Abstract
Brainstem encephalitis (BE) is an uncommon condition. We sought to characterize clinical presentations, etiologies, response to treatment, and predictors of outcome. We performed a retrospective review of non–HIV infected patients diagnosed with BE at Johns Hopkins Hospital (January 1997–April 2010). We characterized clinical and paraclinical features, and used regression models to assess associations with poor outcome. BE was diagnosed in 81 patients. An etiology was identified in 58 of 81 (71.6 %) of cases, most of which were confirmed or probable inflammatory/autoimmune conditions. Of the remaining 23 cases in which a specific diagnosis remained undefined, clinical presentation, CSF, neuroimaging studies, and outcomes were similar to the inflammatory/autoimmune group. Brain biopsy identified a specific diagnosis in 7 of 14 patients (50 %). Fifteen patients (18.5 %) either died or had a poor outcome. In multivariate logistic regression models, a higher CSF protein (per 5 mg/dl, OR = 1.11, 95 % CI: 1.03–1.20), a higher CSF glucose (per 5 mg/dl, OR = 1.36, 95 % CI: 1.09–1.70), and higher serum glucose (per 5 mg/dl, OR = 1.27, 95 % CI: 1.06–1.52) were independently associated with increased odds of poor outcome. Inflammatory and non-infectious conditions accounted for most cases of BE. Higher CSF protein and glucose were independently associated with poor outcome. In immunocompetent patients with BE of undefined etiology despite extensive investigation, a trial of immunosuppressive treatment may be warranted, though deterioration clinically or on magnetic resonance imaging should prompt a brain biopsy.
Keywords: Rhombencephalitis, Brainstem encephalitis, Brain biopsy, Autoimmune, Inflammatory, Glucose
Introduction
Encephalitis affects 1 in 10,000 individuals yearly, often with devastating neurologic consequences [1]. Challenges in the management of patients with encephalitis include identifying the causative agent and defining prognosis. In several large studies, infectious agents comprise the majority of cases in which a cause is identified. Little, however, is known about the typical etiologies of brainstem encephalitis (BE). Several entities, including Listeria rhombencephalitis, Bickerstaff’s brainstem encephalitis, and Ma2-associated paraneoplastic encephalitis, have been described in case reports and series [1–5], but the spectrum of causes and outcomes of BE have not been systematically studied. To better characterize etiologies and prognosis of BE and to aid treatment decisions when physicians are confronted with such patients, we performed a retrospective study of patients with BE in our institution.
Methods
We performed a retrospective review of all adult and pediatric patients presenting with encephalitis at the Johns Hopkins Hospital, a tertiary care medical center, between January 1997 and April 2010. We defined encephalitis as altered mental state, personality change or focal neurological deficits, and ≥2 of the following (a) fever, (b) seizure and/or focal neurologic deficit, (c) CSF pleocytosis, (d) electroencephalography consistent with encephalopathy (focal or generalized slow activities), (e) neuroimaging findings consistent with encephalitis. Exclusion criteria included delirium or encephalopathy secondary to sepsis, toxic or metabolic causes (hypoglycemia, electrolyte disturbances), or primary psychiatric illness. We screened with the following ICD-9 coded diagnoses: encephalopathy, encephalitis, infections of the central nervous system, post-infectious encephalitis and autoimmune encephalitis. Of patients with encephalitis, we then restricted our analysis to those who satisfied our criteria for BE, defined by clinical presentations of predominant brainstem signs (cranial neuropathies and ataxia). Neuroimaging findings of brainstem abnormalities during the admission were also required unless the clinical syndrome was unequivocally localized to the brainstem. Patients with neoplasm, an established diagnosis of multiple sclerosis, or predominantly cerebral lesions on magnetic resonance imaging (MRI) were excluded.
The study was approved by the Johns Hopkins University Institutional Review Board.
We collected demographic and clinical characteristics at presentation and throughout the hospitalization, including presenting symptoms, the initial CSF examination, MRI findings, brain biopsy if performed, diagnosis, treatment and outcome. All patients had HIV testing, Lyme serologies, treponemal testing, brain MRI scans, and CSF examination for cell counts, glucose, protein, gram stain, bacterial cultures, and PCRs for HSV, over 90 % of patients had a serum autoimmune panel (including, but not limited to, ANA, ANCA, anti-dsDNA, anti-Ro/La, anti-cardiolipin, and antithyroid antibodies), chest imaging, and CSF PCRs for EBV and VZV, 75 % of patients had anti-Hu and anti-Ma/Ta testing, 50 % of patients had CSF PCRs sent for enterovirus, and fewer than 50 % underwent testing for a full paraneoplastic antibody panel, GQ1b antibodies, or CSF oligoclonal bands. Patient outcome was dichotomized: a good outcome was assigned if patients recovered completely or had mild residual deficit (ambulatory without assistance), and poor outcome was assigned if severe residual deficit (needed assistance with ambulation) or death occurred.
The diagnosis of a demyelinating syndrome in our cohort was based on consensus definitions [6–9] and, in some cases, evidence of demyelination on brain biopsy. The diagnostic criteria for other conditions are outlined in eTable 1a and b. Only those cases that strictly fit the proposed criteria were assigned diagnoses of either a confirmed or probable etiology.
Statistical analysis
We assessed all potential variables for their associations with outcome in univariate models. Multivariate models included variables we had identified a priori. These variables included age, sex, the presence of ≥50 CSF white blood cells/mm3, the CSF glucose and protein concentrations, and the presence or absence of supratentorial lesions. We also explored whether adding diagnosis (known/undefined) meaningfully altered the conclusions of the primary multivariate model. All analyses were performed with StataVersion 10.0 (StataCorp LP, College Station, Texas USA).
Results
We identified a total of 415 patients who fit the criteria for encephalitis. Eighty-one of these (19.5 %) patients were diagnosed with BE. The etiologies of BE, summarized in Table 1, include infectious causes (4 patients), autoimmune/inflammatory causes (54 patients), and undefined causes (23 patients). Representative neuroimaging and histopathological findings are presented in the Fig. 1.
Table 1.
Etiologies of brainstem encephalitis
| Etiology | Number, n |
|---|---|
| Infectious | 4 |
| Probable Listeria | 3 |
| CNS aspergillosis | 1 |
| Autoimmune/inflammatory | 54* |
| Demyelinating | 37 |
| Paraneoplastic syndrome | 4 |
| Susac syndrome | 3 |
| Neuro-behcet | 3 |
| Neurosarcoidosis | 2 |
| CLIPPERS | 1 |
| Primary CNS vasculitis | 2 |
| Bickerstaff’s encephalitis | 1 |
| Hashimoto’s encephalopathy | 1 |
| Undefined | 23 |
Confirmed or probable cases (see methods)
Fig. 1.
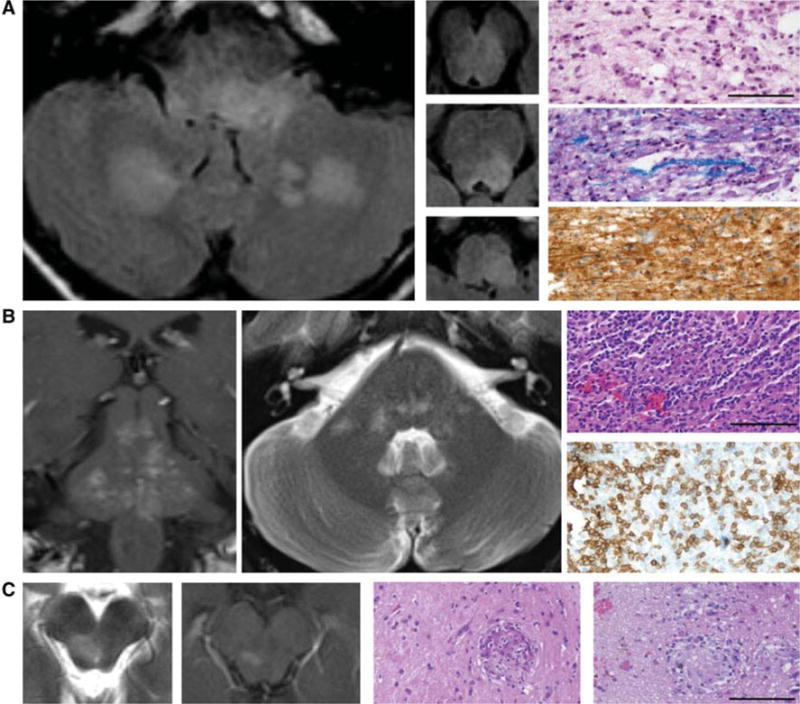
Imaging and histopathological findings in representative cases of brainstem encephalitis. a ADEM left and middle panels, MRI axial FLAIR sequences demonstrating multifocal hyperintensities in the brainstem and cerebellum. Right panels demonstrate macrophage infiltration (upper, hematoxylin and eosin), demyelination (middle, Luxol fast blue), and relative preservation of axons (lower, neurofilament staining). Scale bar 100 μm. b CLIPPERS left panel (MRI coronal T1 post gadolinium) demonstrates punctate and curvilinear enhancing lesions scattered throughout the brainstem. Middle panel (MRI axial T2) demonstrates scattered pontine hyperintensities. Right panels demonstrate marked perivascular and parenchymal inflammation (upper, hematoxylin and eosin) with prominent lymphocytic infiltration (lower, CD3 staining). Macrophages were also present (not shown). Scale bar 100 μm. c Neurosarcoidosis left panels (MRI T2 and T1 post gadolinium) demonstrate an enhancing lesion in the right midbrain. Right panels (hematoxylin and eosin) demonstrate several non-caseating granulomas. Acid fast bacilli and silver staining did not demonstrate any organisms (not shown). Scale bar 100 μm
Demographics
The mean age at presentation for the BE cohort overall was 32.5 years (SD 20.5 years, range: 1.5–72 years), and the male: female ratio was 1:1. Only two patients in our cohort were immunocompromised, and both developed infectious BE: one patient on rituximab therapy for lymphoplasmacytic lymphoma developed presumed Listeria BE, while another patient with failed engraftment of bone marrow transplantation for anaplastic large cell lymphoma developed CNS aspergillosis.
Clinical presentation and serologic evaluation
Antecedent illness was defined as preceding illness occurring within 7–10 days prior to the onset of brainstem dysfunction. These symptoms, typically consisting of respiratory or gastrointestinal complaints, were reported in 34 of the 81 patients (42 %). Antecedent symptoms were significantly more commonly reported in the inflammatory/autoimmune group (51.9 %), as compared to the undefined group (21.7 %). Common clinical manifestations of BE included ataxia (69.1 %), ocular dysfunction (58 %), bulbar dysfunction (58 %) and limb weakness (58 %) (Table 2). ANA titer was greater than 1:640 in one patient diagnosed with CNS vasculitis, and was detected at lower titers (1:40–1:160) in three patients with demyelinating syndromes and one with undefined cause of encephalitis. Antithyroid antibodies were strongly positive (>1,000 WHO units) in one patient, diagnosed with Hashimoto’s encephalopathy. GQ1b IgG was detected at a titer of greater than 1:100 in one patient diagnosed with Bicker-staff’s brainstem encephalitis, and anti-Ma antibodies were found in one patient with paraneoplastic brainstem encephalitis. None of the other antibodies tested were positive in our patients.
Table 2.
Clinical characteristics of patients with brainstem encephalitis
| Total n = 81 | Infectious n = 4 | Autoimmune/inflammatory n = 54 | Undefined n = 23 | p value* | |
|---|---|---|---|---|---|
| Age (years) mean ± SD | 32.5 ± 20.5 | 42.2 ± 16.7 | 26.6 ± 20.2 | 43.5 ± 17.0 | 0.0005 |
| Male, n (%) | 41 (50.6) | 2 (50.0) | 28 (51.9) | 11 (47.8) | 0.81 |
| Clinical presentations | |||||
| Antecedent illness, n (%) | 34 (42.0) | 1 (25.0) | 28 (51.9) | 5 (21.7) | 0.023 |
| Febrile, n (%) | 14 (17.3) | 2 (50.0) | 7 (13.0) | 5 (21.7) | 0.33 |
| Headache, n (%) | 37 (45.7) | 2 (50.0) | 26 (48.1) | 9 (39.1) | 0.62 |
| Encephalopathy, n (%) | 30 (37.0) | 2 (50.0) | 21 (38.9) | 7 (30.4) | 0.61 |
| Ocular complaints, n (%) | 47 (58.0) | 3 (75.0) | 31 (57.4) | 13 (56.5) | 1.00 |
| Bulbar symptoms, n (%) | 47 (58.0) | 4 (100.0) | 25 (46.3) | 18 (78.3) | 0.012 |
| Limb weakness, n (%) | 47 (58.0) | 2 (50.0) | 37 (68.5) | 8 (34.8) | 0.01 |
| Ataxia, n (%) | 56 (69.1) | 2 (50.0) | 35 (64.8) | 19 (82.6) | 0.17 |
| CSF examination | |||||
| WBC (cell/mm3) | 164.8 ± 548.5 | 792.0 ± 1293.1 | 183.4 ± 577.6 | 27.2 ± 38.4 | 0.18 |
| Protein (mg/dL) | 66.6 ± 62.9 | 90.5 ± 92.2 | 62.5 ± 61.4 | 71.1 ± 62.6 | 0.57 |
| Glucose (mg/dL) | 65.6 ± 19.2 | 64.2 ± 15.6 | 67.0 ± 20.0 | 63.1 ± 18.5 | 0.43 |
| Neuroimaging | |||||
| Brainstem abnormality, n(%) | 70 (86.4) | 4 (100.0) | 45 (83.3) | 21 (91.3) | 0.49 |
| Cerebral lesions, n (%) | 42 (51.9) | 2 (50.0) | 32 (59.3) | 8 (34.8) | 0.08 |
| Contrast enhancement, n (%) | 35 (43.2) | 1 (25.0) | 20 (37.0) | 14 (60.1) | 0.08 |
| Treatment | |||||
| Antimicrobial, n (%) | 19 (23.5) | 4 (100.0) | 9 (16.7) | 6 (26.1) | 0.36 |
| Corticosteroid, n (%) | 58 (71.6) | 1 (25.0) | 44 (81.5) | 13 (56.5) | 0.044 |
| Immunotherapy, n (%) | 19 (23.5) | 0 (0) | 14 (25.9) | 5 (21.7) | 0.78 |
| Outcomes | |||||
| Follow-up, months | 22.8 (32.9) | 10.8 (13.1) | 25.0 (34.3) | 18.2 (32.0) | 0.34 |
| Poor outcomes, n (%) | 15 (18.5) | 2 (50.0) | 10 (18.5) | 3 (13.0) | 0.74 |
| Mortality, n (%) | 5 (6.2) | 1 (25.0) | 3 (5.6) | 1 (4.3) | 1.0 |
Bold values indicate significance at p < 0.05
Comparison between undefined and autoimmune/inflammatory groups
CSF examination
Sixty-one of 81 patients (75 %) had abnormal CSF examinations, as defined by CSF pleocytosis (>5 WBC/mm3), elevated protein (>45 mg/dL), or decreased glucose (<50 mg/dL). While CSF pleocytosis was observed in all four patients with infectious BE, it was observed in only two-thirds of patients in the other two groups (Table 2).
Neuroimaging
Imaging abnormalities involving the brainstem were evident in most patients (Table 2). Neither the presence of additional supratentorial lesions nor contrast enhancing lesions discriminated between the inflammatory/autoimmune group and the undefined group.
Tissue diagnosis
Fourteen of the 81 patients underwent brain biopsy (see Table 3; Fig. 1). The underlying pathology was revealed in seven patients, all of whom were diagnosed with inflammatory/autoimmune conditions. These included four patients with demyelination, two patients with neurosarcoidosis and one with CLIPPERS. Seven patients had brain biopsies that did not point to a specific diagnosis; six of these biopsies revealed non-specific inflammatory changes with a lack of evidence of an infectious process. The remaining patient had no apparent abnormalities on biopsy and was subsequently diagnosed with anti-Ma2 paraneoplastic encephalitis. Locations of biopsies included brainstem (n = 7), cerebrum (n = 4), thalamus (n = 1) and cerebellum (n = 1). One of the seven patients who had a brainstem biopsy had mild dysarthria post-operatively that resolved over time, and there was no reported mortality associated with brainstem biopsy.
Table 3.
Brain biopsy in brainstem encephalitis
| No | Age | Sex | Biopsy site | Diagnosis | Influence on treatment |
|---|---|---|---|---|---|
| 1 | 33 | M | Brainstem | Neurosarcoid | Initiate immunosuppressives |
| 2 | 72 | F | Brainstem | Neurosarcoid | Continue corticosteroid |
| 3 | 6 | M | Cerebral | Demyelination | Continue corticosteroid and plasmapheresis |
| 4 | 62 | M | Cerebral | Demyelination | Initiate corticosteroid |
| 5 | 65 | F | Brainstem | Demyelination | Initiate corticosteroid |
| 6 | 65 | F | Brainstem | Demyelination | Initiate corticosteroid |
| 7 | 47 | M | Brainstem | CLIPPERS | Initiate corticosteroid |
| 8 | 23 | M | Cerebral | Inflammation, undefined | Continue corticosteroid |
| 9 | 30 | F | Brainstem | Inflammation, undefined | Initiate corticosteroid |
| 10 | 45 | F | Brainstem | Inflammation, undefined | Initiate corticosteroid |
| 11 | 59 | F | Cerebral | Inflammation, undefined | Initiate corticosteroid |
| 12 | 61 | M | Cerebellum | Inflammation, undefined | Continue corticosteroid |
| 13 | 66 | F | Thalamus | Inflammation, undefined | Initiate immunosuppressives |
| 14 | 39 | M | Cerebral | No inflammation | Final diagnosis: paraneoplastic anti-Ma |
Management
Antimicrobial therapy was utilized in all patients in the infectious group, and in a minority of individuals with inflammatory/autoimmune or undefined BE (Table 3). Corticosteroids were used more frequently in the inflammatory/autoimmune group (81.5 %) as compared to the undefined group (56.5 %) and were typically used as first-line treatment. In individuals with inadequate response, second-line immunotherapies including plasmapheresis, intravenous immunoglobulin, cyclophosphamide, mycophenolate mofetil, were used. These therapies were utilized at about the same rate in both the inflammatory/autoimmune and the undefined group. Following brain biopsy, immunotherapy was either initiated or escalated in 9 of the 14 patients, including in four of nine patients whose biopsy revealed non-specific inflammation. Among those with undefined cause, empiric immunotherapy was utilized in those individuals with either lack of spontaneous regression or with progression of clinical and/or radiological disease.
Outcome
Mean follow-up duration was 22.8 ± 32.9 months (range 0.5–144 months). One of the four patients in the infectious group died; of the three patients with presumed Listeria infection, all of whom were treated with antimicrobials, one recovered completely, the second had mild residual deficits, and the third was left with severe deficits. Rates of poor outcome and mortality were similar between the autoimmune/inflammatory and undefined groups (autoimmune/inflammatory group, poor outcome 18.5 %, mortality 5.6 %; undefined group, poor outcome 13 %, mortality 4.3 %).
We excluded the four patients in the infectious group for subsequent analyses on predictors of outcomes. In the multivariate logistic regression models, the odds of poor outcome were 1.1-fold higher for every 5 mg/dl greater CSF protein (per 5 mg/dL, OR = 1.10, 95 % CI:1.02–1.19) and 1.4-fold higher for every 5 mg/dl greater CSF glucose (per 5 mg/dl, OR = 1.36, 95 % CI: 1.09–1.71) (Table 4).
Table 4.
Multivariate regression analysis of factors associated with poor vs. good outcome in patients with brainstem encephalitis
| Odds ratio, unadjusted | Odds ratio, adjusted | |
|---|---|---|
| Age (per 5 year greater) | 1.13 | 1.16 |
| Male sex | 1.70 | 2.71 |
| CSF cells (per 50/mm3 more) | 1.03 | 0.96 |
| CSF protein (per 5 mg/dL higher) | 1.07* | 1.10* |
| CSF glucose (per 5 mg/dL higher) | 1.25* | 1.36* |
| Cerebral involvement | 1.60 | 1.93 |
p < 0.05
To further define the association between CSF glucose and poor outcome, we examined several additional variables, including blood glucose, history of diabetes, and exposure to corticosteroids. CSF glucose remained a predictor of poor outcome when adjusted for recent prior exposure to corticosteroids or concurrent treatment on the day of lumbar puncture (OR = 1.38, 95 % CI: 1.04–1.83 while steroid exposure did not appear to be meaningfully associated with outcome (OR = 0.33, 95 % CI: 0.03–4.21). Since CSF glucose is, in part, dependent upon serum glucose (correlation between the two in our study was 0.81), we substituted serum glucose for CSF glucose in our multivariate model and found that the odds of poor outcome were 1.3-fold higher for every 5 mg/dl increase of serum glucose (OR = 1.27, 95 % CI: 1.06–1.52). When history of diabetes mellitus was substituted for serum or CSF glucose measures, it was also associated with a poor outcome (OR = 11.25, 95 % CI: 1.23–102.77). Notably, when CSF glucose was added back into the model, the association of diabetes with a poor outcome was no longer apparent (OR = 1.66; 95 % CI: 0.01–28.08); however, CSF glucose was still associated with poor outcome (OR per 5 mg/dL higher = 1.33, 95 % CI 1.03–1.72). Age, gender, greater CSF pleocytosis, and the presence of supratentorial brain MRI lesions did not appear to be meaningfully associated with outcome, nor were the ORs for poor outcome associated with CSF protein and glucose levels meaningfully altered when diagnosis group (inflammatory/autoimmune vs. undefined) was added to multivariate models.
Discussion
The chief findings from this cross-sectional study include: (a) confirmed or probable inflammatory/autoimmune conditions accounted for the majority of BE cases; (b) higher CSF protein and glucose were independently associated with poor outcome; and (c) brain biopsy can prove useful in the establishment of a specific diagnosis and carries limited risks.
To our knowledge, this is one of the largest cohorts examining the diverse etiologies and presentations of BE. Our data reveal that a very high proportion of BE is due to confirmed or probable inflammatory/autoimmune causes, in contrast to cerebral encephalitis in which infections typically account for the majority of established etiologies [1]. Our findings are consistent with the limited available literature of case series or case reports of BE; there are several case series of BE describing specific inflammatory/immune-mediated processes, while only isolated case reports detailing infections, though this may merely reflect a publication bias [1–5]. Potential reasons for the high frequency of inflammatory cases in our group include the frequent involvement of the infratentorial compartment in acute inflammatory demyelinating syndromes such as ADEM [10]. Moreover, encephalitis associated with HSV, the most frequently identified agent of infectious encephalitis, typically affects the temporal and cingulate cortices with only rare involvement of the brainstem [11–13].
Several findings from our study suggest that the majority of patients with undefined cause of BE are also likely to have an autoimmune etiology. Overall, the clinical characteristics of the undefined group were highly similar to that of the inflammatory/autoimmune group (Table 2). In addition, an overwhelming majority of patients without an initial diagnosis who underwent brain biopsy demonstrated evidence of inflammatory changes without identified viral inclusions or microorganisms, including six in the undefined group, suggestive of an immune-mediated process. The one patient who had normal brain biopsy was subsequently found to have an immune-mediated condition. Importantly, among patients treated with immunosuppressive therapies and/or corticosteroids, the proportion that improved in the undefined group (79 %) was similar to that of the inflammatory/autoimmune group (82 %). Thus, the clinical characteristics, histopathological findings of inflammation, and favorable response to immunotherapy strongly suggest that most patients in the undefined group are likely to have inflammatory/autoimmune etiologies. Once infectious etiologies and neoplasm have been excluded, it may be useful to consider immunotherapy for BE patients in whom a specific diagnosis has not been identified. It is important to closely monitor these patients on immunosuppressive therapies, and any deterioration clinically or on neuroimaging should prompt a brain biopsy, to exclude infection or tumor.
Predictors of outcome following BE have not been previously reported. In our study, we found that elevated CSF protein and CSF glucose were associated with a poorer outcome. Elevated CSF protein may reflect a more vigorous inflammatory reaction, potentially resulting in greater cell injury and neurological deficits. We found that elevated CSF glucose was tightly associated with elevated serum glucose, suggesting that poor systemic glycemic control likely drives increased CSF glucose, and that both are predictors of poor outcome in patients with BE. These findings are reminiscent of findings in acute stroke patients, where elevated systemic glucose levels are associated with poor prognosis in diabetic and non-diabetic patients [14], and may provide rationale for aggressive glucose control in those with BE.
More broadly, to our knowledge there has been no study reporting an association of systemic or CSF glucose levels and outcomes in patients with encephalitis. In one study, only elevated CSF IgG index, but not CSF white cell count, CSF/serum glucose ratio or CSF/serum albumin quotient, predicted neurological morbidity in CNS infections, [15] while younger age at presentation, lower Glasgow coma scale, impaired oculocephalic response, and laboratory evidence of CNS viral infection were associated with poor outcome in encephalitis in another study [16]. In a recent study, factors associated with a poor outcome following infectious encephalitis included the presence of comorbid conditions and increasing age [17].
Brain biopsy was useful in identifying the etiologic agent of BE in our cohort. Notably, even when the results of brain biopsy were inconclusive or demonstrated nonspecific inflammation, such information was helpful as it led to initiation or escalation of immunotherapy in four of the six cases, and continuation of corticosteroid in the other two patients. Overall, management was altered in over half of the cases in which brain biopsy was performed, arguing for the utility of brain biopsy in carefully selected patients with BE. In our series, seven biopsies were performed on the brainstem itself, four of which resulted in a specific diagnosis. In one study that demonstrated the utility of brainstem biopsy in patients with brainstem lesions, 18 of 46 patients had their diagnoses revised following brainstem biopsy as compared to relying on diagnostic value of conventional MRI alone [18]. In addition, image-guided stereotactic biopsy of brainstem lesions has been reported to be as safe as supratentorial biopsy [19–21]. Only one of our patients who underwent brainstem biopsy developed new post-operative neurologic signs, and the mild dysarthria that ensued eventually resolved completely. Thus, biopsy of the brainstem itself yielded a specific diagnosis in over 50 % of cases and was quite safe in our cohort.
We identified a single case of CLIPPERS amongst the 81 patients with BE in our cohort. The clinical, radiological and pathological features of this syndrome were first described in eight patients, and the term coined in 2010 [22]. The prevalence of CLIPPERS is unknown. Our data suggest that CLIPPERS is an uncommon cause of BE.
Overall outcomes were favorable for patients with BE, similar to reported mortalities of 4.6–12 % in several large studies of encephalitis patients [1, 23–25]. Thus, encephalitis presenting with predominant brainstem involvement does not appear to result in increased mortality as compared to encephalitis as a whole. The mortality rate of our group was lower than that seen in a recent study of undiagnosed encephalitis patients, where the case fatality rate was 13 % [26]. Thus, although outcomes of BE are variable and can be devastating, the majority of our patients with inflammatory/autoimmune and undefined causes of BE recovered with favorable outcomes.
The proportion of undiagnosed BE cases has not been previously characterized. Despite extensive investigation, a specific etiologic agent was not identified in 30 % of our BE patients. This is comparable to studies of encephalitis in general. In all ten North American studies included in a recent meta-analysis of studies on acute encephalitis, the etiologic agent was not identified in at least 50 % of cases [27].
Limitations of this study include its retrospective nature. The accuracy of the clinical characteristics is dependent upon accurate documentation in the patients’ medical records. As a tertiary referral medical center, our patient population and findings are subject to a referral bias. Although sizeable numbers of patients in the inflammatory/autoimmune and the undefined groups allowed for direct comparison between these groups, data from the infectious group, which included only four patients, should be interpreted with care. Additionally, serological testing for neuromyelitis optica (NMO) antibodies, available only in the last few years, were not performed in many of the patients in the earlier years; thus we may have missed the diagnosis of NMO in this cohort. Also, the overall follow-up time was short, which may bias our results for poor outcome that may improve over time.
In summary, BE has distinct clinical characteristics and etiologies as compared to encephalitis in general. The majority of etiologies in our cohort were of non-infectious, inflammatory causes. Brain biopsy and histopathological diagnosis may play a role in the management of selected patients and can be useful in guiding treatment decisions. CSF protein and glucose may predict those who are at risk for poor outcome. In immunocompetent patients, an empiric trial of steroids and/or other immunosuppressive agents may be warranted when CNS infection and neoplastic/paraneoplastic disorders are excluded; these patients should be followed closely, and clinical or radiologic deterioration should prompt strong consideration of a brain biopsy.
Supplementary Material
Acknowledgments
Ellen M. Mowry: Funding NIH K23NS067055; National MS Society RG4407A2. Receipt of free study medication from Teva Neurological. Justin C. McArthur: Grants from NIH, Biogen-Idec, payment from lectures, speakers’ bureau in various universities, book royalties, stock option from Gliamed. Avindra Nath: Consultant to Biogen Idec and Diogenix. Arun Venkatesan: Grants from NIH, HHMI, Maryland Stem Cell Research Foundation, National Multiple Sclerosis Society. Ik Lin Tan, Sonya Steele, Carlos Pardo-Villamizar report no financial disclosure.
Footnotes
Conflicts of interest On behalf of all authors, the corresponding author states that there is no conflict of interest.
Electronic supplementary material The online version of this article (doi:10.1007/s00415-013-6986-z) contains supplementary material, which is available to authorized users.
Contributor Information
Ik Lin Tan, Johns Hopkins Encephalitis Center, Johns Hopkins University, School of Medicine, Baltimore, MD, USA; Department of Neurology, Johns Hopkins University School of, Medicine, Baltimore, MD 21287, USA.
Ellen M. Mowry, Department of Neurology, Johns Hopkins University School of, Medicine, Baltimore, MD 21287, USA
Sonya U. Steele, Department of Neurology, Johns Hopkins University School of, Medicine, Baltimore, MD 21287, USA
Carlos A. Pardo, Johns Hopkins Encephalitis Center, Johns Hopkins University, School of Medicine, Baltimore, MD, USA Department of Neurology, Johns Hopkins University School of, Medicine, Baltimore, MD 21287, USA.
Justin C. McArthur, Johns Hopkins Encephalitis Center, Johns Hopkins University, School of Medicine, Baltimore, MD, USA Department of Neurology, Johns Hopkins University School of, Medicine, Baltimore, MD 21287, USA.
Avindra Nath, Section of Infections of the Nervous System, National Institute, of Neurological Disorders and Stroke, National Institutes of, Health, Bethesda, MD, USA.
Arun Venkatesan, Email: avenkat2@jhmi.edu, Johns Hopkins Encephalitis Center, Johns Hopkins University, School of Medicine, Baltimore, MD, USA; Department of Neurology, Johns Hopkins University School of, Medicine, Baltimore, MD 21287, USA; Johns Hopkins Neuroimmunology and Neuroinfectious Diseases, 600 N. Wolfe St., Meyer 6-113, Baltimore, MD 21287, USA.
References
- 1.Glaser CA, Honarmand S, Anderson LJ, et al. Beyond viruses: clinical profiles and etiologies associated with encephalitis. Clin Infect Dis. 2006;43(12):1565–1577. doi: 10.1086/509330. [DOI] [PubMed] [Google Scholar]
- 2.Dalmau J, Graus F, Villarejo A, et al. Clinical analysis of anti-Ma2-associated encephalitis. Brain. 2004;127(Pt 8):1831–1844. doi: 10.1093/brain/awh203. [DOI] [PubMed] [Google Scholar]
- 3.Ito M, Kuwabara S, Odaka M, et al. Bickerstaff’s brainstem encephalitis and Fisher syndrome form a continuous spectrum: clinical analysis of 581 cases. J Neurol. 2008;255(5):674–682. doi: 10.1007/s00415-008-0775-0. [DOI] [PubMed] [Google Scholar]
- 4.Mylonakis E, Hohmann EL, Calderwood SB. Central nervous system infection with Listeria monocytogenes. 33 years’ experience at a general hospital and review of 776 episodes from the literature. Medicine (Baltimore) 1998;77(5):313–336. doi: 10.1097/00005792-199809000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Moragas M, Martínez-Yélamos S, Majós C, Fernández-Viladrich P, Rubio F, Arbizu T. Rhombencephalitis: a series of 97 patients. Medicine (Baltimore) 2011;90(4):256–261. doi: 10.1097/MD.0b013e318224b5af. [DOI] [PubMed] [Google Scholar]
- 6.Krupp LB, Banwell B, Tenembaum S, Group IPMS Consensus definitions proposed for pediatric multiple sclerosis and related disorders. Neurology. 2007;68(16 Suppl 2):S7–12. doi: 10.1212/01.wnl.0000259422.44235.a8. [DOI] [PubMed] [Google Scholar]
- 7.Krupp LB, Tardieu M, Amato MP, et al. International pediatric multiple sclerosis study group criteria for pediatric multiple sclerosis and immune-mediated central nervous system demyelinating disorders: revisions to the 2007 definitions. Mult Scler. 2013 doi: 10.1177/1352458513484547. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 8.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69(2):292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sejvar JJ, Kohl KS, Bilynsky R, et al. Encephalitis, myelitis, and acute disseminated encephalomyelitis (ADEM): case definitions and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine. 2007;25(31):5771–5792. doi: 10.1016/j.vaccine.2007.04.060. [DOI] [PubMed] [Google Scholar]
- 10.Schwarz S, Mohr A, Knauth M, Wildemann B, Storch-Hagenlocher B. Acute disseminated encephalomyelitis: a followup study of 40 adult patients. Neurology. 2001;56(10):1313–1318. doi: 10.1212/wnl.56.10.1313. [DOI] [PubMed] [Google Scholar]
- 11.Domingues RB, Fink MC, Tsanaclis AM, et al. Diagnosis of herpes simplex encephalitis by magnetic resonance imaging and polymerase chain reaction assay of cerebrospinal fluid. J Neurol Sci. 1998;157(2):148–153. doi: 10.1016/s0022-510x(98)00069-0. [DOI] [PubMed] [Google Scholar]
- 12.Tan IL, McArthur JC, Venkatesan A, Nath A. Atypical manifestations and poor outcome of herpes simplex encephalitis in the immunocompromised. Neurology. 2012;79(21):2125–2132. doi: 10.1212/WNL.0b013e3182752ceb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wasay M, Mekan SF, Khelaeni B, et al. Extra temporal involvement in herpes simplex encephalitis. Eur J Neurol. 2005;12(6):475–479. doi: 10.1111/j.1468-1331.2005.00999.x. [DOI] [PubMed] [Google Scholar]
- 14.Capes SE, Hunt D, Malmberg K, Pathak P, Gerstein HC. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview. Stroke. 2001;32(10):2426–2432. doi: 10.1161/hs1001.096194. [DOI] [PubMed] [Google Scholar]
- 15.Lackner P, Guengoer E, Beer R, et al. IgG-index predicts neurological morbidity in patients with infectious central nervous system diseases. BMC Infect Dis. 2010;10:202. doi: 10.1186/1471-2334-10-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kennedy CR, Duffy SW, Smith R, Robinson RO. Clinical predictors of outcome in encephalitis. Arch Dis Child. 1987;62(11):1156–1162. doi: 10.1136/adc.62.11.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mailles A, De Broucker T, Costanzo P, et al. Long-term outcome of patients presenting with acute infectious encephalitis of various causes in France. Clin Infect Dis. 2012;54(10):1455–1464. doi: 10.1093/cid/cis226. [DOI] [PubMed] [Google Scholar]
- 18.Rachinger W, Grau S, Holtmannspotter M, Herms J, Tonn JC, Kreth FW. Serial stereotactic biopsy of brainstem lesions in adults improves diagnostic accuracy compared with MRI only. J Neurol Neurosurg Psychiatry. 2009;80(10):1134–1139. doi: 10.1136/jnnp.2009.174250. [DOI] [PubMed] [Google Scholar]
- 19.Fontaine D, Dormont D, Hasboun D, et al. Magnetic resonance-guided stereotactic biopsies: results in 100 consecutive cases. Acta Neurochir (Wien) 2000;142(3):249–255. doi: 10.1007/s007010050032. (discussion 255–246) [DOI] [PubMed] [Google Scholar]
- 20.Apuzzo ML, Sabshin JK. Computed tomographic guidance stereotaxis in the management of intracranial mass lesions. Neurosurgery. 1983;12(3):277–285. doi: 10.1227/00006123-198303000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Samadani U, Judy KD. Stereotactic brainstem biopsy is indicated for the diagnosis of a vast array of brainstem pathology. Stereotact Funct Neurosurg. 2003;81(1–4):5–9. doi: 10.1159/000075097. [DOI] [PubMed] [Google Scholar]
- 22.Pittock SJ, Debruyne J, Krecke KN, et al. Chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids (CLIPPERS) Brain. 2010;133(9):2626–2634. doi: 10.1093/brain/awq164. [DOI] [PubMed] [Google Scholar]
- 23.Granerod J, Ambrose HE, Davies NW, et al. Causes of encephalitis and differences in their clinical presentations in England: a multicentre, population-based prospective study. Lancet Infect Dis. 2010;10(12):835–844. doi: 10.1016/S1473-3099(10)70222-X. [DOI] [PubMed] [Google Scholar]
- 24.Huppatz C, Durrheim DN, Levi C, et al. Etiology of encephalitis in Australia, 1990–2007. Emerg Infect Dis. 2009;15(9):1359–1365. doi: 10.3201/eid1509.081540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mailles A, Stahl JP. Infectious encephalitis in france in 2007: a national prospective study. Clin Infect Dis. 2009;49(12):1838–1847. doi: 10.1086/648419. [DOI] [PubMed] [Google Scholar]
- 26.Schmidt A, Buhler R, Muhlemann K, Hess CW, Tauber MG. Long-term outcome of acute encephalitis of unknown aetiology in adults. Clin Microbiol Infect. 2011;17(4):621–626. doi: 10.1111/j.1469-0691.2010.03276.x. [DOI] [PubMed] [Google Scholar]
- 27.Granerod J, Tam CC, Crowcroft NS, Davies NW, Borchert M, Thomas SL. Challenge of the unknown. A systematic review of acute encephalitis in non-outbreak situations. Neurology. 2010;75(10):924–932. doi: 10.1212/WNL.0b013e3181f11d65. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


