Abstract
Despite availability of guidelines for lung cancer care, variations in lung cancer care among the elderly exist across the nation and are a cause for concern in rural and medically underserved areas. Therefore, the purpose of this study was to evaluate the patterns of lung cancer care and associated health outcomes among elderly residing in a rural and medically underserved area. The authors identified 1924 elderly lung cancer patients from the West Virginia Cancer Registry-Medicare linked database (2002–2007) and categorized them by receipt of guideline-concordant (appropriate and timely) care using guidelines from the American College of Chest Physicians, British Thoracic Society, and the RAND Corporation. Hierarchical generalized logistic models were constructed to identify variables associated with receipt of guideline-concordant care. Kaplan–Meier analysis and log-rank test were used to compare 3-year survival outcomes. Multivariate Cox proportional hazards models were constructed to estimate lung cancer mortality risk associated with nonreceipt of guideline-concordant care. Although guideline-concordant appropriate care was received by fewer than half of all patients (46.5%), of those receiving care, 78.7% received it in a timely manner. Delays in diagnosis and treatment varied significantly. Survival outcomes significantly improved with appropriate care (799 vs. 366 days; P≤0.05), but did not improve with timely care. This study highlights the critical need to address disparities in receipt of guideline-concordant lung cancer care among the elderly residing in rural and medically underserved areas. Although lung cancer diagnostic and management services are covered under the Medicare program, underutilization of these services is a concern. (Population Health Management 2016;19:109–119)
Introduction
Lung cancer is the leading cause of cancer deaths among elderly in the United States.1 Although it is associated with poor prognosis in the elderly, several treatment strategies can cure, or at least prolong survival. Therefore, significant reduction in lung cancer mortality can be achieved if the elderly receive quality cancer care that is both medically effective and timely. To that end, specific strategies for the management and treatment of lung cancer have been recommended in guidelines by the American College of Chest Physicians (ACCP), American Society for Clinical Oncology, National Cancer Institute (NCI), and others.2–7 These guidelines ensure uniformity of care, and are thought to be capable of improving quality and appropriateness of care. Standards for timely lung cancer care also have been recommended through clinical opinion-based guidelines by the British Thoracic Society (BTS), the RAND Corporation, and by the ACCP.4,8,9 However, despite availability of these guidelines, studies of clinical practice patterns in the United States have documented variations in the management of lung cancer by age, race, education, comorbidity, insurance, and hospital type.10–18 Therefore, lack of high-quality cancer care is a continuing concern and is attributable to variations in the use of appropriate standards of care.19
Although variations in lung cancer management and outcomes exist across the nation, it is a cause for major concern in rural areas. Many rural areas of the United States are economically underdeveloped and medically underserved.20 The elderly in these regions carry a higher burden of lung cancer compared to their urban counterparts.21 One such area is the Appalachian region, a population representing 8.1% of the total US population.22 West Virginia is the only state situated entirely within the Appalachian region and is the third most rural state in the nation.22 Fifty of the 55 counties in the state are designated as medically underserved areas, and all or part of 40 counties in the state are classified as health professional shortage areas.23 The age-adjusted lung cancer incidence and mortality rate among the elderly are higher in the state as compared to the United States.24,25 The observed lung cancer disparities in the rural population can be attributed to limited access to quality medical care facilities; less access to or utilization of early cancer detection programs; and increased prevalence of behavioral risk factors, such as tobacco use and sedentary life style, and socioeconomic factors, such as low income and education.26–28 In addition to being medically underserved, the rural population also may experience variations in the quality, availability, and accessibility of services when compared to their urban counterparts.29
Although numerous studies have examined lung cancer treatment variations in the United States, variations in guideline-concordant lung cancer care and their impact on health outcomes in the rural and medically underserved elderly population, such as West Virginia, remain unknown. Therefore, the objectives of this study were to: (1) identify lung cancer treatment patterns among medically underserved elderly patients, (2) determine delays in lung cancer diagnosis and treatment among medically underserved elderly patients, (3) identify the proportion of medically underserved elderly lung cancer patients receiving guideline-concordant care, (4) identify factors associated with the receipt of guideline-concordant care, (5) determine survival benefits associated with the receipt of guideline-concordant care, and (6) determine lung cancer mortality risk associated with the receipt of guideline-discordant care.
Methods
Data source
This study used West Virginia Cancer Registry (WVCR)-Medicare (WVCR-Linked) linked data files from years 2002–2007. The WVCR-Linked data files are similar in structure to the NCI's Surveillance, Epidemiology, and End Results (SEER)-Medicare linked data files, and represent data from the WVCR. Established by the West Virginia Department of Health and Human Resources in 1991, the WVCR is an all-site cancer registry collecting information on all cancers diagnosed and/or treated in the state of West Virginia. Details on the creation of WVCR-Linked data files can be found elsewhere.30 Cancer registry data files provided clinical, demographic, cause of death, and initial treatment information for elderly individuals with lung cancer in the state. The Medicare administrative data files provide the health service claims (utilization and reimbursement) information for care provided by physicians, inpatient hospital stays, hospital outpatient clinics, home health care agencies, skilled nursing facilities, and hospice programs.
Study cohorts
The researchers identified Medicare beneficiaries aged ≥66 years with incident lung cancer diagnosis (International Classification of Diseases for Oncology [ICD-O] codes: C34.0, C34.1, C34.2, C34.3, C34.8, C34.9, and C33.9; American Joint Committee on Cancer Staging [AJCC] Tumor Node Metastasis [TNM] stages: I–IV), between July 1, 2003, and December 31, 2006, (Cohort A), and between years 2003 through 2006 (Cohort B), in the WVCR-Linked data files. Cohort A was used to study appropriateness of cancer care, while Cohort B was used to study timeliness of cancer care. Creation of 2 separate cohorts for analysis was necessary as the guidelines used to study appropriateness and timeliness of cancer care were published by different organizations in 2003 and 1998, respectively. Specifically, the ACCP evidence-based guidelines for diagnosis and management of lung cancer were published in January 2003, and therefore patients were included in Cohort A starting July 1, 2003. Similarly, the clinical opinion-based guidelines for timeliness of lung cancer care were published by the BTS and the RAND Corporation in 1998 and 2000, respectively. Therefore patients were included in Cohort B starting January 1, 2003 (ie, the earliest date of data availability). Beneficiaries were excluded from either cohort if they were diagnosed only at death, had a prior malignancy, were enrolled in a managed care plan, or lacked Part A or B of Medicare.
Given the limited years of follow-up data, for study objectives 5 and 6, Cohort A was limited to patients with cancer diagnosis between July 1, 2003, and December 31, 2004, and Cohort B was limited to patients with cancer diagnosis during the years 2003 and 2004. These cohorts were followed for 3 years following cancer diagnosis to determine lung cancer specific mortality.
Assessing receipt of guideline-concordant appropriate care
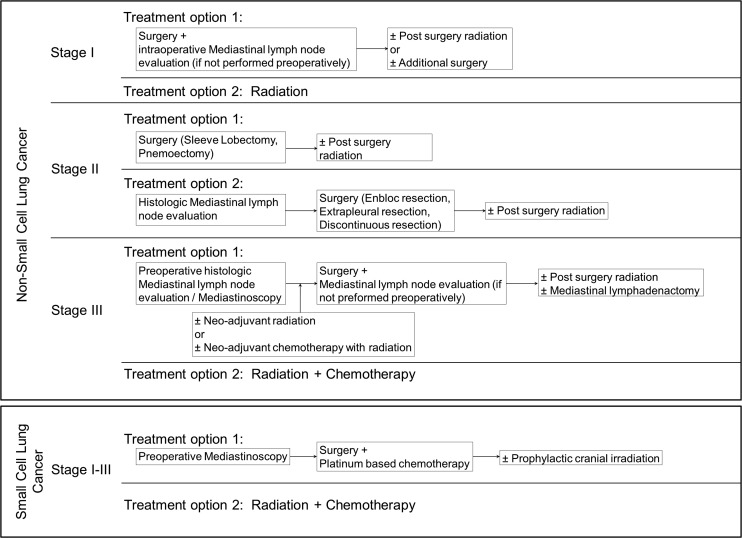
The ACCP evidence-based guidelines for diagnosis and management of lung cancer were incorporated in an algorithm and used to determine receipt of guideline-concordant appropriate care (Fig. 1).4 Specifically, patients with Stage I–III disease were followed for 1 year following their cancer diagnosis to determine receipt of appropriate care. Patients with Stage IV disease were excluded in this analysis, as the data source lacked complete treatment information for these patients. Lung cancer treatments and procedures were identified from Medicare claims data using appropriate International Classification of Diseases, Ninth Revision (ICD-9) diagnosis and procedure codes, Healthcare Common Procedure Coding System (HCPCS) codes, Current Procedural Terminology (CPT) codes, and revenue center codes (see online Appendix 1, available at www.liebertpub.com/pop).
FIG. 1.
Algorithm adapted from American College of Chest Physicians (ACCP) evidence-based guidelines for diagnosis and management of lung cancer published in January 2003, and used to determine receipt of guideline-concordant appropriate lung cancer care.
Assessing delays in diagnosis and treatment
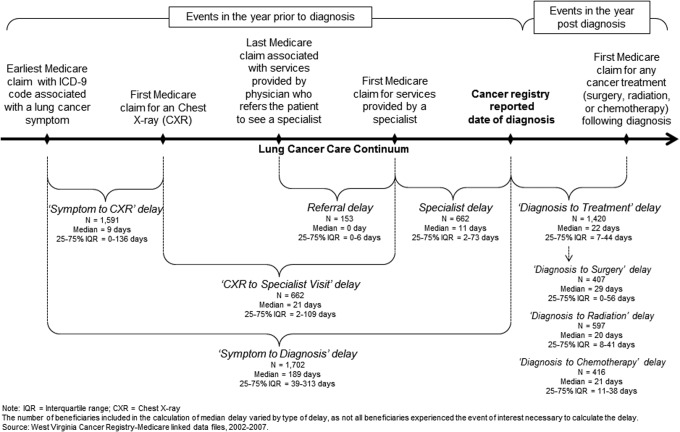
Figure 2 is a pictorial depiction of the approach to assessing delays in diagnosis and treatment. Specifically, delays in diagnosis were determined by following the patients during the year prior to the incident lung cancer diagnosis and were categorized as Symptom to Chest X-Ray (CXR) delay, CXR to Specialist Visit delay, Specialist delay, and Referral delay.
FIG. 2.
Delays in diagnosis and treatment among continuously enrolled Medicare Fee-for-service beneficiaries with incident lung cancer diagnosis in West Virginia, 2003 through 2006.
CXR, chest X-ray; ICD-9, International Classification of Diseases, Ninth Revision; IQR, interquartile range.
The number of beneficiaries included in the calculation of median delay varied by type of delay, as not all beneficiaries experienced the event of interest necessary to calculate the delay.
Source: West Virginia Cancer Registry–Medicare linked data files, 2002–2007.
Given the retrospective nature of the data sources, the research team estimated the occurrence of earliest lung cancer symptoms by identifying the earliest Medicare claim (date) that had an ICD-9 code associated with either: (1) symptoms of primary tumor, (2) symptoms of intrathoracic spread, (3) symptoms of extrathoracic metastases, or (4) paraneoplastic syndromes (see online Appendix 1 for complete list of symptoms). The Symptom to CXR delay was then defined as the time from the earliest Medicare claim date with lung cancer symptom until the date of first Medicare claim for a CXR. The CXR to Specialist Visit delay was defined as the time from the first Medicare claim for a CXR until the date of first Medicare claim on which the service provider was a specialist, such as a respiratory/chest physician, pulmonologist, oncologist, cardiologist, or thoracic/cardiac/regular surgeon. The Specialist delay was defined as the time from the Medicare claim for the first specialist appointment until the date of cancer diagnosis. Among beneficiaries who were referred to a specialist, Referral delay was defined as the time from the last Medicare claim associated with services provided by the referring physician until the date of first Medicare claim on which the service provider was the referred specialist. The overall delay in diagnosis, Symptom to Diagnosis delay, was defined as the time from the earliest Medicare claim date with a lung cancer symptom until the date of cancer diagnosis. Specific delays in diagnosis were identified only among those patients who had Medicare claims associated with events of interest.
Delay in treatment was determined by following the patients for 1 year following incident lung cancer diagnosis. Specifically, Diagnosis to Treatment delay was defined as the time from cancer diagnosis until the date of first Medicare claim for surgery, radiation, or chemotherapy. Lung cancer–specific treatments and procedures were identified from the Medicare claim data files using appropriate ICD-9, HCPCS, CPT, and revenue center codes (see online Appendix 1).
Assessing receipt of guideline-concordant timely care
Guideline-concordant timely lung cancer care was defined using clinical opinion-based guidelines published by the BTS and the RAND Corporation.8,9 The BTS recommends that the duration between cancer diagnosis and initial treatment to be no more than 8 weeks for surgery, 7 weeks for radiotherapy, and 4 weeks for chemotherapy.8 On the other hand, the RAND Corporation recommends any planned treatment to be offered within 6 weeks of the diagnosis date.9 To incorporate recommendations from both guidelines, this study defined timely care by selecting the maximum duration allowed under either guideline for a given type of treatment. Therefore, initial treatment was considered timely if the duration between diagnosis date and treatment date was no more than 8 weeks for surgery, 7 weeks for radiotherapy, and 6 weeks for chemotherapy. Patients receiving no treatment in the year following their cancer diagnosis were excluded from this analysis.
Dependent variables
Treatment patterns were categorized as surgery only, radiation only, chemotherapy only, combination treatment, or no treatment. Primary outcomes of interest, receipt of guideline-concordant appropriate cancer care, was categorized as Yes or No, while receipt of guideline-concordant timely cancer care was categorized as Timely Care or Delayed Care. Survival time in days was calculated from the date of cancer diagnosis to the date of death or the 3-year follow-up cutoff date, whichever came first. Date of death was identified from Medicare enrollment records. To estimate lung cancer–specific survival, patients not found to be deceased by the cutoff date, or who died from causes other than lung cancer were censored at that time and considered to be alive.
Covariates
Based on prognostic significance, covariates included lung cancer type and stage, age at diagnosis, sex, race, urban–rural residence, comorbidity, and measures of socioeconomic status. Lung cancer type was categorized based on cell histology. Patients with ICD-O histology codes 8000–8040 or 8046–9989 were categorized as having non-small cell lung cancer (NSCLC) disease, and those with codes 8041–8045 were categorized as having small cell lung cancer (SCLC) disease. Lung cancer stage was categorized based on the AJCC TNM staging system. Age at diagnosis was categorized as 66–69 years, 70–74 years, 75–79 years, and 80 years and older. Given that the population in West Virginia is predominantly white, race was categorized as white or nonwhite. Urban–rural residence was categorized as metro, urban, or rural, using the Rural–Urban Continuum codes. Comorbidity was estimated using a modified Charlson comorbidity score, based on inpatient claims from the year preceding the cancer diagnosis.31–33 This method of creating a weighted comorbidity score has been reported previously and used for both outcomes and patterns of care analysis.31–33 Given the lack of individual socioeconomic measures in the study data source, median household income and the percentage of individuals with some college education in the census tract of residence were used as markers of socioeconomic status.
Data analysis
Pearson chi-square tests were used to determine unadjusted associations between categorical variables of interest. Median delays (with 25% and 75% interquartiles) in diagnosis and treatment were calculated and compared using nonparametric tests. Specifically, the Mann-Whitney test was used for pairwise comparison of delays and the Kruskal-Wallis test was used for analyses involving multiple groups. Two hierarchical generalized logistic models were constructed with PROC GLIMMIX procedure in SAS 9.2 (SAS Institute Inc., Cary, North Carolina) to identify factors associated with receipt of appropriate care and timely care, respectively, among elderly patients. In the models, the estimated probability of a patient receiving guideline-concordant care conditioned on a set of predictor variables was modeled. The models treated census tract as a random effect to account for potential correlation among patients within the same county. Nonparametric estimates of the survivor function by receipt of appropriate care and timely care were calculated using the Kaplan-Meier method. Survival differences were assessed using the log-rank test. Multivariate Cox proportional hazards models were constructed to estimate lung cancer mortality risk associated with receipt of guideline-discordant care among elderly. To evaluate the proportional hazards assumption, smoothed Schoenfeld residuals were plotted against time and no evidence was found of a systematic deviation from proportional hazards in any model. Variances in models were adjusted to account for patient clustering at the census tract level by the use of the robust inference of Lin and Wei.34 All analyses were performed with SAS 9.2. The study was approved by the West Virginia University Institutional Review Board.
Results
Patient characteristics
Based on the inclusion and exclusion criteria, 1689 (Cohort A) and 1924 (Cohort B) patients were identified from the WVCR-Linked database. Table 1 shows the distribution of clinical and sociodemographic characteristics of these patients by type of lung cancer. The majority of the patients had NSCLC diagnosis and late-stage disease. A majority of the SCLC patients were diagnosed at late stages, as compared to NSCLC patients (P≤0.05).
Table 1.
Characteristics of Continuously Enrolled Medicare Fee-for-Service Beneficiaries with Incident Lung Cancer Diagnosis in West Virginia, July 2003 through December 2006 (Cohort A), and 2003–2006 (Cohort B)
| Proportion (%) | ||||
|---|---|---|---|---|
| Non-Small Cell Lung Cancer | Small Cell Lung Cancer | |||
| Characteristics | Cohort A | Cohort B | Cohort A | Cohort B |
| Overall, n (%) | 1,444 (85.5) | 1,641 (85.3) | 245 (14.5) | 283 (14.7) |
| AJCC-TNM Stage^ | ||||
| I | 26.9 | 27.1 | 6.9 | 7.1 |
| II | 9.8 | 9.4 | - | 4.6 |
| III | 23.3 | 23.6 | 25.3 | 25.8 |
| IV | 40 | 39.9 | 63.3 | 62.5 |
| Age (years) | ||||
| 66–69 | 23 | 22.6 | 24.9 | 25.8 |
| 70–74 | 29.4 | 29.9 | 30.6 | 30 |
| 75–79 | 26 | 26.3 | 23.7 | 23.7 |
| ≥80 | 21.5 | 21.2 | 20.8 | 20.5 |
| Sex | ||||
| Male | 58.2 | 58 | 51.8 | 53 |
| Female | 41.8 | 42 | 48.2 | 47 |
| Race | ||||
| Nonwhite | 2.2 | 2.1 | - | - |
| White | 97.8 | 97.9 | 99.2 | 99.3 |
| Urban–rural residence | ||||
| Metro | 54.8 | 54.2 | 60 | 60.4 |
| Urban | 39.5 | 40.1 | 32.2 | 32.5 |
| Rural | 5.6 | 5.7 | 7.8 | 7.1 |
| Comorbidity, Charlson score | ||||
| 0 | 26.5 | 26.9 | 30.2 | 30 |
| 1 | 29.9 | 30 | 29.4 | 30 |
| ≥2 | 43.6 | 43.1 | 40.4 | 39.9 |
AJCC, American Joint Committee on Cancer; TNM, Tumor Node Metastasis.
Association between characteristic and cancer type among beneficiaries; chi-square tests (P≤0.05).
-Cell size suppressed to meet privacy guidelines.
Source: West Virginia Cancer Registry-Medicare linked data files, 2002–2007.
Treatment patterns
More than a quarter of all patients received no treatment for lung cancer (Table 2). Among those patients receiving any treatment, the most common regimens included combination therapy (30.3%) followed by radiotherapy. Significant variations in treatment patterns were observed by lung cancer type, stage, age, sex, urban–rural residence, and comorbidity score. Specifically, the proportion of patients receiving surgery or radiation was higher among NSCLC patients as compared to SCLC patients (P≤0.05). Similarly, the proportion of patients receiving surgery also was higher among those with early-stage disease, as compared to those with late-stage disease (P≤0.05).
Table 2.
Descriptive Characteristics by Type of Treatment among Continuously Enrolled Medicare Fee-for-Service Beneficiaries with Incident Lung Cancer Diagnosis in West Virginia, July 2003 through December 2006
| Proportion (%)# | |||||
|---|---|---|---|---|---|
| Characteristics | No Treatment | Surgery Only | Radiation Only | Chemotherapy Only | Combination Treatment |
| Overall, n (%) | 453 (26.8) | 228 (13.5) | 321 (19.0) | 176 (10.4) | 511 (30.3) |
| Cancer type^ | |||||
| NSCLC | 26.7 | 15.7 | 20.6 | 8.4 | 28.6 |
| SCLC | 27.8 | - | 9.4 | 22.5 | 40 |
| AJCC TNM stage^ | |||||
| I | 17.5 | 43.2 | 11.4 | - | 24.9 |
| II | 19.6 | 22.2 | 12.4 | - | 43.1 |
| III | 25.9 | - | 18.3 | 11.8 | 40.5 |
| IV | 34 | - | 25 | 15.4 | 25 |
| Age (years)^ | |||||
| 66–69 | 20.6 | 14 | 13 | 10.4 | 42 |
| 70–74 | 21.8 | 14.6 | 18.4 | 11 | 34.2 |
| 75–79 | 27.9 | 14.1 | 21.2 | 10.4 | 26.5 |
| 80 or more | 39.2 | 10.8 | 23.8 | 9.7 | 16.6 |
| Sex^ | |||||
| Male | 29.6 | 12.4 | 18.3 | 9.8 | 29.9 |
| Female | 23.1 | 15 | 19.9 | 11.2 | 30.7 |
| Race | |||||
| Nonwhite | 44.1 | 11.8 | 11.8 | 14.7 | 17.7 |
| White | 26.5 | 13.5 | 19.2 | 10.3 | 30.5 |
| Urban–rural residence^ | |||||
| Metro | 27.6 | 13.4 | 20.7 | 8.4 | 29.9 |
| Urban | 26.8 | 14.2 | 17.1 | 12.2 | 29.8 |
| Rural | 20 | 10 | 16 | 18 | 36 |
| Comorbidity, Charlson score^ | |||||
| 0 | 31.3 | 10.3 | 17.9 | 10.9 | 29.5 |
| 1 | 21.6 | 15.7 | 18.8 | 10.1 | 33.7 |
| ≥2 | 27.6 | 14 | 19.8 | 10.3 | 28.3 |
NSCLC, non-small cell lung cancer; SCLC, small cell lung cancer; AJCC, American Joint Committee on Cancer; TNM, Tumor Node Metastasis.
Proportions reported are row percentages of beneficiaries receiving particular treatment.
Association between characteristic and type of treatment among beneficiaries; chi-square tests (P≤0.05).
-Cell size suppressed to meet privacy guidelines.
Source: West Virginia Cancer Registry-Medicare linked data files, 2002–2007.
Delays in diagnosis and treatment
Earliest lung cancer symptoms commonly reported among patients included chest pain (21.9%), cough (15.4%), weakness (14.9), and dyspnea (14.8%). Although median Symptom to Diagnosis delay was approximately 6 months, the median Diagnosis to Treatment delay was less than a month on average (Figure 2). Median Symptom to CXR, CXR to Specialist Visit, and Specialist delays were less than 2 weeks. Diagnosis to Treatment delays were shortest in patients receiving radiation and were longest in patients receiving surgery. Symptom to Diagnosis delay was longer among patients with early-stage disease, old age, female sex, and high comorbidity score (P≤0.05). However, Diagnosis to Treatment delay was only longer among patients with NSCLC, and early-stage disease (P≤0.05; data not shown).
Receipt of guideline-concordant lung cancer care
Table 3 shows the descriptive characteristics of patients by receipt of guideline-concordant (appropriate and timely) lung cancer care. Fewer than half of all patients (46.5%) received appropriate care in the study population. However, of those patients receiving any cancer care, 78.7% received it in a timely manner. Overall, patients receiving appropriate care were mostly younger and of female sex (P≤0.05). On the other hand, the proportions of patients receiving timely care were higher among those with SCLC diagnosis and late-stage disease (P≤0.05).
Table 3.
Descriptive Characteristics by Receipt of Guideline-Concordant Care among Continuously Enrolled Medicare Fee-for-Service Beneficiaries with Incident Lung Cancer Diagnosis in West Virginia, 2003 through 2006
| Proportion (%)# | ||||
|---|---|---|---|---|
| Appropriateness of Care@ | Timeliness of Care∼ | |||
| Characteristics | Guideline-Concordant | Guideline-Discordant | Timely Care | Delayed Care |
| Overall, n (%) | 444 (46.5%) | 511 (53.5%) | 1,118 (78.7%) | 302 (21.3%) |
| Lung cancer type^ | ||||
| NSCLC | 47.2 | 52.8 | 76.8 | 23.2 |
| SCLC | 40 | 60 | 90.2 | 9.9 |
| AJCC TNM stage^ | ||||
| I | 89.2 | 10.8 | 74 | 26 |
| II | 65.4 | 34.6 | 74.4 | 25.6 |
| III | 84.8 | 15.2 | 79.2 | 20.8 |
| IV | n/a | n/a | 82.8 | 17.2 |
| Age (years)+ | ||||
| 66–69 | 51.8 | 48.2 | 78.6 | 21.4 |
| 70–74 | 53.9 | 46.1 | 80.4 | 19.6 |
| 75–79 | 45 | 55 | 77.8 | 22.2 |
| ≥80 | 30.4 | 69.6 | 77.2 | 22.8 |
| Sex+ | ||||
| Male | 42.9 | 57.1 | 79.9 | 20.1 |
| Female | 51.2 | 48.8 | 77.2 | 22.8 |
| Race | ||||
| Nonwhite | 38.9 | 61.1 | 66.7 | 33.3 |
| White | 46.7 | 53.3 | 78.9 | 21.1 |
| Urban–rural residence | ||||
| Metro | 46.8 | 53.2 | 76.6 | 23.4 |
| Urban | 47.2 | 52.8 | 80.9 | 19.1 |
| Rural | 39.6 | 60.4 | 83.5 | 16.5 |
| Comorbidity, Charlson score | ||||
| 0 | 45 | 55 | 77.2 | 22.8 |
| 1 | 49.3 | 50.7 | 80.2 | 19.8 |
| ≥2 | 45.6 | 54.4 | 78.6 | 21.4 |
NSCLC, non-small cell lung cancer; SCLC, small cell lung cancer; AJCC, American Joint Committee on Cancer; TNM,Tumor Node Metastasis.
Guideline-concordant appropriate care determined using American College of Chest Physicians evidence-based guidelines for diagnosis and management of lung cancer, January 2003.
Guideline-concordant timely lung cancer care determined using British Thoracic Society and RAND Corporation clinical opinion-based guidelines for management of lung cancer.
Row percentages.
Association between beneficiary characteristics and receipt of guideline-concordant appropriate care; chi-square test (P≤0.05).
Association between beneficiary characteristics and receipt of guideline-concordant timely care; chi-square test (P≤0.05).
Source: West Virginia Cancer Registry-Medicare linked data files, 2002–2007.
Factors associated with receipt of guideline-concordant care
Controlling for sociodemographic characteristics, age remained a strong predictor of receipt of appropriate care (Table 4). Compared to patients aged ≥80 years, those aged 66–69 years were more than twice as likely to receive appropriate care, and the odds gradually decreased with increase in age. However, age was not a significant predictor of timely care. Sex was the only other significant predictor of receipt of appropriate care, while lung cancer type and stage were the only other significant predictors of timely care. Specifically, male patients were 27% (P<0.05) less likely to receive appropriate care compared to females. Similarly, NSCLC patients were 60% (P<0.001) less likely to receive timely care compared to SCLC patients.
Table 4.
Factors Associated with Receipt of Guideline-Concordant Care among Continuously Enrolled Medicare Fee-for-Service Beneficiaries with Incident Diagnosis of Lung Cancer in West Virginia, 2003 through 2006
| Receipt of Guideline-concordant care;Odds Ratio (95% Confidence Interval) | ||
|---|---|---|
| Characteristics | Appropriate Care# | Timely Care∼ |
| Lung cancer type | ||
| NSCLC | 1.26 (0.97 to 1.59) | 0.40*** (0.24 to 0.66) |
| SCLC | 1 (Ref) | 1 (Ref) |
| AJCC TNM stage | ||
| I | 1.29 (0.98 to 1.55) | 0.67* (0.48 to 0.93) |
| II | 1.08 (0.73 to 1.37) | 0.69 (0.43 to 1.09) |
| III | 1 (Ref) | 0.83 (0.58 to 1.19) |
| IV | n/a | 1 (Ref) |
| Age (years) | ||
| 66–69 | 2.50*** (1.65 to 3.79) | 1.06 (0.70 to 1.59) |
| 70–74 | 2.68*** (1.81 to 3.98) | 1.24 (0.84 to 1.85) |
| 75–79 | 1.84** (1.22 to 2.77) | 1.06 (0.71 to 1.58) |
| ≥80 | 1 (Ref) | 1 (Ref) |
| Sex | ||
| Male | 0.73* (0.56 to 0.95) | 1.16 (0.89 to 1.52) |
| Female | 1 (Ref) | 1 (Ref) |
| Race | ||
| Nonwhite | 0.77 (0.25 to 2.34) | 0.60 (0.21 to 1.69) |
| White | 1 (Ref) | 1 (Ref) |
| Urban–rural residence | ||
| Metro | 1.50 (0.82 to 2.77) | 0.63 (0.33 to 1.19) |
| Urban | 1.44 (0.78 to 2.66) | 0.91 (0.48 to 1.73) |
| Rural | 1 (Ref) | 1 (Ref) |
| Comorbidity, Charlson score | ||
| 0 | 0.95 (0.68 to 1.32) | 0.85 (0.61 to 1.17) |
| 1 | 1.14 (0.83 to 1.55) | 1.07 (0.78 to 1.47) |
| ≥2 | 1 (Ref) | 1 (Ref) |
| Percentage with some college education (%)^ | ||
| 0.0–0.10 | 0.34 (0.09 to 1.31) | 0.70 (0.24 to 2.08) |
| 0.11–0.20 | 1.20 (0.90 to 1.59) | 1.01 (0.74 to 1.37) |
| ≥0.21 | 1 (Ref) | 1 (Ref) |
| Median household income ($)^ | ||
| 0–25,000 | 1.53 (0.64 to 3.66) | 0.74 (0.33 to 1.63) |
| 25,001–50,000 | 1.58 (0.70 to 3.59) | 1.03 (0.49 to 2.17) |
| ≥50,001 | 1 (Ref) | 1 (Ref) |
NSCLC, non-small cell lung cancer; SCLC, small cell lung cancer; AJCC, American Joint Committee on Cancer; TNM,Tumor Node Metastasis.
Statistical significance: *P≤0.05; **P≤0.01; ***P≤0.001.
Census tract level measure.
Guideline-concordant appropriate care determined using American College of Chest Physicians evidence-based guidelines for diagnosis and management of lung cancer, January, 2003.
Guideline-concordant timely lung cancer care determined using British Thoracic Society and RAND Corporation clinical opinion-based guidelines for management of lung cancer.
Model 1: N=956, Fit Statistics: −2 restricted log pseudo-likelihood=4110.26, Covariance parameter estimates: Intercept=county, estimate=0.33, standard error=0.001.
Model 2: N=1420, Fit Statistics: −2 restricted log pseudo-likelihood=6639.58, Covariance parameter estimates: Intercept=county, estimate=0.14, standard error=0.10.
Source: West Virginia Cancer Registry-Medicare linked data files, 2002–2007.
Survival outcomes by receipt of guideline-concordant care
Three-year median survival time significantly exceeded by 433 days among patients receiving appropriate care (799 vs. 366 days; P≤0.05). However, contrary to expectation, survival outcomes were statistically no different among patients receiving timely care compared to those receiving delayed care (299 vs. 467 days). Stratified analysis by lung cancer type and stage showed similar results (data not shown).
Lung cancer mortality risk associated with receipt of guideline-discordant lung cancer care
In the Cox proportional hazards model assessing appropriateness of care, the adjusted lung cancer mortality risk was significantly higher among patients receiving guideline-discordant care, relative to those receiving guideline-concordant care (Hazard ratio (HR)=1.60, 95% Confidence interval (CI)=(1.23–2.10); P<0.001). Specifically, lung cancer mortality risk among patients receiving guideline-discordant care increased by 60%. However, paradoxical results were observed in the Cox proportional hazards model assessing timeliness of care. Specifically, the adjusted lung cancer mortality risk was found to be significantly lower among patients receiving delayed care relative to those receiving timely care (HR (95% CI)=0.75 (0.60–0.95); P<0.05) (data not shown).
Discussion
In 1990, the seminal report Ensuring Quality Cancer Care by the Institute of Medicine recommended the need for cancer disparities research so as to optimize the delivery of cancer care for all Americans.19 Despite the fervor generated by this report, lung cancer disparities still exist in the rural and medically underserved elderly population, and can be attributed to variations in lung cancer care. To that end, this population-based analysis studied the patterns of guideline-concordant lung cancer care and associated health outcomes among elderly patients residing in a rural and medically underserved state.
Lung cancer treatment patterns varied significantly among elderly patients in the state. Despite the availability of different treatment options, many patients did not receive any treatment. The majority of these patients had late-stage disease and/or were of older age. Therefore, disease severity may partly explain the lack of treatment among these elderly patients. Among patients receiving care, delays in lung cancer diagnosis and treatment also ranged widely in the state. The median Symptom to Diagnosis delay of >6 months was longer than expected and could be minimized if all diagnostic investigations are planned during the initial visit to a physician. On the other hand, the median Diagnosis to Treatment delays were more or less similar to that reported in prior studies (range: 12.5–52 days).17 Surgically treated patients had longer delays than those treated nonsurgically, a difference that likely reflects the extra time needed to refer patents to a thoracic surgeon for additional treatment consideration. A multidisciplinary team approach involving both surgeons and oncologists in the care process may help to minimize such delay.2
Overall, guideline-concordant appropriate care was only received by fewer than half of all patients. This proportion was higher than observed in one study (44.7%),18 but lower than that reported in other previous studies (range: 52%–76%).10,13–16 The comprehensive nature of this study, capturing the appropriateness of lung cancer staging prior to the receipt of treatment, may partly explain the differences in findings. The proportion of patients receiving appropriate care significantly decreased with an increase in age at diagnosis. This finding is similar to that reported in prior studies, and may be attributed to comorbidity burden in patients, physician treatment choice, and/or individual treatment preferences.10–12 Surprisingly, the majority of patients receiving care received it in a timely fashion. Patients with NSCLC or early-stage disease were less likely to receive timely care compared to those with SCLC or late-stage disease. This finding is likely as patients with limited disease may have to wait significantly longer for treatment than those with advanced disease.2 This finding also indicates that severity of disease at presentation may influence the speed of the medical decision-making process. Contrary to expectation, urban–rural disparities in receipt of appropriate care and timely care were not observed in this study. This result may be explained by the fact that the majority of the counties in West Virginia are medically underserved and are classified as health professional shortage areas.23 Similarly, census-tract level measures of patients socioeconomic status also failed to explain the observed disparities in receipt of appropriate care and timely care in this population. This finding was expected as the state population is generally characterized as being poor and undereducated.23
As expected, receipt of guideline-concordant appropriate care significantly improved survival outcomes in patients. Furthermore, the adjusted lung cancer mortality risk was found to be significantly higher among patients not receiving appropriate care. These findings justify the need for uniformity in cancer care through universal adoption of evidence-based guidelines for lung cancer management and treatment. However, contrary to expectation, timely care was not associated with better prognosis in these patients. Although this result corroborates findings from previous studies,35–37 it contradicts findings from 2 US studies.38,39 The observed paradoxical results may be explained by selection bias, as symptomatic patients with advanced stage disease are more likely to receive prompt (timely) care, despite their poor prognosis to begin with. Although further research is needed to explore the association between timely care and survival in these patients, data from this study highlight the opportunities for improvement in cancer care in this population.
Several limitations of this study should be noted. Although cancer registry linked claims data were used, an inherent limitation of using such data is the possibility of misclassification as a result of coding errors.40 However, claims data have been evaluated for their utility as a source of epidemiologic or health services information in cancer patients.40 The results of this study are generalizable to the West Virginia Medicare fee-for-service population aged 66 years and older, as data for Medicare beneficiaries enrolled in the managed care plan were not available for this study. Continuous enrollment in Medicare Part A and B was necessary for this study; therefore, beneficiaries with noncontinuous/intermittent enrollment were excluded. Information on care received by beneficiaries outside of the Medicare system, or through non-Medicare providers, was not available for this study.
The research team acknowledges that various clinical guidelines have been published for lung cancer diagnosis and management, each with recommendations that are more or less the same.3–7 For the purpose of this study, appropriate care was defined using the ACCP guideline for lung cancer management and treatment, as it is the most comprehensive of all available guidelines.4 The algorithm adapted from the guideline takes into account the limitations of the data source. Specifically, information on various lung function test results and lung performance scores were not available in the data source, and were not considered in this analysis. Furthermore, the present study estimates of the proportion of patients receiving appropriate care may be biased slightly upward because it included patients who received appropriate care and additional unproven therapies.
Given the limitations of the data source, delays in diagnosis and treatment were defined using Medicare claim date and may not be exact. The estimates of Symptom to Diagnosis delay may be biased as patients for whom the earliest symptom date could not be identified were excluded when calculating the delay. It is less likely that any reported lung cancer symptom was missed because the research team searched for a comprehensive list of symptoms derived from the ACCP guidelines for management and treatment of lung cancer (see online Appendix 1).41 Overall, date of earliest lung cancer symptom was identified in 90% of the patients in this study. The research team recognizes that the earliest symptom identified in this study may have been unrelated to lung cancer. It is for these reasons Symptom to Diagnosis delay was excluded in the analysis of guideline-concordant timely lung cancer care and prognosis. Overall, the team acknowledges that the definition of appropriate care and timely care may be too narrow, and that given the heterogeneity of patients seen by physicians, no treatment or delayed care may still be considered appropriate. Nonetheless, this study's definition of appropriate care and timely care provides a conceptual framework to assess and compare patterns of care that were prevalent during the years 2002–2007. The research team acknowledges the age of data (2002–2007) used in this study. This resulted from limited data availability from data sources at the time of building the WVCR-Linked data set and the procedural delays in creating the data set for this study. Nonetheless, the team has reason to believe that the results observed in this study using 2002–2007 data would have been unchanged if more recent data had been used. This is because during the past decade, lung cancer incidence and morality rates have remained higher in West Virginia compared to the United States. Specifically, during 2002–2007, age-adjusted lung cancer incidence and morality rates (476.5 and 375.9 per 100,000) among the elderly were higher in West Virginia than in the United States (383.0 and 308.0 per 100,000). 24,25 And based on the most recent data available from the Centers for Disease Control and Prevention, in 2008–2011 the age-adjusted lung cancer incidence and morality rates (442.4 and 361.0 per 100,000) were still higher among elderly in West Virginia than in the United States (365.5 and 286.1 per 100,000). 24,25 Therefore, the results of this study still may be considered relevant for the state population. Future studies can overcome the barriers seen in this study by collecting data on physician treatment choice, patient treatment preferences, clinical test information, and individual-level measures of socioeconomic status.
Conclusions
In summary, variations in lung cancer care exist among the elderly in the rural and medically underserved state population. Although guidelines for management and treatment of lung cancer have been published by various organizations, their adoption in clinical practice is found to be limited. The resulting disparities in receipt of guideline-concordant lung cancer care partly explain the disproportionate burden of lung cancer in this population. Underutilization of lung cancer diagnostic and management services among Medicare beneficiaries also is a cause for concern, as these services are covered under the Medicare program. Interventions aimed at reducing the observed disparities in lung cancer care can help to improve health outcomes in the rural and medically underserved elderly population.
Supplementary Material
Author Disclosure Statement
Drs. Nadpara, Madhavan, and Tworek declared no conflicts of interest with respect to the research, authorship, and/or publication of this article.
The authors received the following financial support for the research, authorship, and/or publication of this article: This study was funded by Agency of Healthcare Research and Quality (AHRQ) (Grant # 1R24HS018622-01 [PI: S. Madhavan]). Research reported in this publication also was partially supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number U54GM104942. The content is solely the responsibility of the authors and does not necessarily represent the official views of AHRQ or the National Institutes of Health.
Acknowledgments
We acknowledge Myra Fernatt, BS, Alana Hudson, PhD, and Loretta Haddy, PhD from West Virginia Cancer Registry; Commissioner Nancy Atkins, RN, MSN, and Nora Antlake from West Virginia Bureau of Medical Services for their administrative and material support.
References
- 1.National Cancer Institute. SEER Cancer Statistics Review, 1975–2009. http://seer.cancer.gov/csr/1975_2009_pops09/index.html Accessed February15, 2015
- 2.Alberts WM, Bepler G, Hazelton T, Ruckdeschel JC, Williams JH., Jr. Lung cancer. Practice organization. Chest. 2003;123(1 suppl):332S–337S [DOI] [PubMed] [Google Scholar]
- 3.Pfister DG, Johnson DH, Azzoli CG, et al. American Society of Clinical Oncology treatment of unresectable non-small-cell lung cancer guideline: Update 2003. J Clin Oncol. 2004;22:330–353 [DOI] [PubMed] [Google Scholar]
- 4.American College of Chest Physicians; Health and Science Policy Committee. Diagnosis and management of lung cancer: ACCP evidence-based guidelines. Chest. 2003;123(1 suppl):D–G, 1S–337S [PubMed] [Google Scholar]
- 5.Clinical practice guidelines for the treatment of unresectable non-small-cell lung cancer. Adopted on May 16, 1997 by the American Society of Clinical Oncology. J Clin Oncol. 1997;15:2996–3018 [DOI] [PubMed] [Google Scholar]
- 6.National Comprehensive Cancer Network and American Cancer Society. Lung Cancer: Treatment Guidelines for Patients. Version 6, April 2015. http://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf Accessed May6, 2015
- 7.National Cancer Institute. Non-small cell lung cancer treatment (PDQ®). General information about non-small cell lung cancer (NSCLC). http://www.cancer.gov/cancertopics/pdq/treatment/non-small-cell-lung/healthprofessional Accessed February15, 2015
- 8.BTS recommendations to respiratory physicians for organising the care of patients with lung cancer. Thorax. 1998;53 suppl 1:S1–S8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reifel JL. Lung cancer. In: Asch SM, Kerr EA, Hamilton EG, Reifel JL, McGlynn EA. Quality of Care for Oncologic Condition and HIV: A Review of the Literature and Quality Indicators. Santa Monica, CA: RAND; 2000:133–171 [Google Scholar]
- 10.Potosky AL, Saxman S, Wallace RB, Lynch CF. Population variations in the initial treatment of non-small-cell lung cancer. J Clin Oncol. 2004;22:3261–3268 [DOI] [PubMed] [Google Scholar]
- 11.Earle CC, Venditti LN, Neumann PJ, et al. Who gets chemotherapy for metastatic lung cancer? Chest. 2000;117:1239–1246 [DOI] [PubMed] [Google Scholar]
- 12.Smith TJ, Penberthy L, Desch CE, et al. Differences in initial treatment patterns and outcomes of lung cancer in the elderly. Lung Cancer. 1995;13:235–252 [DOI] [PubMed] [Google Scholar]
- 13.Salloum RG, Smith TJ, Jensen GA, Lafata JE. Factors associated with adherence to chemotherapy guidelines in patients with non-small cell lung cancer. Lung Cancer. 2012;75:255–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Landrum MB, Keating NL, Lamont EB, Bozeman SR, McNeil BJ. Reasons for underuse of recommended therapies for colorectal and lung cancer in the Veterans Health Administration. Cancer. 2012;118:3345–3355 [DOI] [PubMed] [Google Scholar]
- 15.Zornosa C, Vandergrift JL, Kalemkerian GP, et al. First-line systemic therapy practice patterns and concordance with NCCN guidelines for patients diagnosed with metastatic NSCLC treated at NCCN institutions. J Natl Compr Canc Netw. 2012;10:847–856 [DOI] [PubMed] [Google Scholar]
- 16.Lee JY, Moore PC, Steliga MA. Do HIV-infected non-small cell lung cancer patients receive guidance-concordant care? Med Care. 2013;51:1063–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olsson JK, Schultz EM, Gould MK. Timeliness of care in patients with lung cancer: a systematic review. Thorax. 2009;64:749–756 [DOI] [PubMed] [Google Scholar]
- 18.Nadpara PA, Madhavan SS, Tworek C, Sambamoorthi U, Hendryx M, Almubarak M. Guideline-concordant lung cancer care and associated health outcomes among elderly patients in the United States. J Geriatr Oncol. 2015;6:101–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hewitt M, Simone JV, eds. Ensuring the Quality of Cancer Care. Washington, DC: National Academies Press; 1999 [PubMed] [Google Scholar]
- 20.Behringer B. Health care services in Appalachia. In: Couto RA, Simpson NK, Harris G, eds. Sowing Seeds in the Mountains, Community-based Coalitions for Cancer Prevention and Control. NIH Publication No. 94-3779:62–80. Bethesda, MD: National Institutes of Health, National Cancer Institute; 1994 [Google Scholar]
- 21.Monroe AC, Ricketts TC, Savitz LA. Cancer in rural versus urban populations: a review. J Rural Health. 1992;8:212–220 [DOI] [PubMed] [Google Scholar]
- 22.Cancer death rates—Appalachia, 1994–1998. MMWR Morb Mortal Wkly Rep. 2002;51:527–529 [PubMed] [Google Scholar]
- 23.West Virginia Health Care Authority (WVHCA) West Virginia State Health Plan. 2010. Chapter 3: Health Care Delivery and Financing. http://www.hca.wv.gov/policyandplanning/Documents/State%20Health%20Plan/shpC3.pdf Accessed February15, 2015
- 24.Centers for Disease Control and Prevention. United States Cancer Statistics: 1999–2011 Incidence Request. 2014. http://wonder.cdc.gov/cancer-v2011.html Accessed April6, 2015
- 25.Centers for Disease Control and Prevention. United States Cancer Statistics: 1999–2011 Mortality Request. 2014. http://wonder.cdc.gov/CancerMort-v2011.html Accessed April6, 2015
- 26.State-specific prevalence and trends in adult cigarette smoking—United States, 1998–2007. MMWR Morb Mortal Wkly Rep. 2009;58(9):221–226 [PubMed] [Google Scholar]
- 27.Hall HI, Uhler RJ, Coughlin SS, Miller DS. Breast and cervical cancer screening among Appalachian women. Cancer Epidemiol Biomarkers Prev. 2002;11(1):137–142 [PubMed] [Google Scholar]
- 28.Casey MM, Thiede CK, Klingner JM. Are rural residents less likely to obtain recommended preventive healthcare services? Am J Prev Med. 2001;21:182–188 [DOI] [PubMed] [Google Scholar]
- 29.Amey CH, Miller MK, Albrecht SL. The role of race and residence in determining stage at diagnosis of breast cancer. J Rural Health. 1997;13:99–108 [DOI] [PubMed] [Google Scholar]
- 30.Nadpara PA, Madhavan SS. Linking Medicare, Medicaid, and Cancer Registry data to study the burden of cancers in West Virginia. Medicare Medicaid Res Rev. 2012;2(4):E1–E25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383 [DOI] [PubMed] [Google Scholar]
- 32.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619 [DOI] [PubMed] [Google Scholar]
- 33.Romano PS, Roos LL, Jollis JG. Adapting a clinical comorbidity index for use with ICD-9-CM administrative data: differing perspectives. J Clin Epidemiol 1993;46:1075–1079 [DOI] [PubMed] [Google Scholar]
- 34.Lin DY, Wei LJ. The robust inference for the Cox Proportional Hazards Model. J Am Stat Assoc. 1989;84(408):1074–1078 [Google Scholar]
- 35.Annakkaya AN, Arbak P, Balbay O, Bilgin C, Erbas M, Bulut I. Effect of symptom-to-treatment interval on prognosis in lung cancer. Tumori. 2007;93(1):61–67 [DOI] [PubMed] [Google Scholar]
- 36.Comber H, Cronin DP, Deady S, Lorcain PO, Riordan P. Delays in treatment in the cancer services: impact on cancer stage and survival. Ir Med J. 2005;98(8):238–239 [PubMed] [Google Scholar]
- 37.Myrdal G, Lambe M, Hillerdal G, Lamberg K, Agustsson T, Stahle E. Effect of delays on prognosis in patients with non-small cell lung cancer. Thorax. 2004;59(1):45–49 [PMC free article] [PubMed] [Google Scholar]
- 38.Gould MK, Ghaus SJ, Olsson JK, Schultz EM. Timeliness of care in veterans with non-small cell lung cancer. Chest. 2008;133:1167–1173 [DOI] [PubMed] [Google Scholar]
- 39.Yorio JT, Xie Y, Yan J, Gerber DE. Lung cancer diagnostic and treatment intervals in the United States: a health care disparity? J Thorac Oncol. 2009;4:1322–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Potosky AL, Riley GF, Lubitz JD, Mentnech RM, Kessler LG. Potential for cancer related health services research using a linked Medicare-tumor registry database. Med Care. 1993;31:732–748 [PubMed] [Google Scholar]
- 41.Beckles MA, Spiro SG, Colice GL, Rudd RM. Initial evaluation of the patient with lung cancer: symptoms, signs, laboratory tests, and paraneoplastic syndromes. Chest. 2003;123(1 suppl):97S–104S [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.