Abstract
Background
20–40% of patients with malignant tumors have one or more brain metastases in the course of their illness. Brain metastases are the first manifestation of cancer in 5–10%. Manifestations such as intracranial hypertension or focal neurologic deficits are seen in over 80% of patients with brain metastases. Uncertainty surrounds the treatment of patients with intracranial metastases, as the existing data are derived from trials with low levels of evidence.
Methods
This article is based on a selective literature review and on the authors’ own experience of 100 consecutive patients who underwent surgery at the Department of Neurosurgery at Ruhr University Bochum (RUB), Germany.
Results
Multimodal treatment enables successful surgery for an increasing number of patients with brain metastases. The modalities and goals of treatment are established for each patient individually by an interdisciplinary tumor board. Drug therapy is usually indicated. Surgical resection followed by stereotactic radiotherapy prolongs mean survival by 3–6 months and lowers the risk of recurrence from 40% to 12.5%. In the authors’ own experience, even seriously ill patients can benefit from the resection of brain metastases. The 30-day morbidity was 29%, accounted for mainly by medical complications such as pulmonary embolism, renal failure, and sepsis.
Conclusion
Through the close interdisciplinary collaboration of neurosurgeons, radiation oncologists, and medical oncologists, the symptomatic state and the prognosis of patients with brain metastases can be improved. Longer overall survival implies that further studies will have to pay special attention to the toxicity of treatment.
Among patients with malignant tumors, 20–40% have one or more brain metastases in the course of their illness (1). Brain metastases are the first manifestation of cancer in 5–10% (2); in a neurosurgical patient cohort, they were the initial manifestation in 40% (Table 1). In a review article, Patel et al. showed that more than 80% of patients with a single brain metastasis have symptoms (3). According to another review, the most common primary tumors were lung cancer (31%), melanoma (18%), breast cancer (12%), renal cell carcinoma (12%), and other tumors (26%); 87% of brain metastases were supratentorial (4). The mean interval from the initial diagnosis of cancer to the diagnosis of metastasis was 19.2 months, and from the latter diagnosis to surgery, 0.5 months (4). Median survival is one month without treatment, 2 months with corticosteroid treatment alone, and 3–6 months with radiotherapy. Predictors for longer survival include a high Karnofsky index, young age, control of the primary tumor, a low number of brain metastases, and the histological type of the primary tumor (5– 7).
Table 1. A prospective, single-center case series.
| Parameter | n /100 | |
|---|---|---|
| Clinical manifestations in the four weeks up to the diagnosis of BrM | Asymptomatic | 15 |
| Symptomatic | 85 | |
| Timing of BrM | Metachronous | 54 |
| Synchronous | 46 | |
| Initial diagnosis via BrM | Yes/No | 40/60 |
| Location of BrM | Supra-/ infratentorial | 69/31 |
| Size of BrM | <3 cm/≥3 cm | 40/60 |
| Histology of BrM | Lung cancer (NSCLC/SCLC) | 54 (35/19) |
| Breast cancer | 9 | |
| Malignant melanoma | 13 | |
| Renal-cell carcinoma | 6 | |
| Prostate cancer | 2 | |
| Colorectal cancer | 10 | |
| Gastric cancer | 3 | |
| Esophageal cancer | 2 | |
| Cancer of unknown primary | 1 | |
| Surgical treatment of BrM | Biopsy (open/stereotactic) | 5 (3/2) |
| Resection | 95 | |
| Number of resected BrM = 1 | 87 | |
| Number of resected BrM = 2 | 8 | |
| Outcome | ||
| Recurrence of BrM | Yes | 8 |
| Survival | Dead at final time point | 48 |
| Alive at final time point | 39 | |
| Lost to follow-up | 13 | |
| Perioperative (30-day) morbidity and mortality | Mortality | 17 |
| In-hospital mortality | 4/17 | |
| Morbidity | 29 | |
| Reoperation rate | 4 | |
| Readmission rate | 5 | |
| Complications | Total | 41 |
| – subcategorized: | Neurological | 11/41 (27%) |
| Surgical | 9/41 (22%) | |
| Medical | 21/41 (51%) | |
BrM, brain metastasis; NSCLC, non–smal-cell lung cancer; SCLC, small-cell lung cancer
100 consecutive patients with brain metastases underwent surgery at the Department of Neurosurgery at Ruhr University Bochum, from December 2014 to March 2015. Data were collected prospectively. There were 43 female and 57 male patients, with a mean age of 64 years (range, 45–82). Pre- and postoperative events and survival data were recorded
More than 80% of patients who undergo surgery for brain metastases have a Karnofsky score of >70%, indicating a good clinical condition (3). The percentage of patients with brain metastases who are able to undergo surgery is steadily increasing.
Surgical resection followed by stereotactic radiotherapy prolongs median survival to 19.4 months (7, 8). The median survival after resection of a single brain metastasis without stereotactic radiotherapy is comparable, but with a local recurrence rate of 40% (4, 9). There is no significant dependence of survival time on the number of brain metastases. Rather, the histological type of the primary tumor and the age of the patient are significant predictive factors (7).
In this review, we present the current status of the management of patients with intracranial metastases, with particular attention to treatment algorithms. Each of the participating specialty disciplines carried out a selective literature search in PubMed Central, using the following search terms: brain metastases, surgical treatment, radiotherapy, chemotherapy, and related terms. The primary search was not restricted in any way related to the concept of the study, as we expected the available evidence to be sparse.
In addition, Table 1 contains the findings of our own, prospective, single-center case series, with data on 100 consecutive patients with brain metastases.
Clinical features
Patients with brain metastases can have a variety of clinical manifestations. Headache, a symptom of incrased intracranial pressure, is common, along with focal neurologic deficits, e.g., hemiparesis (3, 10). The differential diagnosis of a primary versus metastatic brain tumor cannot be made from the symptoms and signs alone.
Diagnostic evaluation
Patients with intracranial masses should undergo computed tomography (CT) at the very least; magnetic resonance imaging (MRI) with and without gadolinium enhancement, and with a diffusion-weighted sequence, is more useful and helps distinguish primary brain tumors from metastases. Multiple foci are generally of metastatic origin, as multifocal primary brain tumors are rare.
Tissue should be obtained for histologic study. The decision must be made whether to obtain tissue samples by stereotactic biopsy or to resect the tumor. An appropriate tumor-staging evaluation for the type of primary tumor that is known or suspected to be present should be carried out to determine the extent of disease. If meningeal tumor involvement (neoplastic meningeosis) is suspected, an MRI of the entire neuraxis should be obtained and a lumbar puncture should be performed for cytological analysis of the cerebrospinal fluid. Specific diagnostic procedures are discussed in the relevant guidelines of the specialty societies.
Treatment
The treatment of brain metastases is interdisciplinary and individualized. Each patient’s findings are presented to an interdisciplinary tumor board, which determines the best course of treatment in view of the available scientific evidence. It is important for all participating specialists to know both the potential and the limitations of highly specific tumor therapy. It must also be borne in mind that the evidence supporting the treatment algorithms presented here is generally not of a high level.
The following general factors are important determinants of the optimal form of treatment:
Patient-specific features: biological age and general condition (Karnofsky Performance Status Scale)
Prognostic classification, e.g., with recursive partitioning analysis (RPA) (11): aside from the patient’s age and Karnofsky score, the decisive criterion is control of the primary tumor.
-
The number of brain metastases:
Singular: there is only one brain metastasis, but there may be other metastases outside the central nervous system.
Solitary: there is only one brain metastasis, and there are no other metastases elsewhere in the body.
Multiple: there are more than four brain metastases.
Extracranial metastases
Prior treatments—options for further treatment
-
Temporal interval between initial diagnosis of cancer and diagnosis of brain metastasis
Synchronous (at the same time)
Metachronous (after a delay)
Histologic type of the primary tumor
Location and resectability of the brain metastasis.
Treatment strategies, by specialty
Neurosurgery
The extent of resection depends on the location of the tumor in the brain. All of the established technical methods for the resection of intracranial tumors are used at surgery. In particular, continuous electrophysiological monitoring, supplemented by targeted intraoperative brain stimulation to test individual functional areas, enables the complete resection even of tumors lying in, or adjacent to, functionally important areas of the brain. This has enabled a marked reduction in the frequency of postoperative neurologic deficits, so that only 6–9% of patients are in worse neurologic condition after resection (10, 12, 13).
The indication for surgery depends in part on the patient’s clinical manifestations. A patient presenting with elevated intracranial pressure, perhaps with impending brain herniation, can be helped by surgery. The resection of a symptomatic metastasis or metastases can improve the patient’s neurologic state to such an extent that further antineoplastic treatment is indicated.
Radiation oncology
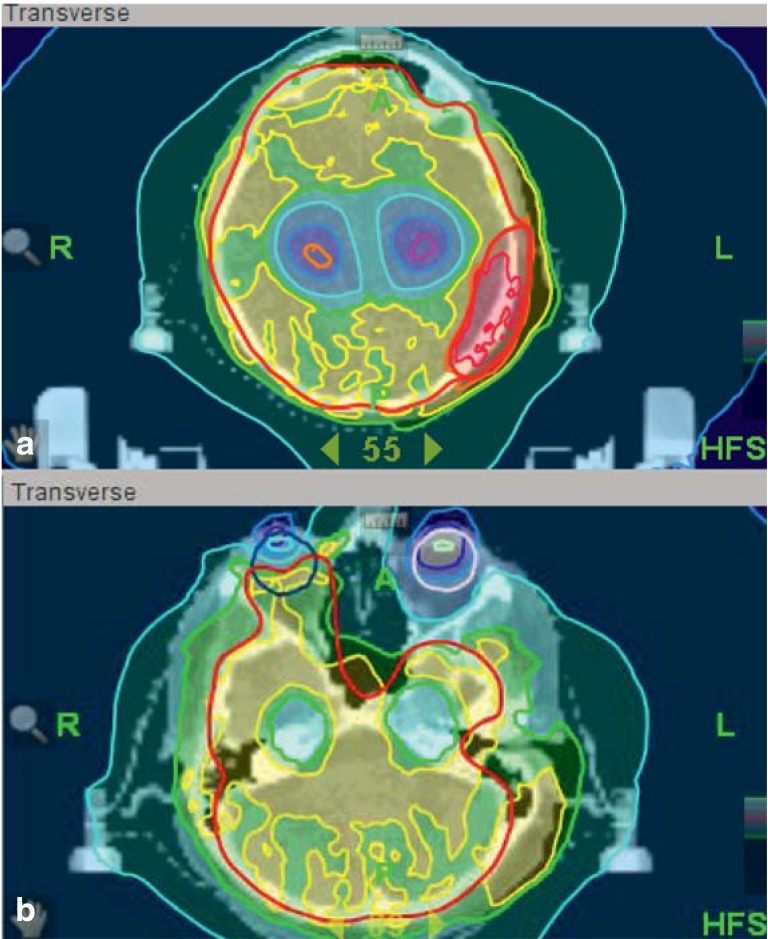
The standard treatment of brain metastases for many years was whole brain radiotherapy (WBRT), generally involving radiation with two lateral fields to cover not only the affected regions, but also other areas of the brain where there might be microscopic tumor invasion. WBRT palliates symptoms and prolongs median survival by three to six months (14– 17). High-precision radiotherapy now makes it possible to apply very high doses of radiation to specific target areas with a steep spatial fall-off of the dose to protect adjacent areas of healthy brain tissue. Radiosurgery, i.e., high-precision radiotherapy delivered in a single treatment session on one day, is now used to treat brain metastases with great success. Because the risk of side effects increases with the treatment volume, radiosurgery cannot be used to treat metastases measuring more than 3–4 cm in diameter. For patients with “multiple” (more than four) brain metastases, WBRT remains the standard treatment (18). A variety of methods are now being tested for combining WBRT with a simultaneous, integrated boost of the radiation dose to brain metastases. These involve special radiotherapeutic techniques such as intensity-modulated radiotherapy (IMRT) or helical IMRT (19, 20). The same techniques can also be used to limit the radiation dose to particular areas of the brain that are in need of protection, e.g., the hippocampus, without endangering local/regional tumor control (21). Prospective trials are now underway to evaluate such methods in comparison to standard treatment and to identify any potential benefit in terms of maintained cognitive function. A typical radiation plan for the treatment of breast cancer metastases is shown in Figure 1. This patient’s metastases in the skull and the right retina were kept under control for nearly one year.
Figure 1.
Radiation plan with (a) an integrated boost to the left-sided skull metastasis and (b) inclusion of the right retinal metastasis, with hippocampal sparing to avoid a cognitive deficit
Chemotherapy
Antineoplastic pharmacotherapy is generally indicated, depending on the histology and, above all, the molecular profile of the primary tumor.
Chemotherapeutic drugs that enter the central nervous system (CNS) are listed in Table 2. Even those that do not can be very effective when the blood–brain barrier is dysfunctional, which is often the case for brain metastases measuring >2 cm. This is indicated by contrast enhancement of the tumor on CT or MRI. Monoclonal antibodies have a highly variable degree of access to tumor cells in brain metastases, but small-molecule signal transduction inhibitors penetrate them well.
Table 2. Cytotoxic agents that cross the blood–brain barrier.
| Cytotoxic agents | Access to brain (high-dose treatment) |
|---|---|
| Nitrosourea | ++ |
| Cytosine arabinoside | ++ |
| Temozolomide | ++ |
| Thiotepa | ++ |
| Ifosfamide | ++ |
| Topotecan | ++ |
| Idarubicin | + |
| Methotrexate | + |
| Cyclophosphamide | + |
+, 1–2% of the serum level
++, ≥ 30% of the serum level
The chemotherapy of synchronous brain metastases is variable and largely based on the primary tumor entity.
The chemotherapy of metachronous brain metastases is based on the same principles as that of synchronous brain metastases, with the additional consideration of possible secondary resistance resulting from prior systemic treatment.
The special situation of “oligometastasis” has attracted attention in recent years. This term refers to hematogenous metastatic disease with a restricted number of lesions (generally no more than five) in only a few (no more than three) organ systems. Multimodal treatment often confers a much better prognosis in such cases than when these numerical limits are exceeded (“multiple metastases”) (22). The outcome of treatment also depends, of course, on the quality of the interdisciplinary team. The goal should always be to control metastases at all sites where they are seen. Thus, for single brain metastases, surgery or modern radiotherapeutic techniques (high-precision radiotherapy) are generally used. The possible indication for systemic chemotherapy as an additional treatment depends on the tumor histology, prior treatment(s), and the results of treatment with local modalities. The available evidence on systemic chemotherapy for brain metastases is limited, although there have been well-studied, large-scale case series from individual institutions (23).
Complications, recurrences, and leptomeningeal seeding in interdisciplinary treatment
Surgery for brain metastases generally carries a low risk of complications, with a 30-day mortality below 5% (3). The focus lies on the reduction of medical complications (Table 1).
Even tumors located at unfavorable sites in the brain, such as the primary motor cortex (pyramidal tract), can be resected without major complications (6). Kellogg et al. reported that 94% of patients had stable or improved neurologic findings three months after surgery (10).
Large-scale studies have shown that preoperative radiotherapy is a negative predictive factor for postoperative worsening of neurologic findings (12, 13).
A high Karnofsky score is generally considered a positive prognostic factor. Advanced age, unfavorable tumor biology (renal-cell carcinoma, triple-negative breast cancer), and pre-existing neurologic deficits have been described as significiant predictive factors for a higher rate of postoperative complications (3).
Early postoperative MRI documents the extent of tumor resection. If MRI is not consistently performed early after surgery, the extent of resection is often incorrectly estimated (13). Methods for better intraoperative visualization of tumor tissue are now being investigated (24).
The local recurrence rate after surgery, without any further treatment, is 15–40% (4, 9).
Multiple studies have refuted the notion that tumor resection promotes leptomeningeal seeding (25, 26). The probability of leptomeningeal seeding is increased only if the tumor is in contact with a CSF space or if tumor cells are strewn intraoperatively, e.g., by a cavitron ultrasonic surgical aspirator (CUSA). The surgical procedure per se is not a risk factor (20, 21).
Current evidence indicates that stereotactic radiotherapy lowers the risk of recurrence from 40% to 12.5% (14). Thus, it may be possible to dispense with WBRT for patients with fewer than four metastases (15 – 17). In particular, patients with extracranial disease who need the rapid initiation of systemic therapy can benefit from stereotactic radiotherapy as a means of treating their brain metastases quickly without any loss of time due to surgery and postoperative recovery.
The radiotherapeutic options for treating recurrent brain metastases depend on prior treatment(s), the number and size of the metastases, and the patient’s general condition. In principle, patients with multiple brain metastases can be offered a second course of WBRT (27). The optimal dosing and fractionation are now being studied in a prospective trial. Moreover, radiosurgery can be offered after prior WBRT as long as no more than three lesions are present with a diameter of up to 4 cm each.
The management of brain metastases depending on the type of primary tumor
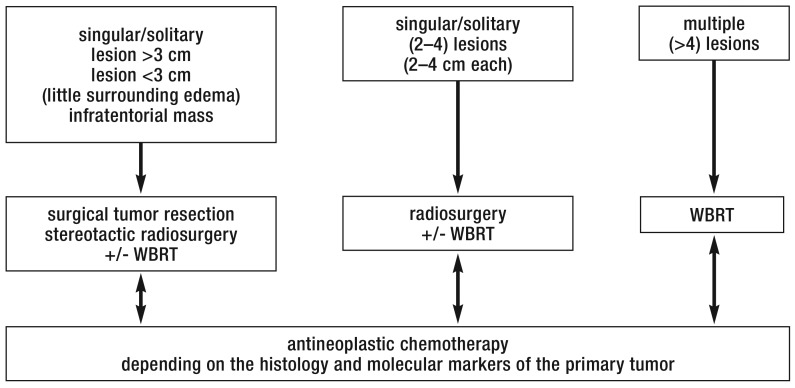
In the following sections, we discuss the algorithm used by the authors to treat brain metastases arising from certain common types of primary tumor (Figure 2). This algorithm can be used as a decision aid in planning individualized therapy.
Figure 2.
Diagram of the treatment algorithms for brain metastases
WBRT, whole-brain radiotherapy
Breast cancer
After lung cancer, breast cancer is the second most common cause of brain metastases, and its incidence is rising (4). Brain metastases of breast cancer are metachronous in over 80% of cases (27, 28). They affect 10–16% of women with breast cancer (28). As cranial imaging is generally not a part of the routine follow-up of women with breast cancer, exact figures on the frequency of brain metastases are not available. 20% of women with brain metastases of breast cancer are asymptomatic (2, 28).
The tumor biology of breast cancer has recently been found to have a decisive influence: human epidermal growth factor receptor 2 (HER2/neu)-positive breast cancers give rise to brain metastases in 14.7% of cases, which is significantly more often than breast cancers of other biologic types. Triple-negative tumor subtypes give rise to brain metastases with a frequency of 10.9% (29). On the other hand, HER2/neu-positive subtypes are associated with markedly better survival after the resection of brain metastases (11.5–16.5 months) than HER2/neu-negative (9.3 months) or triple-negative subtypes (4.9 months) (5, 26, 27, 29). Further treatment after surgery is vitally important in these cases as well (2, 6, 28).
All types of hormone therapy for breast cancer are effective in the treatment of brain metastases from breast cancer, including the combination of capecitabine with the HER2/neu blocker lapatinib (30). Other (cytotoxic) chemotherapeutic agents, however, are of limited efficacy against synchronous cerebral metastases (31).
Lung cancer
Synchronous brain metastases are common in lung cancer. The treatment strategy depends largely on the histologic type of the tumor. For small-cell carcinoma, immediate systemic chemotherapy is indicated and is beneficial against cerebral metastases in almost all cases, even though the substances used (platinum salts and etoposide) do not cross the intact blood–brain barrier (32). Nonetheless, WBRT is still recommended as soon as chemotherapy has brought about a remission, because the risk of recurrent intracranial disease is otherwise high (5, 31, 33).
Brain metastases of non–small-cell lung cancer are resected or irradiated and additionally treated with systemic antineoplastic drugs (or tyrosine kinase inhibitors if there is an epidermal growth factor receptor [EGFR] mutation or an anaplastic lymphoma kinase [ALK] fusion protein). Patients with clinically dominant extracerebral metastases and asymptomatic brain metastases can be treated with systemic chemotherapy and deferred radiotherapy (34).
Multiple brain metastases of lung cancer were previously considered a contraindication to surgical resection. Treatment by chemotherapy and WBRT without resection of the symptomatic metastasis(-es) yields a median survival of 3–6 months, depending on the response to chemotherapy. Recent studies have shown, however, that survival can be significantly prolonged by interdisciplinary treatment (resection of metastases, systemic chemotherapy, stereotactic radiotherapy). A median survival of 18.8 months has been reported (31). More detailed data on quality of life associated with such treatment will be forthcoming from clinical trials that are now in progress.
Malignant melanoma
In the case of malignant melanoma as well, the neuro-surgical resection of metastases can markedly prolong survival (35). Cytotoxic antineoplastic drugs that cross the blood–brain barrier are given after resection (36). Here, too, tumor biology plays a decisive role. If the B-isoform of the rapidly accelerated fibrosarcoma gene (BRAF mutation) is present, as in about half of all cases, highly effective and rapidly acting BRAF inhibitors can be given. When these are given in combination with a mitogen-activated kinase (MEK) inhibitor, the response rate is over 80%, and clinical improvement can be expected within a few days. Overall survival is prolonged (37). Therefore, molecular diagnosis and specific therapy should be initiated as soon as possible. The treatment of choice in the absence of a BRAF mutation is blockade of the immune checkpoints cytotoxic T-lymphocyte–associated antigen 4 (CTLA4) and/or programmed cell death receptor 1 (PD1) (38).
Overview
The improved overall survival of patients with solid tumors has made brain metastases more common. The prognosis of patients with brain metastases can be markedly improved through close interdisciplinary collaboration.
Follow-up imaging is important both for the documentation of the effect of treatment (extent of resection) and for the early detection of recurrence. Symptom control and the lessening of treatment-related complications are paramount aims. Neurosurgical treatment can be further improved by reductions in the frequency of postoperative hemorrhage and wound-healing disturbances necessitating reoperation. As long as perioperative medical complications such as pulmonary, hepatic, and/or renal failure can be avoided, patients can rapidly be given further, targeted treatment. Longer overall survival implies that further studies will have to pay special attention to the toxicity of treatment.
Key Messages.
5-10% of patients with cancer have a brain metastasis as their first manifestation.
All patients with brain metastases should be presented to an interdisciplinary tumor board.
The outcome of patients with brain metastases can be markedly improved through the close collaboration of neurosurgeons, radiation oncologists, and medical oncologists. The individual treatment plan is developed with regard to both patient-specific and tumor-specific factors.
A reduction of treatment-associated complications is essential for the optimization of outcomes.
The goal is complete resection and/or immediate symptom control to improve the patient’s postoperative neurological status so that he or she can undergo further targeted therapy as soon as possible.
Acknowledgments
Translated from the original German by Ethan Taub, M.D.
Footnotes
Conflict of interest statement
The authors declare that no conflict of interests exists.
References
- 1.Sul J, Posner JB. Brain metastases: epidemiology and pathophysiology. Cancer Treat Res. 2007;136:1–21. doi: 10.1007/978-0-387-69222-7_1. [DOI] [PubMed] [Google Scholar]
- 2.Gil-Gil MJ, Martinez-Garcia M, Sierra A, et al. Breast cancer brain metastases: a review of the literature and a current multidisciplinary management guideline. Clin Transl Oncol. 2014;16:436–446. doi: 10.1007/s12094-013-1110-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patel AJ, Suki D, Hatiboglu MA, Rao VY, Fox BD, Sawaya R. Impact of surgical methodology on the complication rate and functional outcome of patients with a single brain metastasis. J Neurosurg. 2015:1–12. doi: 10.3171/2014.9.JNS13939. [DOI] [PubMed] [Google Scholar]
- 4.Patel AJ, Suki D, Hatiboglu MA, et al. Factors influencing the risk of local recurrence after resection of a single brain metastasis. J Neurosurg. 2010;113:181–189. doi: 10.3171/2009.11.JNS09659. [DOI] [PubMed] [Google Scholar]
- 5.Bae MK, Yu WS, Byun GE, et al. Prognostic factors for cases with no extracranial metastasis in whom brain metastasis is detected after resection of non-small cell lung cancer. Lung Cancer. 2015;88:195–200. doi: 10.1016/j.lungcan.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 6.Niikura N, Hayashi N, Masuda N, et al. Treatment outcomes and prognostic factors for patients with brain metastases from breast cancer of each subtype: a multicenter retrospective analysis. Breast Cancer Res Treat. 2014;147:103–112. doi: 10.1007/s10549-014-3090-8. [DOI] [PubMed] [Google Scholar]
- 7.Smith GL, Jiang J, Buchholz TA, et al. Benefit of adjuvant brachytherapy versus external beam radiation for early breast cancer: impact of patient stratification on breast preservation. Int J Radiat Oncol Biol Phys. 2014;88:274–284. doi: 10.1016/j.ijrobp.2013.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schackert G, Lindner C, Petschke S, Leimert M, Kirsch M. Retrospective study of 127 surgically treated patients with multiple brain metastases: indication, prognostic factors, and outcome. Acta Neurochir (Wien) 2013;155:379–387. doi: 10.1007/s00701-012-1606-8. [DOI] [PubMed] [Google Scholar]
- 9.Nieder C, Astner ST, Grosu AL, Andratschke NH, Molls M. The role of postoperative radiotherapy after resection of a single brain metastasis. Combined analysis of 643 patients. Strahlenther Onkol. 2007;183:576–680. doi: 10.1007/s00066-007-1756-4. [DOI] [PubMed] [Google Scholar]
- 10.Kellogg RG, Munoz LF. Selective excision of cerebral metastases from the precentral gyrus. Surg Neurol Int. 2013;4 doi: 10.4103/2152-7806.112189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaspar L, Scott C, Rotman M, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37:745–751. doi: 10.1016/s0360-3016(96)00619-0. [DOI] [PubMed] [Google Scholar]
- 12.Gempt J, Gerhardt J, Toth V, et al. Postoperative ischemic changes following brain metastasis resection as measured by diffusion-weighted magnetic resonance imaging. J Neurosurg. 2013;119:1395–1400. doi: 10.3171/2013.9.JNS13596. [DOI] [PubMed] [Google Scholar]
- 13.Obermueller T, Schaeffner M, Gerhardt J, Meyer B, Ringel F, Krieg SM. Risks of postoperative paresis in motor eloquently and non-eloquently located brain metastases. BMC Cancer. 2014;14 doi: 10.1186/1471-2407-14-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andrews DW. Should surgery followed by whole-brain radiation therapy be the standard treatment for single brain metastasis? Nat Clin Pract Oncol. 2008;5:572–573. doi: 10.1038/ncponc1217. [DOI] [PubMed] [Google Scholar]
- 15.Linskey ME, Andrews DW, Asher AL, et al. The role of stereotactic radiosurgery in the management of patients with newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010;96:45–68. doi: 10.1007/s11060-009-0073-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halasz LM, Rockhill JK. Stereotactic radiosurgery and stereotactic radiotherapy for brain metastases. Surg Neurol Int. 2013;4:185–191. doi: 10.4103/2152-7806.111295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hauswald H, Dittmar JO, Habermehl D, et al. Efficacy and toxicity of whole brain radiotherapy in patients with multiple cerebral metastases from malignant melanoma. Radiat Oncol. 2012;7 doi: 10.1186/1748-717X-7-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hauswald H, Zwicker F, Rochet N, et al. Total skin electron beam therapy as palliative treatment for cutaneous manifestations of advanced, therapy-refractory cutaneous lymphoma and leukemia. Radiat Oncol. 2012;7 doi: 10.1186/1748-717X-7-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hauswald H, Habl G, Krug D, et al. Whole brain helical tomotherapy with integrated boost for brain metastases in patients with malignant melanoma-a randomized trial. Radiat Oncol. 2013;8 doi: 10.1186/1748-717X-8-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levegrun S, Pottgen C, Wittig A, Lubcke W, Abu Jawad J, Stuschke M. Helical tomotherapy for whole-brain irradiation with integrated boost to multiple brain metastases: evaluation of dose distribution characteristics and comparison with alternative techniques. Int J Radiat Oncol Biol Phys. 2013;86:734–742. doi: 10.1016/j.ijrobp.2013.03.031. [DOI] [PubMed] [Google Scholar]
- 21.Gondi V, Tolakanahalli R, Mehta MP, et al. Hippocampal-sparing whole-brain radiotherapy: a „how-to“ technique using helical tomotherapy and linear accelerator-based intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2010;78:1244–1252. doi: 10.1016/j.ijrobp.2010.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palma DA, Louie AV, Rodrigues GB. New strategies in stereotactic radiotherapy for oligometastases. Clin Cancer Res. 2015;21:5198–5204. doi: 10.1158/1078-0432.CCR-15-0822. [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi T, Ichiba T, Sakuyama T, et al. Possible clinical cure of metastatic breast cancer: lessons from our 30-year experience with oligometastatic breast cancer patients and literature review. Breast Cancer. 2012;19:218–237. doi: 10.1007/s12282-012-0347-0. [DOI] [PubMed] [Google Scholar]
- 24.Schebesch KM, Proescholdt M, Hohne J, et al. Sodium fluorescein-guided resection under the YELLOW 560 nm surgical microscope filter in malignant brain tumor surgery—a feasibility study. Acta Neurochir (Wien) 2013;155:693–699. doi: 10.1007/s00701-013-1643-y. [DOI] [PubMed] [Google Scholar]
- 25.Ahn JH, Lee SH, Kim S, et al. Risk for leptomeningeal seeding after resection for brain metastases: implication of tumor location with mode of resection. J Neurosurg. 2012;116:984–993. doi: 10.3171/2012.1.JNS111560. [DOI] [PubMed] [Google Scholar]
- 26.Suki D, Hatiboglu MA, Patel AJ, et al. Comparative risk of leptomeningeal dissemination of cancer after surgery or stereotactic radiosurgery for a single supratentorial solid tumor metastasis. Neurosurgery. 2009;64:664–674. doi: 10.1227/01.NEU.0000341535.53720.3E. [DOI] [PubMed] [Google Scholar]
- 27.Scharp M, Hauswald H, Bischof M, Debus J, Combs SE. Re-irradiation in the treatment of patients with cerebral metastases of solid tumors: retrospective analysis. Radiat Oncol. 2014;9 doi: 10.1186/1748-717X-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leone JP, Lee AV, Brufsky AM. Prognostic factors and survival of patients with brain metastasis from breast cancer who underwent craniotomy. Cancer Med. 2015;4:989–994. doi: 10.1002/cam4.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kennecke HF, Speers CH, Ennis CA, Gelmon K, Olivotto IA, Hayes M. Impact of routine pathology review on treatment for node-negative breast cancer. J Clin Oncol. 2012;30:2227–2231. doi: 10.1200/JCO.2011.38.9247. [DOI] [PubMed] [Google Scholar]
- 30.Bachelot T, Romieu G, Campone M, et al. Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): a single-group phase 2 study. Lancet Oncol. 2013;14:64–71. doi: 10.1016/S1470-2045(12)70432-1. [DOI] [PubMed] [Google Scholar]
- 31.Hong N, Yoo H, Gwak HS, Shin SH, Lee SH. Outcome of surgical resection of symptomatic cerebral lesions in non-small cell lung cancer patients with multiple brain metastases. Brain Tumor Res Treat. 2013;1:64–70. doi: 10.14791/btrt.2013.1.2.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kristensen CA, Kristjansen PE, Hansen HH. Systemic chemotherapy of brain metastases from small-cell lung cancer: a review. J Clin Oncol. 1992;10:1498–1502. doi: 10.1200/JCO.1992.10.9.1498. [DOI] [PubMed] [Google Scholar]
- 33.Putora PM, Ess S, Panje C, et al. Prognostic significance of histology after resection of brain metastases and whole brain radiotherapy in non-small cell lung cancer (NSCLC) Clin Exp Metastasis. 2015;32:143–149. doi: 10.1007/s10585-015-9699-0. [DOI] [PubMed] [Google Scholar]
- 34.Lee DH, Han JY, Kim HT, et al. Primary chemotherapy for newly diagnosed nonsmall cell lung cancer patients with synchronous brain metastases compared with whole-brain radiotherapy administered first: result of a randomized pilot study. Cancer. 2008;113:143–149. doi: 10.1002/cncr.23526. [DOI] [PubMed] [Google Scholar]
- 35.Jones PS, Cahill DP, Brastianos PK, Flaherty KT, Curry WT. Ipilimumab and craniotomy in patients with melanoma and brain metastases: a case series. Neurosurg Focus. 2015;38 doi: 10.3171/2014.12.FOCUS14698. [DOI] [PubMed] [Google Scholar]
- 36.Papadatos-Pastos D, Januszewski A, Dalgleish A. Revisiting the role of systemic therapies in patients with metastatic melanoma to the CNS. Expert Rev Anticancer Ther. 2013;13:559–567. doi: 10.1586/era.13.33. [DOI] [PubMed] [Google Scholar]
- 37.Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372:30–39. doi: 10.1056/NEJMoa1412690. [DOI] [PubMed] [Google Scholar]
- 38.Giacomo AM, Margolin K. Immune checkpoint blockade in patients with melanoma metastatic to the brain. Semin Oncol. 2015;42:459–465. doi: 10.1053/j.seminoncol.2015.02.006. [DOI] [PubMed] [Google Scholar]