Abstract
Anti-infectives, including antibiotics, are essentially different from all other drugs; they not only affect the individual to whom they are given but also the entire community, through selection for resistance to their own action. Thus, their use resides at the intersection of personal and public health. Antibiotics can be likened to a four-edged sword against bacteria. The first two edges of the antibiotic sword were identified immediately after their discovery and deployment in that they not only benefit an individual in treating their infection but also benefit the community in preventing the spread of that infectious agent. The third edge was already recognized by Alexander Fleming in 1945 in his Nobel acceptance speech, which warned about the cost to the community of antibiotic resistance that would inevitably evolve and be selected for during clinical practice. We have seen this cost mount up, as resistance curtails or precludes the activities of some of our most effective drugs for clinically important infections. But the fourth edge of the antibiotic sword remained unappreciated until recently, i.e., the cost that an antibiotic exerts on an individual’s own health via the collateral damage of the drug on bacteria that normally live on or in healthy humans: our microbiota. These organisms, their genes, metabolites, and interactions with one another, as well as with their host collectively, represent our microbiome. Our relationship with these symbiotic bacteria is especially important during the early years of life, when the adult microbiome has not yet formed (1).
For 70 years, antibiotics have been a pillar of medicine and are being used worldwide on an enormous scale. In many countries, antibiotic use exceeds one course per capita per year. In 2010, the top seven antibiotic classes were consumed in an estimated 70 billion individual doses, which equates to about 10 pills, capsules, or teaspoons for every man, woman, and child on earth (2), an annual rate that appears to be rising. This magnitude of use is based at least in part on the perception, among both health professionals and the public, that antibiotics are completely safe. We all are aware of mild, self-limited problems, such as rashes and drug reactions, and doctors know about serious but very rare side effects, yet at a functional level, most of us consider these risks so close to zero that they do not usually factor into the equation about use. There also is the cost of antibiotic resistance, but because it predominantly affects the community rather than the treated individual, its avoidance does not usually affect clinical judgments about whether or not treatment should be given. Parents would rather have their ill child treated with a drug they see as safe and effective than worry about the impact of that single course on the future of humankind, and their doctors generally agree.
However, this construct of essentially complete personal safety is illusory. Shortly after antibiotics began to be used to treat ill people and animals, farmers discovered that adding low doses of antibiotics to the food or water of their livestock would promote their growth; the earlier in life the exposure began, the more profound the effect (3). This observation alone provides an important clue that antibiotic exposure affects metabolic development. Antibiotic use has been widespread because it leads to growth promotion and, therefore, increased profit for farmers. But does this massive decades-long worldwide “experiment” on the farm teach us anything about human health? Is it possible that the antibiotics that we give our children early in life to treat their infections—whether severe (uncommon) or mild (very common)—are influencing a critical window in the development of their own metabolism?
In recent years, scientists have been exploring this question, with mostly consistent results. Observational, clinical, and epidemiologic studies focused on young children are providing a growing body of evidence that antibiotic exposure is associated with increased risk for a variety of diseases including obesity, types 1 and 2 diabetes, inflammatory bowel diseases, celiac disease, allergies, and asthma [see (4, 5) for examples]. Experimental models are providing increasing evidence that these associations are not just correlative but are causal. Studies in mice have found that antibiotic exposure, by disrupting the development of the early-life microbiome, which often causes loss of species and strain diversity (i.e., biodiversity loss), leads to metabolic perturbations that affect adiposity and bone growth and alter normal immunologic development (6, 7).
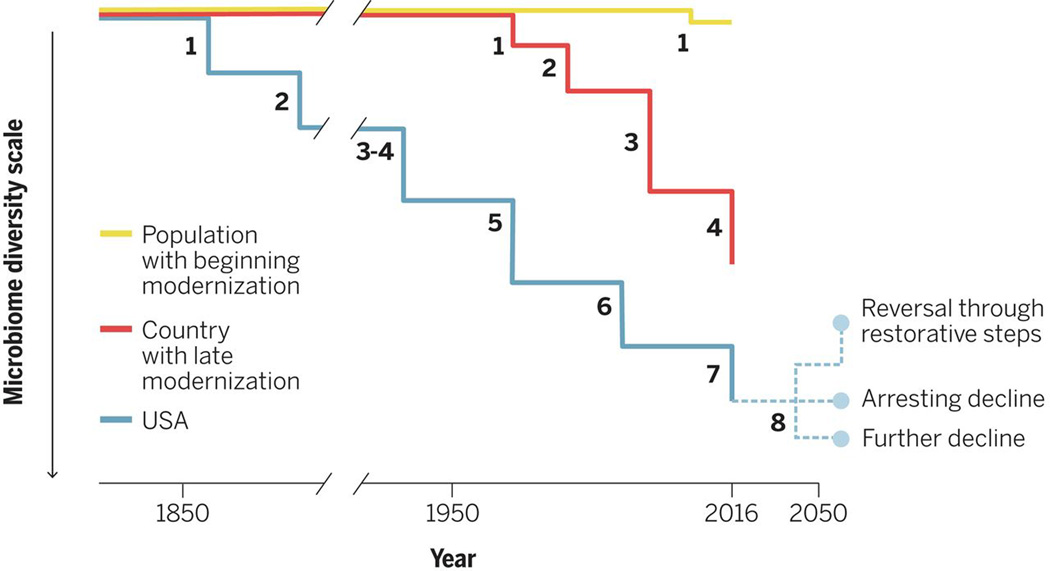
A variety of evidence indicates that the risks appear greatest for young children. Paradoxically, the perinatal period through the first 2 years of life is the time when per capita antibiotic usage is most intensive. An emerging concept to consider is that the effects of antibiotics may be cumulative in an individual, with both epidemiologic and experimental data supporting that view (8). One hypothesis is that antibiotic courses may lead to species loss, especially for taxa that were low in number at that time, yet which may have important metabolic functions. The problem would be most important for taxa with unique functions, although this may not happen in all patients or with all courses. Because we each inherit much of our microbiome from our mother, a further hypothesis is that environmental impacts on the microbiome (including antibiotic and dietary exposures) are cumulative across generations (8, 9) (Fig. 1). We need to carefully assess these hypotheses, because the implications are substantial.
Figure 1. Models of microbiota change in different societies.
The decline in microbiota diversity in the United States happened simultaneously with the early introduction of sanitation, including filtered and chlorinated drinking water, and early antibiotic use. The scale is arbitrary and reflects the aggregate of species and strain losses. The numbers shown represent the generations since the earliest population-wide microbiota species and strain losses and show progressive and cumulative loss of diversity. Each line represents an average; within every generation, there is variation in individual positions, based on their founding populations, exposures, and timing. In a country with late modernization, the diversity loss occurred later, but generation times are shorter, and the steps more irregular and increasing, which reflects the effects of the accelerated pace of modernization in recent years on human microbiota biodiversity loss in developing countries. For the future, three trajectories are shown for the developed country.
Yet antibiotics are vital for health care. It is difficult to imagine optimal health without an umbrella of antibiotics to use when needed. Nevertheless, practitioners have not been taking the biologic cost of antibiotic use into account sufficiently in making treatment decisions. Differences in perceptions about how risk-free antibiotic treatment is may in part account for the enormous variation in rates of their use from practitioner to practitioner, between localities and across countries (10). The emergence of awareness of the biologic costs of a treatment surely requires modulation of its usage. We must clearly understand the real costs, including the differences between particular antimicrobial agents in their effects on the microbiome (11) and, thus, the consequent sequelae on child development (12).
Rather than carpet-bombing germs into submission with broad-spectrum antibiotics, we will need more laser-like approaches to develop drugs against specific pathogens, minimize damage to essential symbiotic microbial species, and preserve community structure and function in the healthy (and developing) microbiome. Future research, based on our extensive and growing knowledge of bacterial genes and genomes, should aim to develop truly narrow-spectrum agents, each ideally targeting a single pathogen. This strategy also requires new diagnostic tests that accurately and economically differentiate between bacterial and viral infections, among specific bacteria, and also distinguish between colonization and infection. Host-specific, indeed individual, differences may require more personalized approaches to antibacterial therapies. Shortening treatment courses is another approach that needs more research, as do the complex trade-offs that arise between emergence of resistance and collateral damage.
There always will be instances in which children must be treated with an antibiotic, but the collateral effects could be mitigated. Should we bank every healthy child’s fecal specimen, so we can chase each antibiotic course with a dose of their own pretreatment microbiota? Or by studying microbiota before and after antibiotic treatment, can we identify a few key organisms to replace in that child and then prescribe well-characterized pharmaceutical-grade standard strains, the probiotics of the future, to be administered in the appropriate vehicle?
We also are learning that other drugs are affecting the microbiome in ways that were not anticipated. For example, metformin, widely used in the treatment of type 2 diabetes, has important effects on microbial populations. It may be that metformin’s antimicrobial actions, which in turn affect short-chain fatty acid metabolism, determine the efficacy of the drug, rather than its direct effects on tissues (13). However, no drugs are as important as antibiotics because of their usage in virtually all children worldwide and because their activities, specifically targeted to bacteria, strongly select and shape microbial community structure. Even later in life, antibiotic exposures may have consequences in terms of risks for metabolic (14) and neoplastic diseases and for acquisition of resistant organisms.
The third and fourth edges of the antibiotic sword—cost to the community and to a person’s future health—are both being driven by antibiotic overuse. First, we must control those excesses, but mitigation will only stabilize the situation not reverse the deterioration that likely has progressively occurred with socioeconomic development (15). Ultimately, we may need to recover the biodiversity lost as a result of these generations of antibiotic use (and other insults). The goal should be to restore the status quo ante, using probiotics, perhaps with accompanying prebiotics, to replace vital missing and/or extinct species and strains that modulate crucial developmental pathways. This critically important next scientific frontier in human health will require much research.
Acknowledgments
Supported in part by R01DK090989, and U01AI122285 from NIH, the Diane Belfer Program in Human Microbial Ecology, and the Knapp Family Fund.
References and Notes
- 1.Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, Magris M, Hidalgo G, Baldassano RN, Anokhin AP, Heath AC, Warner B, Reeder J, Kuczynski J, Caporaso JG, Lozupone CA, Lauber C, Clemente JC, Knights D, Knight R, Gordon JI. Human gut microbiome viewed across age and geography. Nature. 2012;486:222–227. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Boeckel TP, Gandra S, Ashok A, Caudron Q, Grenfell BT, Levin SA, Laxminarayan R. Global antibiotic consumption 2000 to 2010: An analysis of national pharmaceutical sales data. Lancet Infect. Dis. 2014;14:742–750. doi: 10.1016/S1473-3099(14)70780-7. [DOI] [PubMed] [Google Scholar]
- 3.Zimmerman DR. Role of subtherapeutic levels of antimicrobials in pig production. J. Anim. Sci. 1986;62(Suppl 3):6–16. [Google Scholar]
- 4.Arrieta MC, Stiemsma LT, Dimitriu PA, Thorson L, Russell S, Yurist-Doutsch S, Kuzeljevic B, Gold MJ, Britton HM, Lefebvre DL, Subbarao P, Mandhane P, Becker A, McNagny KM, Sears MR, Kollmann T, Mohn WW, Turvey SE, Finlay BB. CHILD Study Investigators, Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci. Transl. Med. 2015;7:307ra152. doi: 10.1126/scitranslmed.aab2271. [DOI] [PubMed] [Google Scholar]
- 5.Azad MB, Bridgman SL, Becker AB, Kozyrskyj AL. Infant antibiotic exposure and the development of childhood overweight and central adiposity. Int. J. Obes. (Lond.) 2014;38:1290–1298. doi: 10.1038/ijo.2014.119. [DOI] [PubMed] [Google Scholar]
- 6.Cho I, Yamanishi S, Cox L, Methé BA, Zavadil J, Li K, Gao Z, Mahana D, Raju K, Teitler I, Li H, Alekseyenko AV, Blaser MJ. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature. 2012;488:621–626. doi: 10.1038/nature11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cox LM, Yamanishi S, Sohn J, Alekseyenko AV, Leung JM, Cho I, Kim SG, Li H, Gao Z, Mahana D, Zárate Rodriguez JG, Rogers AB, Robine N, Loke P, Blaser MJ. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell. 2014;158:705–721. doi: 10.1016/j.cell.2014.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blaser MJ, Falkow S. What are the consequences of the disappearing human microbiota? Nat. Rev. Microbiol. 2009;7:887–894. doi: 10.1038/nrmicro2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sonnenburg ED, Smits SA, Tikhonov M, Higginbottom SK, Wingreen NS, Sonnenburg JL. Diet-induced extinctions in the gut microbiota compound over generations. Nature. 2016;529:212–215. doi: 10.1038/nature16504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hicks LA, Taylor TH, Jr, Hunkler RJUS. U.S. outpatient antibiotic prescribing 2010. N. Engl. J. Med. 2013;368:1461–1462. doi: 10.1056/NEJMc1212055. [DOI] [PubMed] [Google Scholar]
- 11.Nobel YR, Cox LM, Kirigin FF, Bokulich NA, Yamanishi S, Teitler I, Chung J, Sohn J, Barber CM, Goldfarb DS, Raju K, Abubucker S, Zhou Y, Ruiz VE, Li H, Mitreva M, Alekseyenko AV, Weinstock GM, Sodergren E, Blaser MJ. Metabolic and metagenomic outcomes from early-life pulsed antibiotic treatment. Nat. Commun. 2015;6:7486. doi: 10.1038/ncomms8486. 10.1038/ncomms8486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Korpela K, Salonen A, Virta LJ, Kekkonen RA, Forslund K, Bork P, de Vos WM. Intestinal microbiome is related to lifetime antibiotic use in Finnish pre-school children. Nat. Commun. 2016;7:10410. doi: 10.1038/ncomms10410. 10.1038/ncomms10410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forslund K, Hildebrand F, Nielsen T, Falony G, Le Chatelier E, Sunagawa S, Prifti E, Vieira-Silva S, Gudmundsdottir V, Krogh Pedersen H, Arumugam M, Kristiansen K, Voigt AY, Vestergaard H, Hercog R, Costea PI, Kultima JR, Li J, Jørgensen T, Levenez F, Dore J, Nielsen HB, Brunak S, Raes J, Hansen T, Wang J, Ehrlich SD, Bork P, Pedersen O. MetaHIT consortium, Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature. 2015;528:262–266. doi: 10.1038/nature15766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mikkelsen KH, Knop FK, Frost M, Hallas J, Pottegård A. Use of antibiotics and risk of type 2 diabetes: A population-based case-control study. J. Clin. Endocrinol. Metab. 2015;100:3633–3640. doi: 10.1210/jc.2015-2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clemente JC, Pehrsson EC, Blaser MJ, Sandhu K, Gao Z, Wang B, Magris M, Hidalgo G, Contreras M, Noya-Alarcón Ó, Lander O, McDonald J, Cox M, Walter J, Oh PL, Ruiz JF, Rodriguez S, Shen N, Song SJ, Metcalf J, Knight R, Dantas G, Dominguez-Bello MG. The microbiome of uncontacted Amerindians. Sci. Adv. 2015;1:e1500183. doi: 10.1126/sciadv.1500183. [DOI] [PMC free article] [PubMed] [Google Scholar]