Abstract
This review focuses on recent advances in the field of microfluidic liquid chromatography from January 2013 through April 2015. Articles are organized by the type of stationary phase support focusing on device fabrication, column preparation, and use for specific applications. Additionally, a comprehensive table comparing chromatographic figures of merit for the work described is included as Appendix A as a reference for readers.
Keywords: Microfluidics, Liquid Chromatography, Particles, Monoliths, Pillar Arrays
1. Introduction
In their 2012 review on column technology [1], Gritti and Guiochon discussed future directions for small diameter (10 – 300 μm) liquid chromatography (LC) columns used at high pressures and speeds. Because of these small diameters, a 50 mm long column packed with 1 μm particles would have a band variance (for an analyte with k’ = 3 at a reduced plate height of 3) of as small as 3 nL2, which would be unsuitable for standard instrumentation that can have extra-column variances (due to injection, detection, and connecting tubing) on the order of 10,000 nL2 [1]. Indeed, the need to reduce extra-column band broadening in LC microcolumns has been a key challenge to researchers in the field for over thirty years [2]. Additionally, for a column of this size packed with these smaller particles, optimum linear velocities would generate pressures up to 4,000 bar. Higher speed separations with such a column would require endfittings that can handle pressures far exceeding this value. To resolve these issues, they suggested the fabrication of a single device capable of withstanding up to 10,000 bar with integrated injection and detection directly on the column [1]. While such an engineering feat is likely many years away, recent work in microchip liquid chromatography focused toward this ultimate goal is described here, along with an evaluation of how these innovations might be further developed in the future and alternate trends that may sacrifice some of these ambitions to reduce cost and complexity. This review emphasizes advances since previous in-depth reviews [3-6]. Here we will direct our focus to the LC column and separation; further information on coupling such devices to MS has been previously described [7-10].
Literature searches using Web of Science, SciFinder, PubMed, and Google Scholar revealed that over 350 articles pertaining to microchip liquid chromatography were published from January 2013 until April 2015. A large majority of these articles focused on the use of commercial chip-LC systems by Agilent Technologies [11], Waters Corporation [12], and Eksigent Technologies (now part of SCIEX) [13] and emphasized specific applications. Rather than discuss the use of these widely available systems, we highlight technologically-driven investigations that might guide future developments in the field.
2. Discussion
The first experimental demonstration of LC on a chip was reported twenty years ago [14]. The system consisted of a microfabricated silicon-glass fluidic network that incorporated a split-injection tee, a channel packed with 5 μm reversed phase particles adjacent to a series of smaller channels that could act as a particle-retaining frit, and an optical detection flow cell. The chip was placed inside a clamping device that enabled connection of fused silica capillary to and from the chip for connection to an external pump and injector [14]. Although the efficiency of this first chip-LC column was poor, its early design spawned much research into new ways of preparing columns, integrating components, and applications [15-17]. Of particular interest have been the different column types incorporated into chips as particles, monoliths, or pillar arrays have all been used as stationary phase supports. In principle, open-tubular LC columns on chip are also possible; however, few articles have been published on this format in the past 2 years. The advantages and disadvantages of the different supports are summarized in Table 1 [3]. A summary of the column dimensions and performance metrics of several examples of the different column types is given in Appendix A.
Table 1.
Some advantages and disadvantages of three different stationary phase supports. Used with permission from Elsevier from reference [3].
| Support Type | Advantages | Disadvantages |
|---|---|---|
| Particles | • Many stationary phases selectivities
available • High batch-to-batch reproducibility • High loadability |
• Packing quality dependent on packing
skills • Retaining frit required • Higher back pressures generated |
| Monoliths | • Lower back pressure than
particle-packed beds • No packing or frits (mostly) required • Different base chemistries available |
• Inherent variations in synthetic
approach (lower batch-to-bath reproducibility) • Synthesis procedure can depend on chip substrate |
| Pillar Arrays | • Ordered structure can give higher
efficiency than random-packed bed • Can be fabricated by nanoprint lithography (for mass production) |
• Limited loadability • Hard to make porous (and doing so can hurt performance) • Sophisticated fabrication techniques required |
2.1 Particle-Packed Columns
2.1.1 Particle-Retaining Frits
As described in Table 1, one of the drawbacks of packed beds on microchips is the need for retaining frits to keep the particles in place [3]. While a number of fritting techniques have been developed for capillary LC columns [18], most chip-LC frits have relied on the blockage of particles through channel constrictions or weirs (relying on the keystone effect) [3]. One drawback of these types of frits is that usually only the outlet end of the column can have a frit on it, which limits flow to a single direction and can lead to particle loss at the column inlet. Recently, a two-weir column channel was described that eliminates both particle loss and the need for a connecting channel between the sample injector and the head of the column [19]. A side packing channel is used to enable the filling of the channel. This packing channel then is completely closed off to flow using a UV-polymerized monolith solution, eliminating any extra-column broadening that might occur due to this layout [19]. This polymer technique was later modified so that the actual monolith itself could be used as a frit [20]. With these porous polymeric monolith (PPM) frits (Figure 1), beds can be packed in reverse from the column outlet and then fritted in a specific desired position, which allows flexibility in where the column is located on the device as well as facilitating different column lengths with a single chip design [20,21]. Other novel particle retaining techniques for packed beds on chips include the use of an embedded microstructure fiber (containing an array of evenly spaced channels) that acts as both a frit and an emitter spray tip [22] and a stationary phase doped with magnetic nanoparticles that can be controlled by an external magnet to generate a site-specific frit [23].
Figure 1.
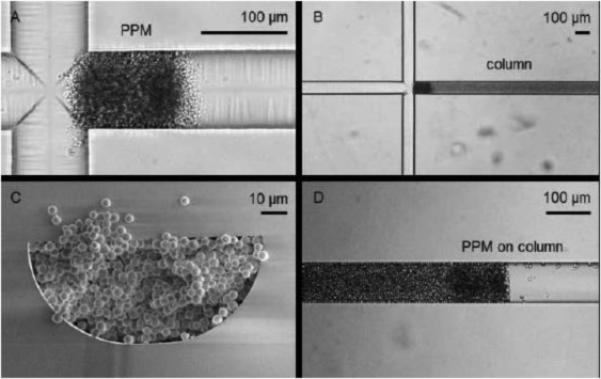
Fabrication of a packed bed utilizing porous polymeric monolithic frits (PPMs). First, the inlet frit is formed adjacent to an injection cross (A) then a column bed is packed in reverse against this frit (B, with column cross-section shown in C). Then, an outlet frit is polymerized at a position equal to the desired length of the column, with any remaining particles then flushed out of the outlet (D). Used with permission from Elsevier from reference [20].
2.1.2 Metal Substrates for Increased Pressure Limitations
Glass is a very common substrate for microfluidics owing to its high chemical resistivity and optical transparency, which are both important characteristics for analytical separations and detection [15]. Many chip-LC columns have been prepared in glass devices [3] including those highlighted in the previous section [19-21]. However, the pressure limits of microfluidic LC columns in glass devices are often far lower than capillary columns. To increase the pressure range of integrated packed beds, the use of metal substrates has been implemented to improve device strength. Titanium microchips containing 320 μm column channels packed with sub-2 μm porous particles that have pressure limits exceeding 1,000 bar have been reported [24,25]. The columns were used to study peak focusing in gradient elution. A selective laser melting (SLM) 3D printing technique for the fabrication of both stainless steel and titanium alloy chip-LC columns (both with particles and monoliths) utilized integrated fitting connections that can withstand over 400 bar [26]. By using a coil design, a 0.9 × 600 mm channel fit on a 5 × 30 × 58 mm chip, demonstrating the possible miniaturization of such columns. However, chromatographic efficiency for these 3D printed metal chips was low (see Appendix A) due to the surface roughness of ~40 μm that results from particles used in the SLM process, a limitation that will have to be overcome to improve performance. While the use of metal substrates limits on-column optical detection, these types of columns could eventually be used for high pressure, high efficiency “omics” separations coupled to MS. Chip-LC separations are especially appealing to couple with MS as the lower flow rates used with these column dimensions are more compatible with electrospray ionization (ESI) than standard-bore LC columns and lower injection volumes can be used (conserving precious biological samples) [7].
2.1.3 PDMS Substrates for Packed Bed Columns
Although metal columns show promise for high pressure applications, many researchers still depend on PDMS (polydimethylsiloxane) as a device substrate due to its relative ease of fabrication. In principle PDMS is a poor choice of column for pressure-driven LC because it is elastomeric and tends to absorb organic solvents; however, several interesting uses have been found [15]. One trend in packed columns in PDMS devices has been the fabrication of several parallel columns on a single device. To enable uniform bed geometry throughout the packing process, over 500 small bypass channels along the side of their chromatographic column (20 mm length) were used to balance the flow impedance of the bypass channels with the growing packed bed (ensuring a consistent flow rate throughout packing) [27]. Once the bed is packed, the column is stabilized using an integrated microvalve which also helps compress the bed and eliminate wall effects (by forcing the particles into microindentations in the substrate). For the simultaneous separation of a fluorescently-tagged 50-mer DNA strand across four parallel columns (all packed with 5 μm strong anion-exchange beads) on the same device, retention times had a standard deviation less than 1% [27]. Gradient elution was then used in such a device to demonstrate the separation of a single-stranded DNA ladder (100-500 base pairs in 50 base pair increments) with near-baseline resolution as well as the capability to purify and recover size-specific strands following PCR with recovery in excess of 90% [28]. A similar but simplified chip design with an integrated bottleneck frit enabled the simultaneous packing of up to 12 parallel columns [29]. This 12-column chip was then used for the preconcentration and analysis of Cr(VI) by flame atomic absorption spectrometry through the connection of a pneumatic nebulizer to chip reservoirs where eluted analytes are collected [30]. There are limits to what PDMS devices will ultimately be used for in LC due to the properties of the material [15]. PDMS is incompatible with organic solvents typically used in LC (especially acetone and toluene commonly used for reversed phase particle packing [27]). Also, the hydrophobic nature of PDMS can lead to the adsorption of biomolecules onto channel walls and flexibility of this elastomer limits pressures to just a few bar [15]. These constraints suggest that ion exchange chromatography methods that rely on pH and salt gradients in aqueous solutions [27,28,31] might be the most promising use of packed beds in PDMS, especially for short lengths used for solid phase extraction that require lower pressures [32].
2.1.4 Component Integration with Packed Beds
Particle-packed columns have also been used in devices designed to integrate other aspects of LC instrumentation (pump, injector, and detector) into a single chip. An interesting direction has been to incorporate elements along the wall of the channel to enable detection. A polyethylene terephthalate chip containing an array of electrodes embedded along an LC column wall was used for whole-column electrochemical detection of neurotransmitters [33]. For the separation of proteomic samples from cell extracts, a chip containing integrated EOF pumps (which are closed to enable pressure-driven flow) for gradient LC was used [34]. By limiting an elution gradient to one column volume, they were able to spread the entire sample across the separation channel and then pump an elution solvent from perpendicular channels directly to an array of reservoirs for MALDI-MS (matrix-assisted laser desorption/ionization-mass spectrometry) detection.
Exciting progress is also being made on a fully integrated, portable LC-MS device by a group from NASA (and their collaborators) developing an Organics Analyzer for Sampling Icy Surfaces (OASIS) [35,36]. The LC chip is fabricated by bonding silicon and Pyrex wafers together to give a cylindrical channel that maintains flow stability beyond 275 bar. With an oval-shaped column shape, they could vary the length from 40-100 mm in a 10 × 5 × 1 mm footprint [35]. The LC column was then integrated with an electrospray tip and electronics that enabled both column temperature control and spray voltage [36] for the eventual coupling to a small (30 × 18 × 13 cm) TOF-MS (time-of-flight MS) analyzer developed separately to create an integrated system that could weigh ~5 kg and only consumes 3 W of power. The ultimate goal with the OASIS instrument is the extraterrestrial analysis of amino acid enantiomers [35] and other small organic molecules [36].
2.1.5 Particle Selectivity for Targeted Analysis
A key advantage of particle-packed beds is the wide availability of different support materials and stationary phase coatings that already exist (see Table 1) and can be used for targeted separation needs. A few recent papers have utilized unique particle types for specific bioanalytical purposes, typically for sample extraction and clean-up prior to analysis. For example, viral particles were purified and enriched 7-fold using hydroxyaptatite particles packed into a PDMS device which was developed to aid diagnosis [37]. A dual-channel device was designed to facilitate two packed beds containing particles for both sample preparation and separation [38]. In the first channel AminoLink particles are used to immobilize glycoproteins, which can then be washed with an enzyme solution to release glycans from the glycoproteins. The released glycans are then washed toward the LC column packed with porous graphitic carbon (PGC) particles (which are commonly used for glycan separations) and analyzed by MALDI-MS [38]. A similar scheme for binding and release of DNA prior to further processing implemented using both ChargeSwitch beads and carboxylated microspheres that were packed utilizing a metering channel that allowed for flexible control of the total bed volume [39]. The particle types described here are just a few of the many supports that can be utilized for specific analysis needs [32, 40], although only very few have been utilized in microfluidic LC thus far. With the widest range of selectivities and chromatographic modes available for targeted analyses, particles are likely the best option for future on-chip column development focused on biomolecular separations.
2.2 Monolithic Columns
2.2.1 Different Monolithic Structures for Microfluidic LC
A wide range of different organic and inorganic monolithic stationary phases have been reported in the past [41] and some have been implemented in different ways for microfluidic LC in recent years. A polymethacrylate monolith photografted into a PDMS device which was then functionalized to act as a weak anion exchange column (5 mm in length) was used to demonstrate the separation of intact proteins and study different separation parameters using this type of monolith [42]. Liquid phase lithography was used to form a poly(ethylene glycol) diacrylate (PEGDA) channel design sandwiched between two glass slides [43]. A butyl acrylate monolithic column was incorporated into the resulting channel. Two-photon excitation fluorescence detection was used demonstrating a way to achieve UV fluorescence detection for polyaromatic hydrocarbons (PAHs) with substrates that would usually block such wavelengths [43]. An organic-inorganic hybrid monolith integrating alumina nanoparticles was used for the extraction and separation of 2-amino-4-chlorophenol in a PMMA (poly(methyl methacrylate)) chip [44]. Following the sample extraction, the analyte was detected using an integrated optical fiber connected to a UV-Vis spectrometer with reported limit of detection values under 10 ppb. Finally, an inorganic UV-polymerized silica sol-gel monolith functionalized with boronic acid (an affinity ligand for carbohydrates) was used for the separation of glycosylated proteins and peptides in a PDMS chip [45].
2.2.2 Cyclic Olefin-Based Polymer Substrates
Another trend in chip-LC monolith columns has been their use in low-cost polymer substrate devices. Although such substrates usually cannot hold pressures as high as glass or silicon substrates [15], monolith columns typically have lower flow resistance than particle-packed beds [41] and as such are more amenable to devices made with these materials. Cyclic olefin polymers (COPs) and cyclic olefin copolymers (COCs) are two such substrates becoming more popular as microfluidic substrates due to their high chemical resistance (including common LC mobile phases), good optical transparency, and ease of fabrication [46-48]. These materials can be especially useful for monolithic columns because of their compatibility with the solvents needed to form monoliths and their high transmission of UV light for photopolymerization [46]. A technique for the ex situ formation of a variety of porous polymer monoliths that can then be integrated into COC chips has been developed [49]. Fabricating trapezoidal shape monoliths allowed for easy alignment into similarly shaped channels which were then bonded and sealed into place with the polymeric substrate (see Figure 2 for a diagram of this process). Although the brittleness of the monoliths limited the aspect ratios of the porous structures that could be implemented (0.5 < L/W < 2), they show promise for use in solid phase extraction and other sample purification techniques [49]. Finally, monoliths in COC substrates have been used for an integrated two-dimensional chromatography device [50]. In their COC chip, a larger (1 mm × 1 mm × 42 mm) 1D column intended for isoelectric focusing is connected perpendicularly to 21 smaller parallel channels (0.5 mm × 0.5 mm × 29 mm) that contain an ester-based methacrylate monolith for the second dimension separation. This initial study focused on chip design that ensured sufficient flow distribution across the device while preventing flow out of the 1D column into the 2D channels during analysis [50]. As 2D-LC is becoming more and more prominent for the analysis of complex biological samples, future iterations of this chip could greatly simplify the complex valving systems currently required for these types of methods. Furthermore, this is a good example of a type of channel arrangement that is not attainable using standard capillary techniques and demonstrates the use of microfabrication techniques for not only miniaturization but also for previously unachievable chromatographic methods.
Figure 2.
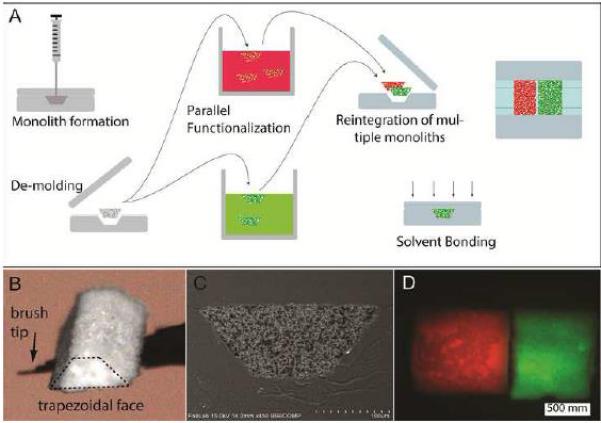
Method for forming an ex situ monolith, functionalizing it, and then integrating it into a microfluidic chip (A). Optical micrograph of an ex situ monolith is shown in (B) with an SEM image of the chip cross-section after the monolith has been integrated into a chip and bonded (C). In (D), two monoliths (with a different dye covalently attached to each) are embedded in the same channel. Used with permission from Elsevier from reference [49].
2.3 Pillar Array Columns
2.3.1 Radially Elongated Pillars
Microfabricated pillar arrays as stationary phase supports for LC columns were first reported in 1998 [51]. These pillar arrays are generated using photolithography and deep reactive-ion etching (DRIE) techniques and have high potential as a stationary phase support because they enable highly reproducible structures (moreso than random-packed beds and synthesized monoliths) and high production throughput [51]. This arrangement has seen a resurgence of interest due to several advances. A major improvement was the use of radially elongated pillars to reduce longitudinal dispersion (B-term) effects by limiting the available diffusion paths in the axial direction (albeit at the expense of higher column flow resistance) [52]. Sidewall effects that can limit column efficiency (due to the change in the fluid velocity at the wall compared to the rest of the pillar array) are reduced in radially elongated pillars (and essentially negligible above an aspect ratio of 9) because the distance covered by the analytes near the wall is lower relative to the rest of the pillar path length than it is with smaller aspect ratio pillars [52,53]. Experimentally, it was shown that plate heights could drop over 80% by increasing the pillar aspect ratio from 1.2 to 15, mainly due to a 25-fold reduction in the measured B-term [52]. The results suggest that the high efficiency of the radially elongated pillar arrays enables shorter column lengths, allowing for smaller column footprints in a miniaturized device. Further fundamental studies on pillar array design have found that transversal dispersion of analyte bands across different inter-pillar flow paths is limited at high linear flow velocities because diffusion steps between adjacent paths are limited in this high velocity regime (even if significant disorder is present in the array) [54] and elongated “foil” pillars (oval-shaped) can be used to improve an electrokinetic injection mechanism and generate flat band profiles onto the pillar array [55].
2.3.2 Sample Loadability and Retention in Pillar Arrays
One of the drawbacks of pillar array columns is their low sample loadability (Table 1) [3]. A variety of methods to increase surface area for stationary phase support have been reported. Electrochemical anodization was used to create 300 nm porous layers on 5 μm pillar arrays to increase the surface area by a factor of 30 compared to non-porous pillars (Figure 3) [56]. This electrochemical method (a progression from techniques reported earlier [57,58]) was found to be beneficial in both its uniformity across the entire column and the tunability in pore size achieved by varying the applied potential. The increased surface area allowed for significantly higher retention than the non-anodized pillars while also demonstrating plate heights on the order of 6 μm [56]. Other methods to help increase sample loadability (and/or analyte retention) have also been either modeled or demonstrated experimentally. A model was proposed that uses similar overall array structure to that utilized by many of the other reports described here but is further modified with pillars that are each made up of smaller nanoarrays with an inter-pillar distance of ~15 nm as a way of increasing surface area to replicate fully and superficially porous chromatographic particles [59]. In this theoretical study it was found that while these nanopillars could slightly decrease efficiency compared to nonporous pillars, they do allow for more structural uniformity in the pores of a stationary phase support than is feasible with a traditional particle. Carbon-nanotubes integrated into a pillar array structure have been shown to increase pillar hydrophobicity analyte retention [60]. Alternatively, COP chips with a pillar array column containing 10 μm diameter diamond pillars (4 μm inter-pillar distance) embossed from a silicon master with the hydrophobic nature of the substrate acting as a stationary phase for LC separation [61]. Bioanalytical applications utilizing pillar array columns with reversed phase functionality have included separations of DNA strands [62] and branched chain amino acids [63].
Figure 3.
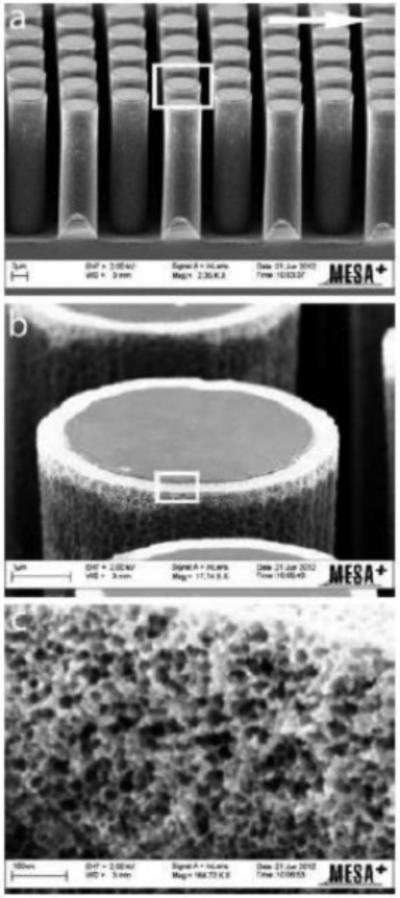
SEM images for an electrochemically anodized pillar with the white rectangular box in (A) indicating the zoomed-in region shown in (B) and the same magnification scheme for (C). The pillar diameter is 5 μm, the pillar height is 18 μm, the inter-pillar distance is 2.5 μm, the porous shell thickness is 300 nm, and the pore diameter is ~30 nm. Used with permission from the Royal Society of Chemistry from reference [56].
2.3.3 Planar Chromatography with Pillar Arrays
Pillar arrays can also be used for planar chromatography. Silicon wafers with 1-3 μm diameter, 15-20 μm tall cylindrical pillars in a 1 cm × 3 cm array (spacing of 2-6 μm between pillars), modified with a C18 reversed phase coating, were tested for efficiency as thin-layer chromatography (TLC) plates [64,65]. Compared to commercial TLC plates, chromatographic efficiency was ~3-5 times better for the pillar array [64]. To further improve performance, pillar dimension were reduced to 0.2-0.4 μm (diameter) by 1-2 μm (height) to which aided in stacking and focusing effects that reduced band broadening.
2.3.4 Future Possibilities Utilizing 3D Printing
In a look at what might be possible in the future utilizing additive manufacturing techniques, a fully printed 3D column structure was demonstrated for the first time [66]. Similar to a pillar array column, the entire stationary phase support structure can be designed and fabricated as desired to ensure ideal flow distributions, although no separations were attempted with this initial report that focused on flow profiles. An advantage of this technique is that an entire column structure (including flow distributors, fittings, etc.) can be printed in a single, integrated device, although improvements to printer resolution will likely be required for high efficiency separations.
3. Conclusions
Although more progress is needed to obtain the type of integrated column device described by Gritti and Guiochon in [1], the papers detailed here demonstrate advancements towards that ultimate goal. Improvements in both device robustness [24-26] and instrument integration [33-35,39,50] are enabling technology that will be required for high efficiency on-chip chromatographic separations. For low-cost separation techniques, monolithic stationary phases in polymeric chips can be implemented [42-50] and it is possible that further advances in embossing techniques to generate pillar array columns [61] could eventually lead to disposable, one-use chromatography columns. Perhaps the most disruptive technology that could impact the future of microfluidic LC is 3D printing [67], whether in substrate fabrication [26] or the manufacturing of a fully integrated instrument-column hybrid [66], although further improvements in printing technology are needed before it supplants the other types of columns and fabrication techniques described here. An important question is if microfluidic LC research will (or should) focus on pushing the limits of separation efficiency or on rugged, portable, fast separations of targeted analytes [3]. While the answer is still not clear, in the papers described here it would seem that industry is driving the technology for the former [11-13,24,25] while the biomedical research community is focused on the latter [37-39], with more traditional separations-focused groups falling somewhere in between. Perhaps it is in this region of overlap where high efficiency, fully portable LC devices may eventually come to fruition.
Highlights.
Advances in on-chip microfluidic LC columns are described.
Stationary phase supports for chip-LC include particles, monoliths, and pillar arrays.
Different substrate materials and selectivities can be used for specific separation needs.
Acknowledgements
Our work in this area is supported by NIH R37 EB003320 to R.T.K. J.P.G. was supported by NIH T32 grant DK007245.
Appendix
Appendix A.
Column Performance Characteristics for Devices Described in this Reviewa
|
Stationary Phase Support |
Device Substrate |
Support Dimension and/or Type b |
Column Dimension c |
Pressure Limit (bar) |
Plate Count d |
Separation Timee (min) |
Analyte Measured f |
Reference Number |
|---|---|---|---|---|---|---|---|---|
| Particles | Silicon-Glass | 5 μm (RP Silica) | wf = 312 μm d = 102 μm L = 20 mm | 140 | 200 | 3 | Organic Dyes | [14] |
| Particles | Glass | 3 μm (RP Silica) | wf = 85 μm d = 40 μm L = 46 mm | 50 | 3,500 | 6 | PAHs | [19] |
| Particles | Glass | 3 μm (RP Silica) | wf = 90 μm d = 40 μm L = 25 mm | 250 | 2,900 | 2 | PAHs | [20] |
| Particles | Glass | 5 μm (RP Silica) | wf = 90 μm d = 40 μm L = 30 mm | 250 | 2,000 | 4 | PAHs | [21] |
| Particles | COC | 3.5 μm (RP Silica) | wf = 150 μm d = 75 μm L = 45 mm | 100 | 5,000 | 8 | Small Organic Drugs | [22] |
| Particles | Stainless Steel | 5 μm (RP Silica) | wf = 150 μm d = 75 μm L = 600 mm | 276 | 2,200 | 50 | Alkylphenones | [26] |
| Particles | PDMS | 5 μm (SAX PS/DVB) | wf = 200 μm d = 100 μm L = 20 mm | 2 | 33,000 | 5 | Single-stranded DNA | [27] |
| Particles | PDMS-Glass | 5 μm (RP Silica) | wf = 100 μm d = 30 μm L = 1 mm | NR | 1,300 | 1 | Organic Dyes | [29] |
| Particles | Glass | 5 μm (RP Silica) | wf = 110 μm d = 50 μm L = 20 mm | NR | 9,000 | 2 | Peptides | [34] |
| Monolith | Glass-PEGDA (Sandwich) | Butyl acrylate | wf = 200 μm d = 25 μm L = 30 mm | NR | 700 | 6 | PAHs | [43] |
| Pillar Array | Silicon-Pyrex | 5 × 75 μm (diamond)/2.5 μm | wf = 1 mm d = 8 μm L = 10 mm | NR | 20,000 | 0.5 | Organic Dyes | [52] |
| Pillar Array | Silicon-Pyrex | 5 × 45 μm (diamond)/3 μm | wf = 1 mm d = 8 μm L = 10 mm | NR | 20,000 | 0.5 | Organic Dyes | [53] |
| Pillar Array | Silicon-Pyrex | 47 × 6 μm (“foil-shape”)/2 μm | wf = 320 μm d = 10 μm L = 10 mm | NR | 10,000 | 1 | Organic Dyes | [55] |
| Pillar Array | Silicon-Pyrex | 5 μm (round)/2 μm | wf = 300 μm d = 18 μm L = 10 mm | 350 | 1,500 | 0.1 | Organic Dyes | [56] |
| Pillar Array | COC | 10 × 10 μm (diamond)/4 μm | wf = 320 μm d = 15 μm L = 40 mm | 6 | 3,600 | 32 | Small Organic Molecules | [61] |
| Pillar Array | Silicon (Planar) | 2 μm (round)/2 μm | wf = 10 mm d = 20 μm L = 4 mm | NR | 8,000 | 0.5 | Organic Dyes | [64] |
| Pillar Array | Silicon (Planar) | 0.4 μm (round)/0.55 μm | wf = NR d = 2 μm L = 2 mm | NR | 1,000 | 0.1 | Organic Dyes | [65] |
Only articles that describe these characteristics for the devices are reported (for specific values, NR = not reported or unable to determine from the information provided)
Particles = particle diameter (RP = reversed phase, SAX = strong anion exchange, PS/DVB = polystyrene divinylbenzene), Pillar arrays = pillar dimensions (shape)/inter-pillar distance
wf = full channel width, d = channel depth, L = column length
Approximate value reported for a specific separation within the given manuscript or calculated based on other efficiency data listed (plate height, reduced plate height, plates/meter, etc.)
Approximate value reported for a specific separation within the given manuscript or calculated based on other data listed (mobile phase velocity, separation length, analyte retention time, etc.)
PAH = polyaromatic hydrocarbon
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gritti F, Guiochon G. The current revolution in column technology: How it began, where is it going? J. Chromatogr. A. 2012;1228:2–19. doi: 10.1016/j.chroma.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 2.Novotny M. Microcolumns in Liquid Chromatography. Anal. Chem. 1981;53:1294A–1308A. [Google Scholar]
- 3.Kutter JP. Liquid phase chromatography on microchips. J. Chromatogr. A. 2012;1221:72–82. doi: 10.1016/j.chroma.2011.10.044. [DOI] [PubMed] [Google Scholar]
- 4.Lavrik NV, Taylor LT, Sepaniak MJ. Nanotechnology and chip level systems for pressure driven liquid chromatography and emerging analytical separation techniques: A review. Anal. Chim. Acta. 2011;694:6–20. doi: 10.1016/j.aca.2011.03.059. [DOI] [PubMed] [Google Scholar]
- 5.Faure K. Liquid chromatography on chip. Electrophoresis. 2010;31:2499–2511. doi: 10.1002/elps.201000051. [DOI] [PubMed] [Google Scholar]
- 6.Pruim P, Schoenmakers PJ, Kok WT. Microfluidic Pressure Driven Liquid Chromatography of Biologically Relevant Samples. Chromatographia. 2012;75:1225–1234. [Google Scholar]
- 7.Ohla S, Belder D. Chip-based separation devices coupled to mass spectrometry. Curr. Opin. Chem. Biol. 2012;16:453–459. doi: 10.1016/j.cbpa.2012.05.180. [DOI] [PubMed] [Google Scholar]
- 8.Lin S-L, Lin T-Y, Fuh M-R. Microfluidic chip-based liquid chromatography coupled to mass spectrometry for determination of small molecules in bioanalytical applications: An update. Electrophoresis. 2014;35:1275–1284. doi: 10.1002/elps.201300415. [DOI] [PubMed] [Google Scholar]
- 9.Oedit A, Vulto P, Ramautar R, Lindenburg PW, Hankemeier T. Lab-on-a-Chip hyphenation with mass spectrometry: strategies for bioanalytical applications. Curr. Opin. Biotechnol. 2015;31:79–85. doi: 10.1016/j.copbio.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 10.Wang X, Yi L, Mukhitov N, Schrell AM, Dhumpa R, Roper MG. Microfluidics-to-mass spectrometry: A review of coupling methods and applications. J. Chromatogr. A. 2015;1382:98–116. doi: 10.1016/j.chroma.2014.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yin H, Killeen K, Brennen R, Sobek D, Werlich M, van de Goor T. Microfluidic Chip for Peptide Analysis with an Integrated HPLC Column, Sample Enrichment Column, and Nanoelectrospray Tip. Anal. Chem. 2005;77:527–533. doi: 10.1021/ac049068d. [DOI] [PubMed] [Google Scholar]
- 12.Gerhardt G. Microfluidics-Based Separations Technology for the Analytical Laboratory. Chromatogr. Today. 2011;4:6–8. [Google Scholar]
- 13.Hebert N, Lin E, van Soest R, Young JB. Approaching 100% LC-MS Uptime for Peptide Analyses using Matched Chip Columns. J. Biomol. Tech. 2010;21:S32. [Google Scholar]
- 14.Ocvirk G, Verpoorte E, Manz A, Grasserbauer M, Widmer HM. High Performance Liquid Chromatography Partially Integrated onto a Silicon Chip. Anal. Meth. Instr. 1995;2:74–82. [Google Scholar]
- 15.Ren K, Zhou J, Wu H. Materials for Microfluidic Chip Fabrication. Acc. Chem. Res. 2013;46:2396–2406. doi: 10.1021/ar300314s. [DOI] [PubMed] [Google Scholar]
- 16.Nge PN, Rogers CI, Woolley AT. Advances in Microfluidic Materials, Functions, Integration, and Applications. Chem. Rev. 2013;113:2550–2583. doi: 10.1021/cr300337x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Culbertson CT, Mickleburgh TG, Stewart-James SA, Sellens KA, Pressnall M. Micro Total Analysis Systems: Fundamental Advances and Biological Applications. Anal. Chem. 2014;86:95–118. doi: 10.1021/ac403688g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheong WJ. Fritting techniques in chromatography. J. Sep. Sci. 2014;37:603–617. doi: 10.1002/jssc.201301239. [DOI] [PubMed] [Google Scholar]
- 19.Thurmann S, Dittmar A, Belder D. A low pressure on-chip injection strategy for high-performance chip-based chromatography. J. Chromatogr. A. 2014;1340:59–67. doi: 10.1016/j.chroma.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 20.Thurmann S, Mauritz L, Heck C, Belder D. High-performance liquid chromatography on glass chips using precisely defined porous polymer monoliths as particle retaining elements. J. Chromatogr. A. 2014;1370:33–39. doi: 10.1016/j.chroma.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 21.Thurmann S, Belder D. Phase-optimized chip-based liquid chromatography. Anal. Bioanal. Chem. 2014;406:6599–6606. doi: 10.1007/s00216-014-8087-y. [DOI] [PubMed] [Google Scholar]
- 22.Mats L, Gibson GTT, Oleschuk RD. Plastic LC/MS microchip with an embedded microstructured fibre having the dual role of a frit and a nanoelectrospray emitter. Microfluid. Nanofluid. 2014;16:73–81. [Google Scholar]
- 23.Kabiri S, Kurkuri MD, Kumeria T, Losic D. Frit-free PDMS microfluidic device for chromatographic separation and on-chip detection. RSC Adv. 2014;4:15276–15280. [Google Scholar]
- 24.Gilar M, McDonald TS, Roman G, Johnson JS, Murphy JP, Jorgenson JW. Repetitive injection method: A tool for investigation of injection zone formation and its compression in microfluidic liquid chromatography. J. Chromatogr. A. 2015;1381:110–117. doi: 10.1016/j.chroma.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 25.Gilar M, McDonald TS, Johnson JS, Murphy JP, Jorgenson JW. Wide injection zone compression in gradient reversed-phase liquid chromatography. J. Chromatogr. A. 2015;1390:86–94. doi: 10.1016/j.chroma.2015.02.057. [DOI] [PubMed] [Google Scholar]
- 26.Sandron S, Heery B, Gupta V, Collins DA, Nesterenko EP, Nesterenko PN, Talebi M, Beirne S, Thompson F, Wallace GG, Brabazon D, Regan F, Paull B. 3D printed metal columns for capillary liquid chromatography. Analyst. 2014;139:6343–6347. doi: 10.1039/c4an01476f. [DOI] [PubMed] [Google Scholar]
- 27.Huft J, Haynes CA, Hansen CL. Fabrication of High-Quality Microfluidic Solid-Phase Chromatography Columns. Anal. Chem. 2013;85:1797–1802. doi: 10.1021/ac303153a. [DOI] [PubMed] [Google Scholar]
- 28.Huft J, Haynes CA, Hansen CL. Microfluidic Integration of Parallel Solid-Phase Liquid Chromatography. Anal. Chem. 2013;85:2999–3005. doi: 10.1021/ac400163u. [DOI] [PubMed] [Google Scholar]
- 29.Nagy A, Gaspar A. Packed multi-channels for parallel chromatographic separations in microchips. J. Chromatogr. A. 2013;1304:251–256. doi: 10.1016/j.chroma.2013.06.065. [DOI] [PubMed] [Google Scholar]
- 30.Nagy A, Baranyai E, Gaspar A. Interfacing microfluidic chip-based chromatography with flame atomic absorption spectrometry for the determination of chromium(VI) Microchem. J. 2014;114:216–222. [Google Scholar]
- 31.Fekete S, Beck A, Veuthey J-L, Guillarme D. Ion-exchange chromatography for the characterization of biopharmaceuticals. J. Pharm. Biomed. Anal. 2015 doi: 10.1016/j.jpba.2015.02.037. In Press. [DOI] [PubMed] [Google Scholar]
- 32.Augusto F, Hantao LW, Mogollón NGS, Braga SCGN. New materials and trends in sorbents for solid-phase extraction. Tr. Anal. Chem. 2013;43:14–23. [Google Scholar]
- 33.Leow PL, Chee PS, Patel BA, O'Hare D. A study of the in-column detection performance for chromatography separation. Microfluid. Nanofluid. 2015 In Press. [Google Scholar]
- 34.Lazar IM, Kabulski JL. Microfluidic LC device with orthogonal sample extraction for on-chip MALDI-MS detection. Lab Chip. 2013;13:2055–2065. doi: 10.1039/c3lc50190f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Getty SA, Dworkin JP, Glavin DP, Martin M, Zheng Y, Balvin M, Southard AE, Feng S, Ferrance J, Kotecki C, Malespin C, Mahaffy PR. Organics Analyzer for Sampling Icy Surfaces: A liquid chromatograph-mass spectrometer for future in situ small body missions. IEEE Aero. Conf. Proc. 2013;2.0807:1–8. [Google Scholar]
- 36.Southard AE, Getty SA, Balvin M, Elsila JE, Melina AE, Kotecki C, Towner DW, Dworkin JP, Glavin DP, Mahaffy PR, Ferrance J. Liquid chromatography-mass spectrometry interface for detection of extraterrestrial organics. IEEE Aero. Conf. Proc. 2014;2.0702:1–7. [Google Scholar]
- 37.Niimi M, Masuda T, Kaihatsu K, Kato N, Nakamura S, Nakaya T, Arai F. Virus purification and enrichment by hydroxyapatite chromatography on a chip. Sens. Actu. B: Chem. 2014;201:185–190. doi: 10.1016/j.snb.2014.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang S, Eshghi ST, Chiu H, DeVoe DL, Zhang H. Glycomic Analysis by Glycoprotein Immobilization for Glycan Extraction and Liquid Chromatography on Microfluidic Chip. Anal. Chem. 2013;85:10117–10125. doi: 10.1021/ac4013013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tan SJ, Phan H, Gerry BM, Kuhn A, Hong LZ, Ong YM, Poon PSY, Unger MA, Jones RC, Quake SR, Burkholder WF. A Microfluidic Device for Preparing Next Generation DNA Sequencing Libraries and for Automating Other Laboratory Protocols That Require One or More Column Chromatography Steps. PLoS One. 2013;8:e64084. doi: 10.1371/journal.pone.0064084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.L Chester T. Recent Developments in High-Performance Liquid Chromatography Stationary Phases. Anal. Chem. 2013;85:579–589. doi: 10.1021/ac303180y. [DOI] [PubMed] [Google Scholar]
- 41.Svec F, Lv Y. Advances and Recent Trends in the Field of Monolithic Columns for Chromatography. Anal. Chem. 2015;87:250–273. doi: 10.1021/ac504059c. [DOI] [PubMed] [Google Scholar]
- 42.Chan AS, Danquah MK, Agyei D, Hartley PG, Zhu Y. A Parametric Study of a Monolithic Microfluidic System for On-Chip Biomolecular Separation. Sep. Sci. Tech. 2014;49:854–860. [Google Scholar]
- 43.Hackl C, Beyreiss R, Geissler D, Jezierski S, Belder D. Rapid Prototyping of Electrochromatography Chips for Improved Two-Photon Excited Fluorescence Detection. Anal. Chem. 2014;86:3773–3779. doi: 10.1021/ac500793e. [DOI] [PubMed] [Google Scholar]
- 44.Zhang J, Chen G, Tian M, Li R, Quan X, Jia Q. A novel organic–inorganic hybrid monolithic column prepared in-situ in a microchip and its application for the determination of 2-amino-4-chlorophenol in chlorzoxazone tablets. Talanta. 2013;115:801–805. doi: 10.1016/j.talanta.2013.06.058. [DOI] [PubMed] [Google Scholar]
- 45.Levy MH, Plawsky J, Cramer SM. Photopolymerized sol–gel monoliths for separations of glycosylated proteins and peptides in microfluidic chips. J. Sep. Sci. 2013;36:2358–2365. doi: 10.1002/jssc.201200990. [DOI] [PubMed] [Google Scholar]
- 46.Nunes PS, Ohlsson PD, Ordeig O, Kutter JP. Cyclic olefin polymers: emerging materials for lab-on-a-chip applications. Microfluid. Nanofluid. 2010;9:145–161. [Google Scholar]
- 47.Ladner Y, Cretier G, Faure K. Electrochromatography on Acrylate-Based Monolith in Cyclic Olefin Copolymer Microchip: An Attractive Technology. In: Van Schepdael A, editor. Microchip Capillary Electrophoresis Protocols. Springer; New York: 2015. pp. 161–167. [DOI] [PubMed] [Google Scholar]
- 48.Yang R, Pagaduan JV, Yu M, Woolley AT. On chip preconcentration and fluorescence labeling of model proteins by use of monolithic columns: device fabrication, optimization, and automation. Anal. Bioanal. Chem. 2015;407:737–747. doi: 10.1007/s00216-014-7988-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kendall EL, Wienhold E, Rahmanian OD, DeVoe DL. Ex situ integration of multifunctional porous polymer monoliths into thermoplastic microfluidic chips. Sens. Actu. B: Chem. 2014;202:866–872. doi: 10.1016/j.snb.2014.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wouters B, De Vos J, Desmet G, Terryn H, Schoenmakers PJ, Eeltink S. Design of a microfluidic device for comprehensive spatial two-dimensional liquid chromatography. J. Sep. Sci. 2015;38:1123–1129. doi: 10.1002/jssc.201401192. [DOI] [PubMed] [Google Scholar]
- 51.He B, Tait N, Regnier F. Fabrication of Nanocolumns for Liquid Chromatography. Anal. Chem. 1998;70:3790–3797. doi: 10.1021/ac980028h. [DOI] [PubMed] [Google Scholar]
- 52.Op De Beeck J, Callewaert M, Ottevaere H, Gardeniers H, Desmet G, De Malsche W. On the Advantages of Radially Elongated Structures in Microchip-Based Liquid Chromatography. Anal. Chem. 2013;85:5207–5212. doi: 10.1021/ac400576s. [DOI] [PubMed] [Google Scholar]
- 53.Op De Beeck J, Callewaert M, Ottevaere H, Gardeniers H, Desmet G, De Malsche W. Suppression of the sidewall effect in pillar array columns with radially elongated pillars. J. Chromatogr. A. 2014;1367:118–122. doi: 10.1016/j.chroma.2014.09.052. [DOI] [PubMed] [Google Scholar]
- 54.De Bruyne S, De Malsche W, Deridder S, Gardeniers H, Desmet G. In Situ Measurement of the Transversal Dispersion in Ordered and Disordered Two-Dimensional Pillar Beds for Liquid Chromatography. Anal. Chem. 2014;86:2947–2954. doi: 10.1021/ac403147q. [DOI] [PubMed] [Google Scholar]
- 55.Sukas S, Desmet G, Gardeniers HJGE. Design and implementation of injector/distributor structures for microfabricated non-porous pillar columns for capillary electrochromatography. J. Chromatogr. A. 2013;1289:80–87. doi: 10.1016/j.chroma.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 56.Callewaert M, Op De Beeck J, Maeno K, Sukas S, Thienpont H, Ottevaere H, Gardeniers H, Desmet G, De Malsche W. Integration of uniform porous shell layers in very long pillar array columns using electrochemical anodization for liquid chromatography. Analyst. 2014;139:618–625. doi: 10.1039/c3an02023a. [DOI] [PubMed] [Google Scholar]
- 57.De Malsche W, Clicq D, Verdoold V, Gzil P, Desmet G, Gardeniers H. Integration of porous layers in ordered pillar arrays for liquid chromatography. Lab Chip. 2007;7:1705–1711. doi: 10.1039/b710507j. [DOI] [PubMed] [Google Scholar]
- 58.De Malsche W, Gardeniers H, Desmet G. Experimental Study of Porous Silicon Shell Pillars under Retentive Conditions. Anal. Chem. 2008;80:5391–5400. doi: 10.1021/ac800424q. [DOI] [PubMed] [Google Scholar]
- 59.Yan X, Li N. Nanopillar array with multi-scale inter-pillar spacing as chromatography stationary phase support: Theoretical performance evaluation. Chem. Eng. Sci. 2014;107:158–164. [Google Scholar]
- 60.Mogenson KB, Delacourt B, Kutter J. Carbon Nanotube-Based Separation Columns for Microchip Electrochromatography. In: Van Schepdael A, editor. Microchip Capillary Electrophoresis Protocols. Springer; New York: 2015. pp. 149–159. [DOI] [PubMed] [Google Scholar]
- 61.Tsougeni K, Ellinas K, Archontaki H, Gogolides E. A microfabricated cyclo-olefin polymer microcolumn used for reversed-phase chromatography. J. Micromech. Microeng. 2015;25:015005. [Google Scholar]
- 62.Zhang L, Majeed B, Lagae L, Peumans P, Van Hoof C, De Malsche W. Ion-pair reversed-phase chromatography of short double-stranded deoxyribonucleic acid in silicon micro-pillar array columns: Retention model and applications. J. Chromatogr. A. 2013;1294:1–9. doi: 10.1016/j.chroma.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 63.Song Y, Takatsuki K, Isokawa M, Sekiguchi T, Mizuno J, Funatsu T, Shoji S, Tsunoda M. Fast and quantitative analysis of branched-chain amino acids in biological samples using a pillar array column. Anal. Bioanal. Chem. 2013;405:7993–7999. doi: 10.1007/s00216-013-7034-7. [DOI] [PubMed] [Google Scholar]
- 64.Kirchner TB, Hatab NA, Lavrik NV, Sepaniak MJ. Highly Ordered Silicon Pillar Arrays As Platforms for Planar Chromatography. Anal. Chem. 2013;85:11802–11808. doi: 10.1021/ac402261p. [DOI] [PubMed] [Google Scholar]
- 65.Kirchner TB, Strickhouser RB, Hatab NA, Charlton JJ, Kravchenko II, Lavrik NV, Sepaniak MJ. Nanoscale pillar arrays for separations. Analyst. 2015 doi: 10.1039/c4an02187h. In Press. [DOI] [PubMed] [Google Scholar]
- 66.Fee C, Nawada S, Dimartino S. 3D printed porous media columns with fine control of column packing morphology. J. Chromatogr. A. 2014;1333:18–24. doi: 10.1016/j.chroma.2014.01.043. [DOI] [PubMed] [Google Scholar]
- 67.Gross BC, Erkal JL, Lockwood SY, Chen C, Spence DM. Evaluation of 3D Printing and Its Potential Impact on Biotechnology and the Chemical Sciences. Anal. Chem. 2014;86:3240–3253. doi: 10.1021/ac403397r. [DOI] [PubMed] [Google Scholar]


