Abstract
Estrogens have been shown to have an inhibitory effect on food intake under free-feeding conditions, yet the effects of estrogens on food-maintained operant responding have been studied to a much lesser extent and, thus, are not well understood. Therefore, the purpose of the present experiment was to use a behavioral economics paradigm to assess differences in demand elasticity between mice with knockout of the estrogen receptor subtype α, knockout of subtype β, and their wild type controls. The mice responded in a closed economy, and the price of food was increased by increasing the fixed-ratio response requirement every four sessions. Overall, we found that mice with the knockout of receptor subtype α had the most elastic demand functions. Therefore, under these conditions, estrogens increased food seeking via activation of the receptor subtype α. The results were inconsistent with those reported by previous studies that employed free-feeding conditions.
Keywords: demand, fixed-ratio schedules, mice, nose poke, sex hormones
In free-feeding female rodents, estrogens have well-documented anorectic actions (e.g., Asarian & Geary, 2006 2013; Butera, 2010). After ovariectomy (OVX), which removes estrogen signaling, females show substantial increases in body weight and food intake relative to sham-operated controls (e.g., Wade & Grey, 1979), and systemic replacement of estradiol reverses these effects (Asarian & Geary, 2002; Butera, Wojcik, & Clough, 2010). Estradiol restores estrogen signaling through two types (α, β) of estrogen receptor (ER), but the inhibitory effects on food intake may be exerted through one or both of these. Genomic knockout (KO) of ERα can expose the effects exerted through ERβ signaling, and vice versa. By 5 months of age, female mice with genomic KO of ERα weigh about 30% more than their wild type (WT) littermates and have almost double the body fat, although their food intake is not elevated (Lemieux, Phaneuf, Labrie, Giguère, Richard & Deshaies, 2005). In males, the effect of ERα KO on body weight is less robust, but the body fat increase is comparable (Heine, Taylor, Iwamoto, Lubahn, & Cooke, 2000; Lemieux et al., 2005; Ohlsson et al., 2000).
ERβ KO mice, however, do not show differences from WT (Ohlesson et al., 2000), which suggests that the effects of estrogens on body composition are mediated mainly through ERα. In regard to administration of either estradiol or drugs that are subtype-selective agonists, it is important to distinguish effects that are mediated by ERs on the cell surface and occur with short latency, from effects that are mediated by receptors inside the cell and alter DNA transcription and occur with a latency of a day or more. Roesch (2006) demonstrated that chronic administration of an agonist selective for ERα prevented overeating and weight gain after OVX in rats, whereas an ERβ selective agonist did not. Santollo, Wiley, and Eckel (2007) showed that the ERα-mediated effect on food intake occurred rapidly and lasted 24 hr. Meal size decreased but meal frequency was unchanged, thus the effect occurred presumably by enhancing satiation within a bout of eating. In contrast, effects of estradiol on food intake often do not occur until >24 hours after treatment (e.g., Asarian & Geary, 2002; Butera et al., 2010; Eckel, 2011; Lampert et al., 2013).
Beyond regulating satiation under free-feeding conditions, estrogens may affect an organism’s sensitivity to operant contingencies for obtaining food reinforcement. Using a discrete-trial task in which OVX rats had to alternate levers across trials, Wang et al. (2008) reported that treatment with estradiol produced more perseveration on the incorrect lever (i.e., a response was not reinforced in the previous trial). Perseveration on the correct lever (i.e., a response had just been reinforced in the previous trial), however, did not differ between estradiol-treated and vehicle-treated rats. Therefore, the perseveration observed following estradiol treatment appeared to be related specifically to insensitivity to extinction, rather than increased motor activity or general indiscriminative responding.
Wang et al. (2008) also trained these same rats to respond under a differential-reinforcement-of-low-rate (DRL) schedule. Estradiol-treated rats made more responses and had shorter interresponse times, thus earned fewer reinforcers compared to vehicle-treated rats. When the DRL contingency later was changed to extinction, estradiol treatment resulted in more responding compared to vehicle treatment, consistent with the perseveration observed in the two-lever alternation task. Because Wang et al. administered estradiol, it is unclear whether ERα or ERβ was responsible for the effects on operant responding.
Uban, Rummel, Floresco, and Galea (2012) attempted to assess whether the roles of ERα and ERβ were distinct in an operant task. First, they arranged a discrete-trial choice task in which intact female rats were given a concurrent choice between one pellet and four pellets. The response requirement for the small reinforcer was always fixed ratio (FR) 1, and the response requirement for the large reinforcer was increased (FR 2, 5, 10, 20) within session. Rats preferred the large reinforcer when its requirement was FR 2, were indifferent at FR 5, and preferred the small reinforcer at FR 10 and 20. The animals were then OVX and retested 6 days later. Compared with their preoperative behavior, and to a group that received sham surgery, OVX rats now preferred the large reinforcer at FR 5 and did not show indifference until FR 10. This, plus the finding that administration of estradiol to OVX rats restored preoperative performance, is consistent with the interpretation that estrogens decreased the preference to work for a large reward. Conversely, after administration of an ERα or an ERβ selective agonist, preference for the larger, more effortful reward was increased. All of these behavioral effects occurred >24 hr after drug administration, suggesting a genomic action is involved. These findings also suggest that, at least in operant choice tasks, ERα and ERβ may interact. Although ERβ selective agonists have shown no effect on food intake (e.g., Roesch, 2006), it may be inappropriate or premature to conclude that ERβ has no role in regulating food intake when both receptor types are activated. Rather, as suggested by Matthews and Gustafsson (2003) the role of ERβ could be to stimulate food intake or to enhance weight gain—to compensate for the inhibitory effects modulated by ERα.
The purpose of the present experiment was to use a behavioral economics paradigm, in which responding for and consumption of food reinforcers was measured at various FR requirements, to assess differences in reinforcer value (indexed by elasticity of demand) for ERαKO, ERβKO, ERαWT, and ERβWT mice. We were interested in testing whether the known inhibitory effects on food intake and body weight under free-feeding conditions could be elucidated when feeding behavior is under operant control and, if so, whether these effects would be differentially modulated by ERα, ERβ, or an interaction. We hypothesized that if tonic suppression of food intake by estradiol were mediated by ERα signaling, then genomic KO of ERα would be associated with greater food intake and lower elasticity of demand. If, on the other hand, an interaction between ERα and ERβ were necessary for action on food intake, then genomic KO of either ERα or ERβ should have little effect on food intake and demand elasticity relative to WT mice.
Method
Subjects
We obtained 24 adult female mice of mixed C57BL/6 and 129 background and homozygous for deletion of either ERα (N = 6) or ERβ (N = 7) and the respective wild types (αWT N = 6; βWT N = 5) from the heterozygote breeding colonies of Dr. T.C. Foster in the McKnight Brain Institute at the University of Florida. These mice were approximately 8 months of age at the start of the study, well below the normal age of cessation of ovarian cycling, and had been genotyped as described elsewhere (e.g., Han et al., 2013).
Prior to study, all mice were housed singly in polycarbonate shoe box cages with free access to Purina 5001 chow pellets and tap water. Cage bedding (SaniChips, Harlan) was changed weekly. Vivarium lights were on a 12:12-hr light:dark cycle, ambient temperature was 23–24°C, and the relative humidity was 40–60%. The University of Florida Animal Care and Use Committee approved all procedures in this protocol with the stipulation that mice be removed from the study if and when body weight loss exceeded 15% (i.e., if an animal dropped below its 85% free-feeding body weight).
Apparatus
The experiment was conducted in 24 standard operant-conditioning chambers (Med Associates, St. Albans, VT) enclosed in ventilated, sound-attenuating cubicles. A 15-w light in the cubicle provided general illumination according to the same 12:12-hr light:dark cycle as the vivarium. Interior dimensions of the chambers measured 14 × 14 × 12 cm. The side walls of the chambers were Plexiglas, and the front and rear panels were aluminum. Steel rods, spaced 0.5 cm apart, comprised the chamber floor. A drop pan was located 4.5 cm below the floor. A nose-poke manipulandum, measuring 1 cm in diameter, was located on the front panel, approximately 3 cm from the right wall and 2 cm above the chamber floor. A 0.75-cm diameter cue light was illuminated white for the duration of the session and was located 5 cm above the nose-poke manipulandum and 4.5 cm from the ceiling. A food trough, through which earned pellets could be accessed, measured 2.5 × 2 cm. The food trough was horizontally centered on the front panel, positioned 1.5 cm to the left of the nose-poke manipulandum and 1 cm above the floor. A computer operating Med-Associates software (St. Albans, VT) controlled experimental events and recorded data in 15-min bins.
Procedure
The purpose of the experiment was to compare demand curves obtained from four groups of female mice (ERα KO, ERβ KO, ERα WT, and ERβ WT). The mice lived in operant chambers for 23 hr per day (i.e., a closed economy). Sessions began at 11:00 a.m., and mice were removed, weighed, and placed in holding boxes without food or water for 1 hr per day while the chambers were serviced. After completing the prevailing FR response requirement, a single 20-mg food pellet was delivered to the trough. The food was a grain-based tablet of similar composition to chow (5TUM: Purina Test Diets). During the 23-hr sessions, mice were free to respond and eat at any time. Water was freely available from a sipper spout on the wall opposite the feeder.
For initial training, the unit price or FR was two nose poke responses per pellet. No shaping procedures were used, and mice were kept in this condition until intake stabilized (approximately 3 days). Mice then entered the formal testing period, which consisted of five contiguous four-session phases between which food price was incremented (FR 5, 10, 25, 50, and 100). Body weights were recorded daily at the end of each session. After the second session at FR 100, some mice had to be removed from the study due to weight loss. These mice included all in the ERα KO group and one each from the ERβ KO and ERα WT groups. Body weight data are thus reported after the 2nd day in each FR phase.
Analyses
The number of pellets earned was averaged across the four sessions at each FR for individual mice, or two sessions for those mice that were removed from the study after two sessions at FR 100. Then, to quantify the relation between consumption and price, Hursh and Silberberg’s (2008) exponential demand equation was fitted to the data:
| (1) |
where Q represents total reinforcer consumption at each FR price (P), Q0 is the demand or consumption when price is infinitely low, k is a scaling parameter that specifies the range of log Q, and α represents the relative rate of exponential decrease in consumption with relative increases in prices across the entire curve. Large α values reflect greater elasticity, and small α values reflect less elasticity. The analyses were conducted using GraphPad Prism (Graph-Pad Software, Inc., San Diego, CA).
Results
Figure 1 shows pellets earned as a function of the FR response requirement and the exponential demand equation (Hursh & Silberberg, 2008) fits to the data. Groups of mice are separated by panels and individual mice in each group are denoted by different symbols. For all mice, the number of pellets earned tended to decrease as the FR increased. This rate of decrease tended to be less steep for the mice in the ERβ WT group and for all but one mouse (F3) in the ERα WT group compared to the KO groups. The highest degree of elasticity was found for ERα KO mice. Even the mouse with the most inelastic demand function in this group (F1) showed more elasticity than almost every single mouse from the other three groups, with the exceptions being F3 in ERα WT and F7 in ERβ KO. There was more between-subject variability in the ERα KO mice, with the variability becoming most evident at the two highest prices tested—FR 50 and FR 100. Notable between-subject variability was also seen for ERβ KO mice at the highest FR.
Fig. 1.
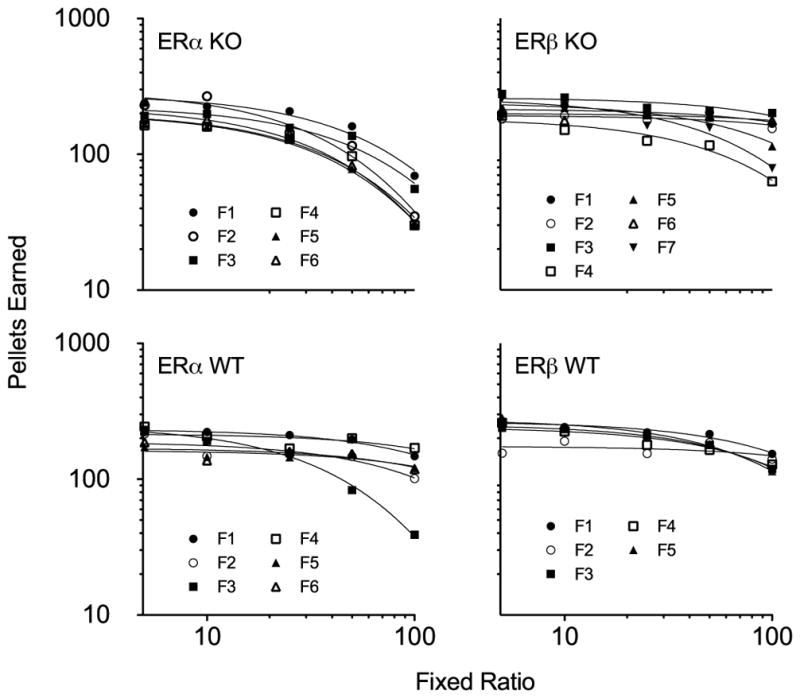
Consumption, expressed as pellets earned, as a function of the FR requirement for the four groups of mice, shown by panel. Individual mice from each group are represented by different symbols, and the exponential demand equation has been fitted to the individual data. Note that the axes are double logarithmic and truncated.
Figure 2 shows individual Q0 (upper panel) and alpha (lower panel) parameters from the exponential demand equation for each group of mice. The horizontal lines and error bars show mean and standard deviation, respectively. The mean and range of Q0 values, which represents consumption at an infinitely low price, appeared to be comparable across groups. Though the individual highest Q0 value observed out of all mice belonged to the ERα KO group, the mean for the ERβ WT mice was slightly higher than the other three groups, and four out of five mice in this group had Q0 values that exceeded the majority of mice from other groups. Though the ranges of Q0 values were comparable, the distribution of values tended to depend on group. That is, ERβ WT mice were clustered near the top of the range (above the mean), with one mouse substantially lower (below the standard deviation) than the rest. The ERα KO mice were clustered towards the lower end of the range (below the mean), with two mice at or above the standard deviation. The Q0 values for both the ERβ KO and ERα WT mice were distributed evenly across the range.
Fig. 2.
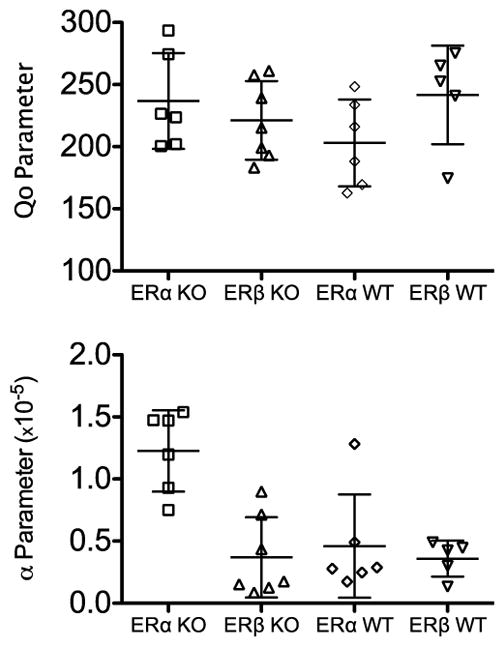
Parameter values obtained from the exponential demand equation fits to the data for the four groups of mice, shown across the x-axis. Each data point represents an individual mouse, and the error bars represent the standard deviation from the mean. Note the truncated y-axis in the top panel.
With respect to the alpha parameter, which indexes elasticity of demand, ERα KO had the highest group mean, with very little overlap in range seen between this group compared to the other three groups. As was already described, but can be seen more clearly here, only two other mice had higher alpha values than the mouse with the lowest alpha value (i.e., least elastic demand) in the ERα KO group. The majority of mice in the other three groups had lower and comparable alpha values, indicating less elastic demand functions, or less sensitivity to increases in price. There was the least between-subject variability for ERβ WT mice, as the individuals were centered around the mean and the standard deviation was small. Most ERα WT mice (five out of six) also had among the lowest alpha values, and all except one were within the range observed for the ERβ WT mice and below the range observed for ERα KO mice. Overall, this supported the conclusions from visual analysis of Figure 1 that ERα KO mice demonstrated the greatest elasticity of demand.
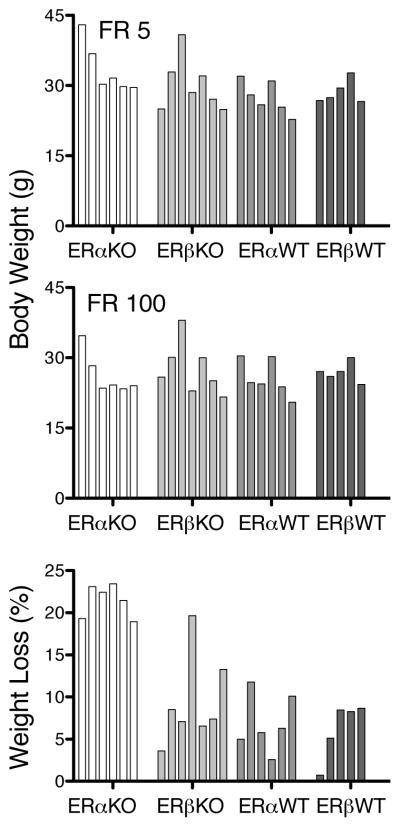
Figure 3 shows individual body weights after the second sessions of FR 5 (upper panel) and FR 100 (middle panel), as well as the percent weight loss that was incurred between those two points (lower panel). After two sessions of FR 5 and again after two sessions of FR 100, there did not seem to be substantial between-group differences in body weights. The two heaviest individual mice were from the ERα KO and ERβ KO groups, however, and the least degree of between-subject variability in weights was evident for the ERβ WT group. When the data were analyzed as percent weight loss, clear between-group differences emerged. All ERα KO mice had a greater percent weight loss (approximately two-fold difference) compared to both WT groups and compared to all but one mouse in the ERβ KO group, whose percent weight loss was comparable to that observed for ERα KO mice. Moreover, all ERα KO mice lost about the same, high percent of weight independent of initial weights, whereas individual mice from other groups that weighed more than 30 g initially, lost less than 10% body weight. In the ERβ KO group, there was one mouse that lost approximately 20% body weight (comparable to ERα KO percent weight loss), but this mouse had a relatively low weight initially.
Fig. 3.
Body weight, expressed in g, after two sessions of FR 5 (top panel) and two sessions of FR 100 (middle panel), as well as percent weight loss from FR 5 to FR 100 (lower panel). Each bar represents an individual mouse, with the shading of the bars corresponding to the four groups of mice shown across the x-axis.
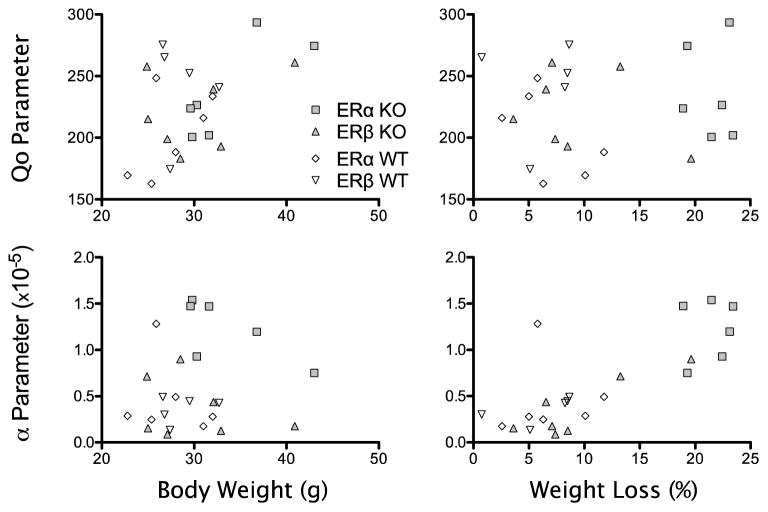
Figure 4 shows scatterplots for the Q0 (upper panels) and α (lower panels) parameters from the exponential demand equation compared to the body weight (left panels) and percent weight loss (right panels) variables. Note that the body weights and percent weight loss measures were previously shown in the top and bottom panel, respectively, of Figure 3. Because there was some heterogeneity of initial weight and weight loss within and between groups, we further examined the data to investigate whether the elasticity parameters were systematically related to either of these variables. Table 1 shows correlation coefficients derived from the scatterplots in Figure 4, both for each genotype and for the entire cohort. For the cohort, there were significant positive correlations between the Q0 parameter and body weight, as well as between the α parameter and percent weight loss. The α parameter was not correlated with body weight, however.
Fig. 4.
Scatterplots showing the relation between the parameters from the exponential demand equation and body weight (left panels) and percent weight loss (right panels). Each data point represents and individual mouse, and each group of mice is represented by a different symbol. Note truncated y-axes in top panels and truncated x-axes in left panels.
Table 1.
Pearson’s correlation coefficients (r) and p-values for body weights and percent weight loss correlated with the parameters Q0 and α from the exponential demand equation.
| Body Weight and Qo
|
Weight Loss (%) and Qo
|
Body Weight and α
|
Weight Loss (%) and α
|
|||||
|---|---|---|---|---|---|---|---|---|
| r | p-value | r | p-value | r | p-value | r | p-value | |
| ER α KO | 0.80 | 0.03 | −0.09 | 0.43 | 0.71 | 0.06 | 0.17 | 0.37 |
| ER β KO | 0.33 | 0.24 | −0.28 | 0.27 | 0.30 | 0.26 | 0.87 | 0.01 |
| ER α WT | 0.56 | 0.12 | −0.54 | 0.14 | 0.25 | 0.31 | 0.06 | 0.46 |
| ER β WT | −0.06 | 0.46 | 0.09 | 0.44 | 0.31 | 0.30 | 0.62 | 0.13 |
| All | 0.48 | 0.01 | 0.05 | 0.40 | 0.11 | 0.31 | 0.79 | <0.0001 |
Note. Bold text represents significant correlations, and bold italicized text represents significant p-values.
Discussion
The purpose of the present experiment was to determine whether known inhibitory effects of estrogen on food intake under free feeding conditions (e.g., Asarian & Geary, 2013) were evident in an operant paradigm. Specifically, we used a behavioral economics paradigm, increasing the price of food across a series of FR response requirements, to assess differences in demand elasticity between ERα KO, ERβ KO, ERα WT, and ERβ WT mice. Overall, we found that, at least under these conditions, estrogens increase food seeking. That is, removal of estrogen signaling through ERα in the ERα KO mice led to decreased responding as price of food increased. ERα KO mice had the most elastic food demand, and this effect could not be accounted for by higher body weights of these individuals compared to individual mice in the other three groups. Moreover, within the ERα KO group, there appeared to be a negative correlation between body weight and the α parameter. These findings were inconsistent with those from nonoperant arrangements. That is, after removal of estrogens by OVX, knockout of receptor subtype ERα, or natural cessation of estrous cycling, female rodents consistently have shown increased food intake and adiposity (Butera, 2010; Clegg, 2012; Sanchez-Mateos et al., 2007).
The initial body weight differences in our experimental groups were not robust, and body composition measures were not taken. Nonetheless, the percent weight loss was substantially greater for ERα KO mice, and as the price of food increased, the ERαKO females showed a precipitous decline of food intake, indexed by high elasticity, despite the fact that weight loss was continuous and relatively severe compared to the groups (i.e., percent weight loss relative to initial weight). ERα KO mice showed no change in response output between FR 50 and FR 100, thus their food intake declined by approximately 50%. The other three groups showed at least a 50% increase in response output, and their corresponding food intake declined by only <25%. One could argue that the ERαKO mice have a physical limitation on high rates of responding, but it may also be the case that the run rates were comparable across the groups and that the overall number of responses was lower because ERα KO females have longer postreinforcement pauses. Unfortunately, one limitation of the present study was that we did not obtain a finer temporal acquisition of data that would have allowed for such an analysis. Regardless, the time necessary to complete responses, rather than number of responses emitted, may have determined the overall pattern of food acquisition, and estrogen may have modulated reinforcer value during “delay” to reinforcement.
Uban et al. (2012) reported that estrogen hormones “reduce the preference to work hard to obtain a larger reward” (p. 395) in a choice task, as preference for a higher FR and larger reinforcer increased (i.e., the discounting function shifted to the right) following OVX and was reversed after estradiol administration. Administration of an ERα or ERβ agonist alone, however, had an opposite effect suggesting an interaction between ERα and ERβ in regulating food-maintained operant responding. In their study, the interaction between ERα and ERβ could have enhanced sensitivity to the large response requirement, reduced sensitivity to the large reinforcer magnitude, or both. In our experiment, however, the outcome measures for ERβKO mice and both groups of WT mice were very similar, meaning that ERα was the primary mechanism through which food demand was enhanced. We used a free-operant paradigm whereas Uban et al. used a discrete-choice paradigm, therefore it is possible that the roles of ERα and ERβ differ depending on the characteristics of the task. Similarly, differential effects of ERα and ERβ activation may also depend on the dimensions along which reinforcement can vary, such as delay to reinforcement, probability of reinforcement, schedule of reinforcement, and reinforcement schedule parameters. It is also possible that mice might differ from rats in various operant protocols (e.g., closed vs. open economies), thus disparate interpretations between rat and mouse studies using ER manipulations may be due to a species difference.
One explanation for why the activation of ERα increased food consumption in our study could be an effect on general motor activity. That is, the essential value (which is inversely related to the α parameter of the exponential demand equation) of the reinforcer was not necessarily greater for ERβ KO and WT mice relative to ERα KO mice, but rather activation of ERα may have led to response persistence. This view would provide an explanation for the contradictory findings between the present study and previous studies using free-feeding subjects (e.g., Lemieux et al., 2005; Roesch, 2006). In those studies, perhaps activation of ERα resulted in decreased food intake and lower body weights because the animals spend more time engaged in alternative, nonfeeding behaviors. This interpretation seems consistent with the finding that meal frequency is generally unaffected (e.g., Santollo et al., 2007) because if food was less reinforcing, then in addition to meal size decreasing, bouts of eating should be less frequent, too.
In an operant paradigm, however, it is unknown whether this response persistence is specific to the contingency or just motor activity in general. Wang et al. (2008) reported greater persistence of responding on an inactive lever, inefficient responding on a DRL schedule, and greater resistance to extinction in OVX rats that received estradiol administration. We did not test either resistance to extinction or responding on a concurrently available inactive manipulandum, but if activation of ERα increased activity or arousal in general, then we should have observed the lowest Q0 values in the ERα KO mice; however, Q0 values did not differ substantially across groups. Whether activation of ERα increases resistance to other disruptor variables (e.g., presession feeding, response-independent food delivery) could be assessed using a behavioral momentum (Nevin, 1974) approach in future studies.
Response persistence associated with activation of ERα may not be specific to food reinforcement. Because females are more vulnerable than males across the acquisition, maintenance, and relapse phases of drug addiction (e.g., Fattore, Fadda, & Fratta, 2009), some studies have tried to identify the role of estrogens in drug addiction. Larson, Anker, Gliddon, Fons, and Carroll (2007) found that estradiol treatment enhanced rats’ escalation of cocaine self-administration: OVX females with hormone replacement escalated to a greater extent than did the OVX controls. Anker, Larson, Gliddon, and Carroll (2007) found that estradiol administration increased reinstatement of previously extinguished responding for cocaine in OVX female rats. Finally, Larson and Carroll (2007) revealed that administration of an ERβ agonist in OVX rats resulted in greater reinstatement, yet, interestingly, an ERα agonist significantly increased responses to the inactive lever despite having no effect on reinstatement (i.e., responses to the lever previously paired with drug). Taken together, the results from these studies using drug reinforcers support the notion of response persistence, with which our results are consistent. Given that steep discounting of delayed rewards (i.e., impulsivity) has been associated with drug addiction (e.g., Bickel, Odum, & Madden, 1999) and, more recently, eating disorders (e.g., Mole et al., 2014), it may be fruitful to explore the role of ERα and ERβ on delay discounting tasks. As delay discounting and demand analyses both assess aspects of reinforcer value, a comparison of performance on these tasks (e.g., Diergaarde, van Mourik, Pattij, Schoffelmeer, & De Vries, 2011) may help to elucidate the distinct and combined roles of ERα and ERβ activation.
Last, a limitation of the present study was that hormone levels throughout estrous cycles were not confirmed. Each FR was in effect for four sessions, but this may not have spanned the entire estrous cycles. Nonetheless, the results of our study were orderly and we did not observe any obvious cyclicity in the data across the four sessions at each FR for each mouse. Any behavioral adjustments from the last session of one FR to the first session at a new FR seemed to occur rapidly. That is, consumption on the first day of a new FR was comparable to the next three sessions at that FR as well. Therefore, the effects of estrogens on food demand appeared to be tonic, rather than acute effects of estrogen. Relatedly, we do not know whether the reduction in food intake at the higher FRs altered the estrous cycle. Tropp and Markus (2001) showed that food deprivation disrupted estrous cycling in some strains of rats, but not others. Whether KO of ERα or ERβ affects food demand similarly in males could also help confirm that the obtained findings were unrelated to estrous cycle. The effects of OVX on elasticity of food demand also would make for an interesting comparison, as OVX reduces signaling of estrogen through ERα and ERβ as well as other hormones such as progesterone. The loss of progesterone and estrogen hormones could affect males and females differentially.
Overall, the present study demonstrated that the removal of estrogen signaling through ERα, but not ERβ, led to decreased responding as price of food increased (i.e., the ERα KO mice had the most elastic food demand). Our results suggest that targeting estrogen as a treatment for various eating disorders and/or obesity may prove fruitful. Although the current study was not intended to be translational, we have identified a variable that should be examined further in future research. Specifically, the focus should be on behavioral mechanisms of action: the way in which a drug (hormone) interacts with factors that are known to normally control responding. In a demand analysis, the controlling variable of behavior is the FR requirement: as the FR is increased, organisms become sensitive to those price increases and thus decrease their consumption. Removal of estrogen signaling through ERα appeared to heighten sensitivity to price increases, suggesting that a potential behavioral mechanism of estrogen signaling through ERα is attenuation of sensitivity to effort requirements.
Acknowledgments
Supported in part by NIH grants AG037984 (TCF) and DK064712 (NER) and the McKnight Brain Research Foundation (TCF).
References
- Anker JJ, Larson EB, Gliddon LA, Carroll ME. Effects of progesterone on the reinstatement of cocaine-seeking behavior in female rats. Experimental and Clinical Psychopharmacology. 2007;15:472–480. doi: 10.1037/1064-1297.15.5.472. [DOI] [PubMed] [Google Scholar]
- Asarian L, Geary N. Cyclic estradiol treatment normalizes body weight and restores physiological patterns of spontaneous feeding and sexual receptivity in ovariectomized rats. Hormones and Behavior. 2002;42:461–471. doi: 10.1006/hbeh.2002.1835. [DOI] [PubMed] [Google Scholar]
- Asarian L, Geary N. Modulation of appetite by gonadal steroid hormones. Philosophical Transactions of the Royal Society. 2006;361:1251–1263. doi: 10.1098/rstb.2006.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asarian L, Geary N. Sex differences in the physiology of eating. American Journal of Physiology: Regulatory, Integrative, and Comparative Physiology. 2013;305:R1215–R1267. doi: 10.1152/ajpregu.00446.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Odum AL, Madden GJ. Impulsivity and cigarette smoking: delay discounting in current, never, and ex-smokers. Psychopharmacology. 1999;146:447–454. doi: 10.1007/pl00005490. [DOI] [PubMed] [Google Scholar]
- Butera PC. Estradiol and the control of food intake. Physiology & Behavior. 2010;99:175–180. doi: 10.1016/j.physbeh.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butera PC, Wojcik DM, Clough SJ. Effects of estradiol on food intake and meal patterns for diets that differ in flavor and fat content. Physiology & Behavior. 2010;99:142–145. doi: 10.1016/j.physbeh.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg DJ. Minireview: The year in review of estrogen regulation of metabolism. Molecular Endocrinology. 2012;26:1957–1960. doi: 10.1210/me.2012-1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diergaarde L, van Mourik Y, Pattij T, Schoffelmeer ANM, De Vries TJ. Poor impulse control predicts inelastic demand for nicotine but not alcohol in rats. Addiction Biology. 2011;17:576–587. doi: 10.1111/j.1369-1600.2011.00376.x. [DOI] [PubMed] [Google Scholar]
- Eckel LA. The ovarian hormone estradiol plays a crucial role in the control of food intake in females. Physiology & Behavior. 2011;104:517–524. doi: 10.1016/j.physbeh.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattore L, Fadda P, Fratta W. Sex differences in the self-administration of cannabinoids and other drugs of abuse. Psychoneuroendocrinology. 2009;34S:S227–S236. doi: 10.1016/j.psyneuen.2009.08.008. [DOI] [PubMed] [Google Scholar]
- Han X, Aenlle KK, Bean LA, Rani A, Semple-Rowland SL, Kumar A, Foster TC. Role of estrogen receptor α and β in preserving hippocampal function during aging. Journal of Neuroscience. 2013;33:2671–2683. doi: 10.1523/JNEUROSCI.4937-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. Increased adipose tissue in male and female estrogen receptor-α knockout mice. Proceedings of the National Academy of Sciences. 2000;97:12729–12734. doi: 10.1073/pnas.97.23.12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursh SR, Silberberg A. Economic demand and essential value. Psychological Review. 2008;115:186–198. doi: 10.1037/0033-295X.115.1.186. [DOI] [PubMed] [Google Scholar]
- Lampert C, Arcego DM, Laureano DP, Diehl LA, da Costa Lima IF, Krolow R, Vendite D. Effect of chronic administration of tamoxifen and/or estradiol on feeding behavior, palatable food and metabolic parameters in ovariectomized rats. Physiology & Behavior. 2013;119:17–24. doi: 10.1016/j.physbeh.2013.05.026. [DOI] [PubMed] [Google Scholar]
- Larson EB, Carroll ME. Estrogen receptor beta, but not alpha, mediates estrogen’s effect on cocaine-induced reinstatement of extinquished cocaine-seeking behavior in overiectomized female rats. Neuropsychopharmacology. 2007;32:1334–1345. doi: 10.1038/sj.npp.1301249. [DOI] [PubMed] [Google Scholar]
- Larson EB, Anker JJ, Gliddon LA, Fons KS, Carroll ME. Effects of estrogen and progesterone on the escalation of cocaine self-administration in female rats during extended access. Experimental and Clinical Psychopharmacology. 2007;15:461–471. doi: 10.1037/1064-1297.15.5.461. [DOI] [PubMed] [Google Scholar]
- Lemieux C, Phaneuf D, Labrie F, Giguère V, Richard D, Deshaies Y. Estrogen receptor α-mediated adiposity-lowering and hypocholesterolemic actions of the selective estrogen receptor modulator acolbifene. International Journal of Obesity. 2005;29:1236–1244. doi: 10.1038/sj.ijo.0803014. [DOI] [PubMed] [Google Scholar]
- Matthews J, Gustafsson JA. Estrogen signaling: a subtle balance between ER alpha and ER beta. Molecular Interventions. 2003;3:281–292. doi: 10.1124/mi.3.5.281. [DOI] [PubMed] [Google Scholar]
- Mole TB, Irvine MA, Worbe Y, Collins P, Mitchell SP, Bolton S, Voon V. Impulsivity in disorders of food and drug misuse. Psychological Medicine. 2014 doi: 10.1017/S0033291714001834. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevin JA. Reinforcement strength in multiple schedules. Journal of the Experimental Analysis of Behavior. 1974;21:389–408. doi: 10.1901/jeab.1974.21-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlsson C, Hellberg N, Parini P, Vidal O, Bohlooly M, Rudling M, Gustafsson JA. Obesity and disturbed lipoprotein profile in estrogen receptor-α deficient male mice. Biochemical and Biophysical Research Communications. 2000;278:640–645. doi: 10.1006/bbrc.2000.3827. [DOI] [PubMed] [Google Scholar]
- Roesch DM. Effects of selective estrogen receptor agonists on food intake and body weight gain in rats. Physiology & Behavior. 2006;87:39–44. doi: 10.1016/j.physbeh.2005.08.035. [DOI] [PubMed] [Google Scholar]
- Sanchez-Mateos S, Alonso-Gonzales C, Gonzals A, Martinez-Campa CM, Mediavilla MD, Cos S, Sanchez-Barcelo EJ. Melatonin and estradiol effects on food intake, body weight, and leptin in ovariectomized rats. Maturitas. 2007;58:91–101. doi: 10.1016/j.maturitas.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Santollo J, Wiley MD, Eckel LA. Acute activation of ERα decreases food intake, meal size, and body weight in ovariectomized rats. American Journal of Physiology—Regulatory, Integrative and Comparative Physiology. 2007;293:R2194–R2201. doi: 10.1152/ajpregu.00385.2007. [DOI] [PubMed] [Google Scholar]
- Tropp J, Markus EH. Effects of mild food deprivation on the estrous cycle of rats. Physiology & Behavior. 2001;73:553–559. doi: 10.1016/s0031-9384(01)00487-5. [DOI] [PubMed] [Google Scholar]
- Uban KA, Rummel J, Floresco SB, Galea LAM. Estradiol modulates effort-based decision making in female rats. Neuropsychopharmacology. 2012;37:390–401. doi: 10.1038/npp.2011.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade GN, Grey JM. Gonadal effects on food intake and adiposity: a metabolic hypothesis. Physiology & Behavior. 1979;22:583–593. doi: 10.1016/0031-9384(79)90028-3. [DOI] [PubMed] [Google Scholar]
- Wang VC, Sable HJK, Ju YH, Allred CD, Helferich WG, Korol DL, Schantz SL. Effects of chronic estradiol treatment on delayed spatial alternation and differential reinforcement of low rates of responding. Behavioral Neuroscience. 2008;122:794–804. doi: 10.1037/a0012513. [DOI] [PMC free article] [PubMed] [Google Scholar]