 corrected for multiple comparisons).
The description of statistical maps obtained from VBM analysis was based on Anatomical Automated Labeling (AAL) Atlas (Tzourio-Mazoyer et al., 2002).
2.4. WM evaluation
Sagittal CUBE T2 FLAIR sequence was also acquired in order to establish recurrence, localization and patterns of distribution of white matter hyperintense lesions in DM1 patients. WM alterations were evaluated with a visual scale according to a modified version of the Fazekas scale (Pantoni et al., 2005) on reformatted FLAIR images. A quantitative measure of the white matter lesions was performed with a ROI based approach by manually contouring the WM lesions. The lesion load (LL%) was calculated through the ratio of lesions volume to brain parenchymal volume and expressed as a percentage. Correlations between lesions indices (Fazekas scale and LL%) and clinical and neuropsychological scores were evaluated by using Spearman and Pearson correlation coefficients, respectively for Fazekas scale and LL%, considering p-value statistically significant at 0.05.
2.4.1. TBSS
DTI sequence was acquired in order to perform tract-based spatial statistical (TBSS) analysis (Smith et al., 2006).
The preprocessing steps on the raw diffusion data consisted in minimizing the distortion relative to gradient application using ‘eddy current correction’ tool and to perform brain-extraction using BET. DTIfit toolbox (Behrens et al., 2003) was used to fit a diffusion tensor model at each voxel of the preprocessed DTI images in order to obtain fractional anisotropy (FA), mean diffusivity (MD) and eigenvalues (L1, L2, L3) maps for each subject. Maps of axial diffusivity (AD) were evaluated (L1 maps) and maps of radial diffusivity (RD) were calculated averaging L2 and L3 maps. All subjects' DTI-derived maps were nonlinear registered to a target image (FMRIB58_FA) using FNIRT. After that, a mean FA image was calculated and thinned (threshold 0.2) in order to create a mean FA skeleton which represented the centres of all tracts common to the group. For each subjects, registered DTI-derived maps were then projected onto this skeleton and the resulting data fed into voxelwise cross-subject statistics.
Group differences between patients and controls in DTI-derived indices were performed using permutation-based non-parametric testing (5000 permutations), correcting for multiple comparisons across space (p < 0.05) by TFCE option (Mazziotta et al., 1995). Age and gender were used as covariates of no interest in the statistical design matrix.
Multiple regression analyses were also performed using neuropsychological variables in different GLM design matrix to evaluate relationship with DTI-derived indices, inserting age and gender as covariates of no interest (TFCE p < 0.05 corrected for multiple comparisons).
JHU DTI-based white-matter atlas (ICBM-DTI-81 white-matter labels atlas) has been superimposed to statistical maps in order to identify the WM tracts (Mori et al., 2005).
2.5. Ethics statement
The research was conducted according to the principles expressed in the Declaration of Helsinki. The study was authorized by the Local Ethics Committee and written informed consent was obtained from all the study participants.
3. Results
3.1. Cognitive profile
TMT-A was impaired in 17.2% of patients, TMT-B in 14.8%, FAB in 20.0%, Stroop test as in bracket (Error Interference in 20.7%, Time Interference in 27.6%) and FAS in 26.7%; impairment in WCST was detected in 36.7% of patients as for Categorization ability, while Perseverations were found in 40.0%.
CBT showed impairments in 40.0% of patients, while 16.7% had impairments in Digit Span. As for delayed recall memory, 3.3% showed RAVLT-DR impaired performances, while 10.0% had impaired ROCF-DR. Motor planning and visuo-spatial abilities, tested using ROCF-copy, ranked below the normal range, in 40.0% of patients.
Neuropsychological findings are summarized in Supplementary Table 1.
3.2. Grey matter atrophy
BPF value in DM1 subjects was 0.760 ± 0.035 and correlated with visuo-spatial and executive performance (TMT-A, TMT-B, ROCF-copy and WCST categorization) (p < 0.05).
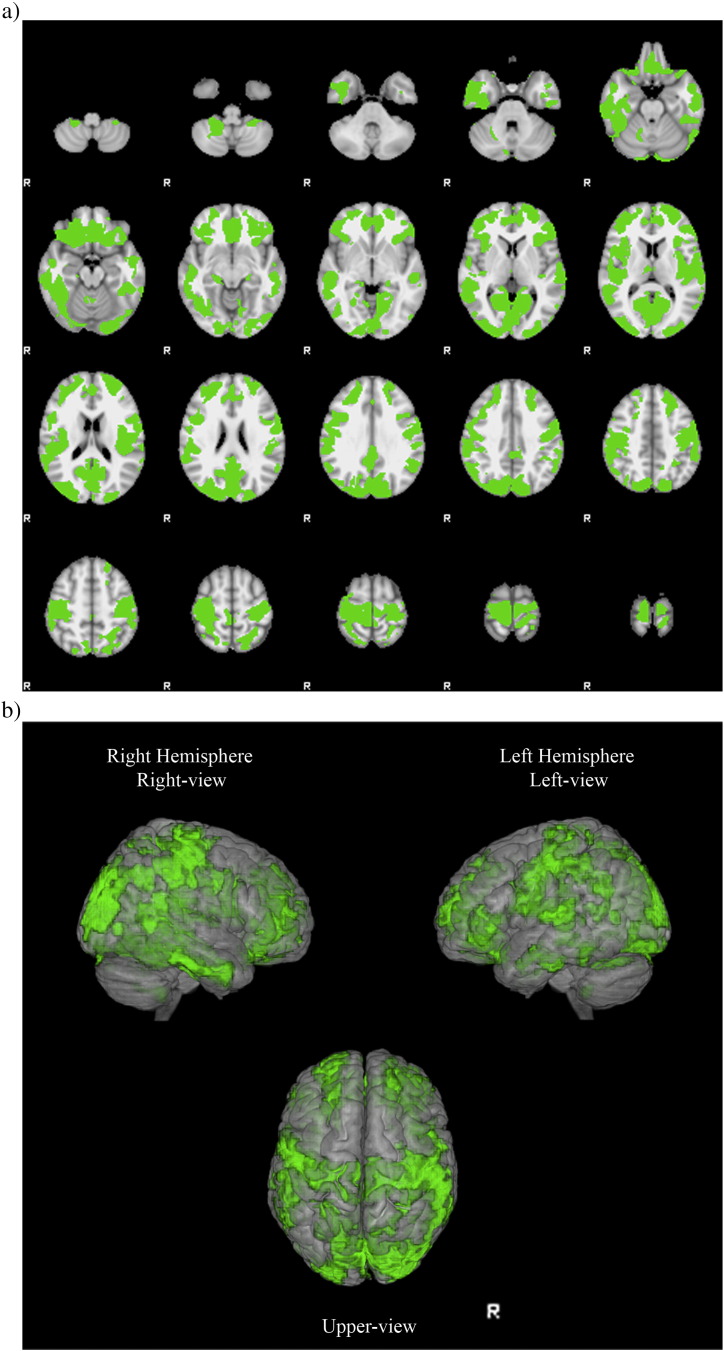
VBM revealed several clusters of reduced cortical GM in DM1 patients compared to healthy controls (TFCE p < 0.001 corrected for multiple comparisons). Atrophy was diffuse in both cerebral hemispheres (Supplementary Table 2) and in particular it was located in perirolandic, orbitofrontal, dorsolateral frontal, insular, temporo occipital, parietomesial, anterior and posterior cingulated areas (Fig. 1).
Fig. 1.
VBM analysis reveals clusters of grey matter atrophy (in green) in DM1 patients compared to healthy controls superimposed on axial slices (a) or volumes (b) of MNI standard brain (TFCE p < 0.001 corrected for multiple comparisons). Clusters are diffuse in both hemispheres, particularly in perirolandic, orbitofrontal, dorsolateral frontal, insular, temporo occipital, parietomesial, anterior and posterior cingulated areas. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
VBM analysis showed a negative relationship between RAVLT-DR scores and the grade of atrophy respectively in left postcentral (AAL 57), left middle and inferior temporal gyri (AAL 85 89) and left supramarginal gyrus (AAL 63) (TFCE p < 0.05 corrected for multiple comparisons) (Fig. 2).
Fig. 2.
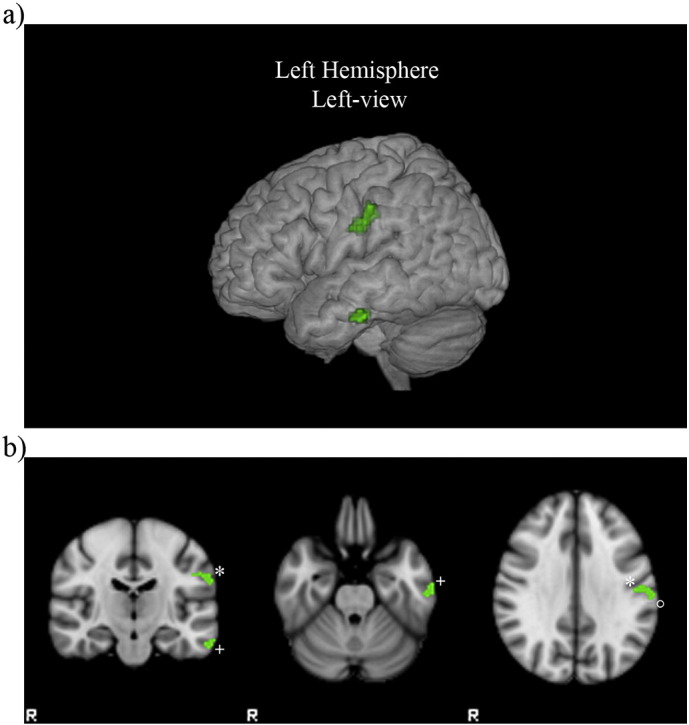
Negative relationship between RAVLT-DR scores and grade of atrophy (in green) in DM1 patients superimposed on volume (a) and coronal and axial slices (b) of MNI standard brain (TFCE p < 0.05 corrected for multiple comparisons). Clusters are located in left postcentral*, left middle and inferior temporal+ gyri and left supramarginal° gyrus. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.3. White matter alterations
WM alterations, evaluated visually with modified Fazekas scale, classified 5 patients (16.7%) as grade 0 (absence of lesions), 11 patients (36.7%) as grade 1 (mild changes), 10 patients (33.3%) as grade 2 (moderate changes), while 4 patients (13.3%) were classified as grade 3 (severe changes). No correlation between Fazekas scale and clinical and neuropsychological scores was demonstrated (p > 0.05).
LL% measured with ROI based method was 0.7 ± 0.9% (range 0–4.1%) and no significant correlation was found with clinical and neuropsychological features (p > 0.05).
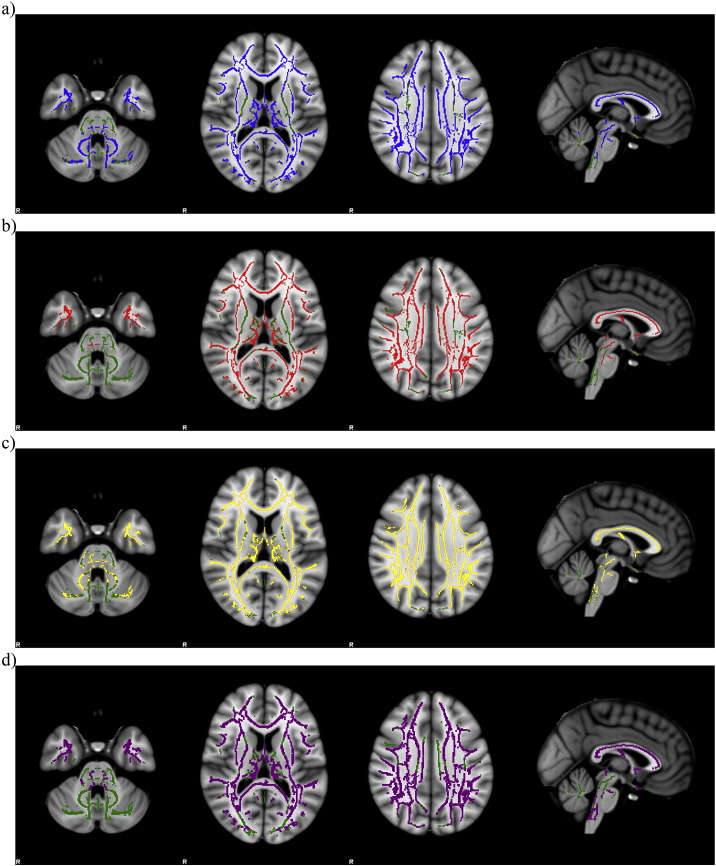
TBSS analysis revealed several areas of decreased FA and increased RD, MD and AD in white matter of DM1 patients compared to healthy controls (TFCE p < 0.05 corrected for multiple comparisons). DTI indexes were extensively impaired in association and projection tracts of both hemispheres (Supplementary Table 3, Supplementary Table 4, Supplementary Table 5, Supplementary Table 6). FA and MD changes involved the brainstem and the cerebellum of the DM1 patients with respect to healthy controls while RD and AD only the brainstem (Fig. 3).
Fig. 3.
TBSS maps show the tracts of significantly decreased FA (a, in blue) and the tracts of significantly increased RD (b, in red), MD (c, in yellow) and AD (d, in purple) in DM1 patients compared to healthy controls superimposed on axial and sagittal slices of MNI standard brain (TFCE p < 0.05 corrected for multiple comparisons). Green indicates the skeleton template. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
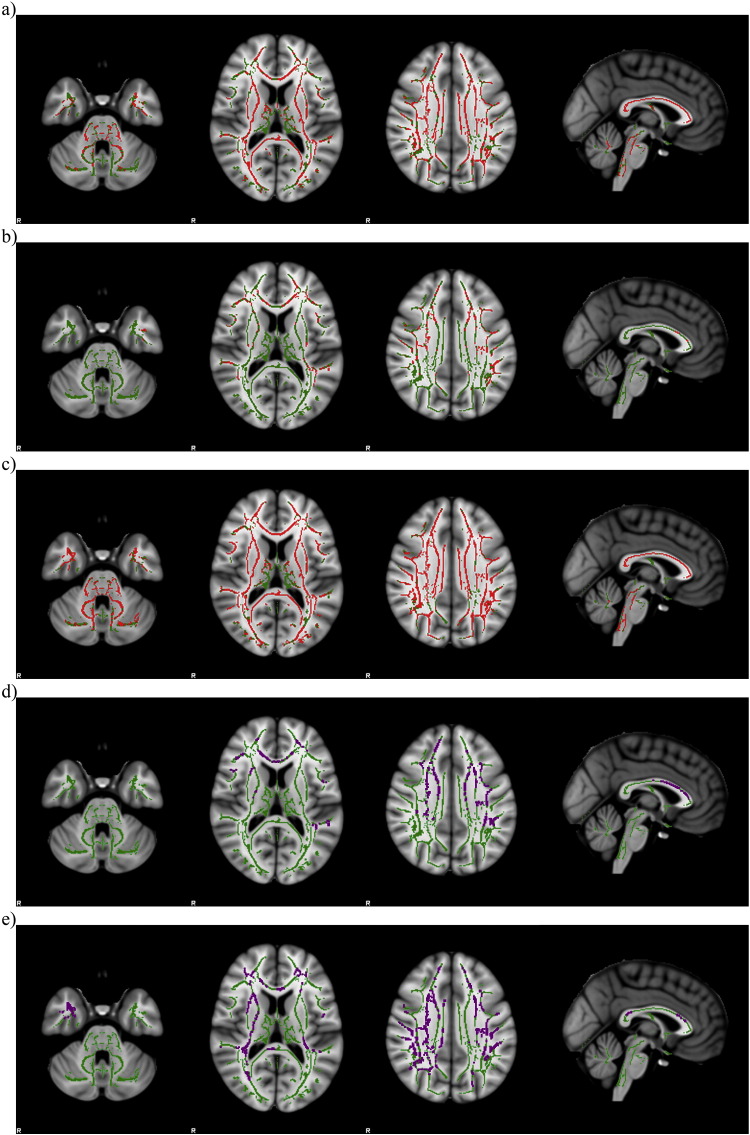
Multiple regression analyses to correlate neuropsychological tests to WM abnormalities showed areas of significant negative relationship between RD and ROCF-copy, RAVLT-DR and CBT scores; moreover, AD had a significant negative relationship with RAVLT-DR and digit span scores (TFCE p < 0.05 corrected for multiple comparisons) (Fig. 4).
Fig. 4.
Multiple regression analyses reveal negative relationship (in red) between RD and ROCF-copy (a), RAVLT-DR (b) and CBT (c) scores in DM1 patients. AD had a significant negative relationship (in purple) with RAVLT-DR (d) and digit span (e) scores in DM1 patients. Results are superimposed on axial and sagittal slices of MNI standard brain (TFCE p < 0.05 corrected for multiple comparisons). Green indicates the skeleton template. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
4.1. Cognitive profile
According to previous evidences about cognitive impairment in DM1 (Meola et al., 2003, Winblad et al., 2010, Minnerop et al., 2011), most of our patients presented diffuse neuropsychological dysfunction, mainly characterized by impairment in executive and mnesic domains with visuo-spatial component (WCST categorization 36.7%, WCST perseverations 40%, ROCF-copy 40.0%, CBT 40.0%). DM1 cognitive performance points out patients' relevant and likely specific difficulties in tasks which represent currently used paradigms to test executive control ability. In a smaller portion of patients, we detected cognitive impairment also in attentive and other executive domains, as showed by FAS, FAB, STROOP, TMT-A and Digit span. Despite wide neuropsychological impairment, opposite to visuo-spatial domain, our study elucidated a preservation of verbal abilities (about 80%) in these patients, this suggesting the need for systematic assessment of memory and cognitive planning in similar studies.
4.2. Grey matter atrophy
The BPF that we obtained was in line with value measured in DM1 patients and reduced respect to the normal population (Kassubek et al., 2003), confirming a global loss of central nervous tissue in this myopathy. The BPF showed a significantly correlation with age (p < 0.001), indicating that the central nervous system atrophy in the adult form of DM1 progresses with age as in the normal elderly and differently from the juvenile form of DM1 where the atrophy is present from the beginning of the disease in early childhood (Caso et al., 2014). Brain volume loss has been correlated with disability progression and cognitive impairment in other neurological disorders, such as multiple sclerosis (MS), but up to now data about DM1 are scarce (Kassubek et al., 2003); a better knowledge about brain volume loss may have important clinical implications for treatment and prognosis in DM1.
Notably in our sample the main executive dysfunction as well as memory and visuo-spatial impairment was not related to focal atrophy in specific brain regions, as already reported by previous studies (Meola et al., 1999), but they were associated to the overall cerebral atrophy expressed by BPF.
The atrophy at VBM is widely distributed and includes the cortical areas pertaining the sensori-motor and cognitive brain networks. The involvement of these brain areas fits with the supposed motor defect in DM1 (Caramia et al., 2010) and with the well-known impairment in cognitive functioning (Meola et al., 2003, Winblad et al., 2010).
Our results were in line with recent reports (Caso et al., 2014) showing similar GM atrophy pattern, but differently from the previous experience, we also reported a significant association between GM atrophy in temporo-parietal areas (left postcentral, left middle and inferior temporal gyri and left supramarginal gyrus) and specific neuropsychological tests investigating verbal learning. Although these tests are not significantly impaired in DM1, the reported correlation could support the putative role of these regions in conceptual representation and recognition of words meaning (Acheson and Hagoort, 2013). Indeed, temporo-parietal cortex includes multimodal associative areas that receive auditory, visual, and somatosensory inputs, and are implicated in processing the phonological and semantic aspect of language.
To our knowledge some studies report controversial associations between cortical atrophy and cognitive deficits, in particular with nonverbal episodic memory and cognitive flexibility (Weber et al., 2010, Schneider-Gold et al., 2015); on the other hand, others studies failed to provide consistent associations in that (Minnerop et al., 2011, Romeo et al., 2010).
4.3. White matter alterations
The WM lesions have been frequently reported in patients with DM1 (Minnerop et al., 2011, Romeo et al., 2010) and predominate at level of frontal and temporal lobes. An important result of our study is the lack of correlation between the LL% and the neuropsychological defects. On the other hand, the TBSS results indicate that projection and associative fibres are extensively affected in DM1 patients with an involvement of the normal appearing white matter beyond the signal changes detected with conventional MR imaging.
We found interesting similarities between the pattern of WM damage revealed in our patients and recent whole-brain DT MRI studies (Minnerop et al., 2011, Wozniak et al., 2014, Caso et al., 2014) consisting in a significant overlap of altered DTI indexes along several fibre bundles.
Greater white matter abnormalities (as indicated by RD or AD changes) were associated with lower score at neuropsychological tests indicating worse functioning. In our study the cognitive performance in visuomotor coordination and working memory tasks (ROCF-copy, CBT, digit span) was associated to microstructural damage detected in major associative tracts as corpus callosum (Reuter-Lorenz, 2003), while scores in visuo-spatial and episodic verbal memory were associated mainly to associative tracts of the internal capsule and corona radiate, that collectively connect neocortex to deeper brain structures.
From a neuropsychological perspective a cognitive-behavioural dysfunction usually depends upon both the size and site of a given lesion. Although certain areas in the brain can be critical for specific cognitive functions, also small but diffuse WM subcortical lesions can produce major effects since they disrupt interconnections between brain regions in distributed neural networks (Lezak et al., 2012).
This is the case of DM1 in which neuropsychological studies suggest that the dysfunction may occur not only in distinct CNS regions but also in complex neuronal networks, this implying that patients' cognitive profile is likely to be the result of a multifactorial process in which genetic and epigenetic factors interact through complex mechanisms related to brain plasticity, compensation, neurodegeneration or neurodevelopmental defects. Since the DTI explores complex neuronal networks and can detect abnormalities in the normal-appearing white matter, this technique has to be tested as a possible marker of the progression of cognitive impairment of DM1.
Finally, it has to be considered that, beside cognitive deficits, DM1 patients suffer from an emotional imbalance expressed by symptoms like depression, lack of interest, decreased emotional participation. While it is accepted that the disruption of white matter tracts, has a clinical relevance in several developmental and psychiatric disorders (Von Der Heide et al., 2013), we cannot exactly evaluate the contribution of the psychiatric component to the cognitive deficit because a specific correlation between psychiatric scales and MR parameters was not performed. This should be the object to be addressed with further studies.
The following are the supplementary data related to this article.
Neuropsychological functioning indicated as means and standard deviations of corrected scores; percentages of subjects with impaired performance are related to available cut-off scores of normality (> 95% of the tolerance limit of the normal population distribution).
VBM results: size of clusters of cortical atrophy in DM1 patients compared to healthy controls defined in AAL map (TFCE p < 0.001 corrected for multiple comparisons). Coordinates are expressed in MNIstandard space. Age and gender were used as covariate of no interest.
TBSS results: size of clusters of FA in DM1 patients compared to healthy controls defined in ICBM-DTI-81 white-matter labels atlas (TFCE p < 0.05 corrected for multiple comparisons). Coordinates are expressed in MNI standard space. Age and gender were used as covariate of no interest.
TBSS results: size of clusters of MD in DM1 patients compared to healthy controls defined in ICBM-DTI-81 white-matter labels atlas (TFCE p < 0.05 corrected for multiple comparisons). Coordinates are expressed in MNI standard space. Age and gender were used as covariate of no interest.
TBSS results: size of clusters of RD in DM1 patients compared to healthy controls defined in ICBM-DTI-81 white-matter labels atlas (TFCE p < 0.05 corrected for multiple comparisons). Coordinates are expressed in MNI standard space. Age and gender were used as covariate of no interest.
TBSS results: size of clusters of AD in DM1 patients compared to healthy controls defined in ICBM-DTI-81 white-matter labels atlas (TFCE p < 0.05 corrected for multiple comparisons). Coordinates are expressed in MNI standard space. Age and gender were used as covariate of no interest.
Acknowledgements
This study was supported by the Association Française contre les Myopathies (AFM Grant #16216).
On behalf of all authors, the corresponding author states that there is no financial or other conflict of interest.
References
- Acheson D.J., Hagoort P. Stimulating the brain's language network: syntactic ambiguity resolution after TMS to the inferior frontal gyrus and middle temporal gyrus. J. Cogn. Neurosci. 2013;25:1664–1677. doi: 10.1162/jocn_a_00430. [DOI] [PubMed] [Google Scholar]
- Andersson J.L.R., Jenkinson M., Smith S. 2007. Non-linear Registration, AKA Spatial Normalisation. FMRIB Technical Report TR07JA2. (Available: www.fmrib.ox.ac.uk/analysis/techrep) [Google Scholar]
- Battaglini M., Jenkinson M., De Stefano N. Evaluating and reducing the impact of white matter lesions on brain volume measurements. Hum. Brain Mapp. 2012;33:2062–2071. doi: 10.1002/hbm.21344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens T.E.J., Woolrich M.W., Jenkinson M., Johansen-Berg H., Nunes R.G., Clare S., Matthews P.M., Brady J.M., Smith S.M. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn. Reson. Med. 2003;50:1077–1088. doi: 10.1002/mrm.10609. [DOI] [PubMed] [Google Scholar]
- Caramia F., Mainero C., Gragnani F., Tinelli E., Fiorelli M., Ceschin V., Pantano P., Bucci E., Barra V., Bozzao L., Antonini G. Functional MRI changes in the central motor system in myotonic dystrophy type 1. Magn. Reson. Imaging. 2010;28:226–234. doi: 10.1016/j.mri.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Caso F., Agosta F., Peric S., Rakočević-Stojanović V., Copetti M., Kostic V.S., Filippi M. Cognitive impairment in myotonic dystrophy type 1 is associated with white matter damage. PLoS One. 2014;9 doi: 10.1371/journal.pone.0104697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conforti R., de Cristofaro M., Cristofano A., Brogna B., Sardaro A., Tedeschi G., Cirillo S., Di Costanzo A. Brain MRI abnormalities in the adult form of myotonic dystrophy type 1: a longitudinal case series study. Neuroradiol. J. 2016;29:36–45. doi: 10.1177/1971400915621325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douaud G., Smith S., Jenkinson M., Behrens T., Johansen-Berg H., Vickers J., James S., Voets N., Watkins K., Matthews P.M., James A. Anatomically related grey and white matter abnormalities in adolescent-onset schizophrenia. Brain. 2007;130:2375–2386. doi: 10.1093/brain/awm184. [DOI] [PubMed] [Google Scholar]
- Draganski B., Ashburner J., Hutton C., Kherif F., Frackowiak R.S., Helms G., Weiskopf N. Regional specificity of MRI contrast parameter changes in normal ageing revealed by voxel-based quantification (VBQ) NeuroImage. 2011;55:1423–1434. doi: 10.1016/j.neuroimage.2011.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekström A.B., Hakenäs-Plate L., Tulinius M., Wentz E. Cognition and adaptive skills in myotonic dystrophy type 1: a study of 55 individuals with congenital and childhood forms. Dev. Med. Child Neurol. 2009;51:982–990. doi: 10.1111/j.1469-8749.2009.03300.x. [DOI] [PubMed] [Google Scholar]
- Good C.D., Johnsrude I.S., Ashburner J., Henson R.N., Friston K.J., Frackowiak R.S. A voxel-based morphometric study of ageing in 465 normal adult human brains. NeuroImage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Harper P.S. third ed. W.B. Saunders; Philadelphia: 2001. Major Problems in Neurology: Myotonic Dystrophy. [Google Scholar]
- Huang C.C., Kuo H.C. Myotonic dystrophies. Chang Gung Med. J. 2005;28:517–526. [PubMed] [Google Scholar]
- IDMC New nomenclature and DNA testing guidelines for myotonic dystrophy type 1 (DM1). The international myotonic dystrophy consortium (IDMC) Neurology. 2000;54:1218–1221. doi: 10.1212/wnl.54.6.1218. [DOI] [PubMed] [Google Scholar]
- Jean S., Richer L., Laberge L., Mathieu J. Comparisons of intellectual capacities between mild and classic adult-onset phenotypes of myotonic dystrophy type 1 (DM1) Orphanet J. Rare Dis. 2014;9:186. doi: 10.1186/s13023-014-0186-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M., Bannister P., Brady J.M., Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Kassubek J., Juengling F.D., Hoffmann S., Rosenbohm A., Kurt A., Jurkat-Rott K., Steinbach P., Wolf M., Ludolph A.C., Lehmann-Horn F., Lerche H., Weber Y.G. Quantification of brain atrophy in patients with myotonic dystrophy and proximal myotonic myopathy: a controlled 3-dimensional magnetic resonance imaging study. Neurosci. Lett. 2003;348:73–76. doi: 10.1016/s0304-3940(03)00740-7. [DOI] [PubMed] [Google Scholar]
- Laberge L., Mathieu J., Auclair J., Gagnon É., Noreau L., Gagnon C. Clinical, psychosocial, and central correlates of quality of life in myotonic dystrophy type 1 patients. Eur. Neurol. 2013;70:308–315. doi: 10.1159/000353991. [DOI] [PubMed] [Google Scholar]
- Lezak M.D., Howieson D.B., Bigler E.D., Tranel D. fifth ed. Oxford University Press; Oxford: 2012. Neuropsychological Assessment. [Google Scholar]
- Mazziotta J.C., Toga A.W., Evans A., Fox P., Lancaster J. A probabilistic atlas of the human brain: theory and rationale for its development. NeuroImage. 1995;2:89–101. doi: 10.1006/nimg.1995.1012. [DOI] [PubMed] [Google Scholar]
- Meola G., Sansone V. Cerebral involvement in myotonic dystrophies. Muscle Nerve. 2007;36:294–306. doi: 10.1002/mus.20800. [DOI] [PubMed] [Google Scholar]
- Meola G., Sansone V., Perani D., Colleluori A., Cappa S., Cotelli M., Fazio F., Thornton C.A., Moxley R.T. Reduced cerebral blood flow and impaired visual-spatial function in proximal myotonic myopathy. Neurology. 1999;53:1042–1050. doi: 10.1212/wnl.53.5.1042. [DOI] [PubMed] [Google Scholar]
- Meola G., Sansone V., Perani D., Scarone S., Cappa S., Dragoni C., Cattaneo E., Cotelli M., Gobbo C., Fazio F., Siciliano G., Mancuso M., Vitelli E., Zhang S., Krahe R., Moxley R.T. Executive dysfunction and avoidant personality trait in myotonic dystrophy type 1 (DM-1) and in proximal myotonic myopathy (PROMM/DM-2) Neuromuscul. Disord. 2003;13:813–821. doi: 10.1016/s0960-8966(03)00137-8. [DOI] [PubMed] [Google Scholar]
- Minnerop M., Weber B., Schoene-Bake J.C., Roeske S., Mirbach S., Anspach C., Schneider-Gold C., Betz R.C., Helmstaedter C., Tittgemeyer M., Klockgether T., Kornblum C. The brain in myotonic dystrophy 1 and 2: evidence for a predominant white matter disease. Brain. 2011;134:3527–3543. doi: 10.1093/brain/awr299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S., Wakana S., Nagae-Poetscher L.M., van Zijl P.C. first ed. Elsevier, Amsterdam; The Netherlands: 2005. MRI Atlas of Human White Matter. [Google Scholar]
- Pantoni L., Basile A.M., Pracucci G., Asplund K., Bogousslavsky J., Chabriat H., Erkinjuntti T., Fazekas F., Ferro J.M., Hennerici M., O'brien J., Scheltens P., Visser M.C., Wahlund L.O., Waldemar G., Wallin A., Inzitari D. Impact of age-related cerebral white matter changes on the transition to disability — the LADIS study: rationale, design and methodology. Neuroepidemiology. 2005;24:51–62. doi: 10.1159/000081050. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz P.A. Parallel processing in the bisected brain: implications for callosal function. In: Zaidel E., Iacoboni M., editors. The Parallel Brain. MIT Press; Cambridge: 2003. pp. 41–54. [Google Scholar]
- Romeo V., Pegoraro E., Ferrati C., Squarzanti F., Sorarù G., Palmieri A., Zucchetta P., Antunovic L., Bonifazi E., Novelli G., Trevisan C.P., Ermani M., Manara R., Angelini C. Brain involvement in myotonic dystrophies: neuroimaging and neuropsychological comparative study in DM1 and DM2. J. Neurol. 2010;257:1246–1255. doi: 10.1007/s00415-010-5498-3. [DOI] [PubMed] [Google Scholar]
- Schneider-Gold C., Bellenberg B., Prehn C., Krogias C., Schneider R., Klein J., Gold R., Lukas C. Cortical and subcortical grey and white matter atrophy in myotonic dystrophies type 1 and 2 is associated with cognitive impairment, depression and daytime sleepiness. PLoS One. 2015;10 doi: 10.1371/journal.pone.0130352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sistiaga A., Urreta I., Jodar M., Cobo A.M., Emparanza J., Otaegui D., Poza J.J., Merino J.J., Imaz H., Martí-Massó J.F., López de Munain A. Cognitive/personality pattern and triplet expansion size in adult myotonic dystrophy type 1 (DM1): CTG repeats, cognition and personality in DM1. Psychol. Med. 2010;40:487–495. doi: 10.1017/S0033291709990602. [DOI] [PubMed] [Google Scholar]
- Smith S.M. Fast robust automated brain extraction. Hum. Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Nichols T.E. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Woolrich M.W., Beckmann C.F., Behrens T.E., Johansen-Berg H., Bannister P.R., De Luca M., Drobnjak I., Flitney D.E., Niazy R.K., Saunders J., Vickers J., Zhang Y., De Stefano N., Brady J.M., Matthews P.M. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Johansen-Berg H., Rueckert D., Nichols T.E., Mackay C.E., Watkins K.E., Ciccarelli O., Cader M.Z., Matthews P.M., Behrens T.E. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. NeuroImage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Spinnler H., Tognoni G. Standardizzazione e taratura italiana di test neuropsicologici. Ital. J. Neurol. Sci. 1987;8(Suppl):1–120. [PubMed] [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., Crivello F., Etard O., Delcroix N., Mazoyer B., Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Udd B., Krahe R. The myotonic dystrophies: molecular, clinical, and therapeutic challenges. Lancet Neurol. 2012;11:891–905. doi: 10.1016/S1474-4422(12)70204-1. [DOI] [PubMed] [Google Scholar]
- Von Der Heide R.J., Skipper L.M., Klobusicky E., Olson I.R. Dissecting the uncinate fasciculus: disorders, controversies and a hypothesis. Brain. 2013;136:1692–1707. doi: 10.1093/brain/awt094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber Y.G., Roebling R., Kassubek J., Hoffmann S., Rosenbohm A., Wolf M., Steinbach P., Jurkat-Rott K., Walter H., Reske S.N., Lehmann-Horn F., Mottaghy F.M., Lerche H. Comparative analysis of brain structure, metabolism, and cognition in myotonic dystrophy 1 and 2. Neurology. 2010;74:1108–1117. doi: 10.1212/WNL.0b013e3181d8c35f. [DOI] [PubMed] [Google Scholar]
- Winblad S., Jensen C., Mansson J.E., Samuelsson L., Lindberg C. Depression in myotonic dystrophy type 1: clinical and neuronal correlates. Behav. Brain Funct. 2010;6:25. doi: 10.1186/1744-9081-6-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak J.R., Mueller B.A., Lim K.O., Hemmy L.S., Day J.W. Tractography reveals diffuse white matter abnormalities in myotonic dystrophy type 1. J. Neurol. Sci. 2014;341:73–78. doi: 10.1016/j.jns.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Brady M., Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation maximization algorithm. IEEE Trans. Med. Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Neuropsychological functioning indicated as means and standard deviations of corrected scores; percentages of subjects with impaired performance are related to available cut-off scores of normality (> 95% of the tolerance limit of the normal population distribution).
VBM results: size of clusters of cortical atrophy in DM1 patients compared to healthy controls defined in AAL map (TFCE p < 0.001 corrected for multiple comparisons). Coordinates are expressed in MNIstandard space. Age and gender were used as covariate of no interest.
TBSS results: size of clusters of FA in DM1 patients compared to healthy controls defined in ICBM-DTI-81 white-matter labels atlas (TFCE p < 0.05 corrected for multiple comparisons). Coordinates are expressed in MNI standard space. Age and gender were used as covariate of no interest.
TBSS results: size of clusters of MD in DM1 patients compared to healthy controls defined in ICBM-DTI-81 white-matter labels atlas (TFCE p < 0.05 corrected for multiple comparisons). Coordinates are expressed in MNI standard space. Age and gender were used as covariate of no interest.
TBSS results: size of clusters of RD in DM1 patients compared to healthy controls defined in ICBM-DTI-81 white-matter labels atlas (TFCE p < 0.05 corrected for multiple comparisons). Coordinates are expressed in MNI standard space. Age and gender were used as covariate of no interest.
TBSS results: size of clusters of AD in DM1 patients compared to healthy controls defined in ICBM-DTI-81 white-matter labels atlas (TFCE p < 0.05 corrected for multiple comparisons). Coordinates are expressed in MNI standard space. Age and gender were used as covariate of no interest.