Abstract
Purpose
To determine the risk factors associated with progression to blindness from primary open-angle glaucoma (POAG) in an African-American population.
Methods
This study examined 2119 patients enrolled in the Primary Open-Angle African-American Glaucoma Genetics (POAAGG) study. A total of 59 eyes were identified as legally blind as a result of POAG (cases) and were age-and sex-matched to 59 non-blind eyes with glaucoma (controls). Chart reviews were performed to record known and suspected risk factors.
Results
Cases were diagnosed with POAG at an earlier age than controls (p = 0.005). Of the 59 eyes of cases, 16 eyes (27.1%) presented with blindness at diagnosis. Cases had worse visual acuity (VA) at diagnosis (p < 0.0001), with VA worse than 20/40 conferring a 27 times higher risk of progression to blindness (p = 0.0005). Blind eyes also demonstrated more visual field defects (p = 0.01), higher pretreatment intraocular pressure (IOP; p < 0.0001), and higher cup-to-disc ratio (p = 0.006) at diagnosis. IOP was less controlled in cases, and those with IOP ≥21 mmHg at more than 20% of follow-up visits were 73 times more likely to become blind (p < 0.0001). Cases missed a greater number of appointments per year (p = 0.003) and had non-adherence issues noted in their charts more often than controls (p = 0.03). However, other compliance data did not significantly differ between groups.
Conclusion
Access to care, initial VA worse than 20/40, and poor control of IOP were the major risk factors associated with blindness from POAG. Future studies should examine earlier, more effective approaches to glaucoma screening as well as the role of genetics in these significantly younger patients who progress to blindness.
Keywords: African, African-Americans, blindness, glaucoma, minority research, primary open-angle glaucoma
Introduction
Primary open-angle glaucoma (POAG) is the leading cause of irreversible blindness worldwide, affecting nearly 70 million people.1 African-Americans are disproportionately affected by POAG, with disease rates 5–6 times higher than those in Caucasians.2 African-Americans also reach adverse endpoints more frequently, including worse visual fields and optic disc cupping,3–7 blindness,8–10 vision-related decrease in quality of life,11–16 and increased mortality.17,18
The cumulative rate of glaucoma-related blindness has been reported to be between 19% and 27% in one eye, and 9% in both eyes.19,20 Elevated intraocular pressure (IOP), less formal education, and more treatment interventions increase the risk of POAG progression,20,21 with greater inter-visit IOP fluctuations and older age at diagnosis being the most consistent predictors of blindness.22 However, there is little consensus on which risk factors account for the increased risk of blindness in African-Americans.2,20
Several clinical trials reported minimal difference in outcome when whites and blacks were provided with equal access to care, suggesting that socio-cultural factors influence POAG progression in African-Americans.20 Another report concurred that African-Americans have less access to examinations and eye care, are less aware of the risks of POAG, and have lower rates of adherence to care recommendations than Caucasians.20,23 However, other studies argue in favor of a biological predisposition to POAG progression. Tielsch and colleagues showed that even when whites and blacks both saw an eye care professional and received treatment, blacks still had increased prevalence of glaucomatous optic nerve damage.2 Overall, the data available for evaluating risk factors for POAG progression remain sparse and inconsistent, especially when concerning the underserved and over-affected African-American population.
This study investigates the risk factors associated with progression to blindness as a result of POAG in the African-American population. We aim to uncover the root causes of this outcome and identify modifiable factors for prevention.
Materials and methods
Study population
This study was a case-control study of a subset of patients enrolled in the Primary Open-Angle African-American Glaucoma Genetics (POAAGG) study at the University of Pennsylvania Health System (UPHS) in Philadelphia, Pennsylvania, and surrounding communities. At the time of this study, the database included 2119 well-characterized patients with comprehensive ophthalmologic histories from UPHS. Additional details about the study design, diagnosis of POAG, and baseline demographics of our population were reported previously.24
Patients eligible for inclusion in the study were over 35 years old, self-identified as African-American, and were diagnosed and treated for POAG exclusively within UPHS. Exclusion criteria included a history of narrow angle glaucoma, closed angle glaucoma, neovascular glaucoma, mixed mechanism glaucoma or plateau iris syndrome, or pseudoexfoliation glaucoma; history of glaucoma secondary to eye surgery or secondary to severe ocular trauma; history of iritis, uveitis, or iridocyclitis; presence of Graves disease with ocular manifestations, optic nerve atrophy from other causes, or advanced proliferative diabetic retinopathy resulting in visual field changes. Each patient and their medical record were reviewed by fellowship-trained glaucoma specialists, and only patients whose blindness was caused primarily by POAG were included in this investigation. The study adhered to the tenets of the Declaration of Helsinki and was approved by the University of Pennsylvania Institutional Review Board. Informed consent was obtained from all participants.
Definition of blindness from glaucoma
Blindness was defined as visual acuity (VA) 20/200 or worse as measured by a Snellen chart.25 Glaucoma specialists screened all patients with VA 20/200 or worse and excluded those who did not meet this criterion.
Classification of glaucoma
Glaucoma was defined as characteristic glaucomatous optic nerve damage diagnosed by fellowship-trained glaucoma specialists and corresponding visual field loss consistent with glaucoma with or without elevated IOP (≥21 mmHg).26
Case-control analysis
Cases were identified as eyes that were blind as a result of POAG. Best-corrected VA (BCVA) measurements were obtained from all eyes in the POAAGG study database (N = 2119) from their most recent visit. Records of all those with VA 20/200 or worse were reviewed to identify those who qualified for the study (59 eyes from 48 subjects). The majority of potential cases were excluded, due to prior treatment for POAG outside UPHS, thereby eliminating unknown variations in care. Overall, 37 patients were unilaterally blind from POAG and 11 patients were bilaterally blind from POAG.
Controls were identified as eyes with POAG which maintained VA better than 20/200 at the time of their most recent visit. Each case was age- and sex-matched to a control randomly selected from the database.
Demographics
Patients who self-identified as widowed, divorced, separated, or other were classified as single. The 2007–2011 American Community Survey 5-year estimates were used to identify mean household income in each patient’s postal code.27
Clinical information
Clinical variables were recorded at diagnosis, throughout treatment, and at study endpoint (Tables 1 and 2). The study endpoint was defined as the date the patient went blind (cases) or the date of the most recent visit (controls). Eyes blind at diagnosis were excluded from analysis at study endpoint (Tables 2 and 3), analysis throughout treatment (Table 4), and risk factors analysis for progression to blindness (Table 5) because they could not contribute meaningful data.
Table 1.
Demographic and clinical characteristics of glaucoma cases in the Primary Open-Angle African-American Glaucoma Genetics study, United States.
| Characteristic | Blind | Non-blind | p-valuea |
|---|---|---|---|
| Subjects (eyes), n | 48 (59)b | 59 (59) | |
| Subject-level characteristics | |||
| Age at end of study, mean ± SD, years | 74.8 ± 12.1 | 75.3 ± 11.8 | 0.32 |
| Sex, n (%) | 1.00 | ||
| Male | 26 (54.2) | 33 (55.9) | |
| Female | 22 (45.8) | 26 (44.1) | |
| Family history of glaucoma in 1st degree relative, n (%) | 15 (31.3) | 26 (44.1) | 0.38 |
| Annual household income, mean ± SD, US$ | 33,000 ± 11,000 | 34,000 ± 14,000 | 0.99 |
| Marital status, n (%) | |||
| Single | 30 (62.5) | 29 (49.2) | 0.24 |
| Married | 18 (37.5) | 30 (50.9) | |
| Comorbidities during the study, n (%) | |||
| Cardiovascular disease | 8 (16.7) | 15 (25.4) | 0.20 |
| Hypertension | 37 (77.1) | 49 (83.1) | 0.17 |
| Diabetes | 14 (29.2) | 26 (44.1) | 0.18 |
| Thyroid disease (hypo/hyper) | 1 (2.1) | 4 (6.8) | 0.18 |
| Sleep apnea | 4 (8.3) | 5 (8.5) | 0.71 |
| Eye-level characteristics | |||
| Central corneal thickness during study, μm mean ± SD | 519.0 ± 37.3 | 523.0 ± 36.4 | 0.46 |
| Myopia, n (%) | 24 (40.7) | 27 (45.8) | 0.47 |
| Cataract, n (%) | 30 (50.9) | 35 (59.3) | 0.40 |
| Classification of glaucoma, n (%) | |||
| Classic glaucoma | 49 (83.1) | 49 (83.1) | 1.00 |
| Treated ocular hypertension | 10 (16.9) | 10 (16.9) |
Paired t-test for comparing means, McNemar’s test for comparing proportions, Wilcoxon signed rank test comparing medians for subject-level characteristics, and generalized estimating equations for eye-specific characteristics.
Eleven blind subjects contributed data from both eyes to the study. SD, standard deviation.
Table 2.
Characteristics of participants with primary open-angle glaucoma at diagnosis (107 subjects, 118 eyes) and at study endpoint (96 subjects, 102 eyes), Primary Open-Angle African-American Glaucoma Genetics study, United States.
| Characteristics at diagnosis of glaucoma | Diagnosis
|
Study endpoint
|
||||
|---|---|---|---|---|---|---|
| Blind | Non-blind | p-valuea | Blindb | Non-blind | p-valuea | |
| Subjects (eyes), n | 48 (59) | 59 (59) | 37 (43) | 59 (59) | ||
| Eyes presenting blind, n (%) | 16 (27.1) | 0 (0) | – | – | – | – |
| Eyes blind within 1 month of diagnosis, n (%) | 19 (32.2) | 0 (0) | – | – | – | – |
| Age at diagnosis, years mean ± SD | 62.2 ± 13.3 | 66.7 ± 10.5 | 0.005 | – | – | – |
| Cup-to-disc ratio at diagnosis, mean ± SDc | 0.81 ± 0.23 | 0.68 ± 0.15 | 0.006 | – | – | – |
| Medications started, n, mean ± SD | 1.19 ± 0.39 | 0.98 ± 0.13 | 0.0003 | – | – | – |
| BCVA, logMAR, mean ± SD | 0.56 ± 0.89 | 0.04 ± 0.10 | <0.0001 | 1.58 ± 0.82 | 0.13 ± 0.16 | <0.0001 |
| BCVA, median (minimum, maximum) Snellen | 20/80 (NLP, 20/20) | 20/20 (CF at face, 20/15) | <0.0001 | 20/400 (NLP, 20/200) | 20/25 (20/100, 20/20) | <0.0001 |
| Category of BCVA, n (%) | ||||||
| Normal (VA≤20/40) | 30 (50.9) | 56 (94.9) | <0.0001 | 0 (0) | 54 (91.5) | – |
| Mild (VA >20/40 but ≤20/70) | 6 (10.2) | 1 (1.7) | – | 0 (0) | 3 (5.1) | – |
| Moderate (VA >20/70 but ≤20/100) | 5 (8.5) | 0 (0) | – | 0 (0) | 2 (3.4) | – |
| Severe (VA >20/100 but <20/200) | 0 (0) | 0 (0) | – | 0 (0) | 0 (0) | – |
| Blind (VA ≥20/200)b | 18 (30.5) | 2 (3.4) | – | 55 (100) | 0 (0) | – |
| IOP, n (%) | ||||||
| <13 mmHg | 0 (0) | 6 (10.2) | 0.005 | 6 (14.0) | 19 (32.2) | 0.008 |
| ≥13 to ≤16 mmHg | 3 (5.1) | 8 (13.6) | – | 16 (37.2) | 32 (54.2) | – |
| >16 to <21 mmHg | 5 (8.5) | 14 (23.7) | – | 6 (14.0) | 4 (6.8) | – |
| ≥21 mmHg | 51 (86.4) | 31 (52.5) | – | 15 (34.9) | 4 (6.8) | – |
| IOP, mmHg, mean ± SD | 29.4 ± 9.0 | 20.7 ± 5.5 | <0.0001 | 19.4 ± 8.1 | 14.0 ± 3.7 | 0.0001 |
| Treatment at final visit, n (%) | ||||||
| Medication | – | – | – | 41(69.5) | 57 (96.6) | 0.0002 |
| Laser | – | – | – | 19 (32.2) | 14 (23.7) | 0.35 |
| Surgery | – | – | – | 9 (15.3) | 5 (8.5) | 0.29 |
| Medications at study | – | – | – | 2.02 ± 1.10 | 1.59 ± 0.81 | 0.02 |
| endpoint, n, mean ± SD | ||||||
| Glaucoma lasers during study period, n, mean ± SD | – | – | – | 0.60 ± 0.79 | 0.32 ± 0.63 | 0.04 |
| Glaucoma surgeries during study period, n, mean ± SD | – | – | – | 0.28 ± 0.70 | 0.10 ± 0.36 | 0.13 |
| Treatment changes during study period, n, mean ± SD | – | – | – | 10.80 ± 10.20 | 3.76 ± 4.67 | 0.0004 |
Paired t-test for comparing means, McNemar’s test for comparing proportions for subject-level characteristics, and generalized estimating equations for eye-specific characteristics.
Two blind eyes and two non-blind eyes were only temporarily blind from a different etiology (blind, refractive error and multifactorial; non-blind, cataract and macular edema).
A total of 55 blind eyes and 49 non-blind eyes had available data on cup-to-disc ratio.
BCVA, best-corrected visual acuity; SD, standard deviation; logMAR, logarithm of the minimum angle of resolution; NLP, no light perception; CF, count fingers; VA visual acuity; IOP, intraocular pressure.
Table 3.
Comparison of visual field data at diagnosis (107 subjects, 118 eyes) and study endpoint (96 subjects, 102 eyes) between blind eyes and non-blind eyes, Primary Open-Angle African-American Glaucoma Genetics study, United States.
| Characteristics at diagnosis of glaucoma | Diagnosis
|
Study endpoint
|
||||
|---|---|---|---|---|---|---|
| Blind | Non-blind | p-valuea | Blind | Non-blind | p-valuea | |
| Eyes, n | 59 | 59 | 43b | 59 | ||
| Type of visual field machine used, n (%) | ||||||
| Humphrey | 23 (39.0) | 44 (74.6) | 0.003 | 25 (58.1) | 33 (55.9) | <0.0001 |
| Goldman | 23 (39.0) | 12 (20.3) | 14 (32.6) | 2 (3.4) | ||
| Octopus | 0 (0) | 2 (3.4) | 0 (0) | 23 (39.0) | ||
| Unknownc | 13 (22.0) | 1 (1.7) | 4 (9.3) | 1 (1.7) | ||
| Global indices | ||||||
| PSD, n | 22 | (45 | 24 | 56 | ||
| Mean (SD) dB | 6.41 (3.23) | 4.00 (2.90) | 0.003 | 7.69 (3.50) | 4.77 (3.18) | 0.0005 |
| Median (min, max) dB | 6.19 (1.56, 12.80) | 2.72 (1.24, 12.00) | 0.003 | 7.81 (2.04, 13.60) | 3.65 (1.14, 14.90) | 0.0008 |
| Mean deviation, n | 22 | 43 | 24 | 56 | ||
| Mean (SD) dB | −14.0 (13.1) | −5.1 (7.71) | 0.006 | −19.9 (8.8) | −6.9 (7.0) | <0.0001 |
| Median (min, max) dB | −15.5 (−32.0, 22.0) | −2.1 (−29.0, 1.7) | 0.0003 | −21.0 (−32.0, −0.5) | −4.7 (−29.0, 2.3) | <0.0001 |
| Visual field interpretation, n (%) | ||||||
| Lens rim artifact | 0 (0) | 3 (5.1) | 0.25 | 0 (0) | 2 (3.4) | 0.51 |
| Cloverleaf artifact | 2 (3.4) | 0 (0) | 0.19 | 1 (2.3) | 1 (1.7) | 1.00 |
| Lid artifact | 2 (3.4) | 1 (1.7) | 0.58 | 0 (0) | 2 (3.4) | 0.52 |
| Any arcuate defect | 27 (45.8) | 21 (35.6) | 0.028 | 32 (74.4) | 30 (50.9) | 0.001 |
| Arcuate defect category | 0.006 | <0.0001 | ||||
| Superior only | 6 (10.2) | 9 (15.3) | 3 (7.0) | 12 (20.3) | ||
| Inferior only | 3 (5.1) | 6 (10.2) | 5 (11.6) | 8 (13.6) | ||
| Both superior and inferior | 18 (30.5) | 6 (10.2) | 24 (55.8) | 10 (17.0) | ||
| Neither superior nor inferior | 18 (30.5) | 36 (61.0) | 6 (14.0) | 28 (47.5) | ||
| Unknown | 14 (23.7) | 2 (3.4) | 5 (11.6) | 1 (1.7) | ||
| Any nasal step | 13 (22.0) | 12 (20.3) | 0.49 | 8 (18.6) | 14 (23.7) | 0.81 |
| Nasal step category | 0.35 | 0.24 | ||||
| Superior only | 5 (8.5) | 6 (10.2) | 5 (11.6) | 4 (6.8) | ||
| Inferior only | 7 (11.9) | 3 (5.1) | 3 (7.0) | 5 (8.5) | ||
| Both superior and inferior | 1 (1.7) | 3 (5.1) | 0 (0) | 5 (8.5) | ||
| Neither superior nor inferior | 32 (54.2) | 45 (76.3) | 30 (70.0) | 44 (74.6) | ||
| Unknown | 14 (23.7) | 2 (3.4) | 5 (11.6) | 1 (1.7) | ||
| Any nasal depression | 1 (1.7) | 1 (1.7) | 1.00 | 2 (4.7) | 1 (1.7) | 0.56 |
| Nasal depression category | 1.00 | 0.15 | ||||
| Superior only | 1 (1.7) | 1 (1.7) | 2 (4.7) | 0 (0) | ||
| Inferior only | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| Both superior and inferior | 0 (0) | 0 (0) | 0 (0) | 1 (1.7) | ||
| Neither superior nor inferior | 44 (74.6) | 56 (94.9) | 36 (83.7) | 57 (96.6) | ||
| Unknown | 14 (23.7) | 2 (3.4) | 5 (11.6) | 1 (1.7) | ||
| Any temporal wedge: | 0 (0) | 1 (1.7) | 1.00 | 1 (2.3) | 1 (1.7) | 1.00 |
| Temporal wedge category | 1.00 | 0.65 | ||||
| Superior only | 0 (0) | 0 (0) | 1 (2.3) | 0 (0) | ||
| Inferior only | 0 (0) | 1 (1.7) | 0 (0) | 0 (0) | ||
| Both superior and inferior | 0 (0) | 0 (0) | 0 (0) | 1 (1.7) | ||
| Neither superior nor inferior | 44 (74.6) | 56 (94.9) | 37 (86.1) | 55 (93.2) | ||
| Unknown | 15 (25.4) | 2 (3.4) | 5 (11.6) | 3 (5.1) | ||
| Any paracentral scotoma | 5 (8.5) | 1 (1.7) | 0.08 | 10 (23.3) | 5 (8.5) | 0.042 |
| Paracentral scotoma category | 0.16 | 0.12 | ||||
| Superior only | 2 (3.4) | 1 (1.7) | 5 (11.6) | 2 (3.4) | ||
| Inferior only | 1 (1.7) | 0 (0) | 2 (4.7) | 1 (1.7) | ||
| Both superior and inferior | 2 (3.4) | 0 (0) | 3 (7.0) | 2 (3.4) | ||
| Neither superior nor inferior | 39 (66.1) | 56 (94.9) | 28 (65.1) | 52 (88.1) | ||
| Unknown | 15 (25.4) | 2 (3.4) | 5 (11.6) | 2 (3.4) | ||
| Any of above visual field interpretation | 35 (59.3) | 31 (52.5) | 0.011 | 37 (86.1) | 41 (69.5) | 0.002 |
Paired t-test for comparing means, McNemar’s test for comparing proportions for subject-level characteristics, and generalized estimating equations for eye-specific characteristics.
A total of 16 eyes presenting blind at diagnosis not included in analysis as they were not able to contribute meaningful data.
No visual field data available.
PSD, pattern standard deviation; SD, standard deviation.
Table 4.
Adherence, IOP, and visual field progression throughout treatment, Primary Open-Angle African-American Glaucoma Genetics study, United States.
| Characteristic | Blind | Non-blinda | p-valueb |
|---|---|---|---|
| Subjects (eyes), n | 34 (40)a | 58 (58)a | |
| Length of follow-up, mean ± SD years | 9.51 ± 8.24 | 8.24 ± 5.97 | 0.43 |
| Visits/year, mean ± SD, n | 5.58 ± 3.20 | 3.97 ± 3.76 | 0.11 |
| Missed visits/year, mean ± SD, n | 2.32 ± 2.02 | 1.18 ± 0.75 | 0.003 |
| Visits missed, mean ± SD, n | 29.0 ± 14.9 | 24.9 ± 14.3 | 0.53 |
| Non-adherence to treatment noted in chart, mean ± SD, % | 19.3 ± 19.3 | 10.7 ± 13.2 | 0.03 |
| IOP maximum, mean ± SD, mmHg | 32.9 ± 10.7 | 22.6 ± 7.0 | <0.0001 |
| IOP minimum, mean ± SD, mmHg | 10.2 ± 5.2 | 10.8 ± 2.8 | 0.61 |
| Average IOP throughout treatment, mean ± SD, mmHg | 18.9 ± 4.9 | 15.5 ± 3.1 | 0.0001 |
| Visits with elevated IOP (≥21 mmHg), mean ± SD, % | 33.9 ± 28.3 | 10.9 ± 17.7 | <0.0001 |
| IOP standard deviation, mean ± SD, mmHg | 5.03 ± 2.60 | 2.77 ± 1.37 | <0.0001 |
| IOP relative standard deviation, mean ± SD, % | 27.6 ± 17.2 | 17.4 ± 6.4 | 0.001 |
| Mean decrease in IOP from pre- treatment, mean ± SD, mmHg | 8.99 ± 7.88 | 5.25 ± 3.98 | 0.002 |
| Eyes, n | 59 | 59 | |
| Number of visits with reliable visual field, n (%) | |||
| 1 | 11 (18.6) | 5 (8.5) | <0.0001 |
| 2 | 3 (5.1) | 6 (10.2) | |
| 3 | 25 (42.4) | 13 (22.0) | |
| 4 | 7 (11.9) | 35 (59.3) | |
| PSD progression rate, mean (SE), dB/year | 0.11 (0.06) | 0.01 (0.04) | 0.004 |
| Mean deviation progression rate, mean (SE), dB/year | −0.25 (0.19) | 0.05 (0.13) | <0.0001 |
Those who presented blind (16 blind eyes) and those who had <2 followup visits (3 blind eyes, 1 non-blind eye) were not included in this analysis as they were not able to contribute any meaningful data to these variables. Exceptions were length of follow-up and mean decrease in IOP from pre-treatment; for these two variables, data from those presenting blind were still excluded (16 blind eyes), but data from those with <2 follow-up visits were included.
Paired t-test for comparing means, McNemar’s test for comparing proportions for subject-level characteristics, and generalized estimating equations for eye-specific characteristics.
SD, standard deviation; SE, standard error; IOP, intraocular pressure; PSD, pattern standard deviation.
Table 5.
Multivariate analysis using backward selectiona to assess risk factors for blindness (96 subjects, 102 eyes), Primary Open-Angle African-American Glaucoma Genetics study, United States.
| N | n (%) | Odds ratio (95% CI) | p-valueb | |
|---|---|---|---|---|
| Best-corrected visual acuity at diagnosis (Snellen) | 0.002 | |||
| 20/20 or better | 55 | 13 (23.6) | 1 (reference) | – |
| 20/25 to 20/40 | 25 | 11 (44.0) | 1.82 (0.44–7.53) | 0.41 |
| Worse than 20/40 | 22 | 19 (86.4) | 27.10 (4.21–174.00) | 0.0005 |
| Missed visits/year, n | 0.001 | |||
| 0–1 | 31 | 7 (22.6) | 1 (reference) | – |
| >1–2 | 36 | 10 (27.8) | 1.23 (0.26–5.88) | 0.79 |
| >2 | 26 | 18 (69.2) | 12.40 (2.32–66.30) | 0.003 |
| Unknownc | 9 | 8 (88.9) | 64.30 (12.40–335.00) | <0.0001 |
| Visits with elevated IOP (≥21 mmHg), % | 0.0002 | |||
| 0 | 32 | 4 (12.5) | 1 (reference) | – |
| >0–20 | 36 | 17 (47.2) | 25.30 (5.46–117.00) | <0.0001 |
| >20 | 34 | 22 (64.7) | 72.60 (15.30–344.00) | <0.0001 |
Initial model included diabetes, age at diagnosis, best-corrected visual acuity category at diagnosis, pre-treatment IOP, initial number of medications started, number of medications at study endpoint, number of glaucoma lasers during study period, number of treatment changes, number of missed appointments/year, percent of times non-adherence noted in chart, percent of visits with elevated IOP, average IOP throughout treatment, and IOP standard deviation.
From generalized linear model using generalized estimating equation to account for correlations from matching and paired eyes of a subject.
Unknown category represents patients whose follow-up predated records for missed appointments (prior to 2000) and from cases who presented blind.
CI, confidence interval; IOP, intraocular pressure.
Visual acuity
BCVA measurements at distance were recorded at diagnosis and end point. All data was converted to Snellen equivalent, including count fingers and hand motion. Five categories of VA loss were defined based on BCVA; normal (BCVA 20/40 or better), mild (BCVA >20/40 but ≤20/70), moderate (BCVA >20/70 but ≤20/100), severe (BCVA >20/100 but <20/200), and blind (BCVA 20/200 or worse).
Visual fields
Humphrey, Goldmann, or Octopus visual fields were recorded for patients at diagnosis and the study endpoint. All visual fields and disc photographs were reviewed by two glaucoma specialists at the University of Pennsylvania. Patients whose visual fields failed to meet minimum performance metrics were excluded from the study. A total of 89 of the 118 eyes had a graded visual field within 1 year of diagnosis, and 68 of 118 eyes had a graded visual field within 1 year of the end date. If a graded visual field did not exist within 1 year of the start or end date, the closest possible date was used.
Intraocular pressure
IOP measurements were recorded at every visit over the study period. Subjects who presented blind at diagnosis only contributed data to the pre-treatment IOP variable. Subjects with <2 follow-up visits before the study endpoint only contributed data to the pretreatment IOP, IOP at final visit, and mean decrease in IOP from pre-treatment variables.
Non-adherence
We measured non-adherence to treatment through four variables; percent of appointments with non-adherence issues noted in patient charts, mean number of visits per year, mean number of missed appointments per year, and percent of appointments missed per year. Non-adherence issues in patient charts were defined as lack of medication compliance or refusal of recommendations. Missed appointments were defined as appointments where the patient cancelled or did not appear for a scheduled visit.
Statistical analysis
Statistical analysis was conducted per-subject for subject-specific measurements and per-eye for eye-specific measurements. For the subject-specific measurements, the comparison of characteristics between cases and matched controls was performed using the paired t-test for comparing means, Wilcoxon signed rank test for comparing medians, and McNemar’s test for comparing proportions. For eye-specific measurements, generalized estimating equations were used to account for correlations from matching and from paired eyes of a patient.28 To identify risk factors for blindness, univariate logistic regression models were first performed, followed by a multivariate logistic regression model that included risk factors with p < 0.10 from the univariate analysis. The multivariate logistic model went through backward model selection to reach the final model that only included risk factors at p < 0.05. In these logistic regression analyses, correlations from matching and from paired eyes of a subject were accounted for using generalized estimating equations.28 All statistical analyses were performed using SAS version 9.2 (SAS Institute Inc, Cary, NC, USA).
Results
Demographics and clinical information
Age at study endpoint, family history of POAG in a first degree relative, annual household income, marital status, and prevalence of comorbidities did not significantly differ between cases and controls (Table 1).
Characteristics of primary open-angle glaucoma at diagnosis and study endpoint
Overall, 16 case eyes (27.1%) presented blind at diagnosis and an additional three eyes (5.1%) progressed to blindness within 1 month (Table 2). Cases were diagnosed with POAG at an earlier age than controls (62.2 ± 13.3 years vs 66.7 ± 10.5 years, p = 0.005).
The median BCVA of cases was worse than that of controls at diagnosis (20/80 vs 20/20, p < 0.0001). Cases also had higher mean pre-treatment IOP (29.4 ± 9.0 mmHg vs 20.7 ± 5.5 mmHg, p < 0.0001), higher cup to disc ratio (0.81 ± 0.23 vs 0.68 ± 0.15, p = 0.006), and were prescribed more ocular medications than controls at diagnosis (1.19 ± 0.39 vs 0.98 ± 0.13, p = 0.0003).
At the study endpoint, cases had worse median BCVA (20/400 vs 20/25, p < 0.0001; Table 2) and higher IOP (19.4 ± 8.1 mmHg vs 14.0 ± 3.7 mmHg, p = 0.0001; Figure 1) than controls. Cases underwent more treatment changes on average during the study period than controls (10.8 ± 10.2 vs 3.8 ± 4.7, p = 0.0004).
Figure 1.
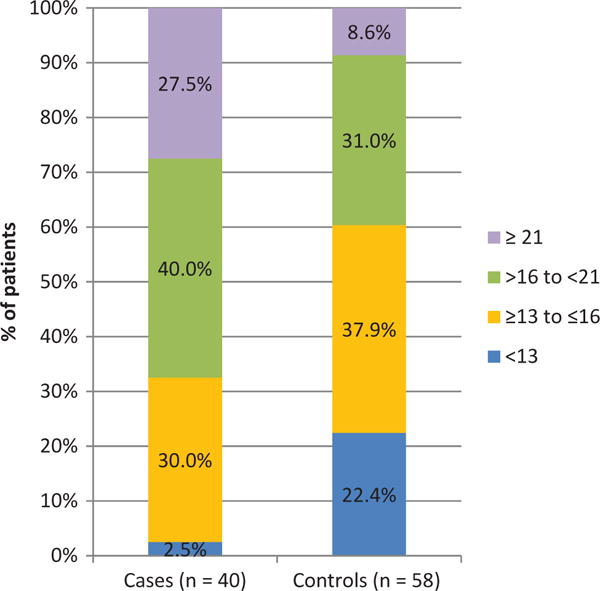
Intraocular pressure (mmHg) at study endpoint, Primary Open-Angle African-American Glaucoma Genetics study, United States.
Visual fields at diagnosis and study endpoint
Cases had a higher mean pattern standard deviation (PSD; 6.41 ± 3.23 dB vs 4.00 ± 2.90 dB, p = 0.003) and more severe mean deviation (−14.00 ± 13.10 dB vs −5.10 ± 7.71 dB, p = 0.006) than controls at diagnosis. Visual field defects as a whole were more common in cases than controls (59.3% vs 52.5%, p = 0.01; Table 3).
Cases had higher mean PSD (7.69 ± 3.50 dB vs 4.77 ± 3.18 dB, p = 0.0005) and more severe mean deviation (−19.90 ± 8.76 dB vs −6.89 ± 7.02 dB, p < 0.0001) at the study endpoint. Cases were also more likely to have an arcuate defect (74.4% vs 50.9%, p = 0.001) and paracentral scotoma (23.3% vs 8.5%, p = 0.04), as well as more visual field defects as a whole (86.1% vs 69.5%, p = 0.002), at study endpoint.
Cases had a faster PSD progression rate (dB/year) and mean deviation progression rate (dB/year) than controls (0.11 ± 0.06 vs 0.01 ± 0.04, p = 0.04; −0.25 ± 0.19 vs 0.05 ± 0.13, p < 0.0001; Table 4).
Adherence to treatment and intraocular pressure measurements throughout treatment
Cases missed a greater absolute number of appointments per year than controls (2.32 ± 2.02 vs 1.18 ± 0.75, p = 0.003; Table 4), but there was no statistical difference for the percentage of missed appointments per year between the groups. Ophthalmologists also noted non-adherence to treatment in the charts of cases more often than in those of controls (19.3 ± 19.3% vs 10.7 ± 13.2%, p = 0.03).
Cases had a higher mean IOP (18.9 ± 4.9 mmHg vs 15.5 ± 3.1 mmHg, p = 0.0001, Figure 1), higher maximum IOP (32.9 ± 10.7 mmHg vs 22.6 ± 7.0 mmHg, p<0.0001), and higher percentage of visits with an elevated IOP (≥21 mmHg; 33.9 ± 28.3% vs 10.9 ± 17.7%, p < 0.0001) than controls throughout treatment.
Multivariate analysis to assess risk factors for blindness
In the multivariate analysis of risk factors for blindness, we considered diabetes, age at diagnosis, BCVA at diagnosis, IOP before or during treatment, number of medications, number of glaucoma laser treatments, number of treatment changes, number of missed appointments per year, and adherence to treatment. By using the backward selection method, we found that worse BCVA at diagnosis (p = 0.002), number of missed appointments per year (p = 0.001), and percentage of visits with an elevated IOP (p = 0.0002) were significantly associated with blindness in the final model (Table 5). Patients with VA worse than 20/40 at diagnosis were 27.1 times more likely to become blind when compared to patients with VA 20/20 or better (odds ratio, OR, 27.10, 95% confidence interval, CI, 4.21–174.00). Those who missed more than two appointments per year also had increased risk of blindness (OR 12.40, 95% CI 2.32–66.30) compared to patients who missed 0–1 appointments per year. Patients with an elevated IOP (≥21 mmHg) at more than 20% of follow-up visits were 72.6 times more likely to become blind than those without elevated IOP (OR 72.60, 95% CI 15.30–344.00).
Discussion
Our study found that cases were diagnosed with POAG earlier (on average 4.5 years) than controls. Interestingly, while positive family history is a known risk factor for POAG,9,29 our study did not find a definitive association between family history and progression to blindness. It is possible that patients with a known positive family history of POAG are more likely to actively seek preventative care, allowing for disease detection and treatment before significant vision loss.
In our study, cases and controls were similar in demographic characteristics, annual household income, and comorbidities. Some studies have demonstrated that diabetes is potentially protective against progression to blindness,26 but our study does not support this finding.
Effects of POAG on VA are often a late manifestation of the disease.19 In this study, patients with BCVA worse than 20/40 were 27 times more likely to progress to blindness than those with BCVA 20/20 or better. This finding highlights the importance of aggressive compliance counseling in patients presenting with reduced vision.
Cases also had worse overall visual field presentation at diagnosis than controls, demonstrated by their higher frequency of arcuate defects, higher PSD, and more severe mean deviation. Arcuate defects, especially superior arcuate defects, are typically found in the early stages of POAG.30,31 Their appearance is especially important to note, as patients with arcuate defects have a low chance of disease stability.32 In addition to having more visual field defects at diagnosis, cases also had a faster mean deviation and PSD progression rate. These findings are supported by Peters and colleagues-33 and Grant and Burke,8 who also reported that visual field status at baseline is a very important risk factor for progression to blindness. These at-risk patients should be more closely monitored with regard to disease progression and compliance.
Studies have repeatedly shown that elevated IOP and greater inter-visit IOP fluctuations are correlated with POAG progression.20,22 This study is consistent with the literature, finding that cases had significantly higher IOP than controls before, during, and after treatment. IOP also fluctuated more in cases, which may be due to poor management of IOP or difficult control of IOP. Multivariate analysis revealed that the percentage of visits with elevated IOP strongly predicted progression to blindness, which is supported by other studies12 and reinforces the well-accepted need for greater IOP control in these most at-risk patients.
Initial medical management was slightly more aggressive in cases versus controls, with cases receiving a significantly higher number of medications at diagnosis. During follow-up, cases underwent a significantly larger number of treatment changes, which may be due to ineffective treatment, progression of disease despite treatment, difficulty following medication regimen, and/or poor adherence to treatment.
When conducting this study, two sub-groups of cases emerged; those who were blind (27.1%) or became blind within 1 month of diagnosis (5.1%), and those progressing to blindness over time despite treatment. Although it is difficult to elucidate exactly why this first subgroup of patients did not seek medical care before becoming blind, we speculate that this likely represents difficulties with access to care or the nature of POAG itself, with no pain and generally no loss of central vision until the end stages. Lack of awareness of disease risk, under-use of primary eye-care services, and low referral levels could also contribute to unnecessary disease progression.34 Notably, financial hardship is unlikely to account for this difference in access to care, demonstrated by the similarity in mean household income between cases and controls. Further qualitative investigation of this patient subgroup could help identify optimal strategies for reaching at-risk patients while their disease is in the early stages.
Previous studies have shown that physician chart notes referencing poor adherence to medication are correlated with pharmacy records13 and that there is a statistically significant relationship between physician assessment of adherence and actual usage.35 Our study found that problems with adherence were noted more frequently in the charts of cases, which suggests that cases may have difficulty with day-to-day medication adherence. However, when patients show disease progression, physicians may be more likely to closely examine non-compliance and comment on this issue in charts. In addition, cases had a significantly higher total number of missed appointments per year than controls. However, there was no significant difference between the total number of completed appointments or the percentage of missed appointments between cases and controls, putting into question the validity of this metric as a predictor for risk of blindness. Although cases may have more adherence issues with day-to-day medications, appointment attendance does not appear to be a significant factor influencing progression to blindness.
Limitations of this study include its retrospective nature, which precluded the standardized documentation of variables throughout the study. The 16 eyes that presented blind at diagnosis were not included in the analysis of study characteristics at endpoint because they could not contribute meaningful data. White-coat syndrome, where the upcoming clinic visit serves as a reminder that enhances compliance for the pre-visit interval, may also affect adherence reporting.36 The study is limited by a lack of information on the duration of glaucoma. Although we recorded length of follow-up, it is not a substitute for disease duration. Some cases may have had glaucoma for years before being diagnosed (demonstrated by the 16 eyes blind at diagnosis), which may translate to a significantly longer disease duration in cases.
This study examined a cross-sectional sample of African-American patients in the Philadelphia area. The geographic isolation of our population, along with referral bias (severe cases are often referred to academic and tertiary care centers), may limit the generalizability of our study to the national African-American population. However, it should be noted that patients who received prior treatment elsewhere were excluded from this study.
In the future, we could also consider a possible genetic component of POAG. We know from prior studies that African-Americans are predisposed to POAG, but this study possibly suggests that there may be subgroups of African-Americans who are more predisposed to disease progression than other subgroups. Cases’ younger age at diagnosis, worse initial disease, and faster progression suggest the possibility of an undefined predisposition towards more severe disease. These cases may demonstrate phenotypes of different genetic variants underlying their disease. Future studies will compare the genetics of patients progressing to blindness with those maintaining their vision.
The strongest risk factors for POAG progression in African-Americans were advanced disease at presentation, missed appointments for glaucoma care, and poor control of IOP. Thus, in order to prevent progression to blindness from POAG, it is important to improve public education, expand access to glaucoma care, closely monitor those with advanced disease at diagnosis, and maintain strict control of IOP throughout treatment.
Acknowledgments
Funding
This work was supported by the National Eye Institute, Besthesda, Maryland (grant #1RO1EY023557-01). Funds also come from the F.M. Kirby Foundation, Research to Prevent Blindness, Fight for Sight, The Paul and Evanina Bell Mackall Foundation Trust, Penn CAREs, The Board of Women Visitors, and the National Eye Institute, National Institutes of Health, Department of Health and Human Services, under eyeGENETM and contract Nos. HHSN260220700001C and HHSN263201200001C. The sponsor or funding organization had no role in the design or conduct of this research.
Footnotes
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the writing and content of this article.
References
- 1.Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet. 2004;363:1711–1720. doi: 10.1016/S0140-6736(04)16257-0. [DOI] [PubMed] [Google Scholar]
- 2.Tielsch JM, Sommer A, Katz J, et al. Racial variations in the prevalence of primary open-angle glaucoma. The Baltimore Eye Survey. JAMA. 1991;266:369–374. [PubMed] [Google Scholar]
- 3.Quigley HA, Enger C, Katz J, et al. Risk factors for the development of glaucomatous visual field loss in ocular hypertension. Arch Ophthalmol. 1994;112:644–649. doi: 10.1001/archopht.1994.01090170088028. [DOI] [PubMed] [Google Scholar]
- 4.Smith SD, Katz J, Quigley HA. Analysis of progressive change in automated visual fields in glaucoma. Invest Ophthalmol Vis Sci. 1996;37:1419–1428. [PubMed] [Google Scholar]
- 5.Lichter PR, Musch DC, Gillespie BW, et al. Interim clinical outcomes in the Collaborative Initial Glaucoma Treatment Study comparing initial treatment randomized to medications or surgery. Ophthalmology. 2001;108:1943–1953. doi: 10.1016/s0161-6420(01)00873-9. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz B, Takamoto T, Martin J. Increased rate of visual field loss associated with larger initial visual field threshold values on follow-up of open-angle glaucoma. J Glaucoma. 2004;13:120–129. doi: 10.1097/00061198-200404000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Wilson MR, Kosoko O, Cowan CL, et al. Progression of visual field loss in untreated glaucoma patients and glaucoma suspects in St. Lucia, West Indies. Am J Ophthalmol. 2002;134:399–405. doi: 10.1016/s0002-9394(02)01585-4. [DOI] [PubMed] [Google Scholar]
- 8.Grant WM, Burke JF. Why do some people go blind from glaucoma? Ophthalmology. 1982;89:991–998. doi: 10.1016/s0161-6420(82)34675-8. [DOI] [PubMed] [Google Scholar]
- 9.Hiller R, Kahn HA. Blindness from glaucoma. Am J Ophthalmol. 1975;80:62–69. doi: 10.1016/0002-9394(75)90870-3. [DOI] [PubMed] [Google Scholar]
- 10.Sommer A, Tielsch JM, Katz J, et al. Racial differences in the cause-specific prevalence of blindness in east Baltimore. N Engl J Med. 1991;325:1412–1417. doi: 10.1056/NEJM199111143252004. [DOI] [PubMed] [Google Scholar]
- 11.Muir KW, Santiago-Turla C, Stinnett SS, et al. Health literacy and vision-related quality of life. Br J Ophthalmol. 2008;92:779–782. doi: 10.1136/bjo.2007.134452. [DOI] [PubMed] [Google Scholar]
- 12.Sherwood MB, Garcia-Siekavizza A, Meltzer MI, et al. Glaucoma’s impact on quality of life and its relation to clinical indicators. A pilot study. Ophthalmology. 1998;105:561–566. doi: 10.1016/S0161-6420(98)93043-3. [DOI] [PubMed] [Google Scholar]
- 13.Janz NK, Wren PA, Lichter PR, et al. Quality of life in newly diagnosed glaucoma patients: The Collaborative Initial Glaucoma Treatment Study. Ophthalmology. 2001;108:887–897. doi: 10.1016/s0161-6420(00)00624-2. [DOI] [PubMed] [Google Scholar]
- 14.Friedman DS, Freeman E, Munoz B, et al. Glaucoma and mobility performance: the Salisbury Eye Evaluation Project. Ophthalmology. 2007;114:2232–2237. doi: 10.1016/j.ophtha.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 15.Wu S-Y, Hennis A, Nemesure B, Leske MC, Barbados Eye Studies Group Impact of glaucoma, lens opacities, and cataract surgery on visual functioning and related quality of life: the Barbados Eye Studies. Invest Ophthalmol Vis Sci. 2008;49:1333–1338. doi: 10.1167/iovs.07-1252. [DOI] [PubMed] [Google Scholar]
- 16.Onakoya AO, Mbadugha CA, Aribaba OT, et al. Quality of life of primary open angle glaucoma patients in Lagos, Nigeria: clinical and sociodemographic correlates. J Glaucoma. 2012;21:287–295. doi: 10.1097/IJG.0b013e31820d7cfd. [DOI] [PubMed] [Google Scholar]
- 17.Bennion JR, Wise ME, Carver JA, et al. Analysis of glaucoma-related mortality in the United States using death certificate data. J Glaucoma. 2008;17:474–479. doi: 10.1097/IJG.0b013e318163bdbd. [DOI] [PubMed] [Google Scholar]
- 18.Wu S-Y, Nemesure B, Hennis A, et al. Open-angle glaucoma and mortality: The Barbados Eye Studies. Arch Ophthalmol. 2008;126:365–370. doi: 10.1001/archophthalmol.2007.77. [DOI] [PubMed] [Google Scholar]
- 19.Kwon Y, Kim C, Zimmerman M, et al. Rate of visual field loss and long-term visual outcome in primary open-angle glaucoma. Am J Ophthalmol. 2001;132:47–56. doi: 10.1016/s0002-9394(01)00912-6. [DOI] [PubMed] [Google Scholar]
- 20.Boland M, Quigley H. Risk factors and open-angle glaucoma: classification and application. J Glaucoma. 2007;16:406–418. doi: 10.1097/IJG.0b013e31806540a1. [DOI] [PubMed] [Google Scholar]
- 21.AGIS-Investigators. The Advanced Glaucoma Intervention Study (AGIS): 12. Baseline risk factors for sustained loss of visual field and visual acuity in patients with advanced glaucoma. Am J Ophthalmol. 2002;134:499–512. doi: 10.1016/s0002-9394(02)01659-8. [DOI] [PubMed] [Google Scholar]
- 22.Nouri-Mahdavi K, Hoffman D, Liu G, et al. Predictive factors for glaucomatous visual field progression in the Advanced Glaucoma Intervention Study. Ophthalmology. 2004;111:1627–1635. doi: 10.1016/j.ophtha.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 23.Schwartz G, Quigley H. Adherence and persistence with glaucoma therapy. Surv Ophthalmol. 2008;53(Suppl. 1):S57–68. doi: 10.1016/j.survophthal.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 24.Charlson E, Sankar P, Miller-Ellis E, et al. The Primary Open-Angle African-American Glaucoma Genetics (POAAGG) study: baseline demographics. Ophthalmology. 2015;122:711–7120. doi: 10.1016/j.ophtha.2014.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Physician’s desk reference for ophthalmology. 26th. Thomson Health Care; 1997. Medical-Economics. [Google Scholar]
- 26.Oliver JE, Hattenhauer MG, Herman D, et al. Blindness and glaucoma: a comparison of patients progressing to blindness from glaucoma with patients maintaining vision. Am J Ophthalmol. 2002;133:764–772. doi: 10.1016/s0002-9394(02)01403-4. [DOI] [PubMed] [Google Scholar]
- 27.United States Census Bureau. 2007–2011 American community survey 5-year estimates summary file. Suitland, MD: Author; 2013. [Google Scholar]
- 28.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 29.Tielsch JM, Katz J, Sommer A, et al. Family history and risk of primary open angle glaucoma. The Baltimore Eye Survey. Arch Ophthalmol. 1994;112:69–73. doi: 10.1001/archopht.1994.01090130079022. [DOI] [PubMed] [Google Scholar]
- 30.Quigley H, Addicks E, Green R. Optic nerve damage in human glaucoma. Arch Ophthalmol. 1982;100:135–146. doi: 10.1001/archopht.1982.01030030137016. [DOI] [PubMed] [Google Scholar]
- 31.Yamagishi N, Anton A, Sample P, et al. Mapping structural damage of the optic disk to visual field defect in glaucoma. Am J Ophthalmol. 1997;123:667–676. doi: 10.1016/s0002-9394(14)71079-7. [DOI] [PubMed] [Google Scholar]
- 32.Aulhorn E, Karmeyer H. Frequency distribution in early glaucomatous visual field defects. Second International Visual Field Symposium. 1976:75–83. [Google Scholar]
- 33.Peters D, Bengtsson B, Heijl A. Factors associated with lifetime risk of open-angle glaucoma blindness. Acta Ophthalmol. 2013;92:421–425. doi: 10.1111/aos.12203. [DOI] [PubMed] [Google Scholar]
- 34.Cross V, Shah P, Bativala R, et al. ReGAE 2: glaucoma awareness and the primary eye-care service: some perceptions among African Caribbeans in Birmingham UK. Eye. 2007;21:912–920. doi: 10.1038/sj.eye.6702461. [DOI] [PubMed] [Google Scholar]
- 35.Kass M, Gordon M, Meltzer D. Can ophthalmologists correctly identify patients defaulting from pilocarpine therapy? Am J Ophthalmol. 1986;101:524–530. doi: 10.1016/0002-9394(86)90940-2. [DOI] [PubMed] [Google Scholar]
- 36.Feinstein A. On white-coat effects and the electronic monitoring of compliance. Arch Intern Med. 1990;150:1377–1378. [PubMed] [Google Scholar]


