Abstract
Background: Acute hypothyroidism induced by thyroid hormone withdrawal (THW) in patients with thyroid cancer after total thyroidectomy can affect mood and quality of life (QoL). While loss or dysregulation of thyroid hormone (TH) has these well-known behavioral consequences, the effects of TH alterations on brain function are not well understood. Resting state functional connectivity (FC) measured by functional magnetic resonance imaging (fMRI) allows non-invasive evaluation of human brain function. This study therefore examined whether THW affects resting state FC and whether changes in FC correlate with the mood or QoL of the patients with THW status.
Methods: Twenty-one patients who had undergone total thyroidectomy for thyroid cancer were recruited. Resting state fMRI scanning of the brain, thyroid function tests, and administration of the 12-Item Short Form Health Survey (SF-12) and the Patient Health Questionnaire-9 (PHQ-9) were performed before and after two weeks of THW. Regional homogeneity (ReHo), one of the measures of resting state FC, was calculated, and each voxel was compared between before and after THW in 19 patients. The ReHo values were extracted from the regions of interest showing within-group differences in ReHo values after THW, and correlations of ReHo values with thyrotropin (TSH) levels, total score of the PHQ-9, and composite scores of the SF-12 were statistically evaluated.
Results: Higher ReHo was observed after THW in the brain cortical regions across primary motor and sensory, visual, and association cortices. Among the regions, the ReHo values in the bilateral pre- and postcentral gyri, bilateral middle occipito-temporal cortices, the left precuneus, and the left lingual gyrus showed positive correlations with serum TSH levels after THW. Higher ReHo values in the bilateral pre- and postcentral gyri, the left middle temporo-occipital cortices, and the left ligual gyrus correlated with the lower mental component summary score from the SF-12, while higher ReHo values in the bilateral pre- and postcentral gyri correlated with higher total scores in the PHQ-9.
Conclusions: Local brain FC is increased in the acute hypothyroid state. Higher FC correlates with a poorer mental QoL and increased depression in the hypothyroid state.
Introduction
Thyroid hormones (THs) are important for the normal function of the human brain (1). A lack of THs during human brain development leads to irreversible mental retardation and neurological deficits (2). The importance of THs has been demonstrated by observations showing that hypothyroidism causes neuropsychiatric problems, including memory disturbances and depression (3–5), and TH enhances the effect of antidepressants used to treat patients with depression (6,7).
Neuroimaging studies suggest that THs are involved in maintaining normal structure and functioning of the adult human brain. Cooke et al. reported that hippocampal volume was lower in adults with hypothyroidism than in age-matched control subjects (8). In a positron emission tomography study, patients with hypothyroidism had lower cerebral blood flow (CBF) than healthy subjects in global or local brain areas (9). Notably, these deficits were normalized by TH replacement (10). Magnetic resonance spectroscopy revealed that TH replacement increases energy metabolism as measured by the increased ratio of phosphocreatine to inorganic phosphate in the frontal cortex of patients with hypothyroidism (11). Higher serum thyrotropin (TSH) levels correlate with lower global and local CBF in patients with depression and bipolar disorder, suggesting a role for hypothyroidism in the brain pathophysiology of mood disorders (12).
While regional brain structure and CBF appear to be sensitive to hypothyroidism, the impact of hypothyroidism on functional connectivity (FC) between brain regions has not been evaluated. Since most cognitive and emotional processes depend on the interaction between spatially distributed brain regions, FC may provide insights into the pathophysiological basis of dysfunction in complex behaviors and mood dysregulation associated with hypothyroidism. Functional magnetic resonance imaging (fMRI) can be used to assess FC. Biswal et al. reported that blood oxygenation level-dependent (BOLD) signals in resting state fMRI showed synchronized fluctuations between brain regions, which is now referred to as FC (13). Regional homogeneity (ReHo) is one measure of FC. Unlike the FC between spatially remote areas, ReHo measures the degree of synchronization of BOLD signals in small volumes within the spatial scale of fMRI (14) and is thought to reflect synchronization of local field potentials produced by neuronal activity within a region (15). ReHo analysis has become a useful tool to detect disturbed neural modulations in the resting state in a variety of neuropsychiatric or metabolic disorders, including Alzheimer's disease (16), depression (17), and type 2 diabetes mellitus (T2DM) (18). For example, Cui et al. reported lower ReHo values in the occipital and postcentral gyri in patients with T2DM than in healthy subjects (18).
A growing number of studies suggest that increased FC may, like decreased FC, be associated with disrupted neural networks in neuropsychiatric disorders (19–21). Hawellek et al. suggested that increased FC observed in multiple sclerosis (MS) patients with deficits in white-matter integrity could represent “less-differentiated patterns of FC” due to the loss of local functional diversity, resulting in increased global patterns of activity during the resting state (20). During maturation of the human brain, short-range FC decreases (22) and ReHo decreases across cortical areas (23). Increased ReHo has also been observed in patients with autism spectrum disorder (24) and in the first episode of medication-naïve patients with schizophrenia (21). In attention deficit hyperactivity disorder (ADHD), decreased symptoms after medication treatment were correlated with decreased ReHo (25).
This study tested whether ReHo values were affected in patients with hypothroidism secondary to thyroidectomy. It was hypothesized that (i) ReHo values would be abnormal after TH withdrawal (THW) in patients with thyroidectomy; and (ii) the ReHo abnormalities would be related to TH-related parameters and psychological and physical symptoms. Because this is the first study to examine ReHo within a longitudinal design, the directionality of the relationship between ReHo values and TH status or related symptoms was not predicted.
Methods
Subjects
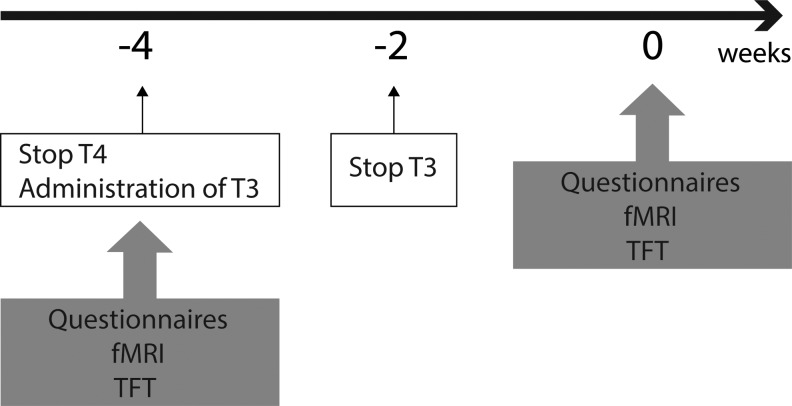
Twenty-one patients who had undergone total thyroidectomy for thyroid cancer and were undergoing THW for diagnostic imaging or for stimulated serum thyroglobulin (Tg) measurements were recruited. After oral and written informed consent, participants were evaluated with thyroid function tests, the 12-Item Short Form Survey (SF-12) (26), the Patient Health Questionnaire-9 (PHQ-9) (27), and resting state fMRI scanning of the brain before and after two weeks of THW (Fig. 1).
FIG. 1.
Schema of the study. Baseline tests including thyroid function tests, questionnaires, and functional brain magnetic resonance imaging (fMRI) were done before thyroid hormone withdrawal (THW). Thyroxine was discontinued for four weeks, and triiodothyronine was administered for the initial two weeks. Thus, the duration of THW was two weeks. Follow-up examinations using the same tests were repeated after the two-week period had elapsed.
The SF-12 is a multipurpose short-form survey with 12 questions. The questions were combined, scored, and weighted to create two scales that provide measures of mental and physical health-related quality of life (Mental Component Summary [MCS] and Physical Component Summary [PCS]) (26). The scale scores were calculated by a standard scoring algorithm summing responses across scale items and then transforming these raw scores to a 0–100 scale for each of the summary scores (with mean of 50 and standard deviation [SD] of 10), with higher scores indicating better health. The PHQ-9 is a multipurpose instrument for screening, diagnosing, monitoring, and measuring the severity of depression (27). The resting fMRI scans of two subjects were of poor quality due to significant head movements, and thus these subjects were removed from the study. The remaining 19 subjects yielded intact and complete pre- and post-deprivation data, and were included in the study. The Institutional Review Board of Asan Medical Center approved the study protocol.
Acquisition of brain MRI data
Data were acquired using a 3 Tesla Intera Achieva MR imaging scanner (Philips Healthcare, Best, The Netherlands). High-resolution T1-weighted anatomical MRI data were obtained with the following parameters: voxel size = 1 mm ×1 mm ×1 mm; TR = 9.9 ms; TE = 4.6 ms; flip angle = 8°; FOV (RL, AP, IS) = 170 mm ×240 mm ×240 mm; matrix = 240 × 240; and 170 slices (slice orientation = sagittal). T2*-weighted MRI data (resting state fMRI) were obtained using a gradient echo planar imaging (EPI) pulse sequence with the following parameters: voxel size (RL, AP, FH) = 1.65 mm ×1.65 mm ×3 mm; TR = 3000 ms; TE = 30 ms; flip angle = 90°; FOV (RL, AP, IS) = 237 mm ×237 mm ×126 mm; matrix = 144 × 144; and 42 slices (slice orientation = transverse). A total of 150 volumes of T2*-weighted images were acquired.
Preprocessing of resting state fMRI
The AFNI software package (28) was used to preprocess all the data and perform the statistical analysis. EPI images were despiked, slice-time corrected, deobliqued, and realigned with the first volume of the run to correct for head movements. Studies with an estimated maximum head motion >1 mm and/or 1° were excluded. EPI and structural images were transformed into the standard Talairach space using the TT_N27 template included in AFNI. To remove BOLD signal fluctuations unrelated to neuronal activity, signals originating from mechanical noises, heartbeats, and respirations were removed using ANATICOR (29). A temporal filter (0.01–0.08 Hz) was used to minimize the effects of undesired fluctuations, including higher frequency noise.
ReHo was computed based on unsmoothed BOLD time series using Kendall's coefficient of concordance (KCC) (30) as a homogeneity metric over a cluster of 27 neighboring voxels (31,32). The script in the AFNI package, 3dReHo, was used for the calculation of KCC. For statistical analysis, the ReHo maps were smoothed using a Gaussian kernel with a full-width at half-maximum (FWHM) value of 7.0 mm.
Statistical analysis
Demographic and clinical variables were compared before and after THW using a paired t-test in R (33). A p-value of <0.05 was considered to be statistically significant.
To evaluate changes in ReHo patterns, paired t-tests were performed on the voxel-wise ReHo maps before and after THW using 3dttest++ in AFNI. Multiple comparisons in ReHo analysis were family-wise error corrected at the whole brain level with 124 and more contiguous voxels were determined using Monte Carlo simulations (voxel size = 2 mm ×2 mm ×2 mm; cluster significance level = 0.05).
To investigate the relationship between regional ReHo abnormalities and clinical variables, correlation analysis was performed based on regions of interest (ROIs). The ReHo values were extracted from each ROI where a within-group difference was found in the paired t-tests. The extracted ReHo values after THW in each ROI were correlated with the TSH serum level to confirm the relationship between ReHo and thyroid function in the region. The changes in ReHo values after THW in each ROI were correlated with changes in the total score of the PHQ-9 and in the MCS and PCS of the SF-12 after THW. Spearman's rank correlation tests, rather than Pearson coefficients, were used due to the non-linear pattern of the distribution of scatter plot between the variables. All correlation analyses performed on each ROI were corrected for multiple comparisons (Bonferroni correction across four variables; total score of the PHQ-9, the MCS and PCS of the SF-12, and TSH), and results were considered significant when p < 0.0125. R was used for correlation analysis and related plotting.
Results
Clinical variables
Table 1 shows clinical variables, including weight, blood pressure (BP), pulse rate (PR), TSH, free thyroxine (fT4), composite score of the SF-12, and total score of the PHQ-9 before and after THW. After two weeks of THW, the mean serum TSH increased from 0.45 ± 0.73 mIU/L to 66.05 ± 17.45 mIU/L (p < 0.001), and the mean serum fT4 decreased from 1.63 ± 0.20 ng/dL to 0.13 ± 0.06 ng/dL (p < 0.001).
Table 1.
Demographic and Clinical Characteristics of Patients Before and After Thyroid Hormone Withdrawal (n = 19)
| Before | After | |
|---|---|---|
| Age (years) | 49.95 ± 7.40 | |
| Women, n (%) | 15 (79%) | |
| Weight (kg) | 63.65 ± 11.52 | 63.03 ± 11.07 |
| BP | ||
| Systolic (mmHg)* | 127.79 ± 14.97 | 120.42 ± 15.80 |
| Diastolic (mmHg) | 75.53 ± 8.54 | 72.95 ± 10.77 |
| PR (n/min)* | 77.37 ± 9.97 | 71.10 ± 12.09 |
| fT4 (ng/dL)** | 1.63 ± 0.20 | 0.13 ± 0.06 |
| TSH (mU/L)** | 0.45 ± 0.73 | 66.05 ± 17.45 |
| SF-12 | ||
| Physical Component Summary (PCS) | 46.5 ± 6.6 | 47.0 ± 1.5 |
| Mental Component Summary (MCS)* | 49.5 ± 5.8 | 45.8 ± 8.7 |
| PHQ-9a total score | 4 ± 3.37 | 6.53 ± 5.91 |
The PHQ-9 scores showed a trend toward a difference (p = 0.06).
p < 0.05; **p < 0.001.
BP, blood pressure; fT4, free thyroxine; PHQ-9, Patient Health Questionnaire; PR, pulse rate; TSH, thyrotropin; SF-12, 12-Item Short Form Health Survey.
Systolic BP decreased from 127.79 ± 14.97 mmHg to 120.42 ± 15.80 mmHg, and the PR from 77.37 ± 9.97 beats per minute to 71.10 ± 12.09 after THW (p < 0.05). The MCS score decreased from 49.5 ± 5.8 to 45.8 ± 8.7 after THW (p < 0.05). The total score of the PHQ-9 showed a trend of increase from 4.00 ± 3.37 points to 6.53 ± 5.91 points after THW (p = 0.06). There was no change in weight, diastolic BP, or PCS after THW.
ReHo analysis
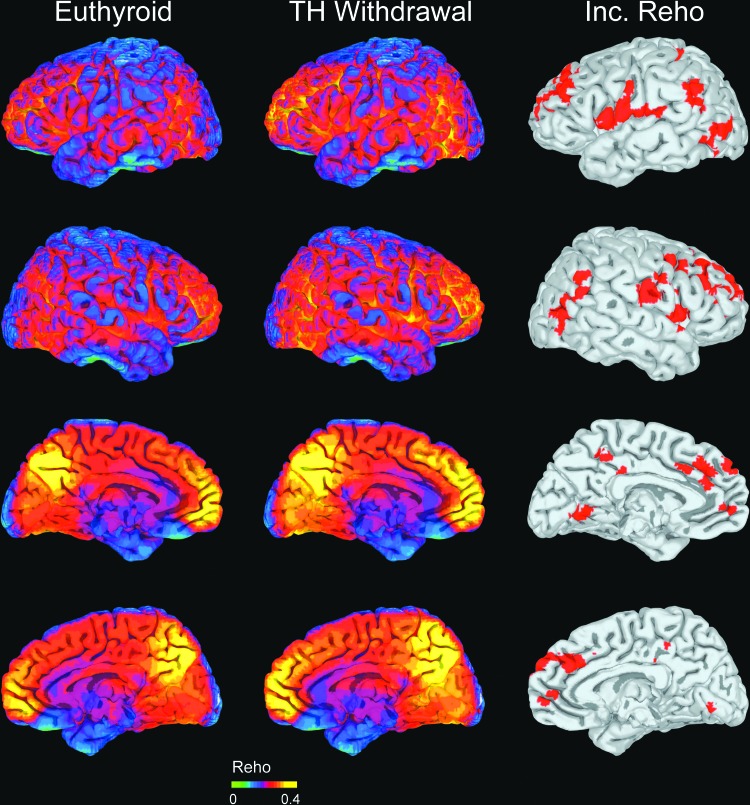
As shown in Figure 2, ReHo values were mapped onto cortical surfaces. Visual inspection showed that several brain regions, such as the precuneus and medial prefrontal gyrus, had higher ReHo values than other areas of the brain before and after THW.
FIG. 2.
The regional homogeneity (ReHo) of the resting fMRI signal was quantified by Kendall's coefficient concordance (KCC). The KCC was plotted on a template brain surface before (first column) and after (second column) THW. A paired t-test revealed significantly higher KCC values (p < 0.05 corrected at the cluster level; third column) after THW than before THW in several brain regions, including the superior frontal cortex bilaterally, the left medial frontal cortex, the pre- and postcentral gyri bilaterally, the middle occipito-temporal cortices bilaterally, the left precuneus, the left lingual gyrus, the left inferior parietal cortex, and the right putamen. Color images available online at www.liebertpub.com/thy
A higher ReHo (KCC) was observed after THW in spatially distributed brain regions, including the superior frontal gyrus bilaterally, the left medial frontal gyrus, the pre- and postcentral gyri bilaterally, the middle temporo-occipital gyrus bilaterally, the left precuneus, the left lingual gyrus, the left inferior parietal gyrus, and the right putamen (Fig. 2 and Table 2).
Table 2.
Talairach Coordinates and t-Scores for Peak Voxels
| Brain region | Laterality | x | y | z | Cluster size | t-Score |
|---|---|---|---|---|---|---|
| Superior frontal gyrus | R | 23 | 45 | 32 | 2474 | 4.83 |
| L | −21 | 45 | 32 | 1136 | 3.51 | |
| Medial frontal gyrus | L | −1 | 51 | 4 | 194 | 4.17 |
| Post/precentral gyrus | R | 59 | −11 | 22 | 845 | 5.46 |
| L | −57 | −25 | 16 | 650 | 3.51 | |
| Middle/Inferior occipito-temporal gyrus | R | 47 | −67 | 6 | 449 | 3.35 |
| L | −45 | −69 | 4 | 303 | 3.63 | |
| L | −49 | −61 | 14 | 212 | 3.37 | |
| Precuneus | L | −3 | −45 | 50 | 245 | 3.27 |
| Lingual gyrus | L | −11 | −53 | 4 | 219 | 3.59 |
| Inferior parietal gyrus | L | −29 | −41 | 52 | 166 | 3.73 |
| Putamen | R | 23 | 9 | 6 | 128 | 4.77 |
Family-wise error corrected across the whole brain volume with a cluster significance level of p < 0.05 and >124 voxels.
ROI correlational analysis
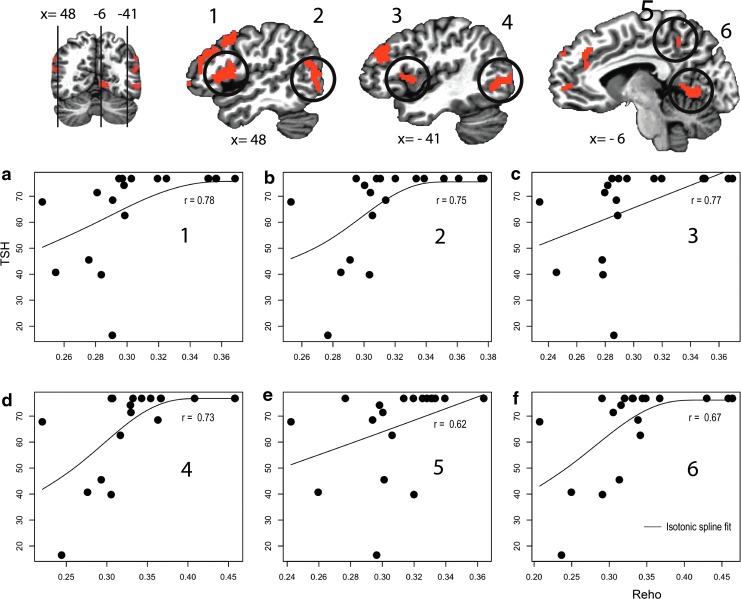
Among 12 cortical regions showing significant increases in ReHo after THW, six regions showed significant positive correlations between the levels of TSH and the mean ReHo values measured after THW, including pre- and postcentral gyri bilaterally, middle temporo-occipital gyri bilaterally, left precuneus, and left lingual gyrus (p < 0.005; Fig. 3).
FIG. 3.
Among the regions of interest (ROIs) showing significantly higher ReHo values after THW in resting state fMRI, 6/12 ROIs (1–6 in first row) showed significant correlations between ReHo values and serum thyrotropin (TSH) levels after THW. After THW, the ReHo values in (a) the right and (c) the left pre- and postcentral gyri, (b) the right and (d) the left middle temporo-occipital cortices, (e) the left precuneus, and (f) the left ligual gyrus showed positive correlations with the serum TSH level. Color images available online at www.liebertpub.com/thy
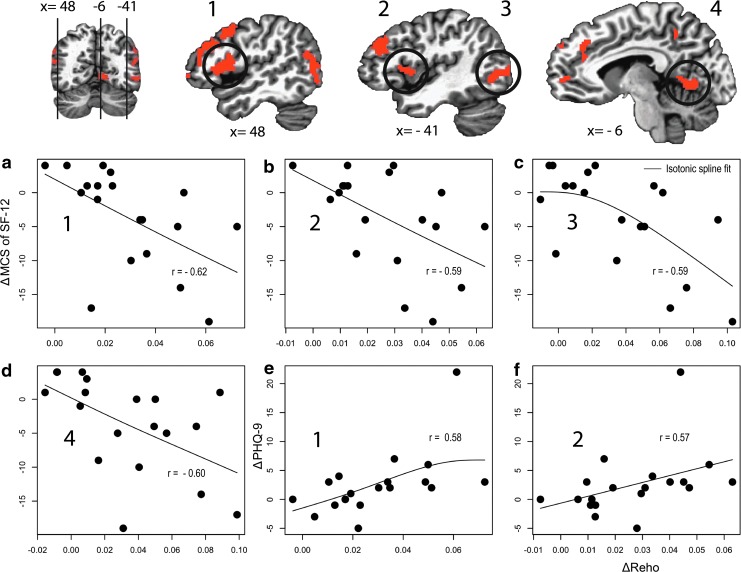
For the six ROIs, changes in ReHo values were correlated with changes in the PCS and MCS of the SF-12 and in the PHQ-9, which reflect physical and mental functioning and depressive symptoms, respectively. There was a significant negative correlation between changes in the MCS of the SF-12 and changes in ReHo values in the pre- and postcentral gyri bilaterally, the left middle temporo-occipital cortices, and the left ligual gyrus (p < 0.005), and changes in the total score of the PHQ-9 correlated significantly with changes in ReHo values in the pre- and postcentral gyri bilaterally (p < 0.0125; Fig. 4).
FIG. 4.
Among the ROIs showing significantly higher ReHo values after THW in resting state fMRI, several ROIs (1–4 in first row) showed significant correlations between changes in ReHo values and changes in total mental component summary score of the 12-Item Short Form Health Survey (SF-12) (a–d) and the total score of Patient Health Questionnaire-9 (PHQ-9) (e and f). After THW, higher ReHo values in (a) the right and (b) the left pre- and postcentral gyri, (c) the left middle temporo-occipital cortices, and (d) the left ligual gyrus showed correlations with lower total mental component summary (MCS) of the SF-12. Higher ReHo values in (e) the right and (f) the left pre- and postcentral gyri showed correlations with higher total scores of the PHQ-9. Color images available online at www.liebertpub.com/thy
Discussion
This study found that ReHo increased in various brain areas after THW. The brain regions affected by THW, which were extensive, included both sides of the superior frontal, medial frontal, pre- and postcentral and middle temporo-occipital gyri, the left lingual gyrus, the left inferior parietal gyrus, the left precuneus, and the right putamen, which together encompass the motor, sensory, visual, and association cortices (34–37). The superior frontal gyrus is reported to be associated with attention and response inhibition (38–41). The middle temporo- occipital gyrus has a major role in visual motion perception (42). The left medial frontal cortex and the left precuneus are part of a default mode network, a system involved in self-referential processes (43–46). The putamen regulates movement and motion related learning (47,48). The findings of this study suggest that THW affects multiple domains of brain function, including motor and sensory functions, motor-related learning, attention, response inhibition, and memory.
The mean ReHo values in many of the regions exhibiting higher ReHo values after THW correlated with serum TSH levels. In these areas, subjects with higher ReHo values had higher serum TSH levels after THW than before, suggesting a strong relationship between hypothyroidism and the degree of local connectivity, as measured by ReHo. The regions included pre- and postcentral gyri bilaterally, middle temporo-occipital gyri bilaterally, the left precuneus, and the left lingual gyrus, which correspond mostly to motor, somatosensory, and visual cortices. Changes in ReHo values in these regions correlated with changes in QoL measures and the depressive symptoms of the patients, suggesting that higher local connectivity had an effect on patients' psychological functions. Higher ReHo values in the bilateral pre- and postcentral and middle temporo-occipital gyri and the left lingual gyrus correlated with poorer mental QoL measures, as measured by the MCS of the SF-12. Higher ReHo values in the bilateral pre- and postcentral gyri correlated significantly with an aggravation of depressive symptoms, as measured by the PHQ-9.
In a previous study, higher ReHo correlated with higher regional CBF (49), which is consistent with the present findings showing that regions with higher ReHo, such as the medial prefrontal cortex and the precuneus, overlapped with the default mode network, which is known to have higher CBF during the resting state (50). However, CBF is not the only factor determining ReHo. For example, ReHo decreases during the anesthetized state in brain areas, while anesthesia usually results in lower CBF (51,52). In the current study, ReHo reflects synchronicity of BOLD signals within a scale of approximately a cubic centimeter. In a cubic centimeter of the human visual cortex, for example, >50 million neurons are found (53). The higher ReHo values could therefore indicate functional hyperconnectivity of subsets of neurons. Consistent with this hypothesis, higher ReHo in the frontal and occipital cortices has been observed in patients with autism spectrum disorder, possibly because cortical differentiation is reduced in this disorder (24).
Although the effects of THs on the adult human brain are still largely unknown, evidence from animal studies show that TH modulates neurotransmission and neuroplasticity. TH can act like a neurotransmitter (54,55) inhibiting GABA uptake (56) or enhancing presynaptic flux of Ca2+ in the adult rat brain (57). In rats, THs have been implicated in brain plasticity (58) and synaptogenesis (59,60). THW appears to disrupt synaptogenesis or neural plasticity, leading to alterations in the local functional brain network. It is also probable that TSH would have a direct effect on brain function since TSH receptors are found on the surface of some brain cells as well as the thyroid gland (61). In the TSH receptor (TSHR) knockout mouse, TSH-TSHR dysregulation was associated with behaviors similar to those observed in ADHD (62). In humans, a future study using exogenous administration of recombinant human TSH (rhTSH) to elevate rhTSH levels in the euthyroid state would be an informative control condition for comparison with the hypothyroid status in thyroidectomy patients. This comparison would help to clarify whether the increased FC in the present results is due to low TH or increased TSH.
There are several limitations in this study. The brain regions showing higher ReHo values did not overlap with those frequently reported in patients with depression, except for the superior frontal gyrus. Significant correlations between ReHo values and depressive symptoms measured by the PHQ-9 were only found in the bilateral pre- and postcentral gyri. The present study had sufficient power to detect small to moderate effect sizes, and a larger sample size may have detected additional impairments or changes in FC. The patients in this study only showed a trend toward more pronounced depressive symptoms after THW. Unlike the SF-12, which measures the multidimensional aspects of daily psychological symptoms, the PHQ-9 was devised mainly to diagnose major depression. Therefore, it may not have the sensitivity to evaluate mild mood symptoms, such as those reported in the current study. After THW, systolic BP, PR, and fT4 decreased as TSH increased. Decreased systolic BP and PR are consistent with the finding that hypothyroid patients have depressed inotropic and chronotropic cardiac function (63,64). The function of THs in increasing contractile force of cardiac myocytes is well known (63–65). However, the influence of hypothyroidism on systolic BP is still controversial. The lower systolic BP observed in this study is inconsistent with reports showing that patients with hypothyroidism have normal or higher systolic BP than healthy volunteers (66–68).
To the authors' knowledge, this is the first study comparing the FC of the brain before and after THW in patients with total thyroidectomy. It was found that two weeks of THW increased the synchronization of BOLD signals as measured by ReHo in various brain areas. The brain areas with higher ReHo values are known to be involved in motor, somatosensory, and visual functions, as well in various cognitive functions, including attention, memory, and learning. Among these regions, the motor, somatosensory, and visual cortices showed strong positive correlations between ReHo and TSH after THW. It is uncertain whether higher local connectivity after THW is a compensatory mechanism for lower neuronal function or over-connectivity, or a reflection of reduced cortical differentiation after THW. The observation that increased ReHo values in those brain areas correlated with lower QoL measures and more pronounced depressive symptoms favors the latter explanation.
In conclusion, this study has shown that local brain FC evaluated by resting fMRI is higher in multiple brain areas in the acute hypothyroid state and that higher brain FC correlates with poorer psychological QoL measures and increased depression in patients in the hypothyroid state. In future studies, utilization of comprehensive neurocognitive assessments in conjunction with QOL and depressive symptoms could yield valuable insights into the impact of adult hypothyroidism on cognitive performance and mood, the relationship of these functional measures to neural changes measured by FC, and the degree to which these disturbances are reversible with TH replacement.
Acknowledgments
This study was supported by a Research Grant (# GZ-RES12-RF003) from Genzyme Korea Co. Ltd., Seoul, Korea. The funders had no role in study design, data collection and analysis, or preparation of the manuscript.
Author Disclosure Statement
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- 1.Bernal J. 2007. Thyroid hormone receptors in brain development and function. Nat Clin Pract Endocrinol Metab 3:249–259 [DOI] [PubMed] [Google Scholar]
- 2.Fierro-Benitez R, Ramirez I, Garces J, Jaramillo C, Moncayo F, Stanbury JB. 1974. The clinical pattern of cretinism as seen in highland Ecuador. Am J Clin Nutr 27:531–543 [DOI] [PubMed] [Google Scholar]
- 3.Gold MS, Pottash AL, Extein I. 1981. Hypothyroidism and depression. Evidence from complete thyroid function evaluation. JAMA 245:1919–1922 [DOI] [PubMed] [Google Scholar]
- 4.Lass P, Slawek J, Derejko M, Rubello D. 2008. Neurological and psychiatric disorders in thyroid dysfunctions. The role of nuclear medicine: SPECT and PET imaging. Minerva Endocrinol 33:75–84 [PubMed] [Google Scholar]
- 5.Dow KH, Ferrell BR, Anello C. 1997. Quality-of-life changes in patients with thyroid cancer after withdrawal of thyroid hormone therapy. Thyroid 7:613–619 [DOI] [PubMed] [Google Scholar]
- 6.Bauer M, Heinz A, Whybrow PC. 2002. Thyroid hormones, serotonin and mood: of synergy and significance in the adult brain. Mol Psychiatry 7:140–156 [DOI] [PubMed] [Google Scholar]
- 7.Joffe RT.2002Should thyroid replacement therapy be considered for patients with treatment-refractory depression? J Psychiatry Neurosci 27:80. [PMC free article] [PubMed] [Google Scholar]
- 8.Cooke GE, Mullally S, Correia N, O'Mara SM, Gibney J. 2014. Hippocampal volume is decreased in adults with hypothyroidism. Thyroid 24:433–440 [DOI] [PubMed] [Google Scholar]
- 9.Constant EL, de Volder AG, Ivanoiu A, Bol A, Labar D, Seghers A, Cosnard G, Melin J, Daumerie C. 2001. Cerebral blood flow and glucose metabolism in hypothyroidism: a positron emission tomography study. J Clin Endocrinol Metab 86:3864–3870 [DOI] [PubMed] [Google Scholar]
- 10.Bauer M, Silverman DH, Schlagenhauf F, London ED, Geist CL, van Herle K, Rasgon N, Martinez D, Miller K, van Herle A, Berman SM, Phelps ME, Whybrow PC. 2009. Brain glucose metabolism in hypothyroidism: a positron emission tomography study before and after thyroid hormone replacement therapy. J Clin Endocrinol Metab 94:2922–2929 [DOI] [PubMed] [Google Scholar]
- 11.Smith CD, Ain KB. 1995. Brain metabolism in hypothyroidism studied with 31P magnetic-resonance spectroscopy. Lancet 345:619–620 [DOI] [PubMed] [Google Scholar]
- 12.Marangell LB, Ketter TA, George MS, Pazzaglia PJ, Callahan AM, Parekh P, Andreason PJ, Horwitz B, Herscovitch P, Post RM. 1997. Inverse relationship of peripheral thyrotropin-stimulating hormone levels to brain activity in mood disorders. Am J Psychiatry 154:224–230 [DOI] [PubMed] [Google Scholar]
- 13.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. 1995. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med 34:537–541 [DOI] [PubMed] [Google Scholar]
- 14.Anderson JS, Zielinski BA, Nielsen JA, Ferguson MA. 2014. Complexity of low-frequency blood oxygen level-dependent fluctuations covaries with local connectivity. Hum Brain Mapp 35:1273–1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rao JS, Ma M, Zhao C, Liu Z, Yang ZY, Li XG. 2015. Alteration of brain regional homogeneity of monkeys with spinal cord injury: a longitudinal resting-state functional magnetic resonance imaging study. Magn Reson Imaging 33:1156–1162 [DOI] [PubMed] [Google Scholar]
- 16.He Y, Wang L, Zang Y, Tian L, Zhang X, Li K, Jiang T. 2007. Regional coherence changes in the early stages of Alzheimer's disease: a combined structural and resting-state functional MRI study. NeuroImage 35:488–500 [DOI] [PubMed] [Google Scholar]
- 17.Spati J, Hanggi J, Doerig N, Ernst J, Sambataro F, Brakowski J, Jancke L, Grosse Holtforth M, Seifritz E, Spinelli S. 2015. Prefrontal thinning affects functional connectivity and regional homogeneity of the anterior cingulate cortex in depression. Neuropsychopharmacology 40:1640–1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cui Y, Jiao Y, Chen YC, Wang K, Gao B, Wen S, Ju S, Teng GJ. 2014. Altered spontaneous brain activity in type 2 diabetes: a resting-state functional MRI study. Diabetes 63:749–760 [DOI] [PubMed] [Google Scholar]
- 19.Sheline YI, Price JL, Yan Z, Mintun MA. 2010. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proc Natl Acad Sci U S A 107:11020–11025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hawellek DJ, Hipp JF, Lewis CM, Corbetta M, Engel AK. 2011. Increased functional connectivity indicates the severity of cognitive impairment in multiple sclerosis. Proc Natl Acad Sci U S A 108:19066–19071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo W, Liu F, Xiao C, Liu J, Yu M, Zhang Z, Zhang J, Zhao J. 2015. Increased short-range and long-range functional connectivity in first-episode, medication-naive schizophrenia at rest. Schizophr Res 166:144–150 [DOI] [PubMed] [Google Scholar]
- 22.Dosenbach NU, Nardos B, Cohen AL, Fair DA, Power JD, Church JA, Nelson SM, Wig GS, Vogel AC, Lessov-Schlaggar CN, Barnes KA, Dubis JW, Feczko E, Coalson RS, Pruett JR, Jr., Barch DM, Petersen SE, Schlaggar BL. 2010. Prediction of individual brain maturity using fMRI. Science 329:1358–1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lopez-Larson MP, Anderson JS, Ferguson MA, Yurgelun-Todd D. 2011. Local brain connectivity and associations with gender and age. Dev Cogn Neurosci 1:187–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maximo JO, Keown CL, Nair A, Muller RA. 2013. Approaches to local connectivity in autism using resting state functional connectivity MRI. Front Hum Neurosci 7:605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.An L, Cao XH, Cao QJ, Sun L, Yang L, Zou QH, Katya R, Zang YF, Wang YF. 2013. Methylphenidate normalizes resting-state brain dysfunction in boys with attention deficit hyperactivity disorder. Neuropsychopharmacology 38:1287–1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ware J, Jr, Kosinski M, Keller SD. 1996. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care 34:220–233 [DOI] [PubMed] [Google Scholar]
- 27.Kroenke K, Spitzer RL, Williams JB. 2001. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 16:606–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cox RW. 1996. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 29:162–173 [DOI] [PubMed] [Google Scholar]
- 29.Jo HJ, Saad ZS, Simmons WK, Milbury LA, Cox RW. 2010. Mapping sources of correlation in resting state FMRI, with artifact detection and removal. Neuroimage 52:571–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kendall MG, Gibbons JD. 1990. Rank Correlation Methods. Fifth edition. Oxford University Press, New York, NY [Google Scholar]
- 31.Zang Y, Jiang T, Lu Y, He Y, Tian L. 2004. Regional homogeneity approach to fMRI data analysis. NeuroImage 22:394–400 [DOI] [PubMed] [Google Scholar]
- 32.Zuo XN, Xu T, Jiang L, Yang Z, Cao XY, He Y, Zang YF, Castellanos FX, Milham MP. 2013. Toward reliable characterization of functional homogeneity in the human brain: preprocessing, scan duration, imaging resolution and computational space. NeuroImage 65:374–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Team RDC 2009. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria [Google Scholar]
- 34.Sherrill KR, Erdem UM, Ross RS, Brown TI, Hasselmo ME, Stern CE. 2013. Hippocampus and retrosplenial cortex combine path integration signals for successful navigation. J Neurosci 33:19304–19313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dukelow SP, DeSouza JF, Culham JC, van den Berg AV, Menon RS, Vilis T. 2001. Distinguishing subregions of the human MT+ complex using visual fields and pursuit eye movements. J Neurophysiol 86:1991–2000 [DOI] [PubMed] [Google Scholar]
- 36.Dresel C, Parzinger A, Rimpau C, Zimmer C, Ceballos-Baumann AO, Haslinger B. 2008. A new device for tactile stimulation during fMRI. NeuroImage 39:1094–1103 [DOI] [PubMed] [Google Scholar]
- 37.Piefke M, Kramer K, Korte M, Schulte-Ruther M, Korte JM, Wohlschlager AM, Weber J, Shah NJ, Huber W, Fink GR. 2009. Neurofunctional modulation of brain regions by distinct forms of motor cognition and movement features. Hum Brain Mapp 30:432–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dambacher F, Sack AT, Lobbestael J, Arntz A, Brugmann S, Schuhmann T. 2014. The role of right prefrontal and medial cortex in response inhibition: interfering with action restraint and action cancellation using transcranial magnetic brain stimulation. J Cogn Neurosci 26:1775–1784 [DOI] [PubMed] [Google Scholar]
- 39.McNab F, Leroux G, Strand F, Thorell L, Bergman S, Klingberg T. 2008. Common and unique components of inhibition and working memory: an fMRI, within-subjects investigation. Neuropsychologia 46:2668–2682 [DOI] [PubMed] [Google Scholar]
- 40.Menon V, Adleman NE, White CD, Glover GH, Reiss AL. 2001. Error-related brain activation during a Go/NoGo response inhibition task. Hum Brain Mapp 12:131–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Neta M, Miezin FM, Nelson SM, Dubis JW, Dosenbach NU, Schlaggar BL, Petersen SE. 2015. Spatial and temporal characteristics of error-related activity in the human brain. J Neurosci 35:253–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Kemenade BM, Seymour K, Wacker E, Spitzer B, Blankenburg F, Sterzer P. 2014. Tactile and visual motion direction processing in hMT+/V5. NeuroImage 84:420–427 [DOI] [PubMed] [Google Scholar]
- 43.Milton F, Muhlert N, Butler CR, Benattayallah A, Zeman AZ. 2011. The neural correlates of everyday recognition memory. Brain Cogn 76:369–381 [DOI] [PubMed] [Google Scholar]
- 44.Henson RN, Burgess N, Frith CD. 2000. Recoding, storage, rehearsal and grouping in verbal short-term memory: an fMRI study. Neuropsychologia 38:426–440 [DOI] [PubMed] [Google Scholar]
- 45.Botzung A, Denkova E, Manning L. 2008. Experiencing past and future personal events: functional neuroimaging evidence on the neural bases of mental time travel. Brain Cogn 66:202–212 [DOI] [PubMed] [Google Scholar]
- 46.Gutchess AH, Kensinger EA, Schacter DL. 2010. Functional neuroimaging of self-referential encoding with age. Neuropsychologia 48:211–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Buccino G, Vogt S, Ritzl A, Fink GR, Zilles K, Freund HJ, Rizzolatti G. 2004. Neural circuits underlying imitation learning of hand actions: an event-related fMRI study. Neuron 42:323–334 [DOI] [PubMed] [Google Scholar]
- 48.Lehericy S, Bardinet E, Tremblay L, Van de Moortele PF, Pochon JB, Dormont D, Kim DS, Yelnik J, Ugurbil K. 2006. Motor control in basal ganglia circuits using fMRI and brain atlas approaches. Cereb Cortex 16:149–161 [DOI] [PubMed] [Google Scholar]
- 49.Li Z, Zhu Y, Childress AR, Detre JA, Wang Z. 2012. Relations between BOLD fMRI-derived resting brain activity and cerebral blood flow. PLoS One 7:e44556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. 2001. A default mode of brain function. Proc Natl Acad Sci U S A 98:676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang Z, Wang Z, Zhang J, Dai R, Wu J, Li Y, Liang W, Mao Y, Yang Z, Holland G, Zhang J, Northoff G. 2014. Altered temporal variance and neural synchronization of spontaneous brain activity in anesthesia. Hum Brain Mapp 35:5368–5378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Qiu M, Ramani R, Swetye M, Rajeevan N, Constable RT. 2008. Anesthetic effects on regional CBF, BOLD, and the coupling between task-induced changes in CBF and BOLD: an fMRI study in normal human subjects. Magn Reson Med 60:987–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Granit R. 1977. The Purposive Brain. MIT Press, Cambridge, MA [Google Scholar]
- 54.Dratman MB, Gordon JT. 1996. Thyroid hormones as neurotransmitters. Thyroid 6:639–647 [DOI] [PubMed] [Google Scholar]
- 55.Sarkar PK, Durga ND, Morris JJ, Martin JV. 2006. In vitro thyroid hormone rapidly modulates protein phosphorylation in cerebrocortical synaptosomes from adult rat brain. Neuroscience 137:125–132 [DOI] [PubMed] [Google Scholar]
- 56.Mason GA, Walker CH, Prange AJ., Jr. 1987. Modulation of gamma-aminobutyric acid uptake of rat brain synaptosomes by thyroid hormones. Neuropsychopharmacology 1:63–70 [DOI] [PubMed] [Google Scholar]
- 57.Chakrabarti N, Ray AK. 2000. Rise of intrasynaptosomal Ca2+ level and activation of nitric oxide synthase in adult rat cerebral cortex pretreated with 3-5-3′-L-triiodothyronine. Neuropsychopharmacology 22:36–41 [DOI] [PubMed] [Google Scholar]
- 58.Alva-Sanchez C, Sanchez-Huerta K, Arroyo-Helguera O, Anguiano B, Aceves C, Pacheco-Rosado J. 2009. The maintenance of hippocampal pyramidal neuron populations is dependent on the modulation of specific cell cycle regulators by thyroid hormones. Brain Res 1271:27–35 [DOI] [PubMed] [Google Scholar]
- 59.Sala-Roca J, Estebanez-Perpina E, Balada F, Garau A, Marti-Carbonell MA. 2008. Effects of adult dysthyroidism on the morphology of hippocampal neurons. Behav Brain Res 188:348–354 [DOI] [PubMed] [Google Scholar]
- 60.Orford M, Mazurkiewicz D, Milligan G, Saggerson D. 1991. Abundance of the alpha-subunits of Gi1, Gi2 and Go in synaptosomal membranes from several regions of the rat brain is increased in hypothyroidism. Biochem J 275:183–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Davies T, Marians R, Latif R. 2002. The TSH receptor reveals itself. J Clin Invest 110:161–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mouri A, Hoshino Y, Narusawa S, Ikegami K, Mizoguchi H, Murata Y, Yoshimura T, Nabeshima T. 2014. Thyrotoropin receptor knockout changes monoaminergic neuronal system and produces methylphenidate-sensitive emotional and cognitive dysfunction. Psychoneuroendocrinology 48:147–161 [DOI] [PubMed] [Google Scholar]
- 63.Biondi B, Klein I. 2004. Hypothyroidism as a risk factor for cardiovascular disease. Endocrine 24:1–13 [DOI] [PubMed] [Google Scholar]
- 64.Klein I, Ojamaa K. 2001. Thyroid hormone and the cardiovascular system. N Engl J Med 344:501–509 [DOI] [PubMed] [Google Scholar]
- 65.Axelband F, Dias J, Ferrao FM, Einicker-Lamas M. 2011. Nongenomic signaling pathways triggered by thyroid hormones and their metabolite 3-iodothyronamine on the cardiovascular system. J Cell Physiol 226:21–28 [DOI] [PubMed] [Google Scholar]
- 66.Kotsis V, Alevizaki M, Stabouli S, Pitiriga V, Rizos Z, Sion M, Zakopoulos N. 2007. Hypertension and hypothyroidism: results from an ambulatory blood pressure monitoring study. J Hypertens 25:993–999 [DOI] [PubMed] [Google Scholar]
- 67.Fletcher AK, Weetman AP. 1998. Hypertension and hypothyroidism. J Hum Hypertens 12:79–82 [DOI] [PubMed] [Google Scholar]
- 68.Regalbuto C, Alagona C, Maiorana R, Di Paola R, Cianci M, Alagona G, Sapienza S, Vigneri R, Pezzino V. 2006. Acute changes in clinical parameters and thyroid function peripheral markers following L-T4 withdrawal in patients totally thyroidectomized for thyroid cancer. J Endocrinol Invest 29:32–40 [DOI] [PubMed] [Google Scholar]